Abstract
During malignant invasion tumor cells establish contact with extracellular matrix proteins, including fibrillar collagen. In addition to providing a physical barrier against invasion, fibrillar collagen also restricts cell proliferation. It has been assumed that the growth regulatory activity of fibrillar collagen is the result of an indirect restrictive effect on cell spreading and cytoskeletal organization. Here we provide evidence for a direct inhibitory effect of fibrillar collagen on proliferation of human melanoma and fibrosarcoma cells that involves activation of the tyrosine kinase discoidin domain receptor 2 and is independent of effects on cell spreading. Cells plated in the presence of fibrillar collagen were growth arrested in the G0/G1 phase of the cell cycle. However treatment with the tyrosine kinase inhibitor genistein, down-regulation of discoidin domain receptor 2, or collagen deglycosylation that prevents discoidin domain receptor 2 activation allowed cells to enter the cell cycle in the presence of fibrillar collagen without a requirement for spreading and actin organization. Our data provide evidence for a novel direct mechanism by which cell contact with fibrillar collagen restricts proliferation.
The extracellular matrix (ECM)2 is a complex mixture of structural and functional proteins that provides cell scaffolding and is critical in regulating cell survival, differentiation, migration, and proliferation (1). The ECM can regulate cell proliferation via two major mechanisms. The first mechanism is providing a reservoir of ECM-bound growth factors, therefore controlling their bioavailability and their interaction with their receptors. The second and growth factor-independent mechanism is interacting with integrins that through adhesion and spreading generate growth stimulatory signals (2, 3). Upon contact with specific domains on ECM proteins, integrins are clustered and activated to induce cytoskeletal reorganization, formation of focal adhesions, and activation of the focal adhesion kinase p125 (p125FAK). As a result, signaling pathways such as the extracellular regulated kinase and the phosphatidylinositol 3-kinase pathways become active, inducing changes in expression of cell cycle regulatory proteins (4, 5). Most ECM proteins, such as fibronectin, laminin, vitronectin, and the proteoglycan hyaluronic acid, stimulate cell proliferation by their ability to promote cell spreading and induce formation of focal adhesions and activation of p125FAK (6–10). However, a remarkable exception to this “rule” is type I collagen, which can have either a stimulatory or an inhibitory effect on cell proliferation, dependent on its native structure. When present in a monomeric fibrillar or denatured form (gelatin), type I collagen acts as a growth stimulatory protein. In contrast, when present in an organized fibrillar form, type I collagen inhibits the proliferation of a variety of normal and malignant cells, including vascular and bladder smooth muscle cells (11, 12), endothelial cells (13), and melanoma cells (14). Furthermore, alteration of the structure of type I collagen by oxidation (15) or proteolytic degradation (16) changes its activity from growth restrictive to growth promoting.
The mechanism by which fibrillar collagen (FC) inhibits cell proliferation is, however, poorly understood. Because cells cultured in the presence of FC typically fail to spread, it has been generally assumed that the growth inhibitory activity of FC is the result of an indirect inhibitory effect on cell spreading, cytoskeletal organization, and formation of focal adhesions (17). Here we have explored whether FC could also inhibit the proliferation of tumor cells by a direct mechanism that involves a receptor-mediated signal, independent of cell spreading and cytoskeletal organization. Our experiments identify the discoidin domain receptor 2 (DDR2), a cell surface-associated tyrosine kinase receptor that binds to FC, as providing a new mechanism responsible for the G0/G1 cell cycle arrest observed when tumor cells are in contact with FC.
MATERIALS AND METHODS
Antibodies
The primary polyclonal antibody for DDR1 (C20) and the monoclonal antibody for FAK (A17, C20) were purchased from Santa Cruz Biotechnology. The monoclonal anti-β-tubulin (Clone Tub 2.1) was from Sigma, the polyclonal anti-FAKpY397 antibody was from BIOSOURCE International, and the Texas Red-X phalloidin was from Molecular Probes. The primary polyclonal DDR2 (R2-JM) antibody was provided by Regeneron Pharmaceuticals, Inc.
Cell Lines
Human melanoma cell lines M24met and A2058 and the human fibrosarcoma cell line HT1080 were from the American Tissue Culture Collection and were maintained under the recommended conditions. M24met and A2058 cells were cultured with RPMI 1640 (Cellgro) plus 10% fetal bovine serum and 2 mM L-glutamine, whereas HT1080 cells were cultured with Dulbecco’s modified Eagle’s medium (Cellgro) plus 10% fetal bovine serum and 2 mM L-glutamine.
Preparation of Collagen
To prepare monomeric-fibrillar collagen (MFC), 0.01 M HCl was used to dilute a collagen stock (Angiotech Bio-materials) to a final concentration of 1 mg/ml. FC was prepared by neutralizing the acidic collagen with 1 M NaOH according to the manufacturer’s procedure to a final concentration of 1.5 mg/ml. Heat-denatured collagen (HDC) was prepared by heat treating FC for 30 min at 60 °C before plating. Collagen was incubated at 37 °C for a minimum of 5 h and rinsed with phosphate-buffered saline (PBS; 137 mM NaCl, 2.6 mM KCl, 10 mM Na2HPO4, and 1.7 mM KH2PO4, pH 7.4) before cell addition. Collagenase-resistant (r/r) collagen was a gift from Dr. Lisa Coussens (University of California San Francisco, San Francisco, CA). For deglycosylation, collagen was incubated with freshly prepared 10 mM sodium m-periodate for 20 min in the dark at room temperature. Excess periodate was removed by adding 20 mM sodium bisulphate. The collagen was dialyzed overnight at 4 °C against 10 mM acetic acid.
