Abstract
It is well established that the dopamine (DA) and serotonin (5-HT) systems have extensive and complex interactions. However, the effects of specific 5-HT receptor agonists on traditionally DA-related behaviors remain unclear. Our goal in these studies was to characterize the effects of 5-HT receptor agonists on measures of locomotor activity and vertical rearing. The SSRIs fluoxetine and citalopram produced significant decreases in locomotor activity and vertical rearing at the highest doses used with females significant more sensitive to citalopram. The 5-HT1A agonist 8-OH-DPAT and the 5-HT2C agonist MK 212 significantly decreased activity in both male and female mice, with females more sensitive to 8-OH-DPAT. In contrast, the 5-HT1B agonist RU 24969 and the 5-HT2A agonist DOI both increased activity, with DOI exhibiting differential effects with regard to sex. Finally, the 5-HT3 agonist SR 57227 produced significant locomotor increases only in female mice at the lowest dose. The results of these experiments define locomotor profiles of several 5-HT agonists in male and female C57BL/6J mice, providing a foundation for further explorations of 5-HT receptor effects on activity.
Keywords: Serotonin, dopamine, locomotor activity, fluoxetine, citalopram, C57Bl/6J, sex, 8-OH-DPAT, RU 24969, MK 212, DOI, SR57227
Introduction
It is well established that dopamine (DA) and serotonin (5-HT) systems have extensive and complex interactions, especially within the basal ganglia (1). The activity of the striatum, a sensory-motor-limbic integration region of the basal ganglia that controls locomotor and vertical activity in rodents and includes the caudate-putamen and nucleus accumbens, is modulated by many factors, in particular DA and 5-HT projections arising from the ventral midbrain and brainstem (21). While the effects of direct and indirect DA receptor agonists on locomotor and vertical activity have been well-characterized (4), the effects of 5-HT receptor agonists remain unclear. The ventral midbrain, where DA cell bodies are located, receives the heaviest 5-HT innervations in the brain (25), and 5-HT receptors regulate the activity of DA and GABA neurons in this region (43);(40). There is also a strong 5-HT projection to the striatum which provides presynaptic 5-HT regulation of DA release (1). In light of these extensive interactions, the effects of 5-HT receptor agonists on behaviors traditionally associated with activation of the dopaminergic system, such as locomotor and vertical activity (48);(15;16), merit further study.
Fourteen 5-HT receptor subtypes have been identified to date, and at least five of these are known to have extensive interactions with the DA system, including the 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, and 5-HT3 receptors (1). Many 5-HT receptor agonists are well characterized with regard to anxiety-related, anti-depressant, hallucinogenic and other properties. However, the effects of these compounds on locomotor activity in mice remain relatively unknown. Although some locomotor studies of drugs such as the 5-HT1A agonist, 8-OH-DPAT, have been performed in mice and rats, the doses used are limited, and studies center almost exclusively on examining the modulatory influences of specific 5-HT receptors on stimulant-induced behaviors. Thus, a study of the effects of 5-HT receptor agonists on mouse locomotor activity could prove useful in further studies of drug-induced behaviors, as well as providing insight into the direct locomotor effects of the 5-HT agonists.
It is known that male and female mice differ in their responses to some psychoactive drugs that activate both the 5-HT and DA systems, such as amphetamine (11;45). Thus, it is possible that 5-HT receptor agonists with actions in the DA system might show sex differences. Additionally, the DA and 5-HT systems are known to have extensive interactions, particularly in the area of the striatum and nucleus accumbens, brain areas linked with the locomotor- and vertical activity- stimulating effects of drugs (29). It is therefore likely that stimulation of specific 5-HT receptor subtypes in this area might have significant effects on locomotor and vertical activity, effects often mediated through the dopaminergic system (30), (16), (49), but which are not necessarily mediated exclusively by increases in DA (36). Therefore, although locomotor and vertical activation or attenuation may be due to changes in DA via 5-HT receptor stimulation, 5-HT receptor stimulation alone may also alter locomotor and vertical activity.