Cell Collection, Protein Isolation, and Fluorescence-activated Cell Sorting (FACS) Analysis
Subconfluent cells were transferred onto the specific matrix and collected after the desired time. Cells on MFC or HDC were collected by trypsin/EDTA dissociation, whereas cells on FC were collected using collagenase A (2.5 mg/ml) (Roche Applied Science). The cell number was determined with a hemacytometer. Following collection cells were rinsed with PBS, pelleted, and lysed with modified radioimmunoprecipitation buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 10 mM sodium pyrophosphate containing 1.5 mM MgCl2, 1 mM EGTA, 1% sodium deoxycholate, 0.25 mM Na3VO4, 100 mM NaF, and 10 mg/ml each of leupeptin and aprotinin and 1 mM phenylmethylsulfonyl fluoride). Samples for DDR2 Western blotting were isolated by direct cell lysis using modified radio-immunoprecipitation buffer. Lysis entailed vortexing for three sets of 10 s with a 10-min incubation on ice between each, followed by centrifugation (14000 rpm) and transferring supernatant to a fresh tube. Protein concentration was determined using the BCA protein assay kit (Pierce), following the manufacturer’s instructions. Cells for FACS analysis were collected, rinsed with PBS, and fixed in 70% ethanol (in PBS) overnight at 4 °C. Following fixation nuclei were incubated for 30 min at 37 °C with 20 μg/ml RNase A in PBS, rinsed with PBS, and then resuspended to 3 × 106 cells/ml in 40 μg/ml propidium iodide in PBS. The nuclei were then passed through a cell strainer and analyzed on an EPICS Elite ESP cell sorter (FACS) (Beckman Coulter, Inc.) using Expo 32 (version 1.2) software. The results are expressed as percentages of total cells excluding apoptotic/necrotic cells, which contributed to an average of 6.5 ± 0.8% of the total cells. Exceptions to this were Lipofectamine 2000-treated cells, with or without siRNA, which had an average apoptotic/necrotic rate of 14.2 ± 1.3%.
Immunocytochemistry
The cells were washed once with PBS, fixed for 10 min at room temperature with 4% paraformaldehyde in PBS, and then washed again with PBS. The cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min and blocked with 5% bovine serum albumin in PBS for 10 min and the primary antibody (anti-FAKpY397) at a 1:200 dilution was added for 1 h at 37 °C. After washing twice with 0.1% Triton X-100 in PBS, a fluorescein isothiocyanate-conjugated anti-rabbit IgG was added (dilution 1:500) for 45 min. For actin staining, Texas Red-X phalloidin (1:80 dilution in PBS) was added to the cells for 1 h at 37 °C and then rinsed twice with PBS. 4′,6′-Diamidino-2-phenylindole (5 μg/ml) was added as a nuclear counter stain for 3 min at room temperature. The cells were then rinsed with PBS, mounted using Vectashield (Vector), and viewed with a Zeiss Axioplan microscope, an OptiQuip 100 W Hg arc lamp, and a triple pass filter set. The images were captured with a SPOT Insight QE color digital camera controlled by SPOT 3.5.6 software (Diagnostic Instruments).
Immunoprecipitation and Western Blotting
Immunoprecipitation for FAK was done according to the method of Sieg et al. (18, 19). Briefly the cells were lysed in modified radioimmunoprecipitation buffer. The lysates were precleared in the presence of 20 μl of Sepharose and incubated in the presence of two monoclonal anti-FAK antibodies (4 μg/ml) overnight at 4 °C. For DDR2 immunoprecipitation, 250 μg of cell lysate was incubated with 1 μl R2-JM anti-DDR2 antibody for 1 h at 4 °C. As control an equal amount of lysate was incubated with normal IgG of the species in which the primary antibody was raised. All of the controls were blank (data not shown). To compensate for a higher amount of DDR2 in cells cultured on FC, a larger amount of MFC cell lysate was immunoprecipitated for Tyr(P) detection. The immunocomplexes were collected with Gammabind Plus Sepharose (Amersham Biosciences) for FAK, and protein A-Sepharose 4B Fast Flow for DDR2 at 4 °C for 1 h, washed with lysis buffer, and boiled prior to analysis by SDS-PAGE. For immunoblotting, proteins processed by SDS-PAGE, were transferred to nitrocellulose membrane. The membrane was blocked overnight at 4 °C with 5% milk powder in washing solution (25 mM Tris, 137 mM NaCl, 2.7 mM KCl, and 0.1% Tween 20). DDR1 and DDR2 blots were blocked for 1 h at room temperature, and the primary antibody was incubated at 4 °C overnight. All other primary antibodies were incubated for 2 h at room temperature. The monoclonal β-tubulin antibody was used at a 1:1000 dilution, and all other primary antibodies were used at 1 μg/ml. The blots were developed using a horseradish peroxidase-coupled secondary antibody at a 1:10,000 dilution for 1 h at room temperature and Enhanced Chemiluminescence (Amersham Biosciences). The blots were quantified using Labworks Imaging and Analysis Software (UVP).
Small Interfering RNA
The following siRNA sequences were used: DDR1, 5′-AATGTGCGTAAGGGACACCCT-3′ (NM_013993, base pairs 2263–2283); and DDR2, 5′-AACCTGATGACCTGAAGGAGT-3′ (NM_006182, base pairs 621–641). The cells were cultured in T150 culture flasks (Corning) in RPMI 1640, 10% fetal calf serum, without antibiotics until 80% confluence was reached. The cells were rinsed twice with PBS, and siRNA was added for 5 h at 37 °C, 5% CO2, then replaced with fresh RPMI 1640 containing 10% fetal calf serum, and incubated for a further 72 h before being used in specific experiments. For siRNA transfection we prepared the following: tube 1, 61 μl of siRNA (20 μM stock; Qiagen) + 2.2 ml of RPMI 1640; and tube 2, 90 μl of Lipofectamine 2000 (Invitrogen) + 2.16 ml of RPMI 1640. The tubes were incubated for 5 min at room temperature, then combined, and incubated for a further 20 min. Next, 13.5 ml of RPMI 1640 was added, and the siRNA mix was added to the cells. Final siRNA concentration was 68 nM. Transfection efficiency was assessed using fluorescein isothiocyanate-labeled siRNA and viewing cells after the 5-h siRNA incubation with a Leica MZ FL III fluorescence stereomicroscope and 75 W xenon arc lamp and triple pass filter set. Transfection efficiency was 90 ± 2.6%.
Gelatin Zymography
The cell conditioned media were assessed for matrix metalloproteinase-2 (MMP-2) by gelatin zymography, according to the method of Heussen and Dowdle (20), with loading normalized for cell number and the nonreduced sample being loaded onto a 7% SDS-PAGE containing 0.5 mg/ml gelatin.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
RNA was isolated from cell pellets using TRIzol (Invitrogen), following the manufacturer’s instructions. RNA concentration and purity was determined by the A260/280 ratio, and the integrity was checked by gel electrophoresis. Per 20-μl reaction 500 ng of total RNA was used: 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 500 μM each dNTP, 10 mM dithiothreitol, 50 units of RNase inhibitor, 500 ng of N9-random oligonucleotide, and 200 units of Moloney murine leukemia virus reverse transcriptase (Invitrogen). The reaction was performed according to the manufacturer’s instructions. 2.5 μl of the reverse transcriptase reaction mix was used per 25-μl PCR: 20 M Tris-HCl (pH 8.4), 50 mM KCl, 200 μM each dNTP, 1.5 mM MgCl2, 1.25 units of Taq polymerase, and 500 pM forward and reverse primers (Invitrogen). PCR cycle conditions for DDR2 primers were 1 cycle at 94 °C for 1 min, 35 cycles of 95 °C for 30 s, 51 °C for 30 s, 72 °C for 30 s, and then 1 cycle at 72 °C for 10 min. Glyceraldehyde-3-phosphate dehydrogenase reaction conditions were the same except the annealing temperature was 55 °C. PCR products were analyzed by gel electrophoresis. DDR2 mRNA levels were normalized for glyceraldehyde-3-phosphate dehydrogenase levels. The primer sequences are available upon request.
Statistical Analysis
The data were considered to have a parametric distribution. Therefore, a Student’s t test (two-tail) assuming equal variance was used, with p < 0.05 being considered significant.
RESULTS
FC Induces a G0/G1 Growth Arrest in Human Melanoma Cells Independently of Cell Shape or Actin Organization
We had previously observed that when M24met human melanoma cells are cultured on FC the percentage of cells in the G0/G1 phase of the cell cycle increases after 24 h (14). To determine whether this increase was due to a delay in progression or to a true arrest at the G1/S checkpoint, we synchronized M24met cells by serum starvation (24 h) before we cultured them on FC or MFC in the presence of serum and analyzed their cell cycle profile over time by propidium iodide staining and FACS. In the presence of MFC, cells entered the cell cycle as indicated by a progressive decrease in the percentage of cells in G0/G1 phase (from 82 to 65% at 24 h) and a corresponding increase in the percentage of cells in S phase (from 10.8 to 24.4%). In contrast, in the presence of FC, the percentage of cells in G0/G1 increased (from 82 to 88% at 24 h), and no increase in cells in S phase was observed (Fig. 1A). That FC inhibited rather than delayed cell growth was verified by performing cell counts over 72 h. Consistent with a growth arrest, the number of M24met cells plated on FC did not increase over 72 h, whereas the cells plated on MFC increased in number by 4-fold (Fig. 1B). In addition to undergoing cell cycle arrest, M24met cells underwent a change in morphology when cultured on FC. Whereas cells plated on MFC spread and developed an organized cytoskeleton with bundles of actin filaments, cells plated on FC were rounded, developed long cytoplasmic “dendrite-like” processes, failed to spread, and showed no evidence of cytoskeletal organization (Fig. 1C). These changes did not involve differences in adhesion (data not shown) and were observed in the presence of rigid as well as relaxed (floating) FC gels (data not shown). Consistent with the concept that cell spreading promotes proliferation through the formation of active focal adhesions, cells plated on MFC expressed the phosphorylated form of p125FAK, whereas there was no evidence for p125FAK activation in M24met cells plated on FC (Fig. 1, D and E). We then used HDC as a source of MFC, which was then equally mixed with FC to determine whether MFC could circumvent the growth inhibitory effect of FC. This experiment indicated a similar level of arrest at the G1/S checkpoint when M24met cells were plated on an equal mixture of HDC and FC compared with FC. The data suggest that FC may have a dominant effect on cell proliferation (Fig. 1F). However, because cells failed to spread in both conditions (FC and a mixture of FC and HDC), we could not determine from these experiments whether FC acted independently of its restrictive effect on cell spreading.
FIGURE 1. FC induces a G0/G1 growth arrest in human melanoma cells independently of cell shape or actin organization.

A, M24met cells were serum-starved for 24 h and then cultured in the presence of 10% serum on either MFC or FC and collected every 4 h. The cells were then processed for FACS analysis to determine cell cycle distribution. The results are expressed as the means ± S.E. percentage of cells in G0/G1 or S phase versus time (n = 3). B, M24met cells were cultured on MFC or FC and collected every 24 h, and the cell number was determined using a hemocytometer (n = 3). C, actin staining of M24met cells cultured for 24 h on MFC or FC. D and E, M24met cells were cultured for 24 h on MFC or FC and assessed for FAK pY397 expression by immunoprecipitation (D) and immunocytochemistry (E). F, M24met cells were cultured for 24 h on MFC, HDC, HDC + FC mix, or FC. The cells were collected, and cell cycle distribution was determined by FACS analysis. The results are expressed as the means ± S.E. percentage of cells in G0/G1 (n = 3). G, M24met cells were cultured for 24 h on MFC or FC, with or without latrunculin B (1 μM), and collected, and cell cycle distribution was determined by FACS analysis. The results are expressed as the means ± S.E. percentage of cells in G0/G1 (n = 3). The cells were also examined for actin cytoskeleton organization. IP, immunoprecipitation; WB, Western blot.
To determine whether the lack of actin filament organization and cell spreading was responsible for FC-induced arrest in G0/G1, we treated cells with latrunculin B (1 μM), a naturally occurring toxin that inhibits actin polymerization and disrupts microfilament organization (21). As anticipated, treatment of M24met cells on MFC with latrunculin B inhibited spreading and actin polymerization, but it did not significantly affect the percentage of cells in the G0/G1 phase of the cell cycle (Fig. 1G). However, it did result in a time-dependent accumulation of cells at the G2/M phase (data not shown). Latrunculin B did not alter cell cycle distribution of cells plated on FC (Fig. 1G). Thus, whereas latrunculin B and FC both inhibited cytoskeletal organization and cell cycle progression, the phase of the cell cycle where the arrest occurred was strikingly different. Contact between FC and M24met cells arrested cells at the G1/S checkpoint, whereas latrunculin B arrested cells at the G2/M phase. These data suggest that the blocking effect of FC at the G1/S checkpoint could be independent from its inhibitory effect on cytoskeletal organization and that there could be a more direct, surface receptor-mediated mechanism involved.