Although some 5-HT receptors are relatively well-characterized with regard to sex differences in brain distribution and the behavioral effects of stimulation, such as the 5-HT1A, 5-HT2A, and 5-HT2C receptors (53);(6);(3), others remain poorly characterized, and no studies have examined sex differences in the effects of specific 5-HT receptor agonists on locomotor activity.
Despite what is known about the effects of 5-HT receptor agonists on mesolimbic DA firing rates and control of such behaviors such as drug self-administration (46), the specific function of these receptors in DA-related behaviors, such as locomotor activity in mice, remain unclear. As locomotor and vertical activity are behaviors known to be regulated by DA signaling, our goal in these studies was to characterize the effects of several 5-HT receptor agonists on DA\5-HT interactions in mice. C57BL/6J is the most commonly used mouse strain in neuropharmacological research, and thus we chose to use this strain to characterize the locomotor effects of seven different serotonergic compounds; the selective serotonin reuptake inhibitors (SSRIs) fluoxetine and citalopram, the 5-HT1A agonist 8-OH-DPAT, the 5-HT1B agonist RU 24969, the 5-HT2A/2C agonist DOI, the selective 5-HT2C agonist MK 212, and the 5-HT3 agonist SR 57227. Naïve mice were tested with a variety of drug doses for locomotor distance traveled and measures of vertical rearing activity.
Methods
Animals
C57Bl/6J mice were obtained from Jackson laboratories (Bar Harbor, ME), and a breeding colony was established. Mice were housed in groups of three or four per cage with food and water ad libitum on a 12-hr light-dark cycle with lights on at 7 am. All experiments used both male and female mice that were between 2 and 6 months old, and for each drug, the male and female mice being tested were age matched. Experimental protocols adhered to National Institutes of Health Animal Care Guidelines and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Activity Monitoring
Locomotor activity and vertical rearing were assessed using open field activity monitors (Med Associates, St. Albans, VT). The open field consisted of a square plexiglass container (27.0cm × 27.0cm × 20.3 cm) with three sixteen beam infrared arrays. Two arrays were placed on the periphery of the chamber at floor level for detection of locomotor activity (X and Y planes, measured approximately 0.25” off the floor), while the third array was placed 2” above the X and Y arrays to obtain measures of vertical activity (Z plane, measured 2.25” off the floor). Data was collected using Med Associates Activity Monitoring Software (Med Associates, St. Albans, VT), and distance traveled was measured in cm over a given length of time. Vertical rearings were measured as number of beam breaks in the vertical plane over a given length of time. Behavioral analyses were conducted during the light phase between 9 am – 5 pm. The locomotor chambers contained no bedding, and were cleaned with 70% EtOH and dried thoroughly between testings.
Drug Administration
Following a two-hour period where mice were allowed to habituate to the chambers, animals received either saline (0.1 mL injection volume) or drug dissolved in saline (unless specified otherwise), administered in a 0.1 mL volume i.p., by weight, at the doses described below. Separate injections were prepared for male and female mice based on average male and female weight for the cohort. Animals were divided into cohorts, each of which received all doses of a single drug type. Doses were randomized in a Latin-Square design. Data was collected using Med Associates proprietary software (Med Associates, St. Albans, VT) in 5-minute bins for a period of two hours following injection.
Statistics
Data was analyzed for distance traveled in cm and the number of vertical rears performed during the activity profile of the drug. Locomotor activity was grouped in bins for either the first 20 minutes after drug injection (fluoxetine, citalopram, MK 212), the first 30 minutes after drug injection (8-OH-DPAT), or the first 60 minutes after drug injection (RU 24969, DOI, SR 57227), depending on the active period of the drug. Active period of the drug was determined by AUC analysis of activity curves measured over two hours following drug injection (for a series of representative locomotor activity traces over the full two hours of recording following drug injection, see supplemental data). The time course of each drug tested can be seen at representative doses for locomotor and vertical activity in Supplemental figures 1–9. Following summation of data over 20, 30 or 60 minutes, all groups were tested for outliers using the Grubb's Test for Outliers. Data was then grouped by sex and analyzed by a one-way ANOVA for effect of drug in either male or female mice, with a corrected Bonferroni post-hoc analysis to determine specific effects of dose. For comparisons between sex, activity was compared as percent of saline values, to control for differences in saline response between sex. Data was analyzed for between sex effects and interactions between drug and sex by repeated-measures ANOVA with corrected Bonferroni post-hoc analysis. P<0.05 was considered significant.