Involvement of the Discoidin Domain Receptors in FC-mediated Cell Cycle Arrest
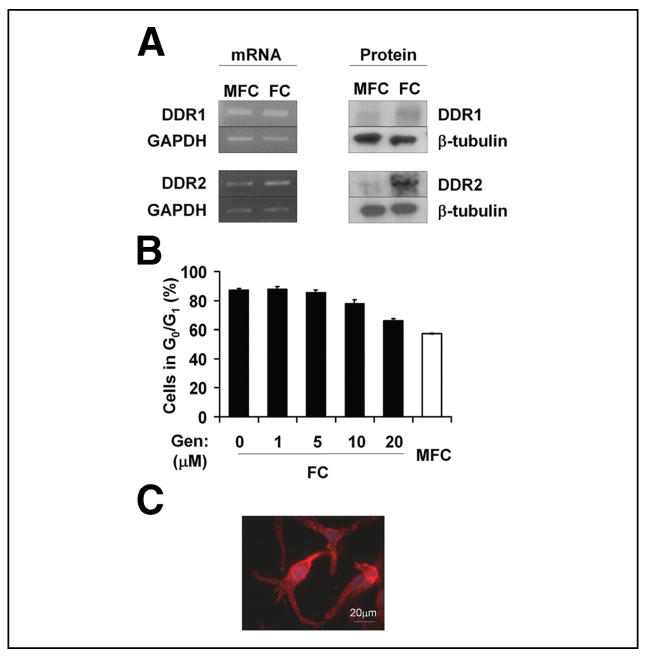
Known receptors for type I collagen include integrins α1β1, α2β1, α10β1, and α11β1 and the DDR family of tyrosine kinases (22, 23). We had previously demonstrated that clustering and activation of β1 integrins stimulated cell proliferation and that blocking β1 binding to FC or MFC, inhibited proliferation (14). These observations eliminated the possibility that β1 integrins were responsible for the cell cycle arrest induced by FC. The DDR family of collagen receptors is comprised of two tyrosine kinase receptors, DDR1 and DDR2, which are both activated by contact with FC. In contrast to DDR2, DDR1 can also be activated by contact with non-fibril-forming collagens (e.g. collagen IV) (24). To determine a possible involvement of the DDRs, we initially examined their mRNA and protein expression on MFC and FC. The data indicated that both receptors were expressed at the mRNA and protein levels in M24met cells and that their expression was significantly elevated in M24met cells cultured on FC compared with MFC for 24 h (2 ± 0.25-fold, p = 0.009 for DDR1; and 3.6 ± 0.4-fold, p = 0.003 for DDR2) (Fig. 2A). To further investigate the possible involvement of the DDRs, we used a broad tyrosine kinase inhibitor, genistein, to determine whether this would block FC-induced cell cycle arrest. Genistein did block FC-induced cell cycle arrest in a dose-dependent manner, with the percentage of cells in G0/G1 on FC at 20 μM genistein being similar to that of MFC (66.1 ± 1.3 and 57.4 ± 0.4%, respectively) (Fig. 2B). Interestingly, although genistein treatment allowed cell cycle progression, it had no effect on cell spreading or actin reorganization, providing more definitive evidence that the G0/G1 growth arrest caused by contact with FC is independent of inhibition of spreading and cytoskeletal organization (Fig. 2C). These data further support the concept that FC-induced cell cycle arrest involves a direct receptor-mediated mechanism.
FIGURE 2. Involvement of the discoidin domain receptors in FC-mediated cell cycle arrest.
A, expression of DDR1 and DDR2 mRNA and protein in M24met cells after 24 h cultured on MFC and FC (representative blots shown, n = 3). B, M24met cells were incubated for 24 h on MFC or FC in the presence of the indicated concentrations of genistein and analyzed for cell cycle distribution by FACS. The results are expressed as the means ± S.E. percentage of cells in G0/G1 (n = 3). C, actin staining of M24met cells cultured for 24 h on FC in the presence of genistein (20 μM). GAPDH, glyceraldehyde-3-phosphate dehydrogenase
FC-induced Cell Cycle Arrest Is Mediated by the Receptor DDR2
Because genistein is a broad spectrum tyrosine kinase inhibitor, this experiment did not conclusively prove that the DDRs were responsible for the FC-induced cell cycle arrest, nor did it determine whether only one or both of the DDRs were required. To investigate this we used siRNA to specifically down-regulate either DDR1 or DDR2. Transfection of M24met cells with a DDR1 siRNA inhibited DDR1 protein expression by 72%, whereas a scrambled sequence had no effect (Fig. 3A). However, down-regulation of DDR1 expression had no effect on cell cycle progression and cytoskeletal organization (Fig. 3B and data not shown), thus eliminating DDR1 as a collagen receptor mediating the FC-induced cell cycle arrest.
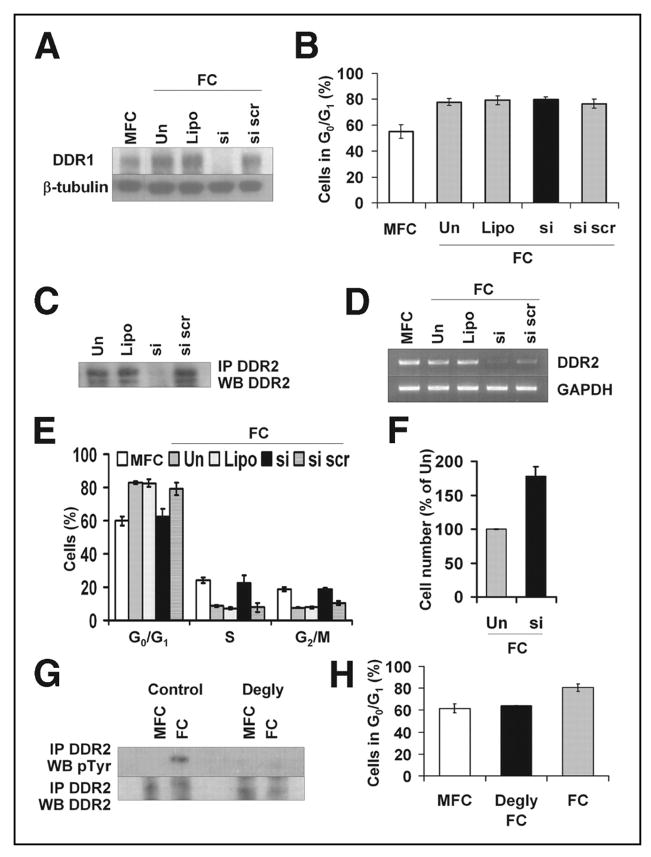
FIGURE 3. FC-induced cell cycle arrest is mediated by DDR2.
A, DDR1 protein expression by Western blotting of M24met cells after treatment with DDR1 siRNA and 24 h on FC (representative blots shown, n = 3). B, M24met cells treated with DDR1 siRNA were incubated for 24 h on FC and examined for cell cycle distribution by FACS. The results are expressed as the means ± S.E. percentage of cells in G0/G1 (n = 3). C, DDR2 protein level of M24met cells after treatment with DDR2 siRNA and indicated controls (representative Western blot shown, n = 4). D and E, M24met cells treated with DDR2 siRNA were incubated for 24 h on FC and examined for DDR2 mRNA expression (D, representative gels shown, n = 4) and cell cycle distribution by FACS (E). The FACS results are expressed as the means ± S.E. percentage of cells in G0/G1 (n = 4). F, M24met cells treated with DDR2 siRNA were cultured on MFC or FC for 24 h, and the cell number was determined using a hemocytometer (n = 3). G, M24met cells were cultured on normal or deglycosylated MFC and FC for 24 h, and DDR2 activation was assessed by DDR2 immunoprecipitation followed by phosphotyrosine Western blot (representative Western blot shown, n = 3). Please note that because of elevated total DDR2 in FC samples (Fig. 2A) more MFC lysate was used for immunoprecipitation to enable comparison of approximate equal amounts of total DDR2 when assessing activation. H, M24met cells were cultured for 24 h on MFC and FC (deglycosylated and normal) and examined for cell cycle distribution by FACS. The results are expressed as the means ± S.E. percentage of cells in G0/G1 (n = 3). Un, untreated; Lipo, Lipofectamine; si, DDR2 siRNA; si scr, scrambled siRNA; IP, immunoprecipitation; WB, Western blot.
We then transfected M24met cells with a DDR2 siRNA, which reduced DDR2 protein levels to 31 ± 6% (p = 0.0004) of control levels (Fig. 3C). Because the process of cell extraction for cell cycle analysis resulted in DDR2 protein degradation (data not shown), we verified the effect of DDR2 siRNA on DDR2 expression by reverse transcription-PCR, which revealed an inhibition of 78.5 ± 9.6%, whereas a scrambled siRNA had little effect (Fig. 3D). In contrast to DDR1, down-regulation of DDR2 in M24met cells in contact with FC allowed cells to re-enter the cell cycle, as indicated by the significant decrease in the percentage of cells in G0/G1 (62.7 ± 4.2% versus 82.9 ± 1% with scramble siRNA, p = 0.003) and the corresponding increase in the percentage of cells in S and G2/M (Fig. 3E). We also observed that when plated on FC DDR2 siRNA-treated cells proliferated more than control cells because their number after 24 h in culture was 1.78 ± 0.14-fold higher than controls (p = 0.005; n = 3) (Fig. 3F). This is comparable with the 1.7 ± 0.07-fold increase in cell number observed when parent cells were plated on MFC compared with FC at 24 h (Fig. 1B). Consistent with the concept that FC exerts a direct effect on cell proliferation that is independent of spreading and cytoskeletal organization, we found that down-regulation of DDR2 expression did not alter cell morphology and was not associated with actin polymerization (data not shown). To further confirm the involvement of DDR2 in FC-induced cell cycle arrest, we investigated DDR2 phosphorylation and confirmed increased DDR2 tyrosine phosphorylation in M24met cells plated on FC compared with MFC in approximately equal amounts of DDR2 recovered by immunoprecipitation (Fig. 3G). Vogel et al. (25) demonstrated that DDR2 activation requires glycosylated collagen. To obtain additional evidence in support of the role of DDR2, we cultured M24met cells on deglycosylated FC for 24 h and analyzed DDR2 activation and cell cycle distribution. We observed an absence of DDR2 activation in cells in contact with deglycosylated FC (Fig. 3G). Consistent with the requirement for DDR2 activation to arrest cells in G0/G1 on FC, M24met cells cultured on deglycosylated FC re-entered the cell cycle and had a profile similar to MFC (Fig. 3H). These data further confirm the involvement of DDR2 in FC-induced G0/G1 cell cycle arrest.
Because we had previously shown that FC induced growth arrest was similar whether M24met cells were plated on FC (two-dimensional) or were embedded in a three-dimensional FC (14), we also examined whether down-regulation of DDR2 expression by siRNA would enable M24met cells to re-enter the cell cycle when cultured in a three-dimensional FC. The data revealed that DDR2 siRNA-treated cells embedded within a three-dimensional FC re-entered the cell cycle in a similar fashion as when they were cultured on a two-dimensional FC (data not shown). As observed in two-dimensional FC, these changes in proliferation were not associated with changes in morphology (data not shown).
DDR2 Mediates FC-induced Cell Cycle Arrest in Other Cancer Cell Lines
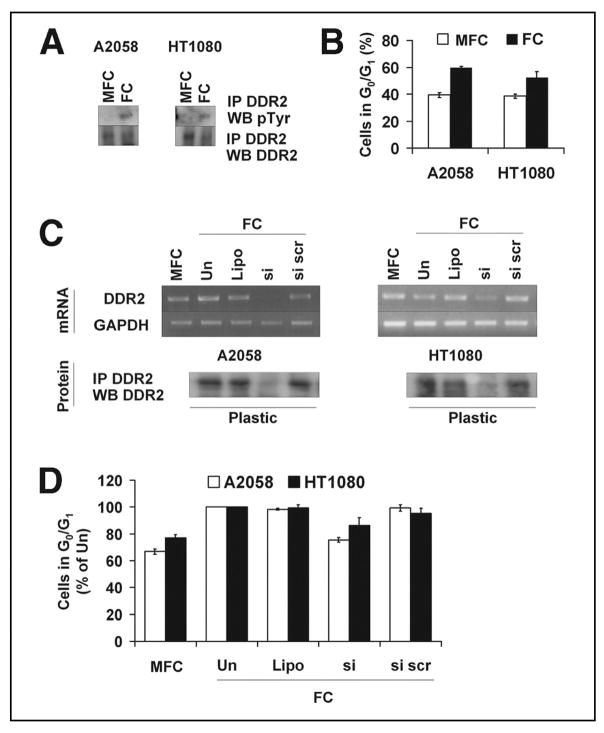
We then examined whether other human cancer cell lines would similarly respond to contact with FC. As with M24met cells, the melanoma cell line A2058 and the human fibrosarcoma cell line HT1080 expressed DDR2, and the receptor was activated (tyrosine phosphorylation) when cells were cultured on FC (Fig. 4A). We observed a significant increase in the percentage of cells in G0/G1 when they were plated in contact with FC in comparison to MFC (p = 0.0006 and p = 0.02 respectively) (Fig. 4B). Down-regulation of DDR2 in these cells by siRNA transfection resulted in a decrease in the percentage of cells in G0/G1 relative to control cells (Fig. 4, C and D) but did not alter cell morphology (data not shown) as previously demonstrated with M24met cells. Thus FC-mediated cell cycle arrest via DDR2 is a growth regulatory mechanism common to several DDR2 expressing cancer cell lines.
FIGURE 4. DDR2 mediated FC-induced cell cycle arrest in A2058 and HT1080 cells.