Drugs
The SSRIs (±)-N-methyl-γ-[4-(trifluoromethyl)phenoxy]benzenepropanamine hydrochloride (fluoxetine) and 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile hydrobromide (citalopram), the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tertraline (8-OH-DPAT), and the 5-HT2A/2C receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2 amino propane ((±) DOI-hydrochloride) were purchased from Sigma (Sigma Aldrich, St. Louis, MO). The 5-HT1B agonist 5-methoxy-n1N-dimethyltryptamine and 5-methoxy-3(1,2,3,6,-tetrahydro-4-pyrindinyl)-1H-indole (RU 24,969), the 5-HT2C agonist 6-chloro-2-(1-piperazinyl)pyrazine hydrochloride (MK 212),and the 5-HT3 agonist 1-(6-chloro-2-pyridinyl)-4-piperidinamine hydrochloride (SR 57227) were purchased from Tocris (Ellisville, MO). All drugs were given in a volume of 0.1 mL with concentrations determined by animal weight averages. Fluoxetine HCl was dissolved in ultra-pure water, while all other drugs were dissolved in 0.9% isotonic sterile saline.
Results
Saline injection produces greater locomotor and vertical responses in male mice
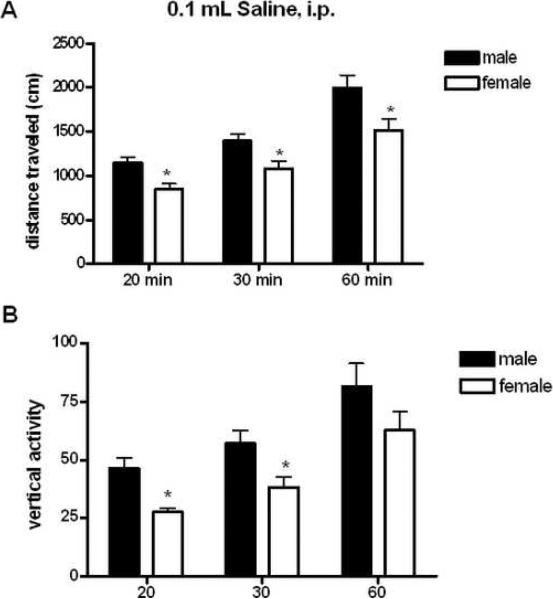
All animals were habituated and monitored for locomotor activity for two hours prior to injection of drug. In all cohorts and with all drugs tested, no difference between male and female mice was observed in measures of spontaneous locomotor and vertical activity during the two hours of habituation to the test chamber (see Supplemental Figure 1). Following habituation, animals were injected with 0.1mL saline, and locomotor activity was monitored for two hours (Representative time course of saline injection shown in Supplemental Figure 2). Male mice showed significantly greater locomotor activity compared to female mice during the first 20 (t=3.022, p<0.01), 30 (t=2.513, p<0.05), and 60 minutes following saline injection (t=2.383, p<0.05) (Figure 1A). Similarly, in comparison to females, male mice performed more vertical rears, particularly during the first 20 (t=4.183, p<0.001) and 30 minutes (t=4.391, p<0.05), (Figure 1B). These data suggest that male mice overall may be more sensitive to the locomotor-activating effects of acute injections than female mice.
Figure 1. Locomotor Responses to 0.1 mL Saline, i.p.
A: Effects of 0.1 mL saline injection on locomotor activity across all cohorts of male and female mice for 20, 30 and 60 minutes post-injection (n=67 male, 72 female). *p<0.01 effect of sex at 20 minutes post injection, *p<0.05 effect of sex at all other points. B: Effects of 0.1 mL saline injection on vertical rearing across all cohorts of male and female mice for 20, 30 and 60 minutes post-injection. *p<0.001 effect of sex at 20 minutes post injection, *p<0.05 effect of sex at 30 minutes post injection (n=67 male, 72 female).