A, total and tyrosine phosphorylated DDR2 protein levels in A2058 and HT1080 cells cultured on MFC and FC for 24 h (representative Western blots shown, n = 3). Please note that as in Fig. 3G, more MFC lysate was used for immunoprecipitation to enable comparison of approximate equal amounts of total DDR2 when assessing activation. B, A2058 and HT1080 cells were cultured for 24 h on MFC or FC, collected, and examined for cell cycle distribution (n = 3). C and D, A2058 and HT1080 cells treated with DDR2 siRNA and the indicated controls, and the protein levels determined by Western blotting, or the cells were incubated for 24 h on FC, collected, and examined for DDR2 mRNA expression by reverse transcription-PCR (C, representative Western blots or gels shown, n = 4). In reverse transcription-PCR experiments, the cells were also collected for cell cycle analysis (D) (n = 4). Un, untreated; Lipo, Lipofectamine; si, DDR2 siRNA; si scr, scrambled siRNA; IP, immunoprecipitation; WB, Western blot.
Inhibition of MMP Activity Prevents Cells from Escaping FC-induced Cell Cycle Arrest
The observation that the growth inhibitory effect of type I collagen requires a polymerized structure and is not observed in the presence of HDC or MFC suggests that alteration of collagen by proteases expressed by tumor cells may provide a mechanism to escape the restriction of FC on cell proliferation. Because M24met cells produce several MMPs, it would be expected that the growth inhibitory effect observed over 24 h would not be sustained over a longer period of time. This was explored by examining the proliferation of M24met cells on FC over a 10-day period in the presence or absence of AG3340 or BB3103, two synthetic MMP inhibitors, or by testing the growth of M24met H1.11, a subclone of M24met cells overexpressing tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) (26). M24met cells were able to proliferate in the presence of FC as shown by significantly higher cell numbers at days 7 and 10. However, consistent with the concept that proliferation requires proteolytic modification and degradation of FC, the growth of these cells was markedly reduced in the presence of AG3340 or BB3101, or when cells over expressed TIMP-2 (Fig. 5A). We also observed an increase in MMP-2 expression and activation over time in M24met cells plated on FC, and activation was inhibited in the presence of MMP inhibitors, confirming their activity (Fig. 5B). Ferri et al. (27) have previously demonstrated that overexpression of DDR2 in smooth muscle cells results in increased MMP-2 activation when cells are plated on FC. To investigate whether loss of DDR2 would also affect MMP-2 in our system, we collected conditioned medium from M24met cells treated with DDR2 siRNA after 5–10 days being cultured on FC. We observed a slight reduction in MMP-2 activation in cells treated with DDR2 siRNA as well as a small reduction in total MMP-2 (Fig. 5C). Our data thus suggest that DDR2 is not the only regulator of MMP-2 expression and activation in the presence of FC. A final confirmation that proteolytic degradation is critical to allow these cells to proliferate in the presence of FC was obtained by testing the proliferation of M24met cells in the presence of a mutant collagenase-resistant (r/r) (28) FC for 10 days. The data (Fig. 5D) indicated that cells plated on r/r FC are growth inhibited when compared with cells plated on normal “cleavable” FC. Thus prevention of FC degradation maintains the growth inhibitory signal of FC and prevents tumor cell proliferation.
FIGURE 5. Inhibition of MMP activity prevents cells from escaping FC-induced cell cycle arrest.

A, M24met cells and M24met H1.11 (clone overexpressing TIMP-2) were cultured on FC and at defined time points cell numbers were determined by counting with a hemocytometer. When indicated AG3340 (100 ng/ml) or BB3101 (1 μg/ml) was added every 48 h to the culture medium. The results are expressed as the means ± S.E. percentage of cells at 0 h (no inhibitor, n = 7; AG3340, n = 5; H1.11, n = 4; and BB3101, n = 2). B, conditioned culture medium was collected from the above time course, and aliquots were examined by gelatin zymography as indicated under “Material and Methods ” (representative gel shown, n = 3). C, DDR2 siRNA treated M24met cells were cultured on FC for 7 days, and conditioned medium was collected and examined by gelatin zymography as indicated under “Material and Methods ” (representative gel shown, n = 3). Un, untreated; Lipo, Lipofectamine; si, DDR2 siRNA; si scr, scrambled siRNA. D, M24met cells were cultured on normal or collagenase-resistant (r/r) FC and at defined time points cell numbers were determined by counting with a hemocytometer. The results are expressed as the means ± S.E. percentage of cells at 0 h (n = 3).
In summary, we have demonstrated that FC induces a G0/G1 cell cycle arrest in tumor cells by a direct receptor-mediated mechanism that involves DDR2 and that this mechanism is independent of cell spreading and cytoskeletal organization. The data provide evidence for a novel receptor-mediated mechanism by which contact between cells and FC inhibits tumor cell proliferation.
DISCUSSION
As melanoma cells switch from a radial growth pattern in the cell-rich environment of the epidermis to a vertical growth pattern, they penetrate the collagen-rich milieu of the dermis. The presence of collagen fibers in tissue penetrated by malignant cells is a physical barrier against their invasive behavior (29). However, it has recently been suggested that collagen fibers also restrict cell proliferation. FC has been shown by a number of groups of investigators to inhibit the proliferation of normal as well as malignant cells (11–13, 16), and we had previously reported that contact between M24met human melanoma cells and FC inhibits their proliferation at the G1/S checkpoint of the cell cycle (14). In this study we provide a novel insight on the mechanism involved in this cell cycle arrest. First, our kinetic data in synchronized cells (Fig. 1A) confirm that the FC-induced elevation of cells in G0/G1 is due to a true arrest at the G1/S checkpoint rather than a delay in cell cycle progression as shown by a progressive accumulation of cells in G0/G1 over 24 h in the presence of FC with a total absence of reentry in the S phase. The absence of significant cell proliferation over 72 h in the presence of FC is also consistent with an arrest at the G1/S checkpoint.
The mechanism by which contact between cells and FC inhibits cell proliferation has been so far poorly understood. It has generally been assumed that it is the result of an indirect inhibitory effect of FC on cell spreading (16, 17). Consistent with this concept, we observed an absence of cell spreading and activation of p125FAK when melanoma cells were plated on FC. However, two experiments suggested that inhibition of cell spreading may not be the only factor responsible for the growth arrest and that contact with FC could have a direct, receptor-mediated effect on cell proliferation. We observed that disruption of the cytoskeleton and cell spreading, although inhibiting cell proliferation like FC, resulted in accumulation of cells at the G2/M phase rather than the G0/G1 phase. Secondly, we documented that treatment of M24met cells plated on FC with the broad spectrum tyrosine kinase inhibitor genistein allowed cells to reenter the cell cycle without the need to spread and organize their cytoskeleton, thus providing evidence for a dissociation between spreading and proliferation. Fornaro et al. (30) also demonstrated a dissociation between spreading and proliferation by showing that down-regulation of the integrin β1C in endothelial cells allows cells to proliferate without spreading. Similarly, exposure of vascular smooth muscle cells to the secreted protein acidic and rich in cysteine inhibits proliferation independently of an effect on cell shape (31).
These observations led us to postulate the existence of a receptor-mediated mechanism of cell cycle regulation mediated by contact with FC. Among the cell surface-associated receptors that can interact with FC are the integrins α1β1, α2β1, α10β1, and α11β1 and the members of the receptor tyrosine kinase DDR family. We were able to eliminate the involvement of integrins because we had previously demonstrated that stimulation of β1 integrin subunit enhanced, rather than inhibited, cell proliferation (14), an observation that is consistent with the concept that contact between integrins and ECM proteins provides a growth stimulatory signal to cells (3). Our observation that genistein prevented FC-induced cell cycle arrest pointed to a potential role for DDRs, which, in contrast to integrins, are tyrosine kinase receptors. We observed that this family of receptors is expressed in M24met cells and is up-regulated when cells are cultured on FC. Interestingly, although DDR1 is activated by both fibrillar and nonfibrillar collagens, DDR2 is exclusively stimulated by fibrillar collagens (25), and we consistently demonstrated DDR2 activation in M24met cells plated on FC by showing its phosphorylation. We obtained evidence for a specific role for DDR2 in FC-mediated cell cycle arrest by demonstrating that down-regulation of DDR2 by siRNA prevents FC-induced cell cycle arrest and stimulates proliferation, without a requirement for cell spreading. In contrast, down-regulation of DDR1 had no effect on cell proliferation in the presence of FC. The involvement of DDR2 in controlling cell proliferation has been previously reported in skin fibroblasts and hepatic stellate cells grown on FC and Matrigel (32, 33). Although our DDR2 data is different from those of Ikeda et al. (34) and Olaso et al. (32, 33), which demonstrated that DDR2 activation stimulates cell proliferation, these authors have examined nonmalignant cell lines (skin fibroblasts or stellate cells), whereas we used cancer cell lines (melanoma and fibrosarcoma). A single kinase having opposite effects on cell proliferation in different cell types is not without precedent, because protein kinase Cα has been demonstrated to stimulate proliferation in MCF-7 cells (breast cancer cell line) but has very little effect in fibroblasts (35). Additionally, other factors including cAMP, transforming growth factor β, v-Abl, and parathyroid hormone have been shown to stimulate or inhibit proliferation depending on cell type (36–40). It is therefore conceivable that DDR2 can act as a growth inhibitory or growth stimulatory signal as a function of the cell type.
The observation that the growth inhibitory effect of FC on M24met cells was equally observed in three-dimensional and two-dimensional collagen models is consistent with the effect being primarily mediated by a receptor-ligand mechanism independent of an effect of the three-dimensional cell architecture. Prevention of FC-induced cell cycle arrest by DDR2 reduction was not limited to M24met cells and was also observed in the human melanoma cell line A2058 and the human fibro-sarcoma cell line HT1080, thus indicating that restriction in proliferation by DDR2 contact with FC is a more general phenomenon in cancer cells. Taken together, these experiments identify DDR2 as a collagen-binding cell surface receptor responsible for the growth inhibitory signal generated upon contact with FC, but not MFC. Our data, however, do not rule out the additional presence of an indirect growth inhibitory effect of FC through the control of cell spreading at the G2/M phase.
The importance of the growth inhibitory effect of FC on tumor cell proliferation and its relevance to cancer progression was recently emphasized by Hotary et al. (16), who demonstrated that cleavage of FC by membrane type I MMP (MMP-14), a proteinase that cleaves FC and activates pro-MMP-2, enables cells to proliferate and to escape a growth-restrictive ECM environment (17). It is unlikely that MMP production is sufficient to degrade the entire FC; however, because MMP-14 is localized to the cell surface, FC cleavage is limited to the interface between cells and collagen, creating a microenvironment of cleaved FC and enabling cells to proliferate. Consistent with this concept, we did not observe a single time point when cells escaped growth arrest but rather observed a gradual growth as cells degraded their immediate surroundings. Consistent with the observations by Hotary et al. (16), we also demonstrated that M24met cells plated on FC did not proliferate if collagen degradation was inhibited by MMP inhibitors or in the presence of a mutant form of FC (r/r) that is resistant to proteolytic cleavage by MMPs.
The observation that upon contact with FC M24met cells activated pro-MMP-2 is also consistent with a recent report (27) demonstrating that overexpression of DDR2 in human smooth muscle cells enhances MMP-2 activation. However, loss of DDR2 expression had only a limited effect on MMP-2 expression and activation, indicating that DDR2 is not the sole regulator of MMP-2 expression and activation in the presence of FC. Alternatively, these differences may be attributed to the use of different cells, ours being a melanoma, whereas Ferri et al. (27) used smooth muscle cells. These data raise the question of whether MMP-14 or other type I collagen-degrading MMPs, like MMP-1, MMP-8 or MMP-13, could be up-regulated upon DDR2 activation in cells in contact with FC. This question is currently investigated in our laboratory.
The integrins α1β1, α2β1, α10β1, and α11β1 are the main established receptors for collagen, and we had previously shown that the α2β1 integrin is involved in M24met adhesion to FC (14). However, we found no evidence for activation of this integrin upon contact with FC as documented by an absence of focal adhesions and FAK phosphorylation in M24met cells plated on FC, indicating that DDR2 does not signal through these integrins. This is consistent with previous observations from Ikeda et al. (34), who could not establish a direct relationship between integrins and DDR2, and studies demonstrating that integrins and DDR1 act independently of each other (41, 42). The observation that upon contact with FC DDR2 is activated, whereas integrin collagen receptors are not, is intriguing. Although we do not have a complete explanation for this fact, it is possible that the rigid polymeric structure of FC prevents collagen-binding integrins from clustering and being activated, whereas DDR2 activation can occur, resulting in a dominant growth restrictive signal. Upon denaturation and degradation of FC, DDR2 binding would be lost, whereas integrin clustering would be favored, resulting in a shift toward integrin mediated growth-promoting signals.
In summary, our observations provide evidence for the existence of a direct, cell receptor-mediated mechanism by which the ECM controls the proliferation of malignant cells that, in contrast to previously reported indirect mechanisms, is independent of cell spreading and cytoskeletal organization. They also confirm that degradation of FC by invading tumor cells in addition to providing a mechanism to remove a physical barrier is an important way to escape growth-limiting signals.