Fluoxetine decreases locomotor activity in male and female mice
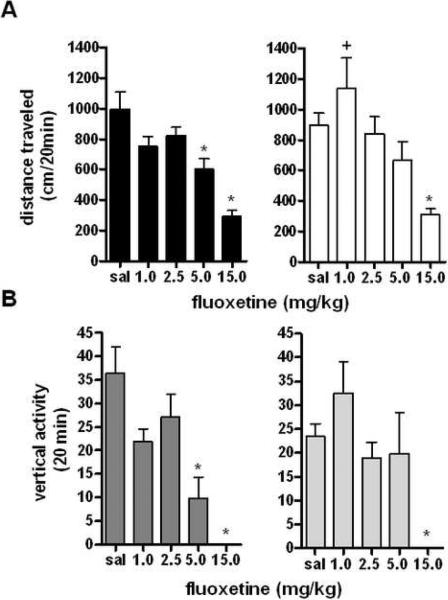
It has been generally agreed that fluoxetine produces decreases in locomotor activity at high doses (43;19), but as yet, no studies have been performed analyzing the effect of sex on locomotor activity following fluoxetine administration. Following two hours of monitored habituation to the locomotor chamber, animals were given either saline or fluoxetine (1.0–15.0 mg/kg) (8;34) in a 0.1mL volume by weight. Data was then analyzed for sex differences and overall effect of drug on locomotor activity using a repeated measures two-way ANOVA with corrected Bonferroni post-doc analysis during the first 20 minutes following injection of drug (for full time course of fluoxetine injection at a representative dose, see Supplemental Figure 3A) using percent change from saline control to normalize for differences in saline response. There was no significant effect of sex on overall fluoxetine response (F1,109=1.450, p=0.2311), or significant interaction between sex and fluoxetine response (F4,109=1.610, p=0.1769), although both sexes exhibited a significant decrease in locomotor activity in response to fluoxetine (F1,109=14.14, p<0.001 Figure 2A). When analyzed for specific effects of dose by sex, results confirmed that males and females showed decreased activity across all doses of drug.
Figure 2. Locomotor and Vertical Responses to the SSRI Fluoxetine.
A: Effects of fluoxetine on locomotor activity for 20 min post injection in male and female mice. *p<0.05 effect of fluoxetine at 15 mg/kg in females, *p<0.01 effect of drug at 5.0 mg/kg in males, *p<0.001 effect of fluoxetine at 1.0 mg/kg in males, +p<0.001 effect of sex on locomotor response to fluoxetine (n=12 per group). B: Effects of fluoxetine on vertical rearing for 20 min post injection in male and female mice. *p<0.001 effect of drug, no difference between sexes (n=12 per group).
Fluoxetine decreases vertical activity in male and female mice
Recordings were also conducted for vertical activity in response to fluoxetine. Not surprisingly, there was a significant effect of fluoxetine on vertical activity in all mice (F4,108=13.55, p<0.001). Analyzed for sex differences during the first 20 minutes following injection as percent of saline control, there was no significant effect of sex on vertical activity (F1,108=0.002597, p=0.9595), although a significant interaction between sex and fluoxetine dose (F4,108=2.607, p<0.05, Figure 2B) was observed. Additional one-way ANOVA analysis for dose of drug separated by sex confirmed the effects of fluoxetine on vertical activity, and it is possible that the drug by sex interaction is due to differences in saline locomotor response. (For full time course of fluoxetine injection and vertical activity recording at a representative dose, see Supplemental Figure 3B) Taken together, our data show that fluoxetine administration produces significant decreases in locomotor and vertical activity, but overall effects do not differ between male and female mice.