Acknowledgments
We thank Dr. Marc Lafleur (St. Vincent’s Institute of Medical Research, Australia) for scientific discussions, Jerry Barnhart (The Saban Research Institute, FACS Core Facility) for processing the FACS samples, and Jackie Rosenberg for assistance in preparing this manuscript. We also thank Dr. Lisa Coussens (University of California San Francisco) for the collagenase-resistant collagen (r/r) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY) for the DDR2 (R2-JM) antibody.
Footnotes
The abbreviations used are: ECM, extracellular matrix; DDR, discoidin domain receptor; FC, fibrillar collagen; HDC, heat-denatured collagen; MMP, matrix metalloproteinase; MFC, monomeric fibrillar collagen; siRNA, small interfering RNA; TIMP, tissue inhibitor of matrix metalloproteinase; PBS, phosphate-buffered saline; FACS, fluorescence-activated cell sorting.
This work was supported by Grants R01 CA42919 and R01 CA98469 (to Y. A. D.) and Grants R01 CA57621 and P50 CA58207 (to Z. W.) from the NCI, National Institutes of Health.
References
- 1.Lukashev ME, Werb Z. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau NJ, Jones PL. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- 3.Giancotti FG, Ruoslahti E. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 4.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Pestell R, Guan JL. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron-Van Evercooren A, Kleinman HK, Seppa HE, Rentier B, Dubois-Dalcq M. J Cell Biol. 1982;93:211–216. doi: 10.1083/jcb.93.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ocalan M, Goodman SL, Kuhl U, Hauschka SD, von der MK. Dev Biol. 1988;125:158–167. doi: 10.1016/0012-1606(88)90068-1. [DOI] [PubMed] [Google Scholar]
- 8.Inoue M, Katakami C. Investig Ophthalmol Vis Sci. 1993;34:2313–2315. [PubMed] [Google Scholar]
- 9.Zhu X, Assoian RK. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitale M, Illario M, Di Matola T, Casamassima A, Fenzi G, Rossi G. Endocrinology. 1997;138:1642–1648. doi: 10.1210/endo.138.4.5052. [DOI] [PubMed] [Google Scholar]
- 11.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 12.Herz DB, Aitken K, Bagli DJ. J Urol. 2003;170:2072–2076. doi: 10.1097/01.ju.0000091810.33953.13. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JM, Forrester JV. Exp Eye Res. 1990;50:165–172. doi: 10.1016/0014-4835(90)90227-l. [DOI] [PubMed] [Google Scholar]
- 14.Henriet P, Zhong ZD, Brooks PC, Weinberg KI, DeClerck YA. Proc Natl Acad Sci U S A. 2000;97:10026–10031. doi: 10.1073/pnas.170290997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacakova L, Wilhelm J, Herget J, Novotna J, Eckhart A. Exp Mol Pathol. 1997;64:185–194. doi: 10.1006/exmp.1997.2219. [DOI] [PubMed] [Google Scholar]
- 16.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 17.Yamada KM. Nature. 2003;424:889–890. doi: 10.1038/424889a. [DOI] [PubMed] [Google Scholar]
- 18.Sieg DJ, Hauck CR, Schlaepfer DD. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 19.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 20.Heussen C, Dowdle EB. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 21.Spector I, Shochet NR, Kashman Y, Groweiss A. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 22.Heino J. Matrix Biol. 2000;19:319–323. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- 23.Leitinger B. J Biol Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 24.Leitinger B, Steplewski A, Fertala A. J Mol Biol. 2004;344:993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- 25.Vogel W, Gish GD, Alves F, Pawson T. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery AM, Mueller BM, Reisfeld RA, Taylor SM, DeClerck YA. Cancer Res. 1994;54:5467–5473. [PubMed] [Google Scholar]
- 27.Ferri N, Carragher NO, Raines EW. Am J Pathol. 2004;164:1575–1585. doi: 10.1016/S0002-9440(10)63716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu H, Byrne MH, Stacey A, Goldring MB, Birkhead JR, Jaenisch R, Krane SM. Proc Natl Acad Sci U S A. 1990;87:5888–5892. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liotta LA. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 30.Fornaro M, Zheng DQ, Languino LR. J Biol Chem. 1995;270:24666–24669. doi: 10.1074/jbc.270.42.24666. [DOI] [PubMed] [Google Scholar]
- 31.Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. J Cell Biochem. 2002;84:759–771. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- 32.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. J Clin Investig. 2001;108:1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, Lovett DH, Lin HC, Friedman SL. J Biol Chem. 2002;277:3606–3613. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, Labrador P, Klein R, Lovett D, Yancopoulos GD, Friedman SL, Lin HC. J Biol Chem. 2002;277:19206–19212. doi: 10.1074/jbc.M201078200. [DOI] [PubMed] [Google Scholar]
- 35.Fishman DD, Segal S, Livneh E. Int J Oncol. 1998;12:181–186. doi: 10.3892/ijo.12.1.181. [DOI] [PubMed] [Google Scholar]
- 36.Kanno N, Lesage G, Phinizy JL, Glaser S, Francis H, Alpini G. Hepatology. 2002;35:1329–1340. doi: 10.1053/jhep.2002.33330. [DOI] [PubMed] [Google Scholar]
- 37.Partridge M, Green MR, Langdon JD, Feldmann M. Br J Cancer. 1989;60:542–548. doi: 10.1038/bjc.1989.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutty G, Ikeda K, Chandler C, McLeod DS. Curr Eye Res. 1991;10:61–74. doi: 10.3109/02713689109007611. [DOI] [PubMed] [Google Scholar]
- 39.Renshaw MW, Kipreos ET, Albrecht MR, Wang JY. EMBO J. 1992;11:3941–3951. doi: 10.1002/j.1460-2075.1992.tb05488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misiano P, Scott BB, Scheideler MA, Garnier M. Eur J Pharmacol. 2003;468:159–166. doi: 10.1016/s0014-2999(03)01673-x. [DOI] [PubMed] [Google Scholar]
- 41.Vogel W, Brakebusch C, Fassler R, Alves F, Ruggiero F, Pawson T. J Biol Chem. 2000;275:5779–5784. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- 42.Wang YK, Wang YH, Wang CZ, Sung JM, Chiu WT, Lin SH, Chang YH, Tang MJ. J Biol Chem. 2003;278:21886–21892. doi: 10.1074/jbc.M300092200. [DOI] [PubMed] [Google Scholar]