Citalopram decreases locomotor activity at high doses
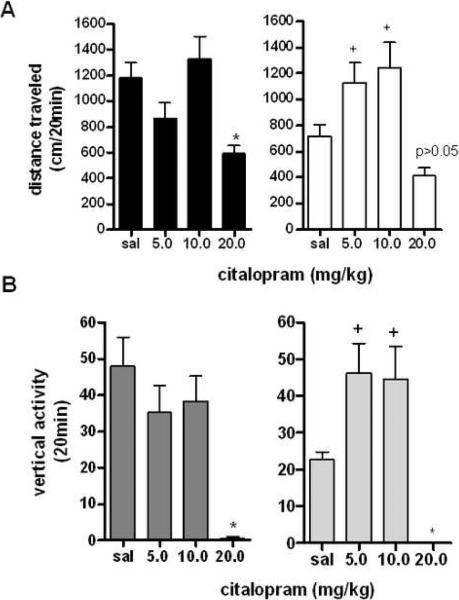
Although previous studies have shown few effects of citalopram on locomotor activity (7;15), other studies have shown significant effects of citalopram on locomotor activity at higher doses (16). Thus, it would be useful to re-examine the locomotor effects of citalopram, and, if possible, explore the effects of sex on locomotor response. Following two hours of monitored habituation to the locomotor chambers, animals were given either saline or the SSRI citalopram (5.0 –20.0 mg/kg) (34;17). For a full time course of locomotor effects of citalopram on male and female mice at a representative dose, see Supplemental Figure 4A. When analyzed for sex differences during the first 20 minutes, it was apparent that there was a significant difference between the males and females (effect of sex F1,65=13.16, p<0.001, analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis), with females showing significant increases in activity at 5.0 and 10.0 mg/kg (Figure 3A), although no significant interaction effect was observed (F2,65=2.640, p=0.079). When separated and analyzed further for effects of citalopram dose on sex, male and female mice showed overall effects of citalopram, with significant decreases in locomotor activity only at the highest dose (overall effect of citalopram in males F3,46=6.511, p<0.001, overall effect in females F3,47=7.544, p<0.001, analyzed by one-way ANOVA for each sex relative to saline control).
Figure 3. Locomotor and Vertical Responses to the SSRI Citalopram.
A: Effects of citalopram on locomotor activity for 20 min post injection in male and female mice. *p<0.05 effect of citalopram at 20 mg/kg. +p<0.05 effect of sex on locomotor response to 10 mg/kg citalopram, +p<0.01 effect of sex on locomotor response (n=12 per group). B: Effects of citalopram on vertical activity for 20 min post injection in male and female mice. *p<0.01 effect of citalopram at 20 mg/kg. +p<0.01 effect of sex on vertical activity at 5.0 and 10.0 mg/kg citalopram (n=12 per group).
Citalopram decreases vertical activity at high doses in male and female mice
In measures of vertical activity in response to the SSRI cirtalopram, analysis revealed a significant effect of sex (F1,65=13.16, p<0.001, analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis). There was also a significant effect of sex for measures of vertical activity (F2,62=4.222, p<0.05 analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis), although no interaction between sex and citalopram dose was detected (F3,79=0.9842, p=0.405). Analyzed separately for effects of citalopram on males and female, citalopram dramatically reduced the number of vertical rears during the first 20 minutes at the highest dose tested in both sexes (males: F3,44=9.924, p<0.001, females: F3,46=11.59, p<0.001), although females showed increases in vertical activity at 5.0 and 10.0 mg/kg, possibly related to a low initial response to saline injection (Figure 3B). For a full time course of the vertical effects of citalopram at a representative dose, see Supplemental Figure 4B.
In the case of vertical responses to citalopram in females, the effect of 20 mg/kg citalopram was a drastic decrease in vertical activity, compared to significant increases in vertical activity at lower doses. Because the 20 mg/kg dose resulted in vertical activity without sufficient variance to allow for a Bonferroni post-hoc test, we ran a one sample t-test comparing the mean value of the 20 mg/kg dose to saline, and this showed significant effect of drug at 20 mg/kg (t=11.86, df=11, p<0.0001). Overall, it appears that citalopram produced decreases in locomotor and vertical activity at the highest doses used, but that effects at intermediate doses vary with sex of the animal.
The 5-HT1A agonist 8-OH-DPAT decreases locomotor activity in male mice
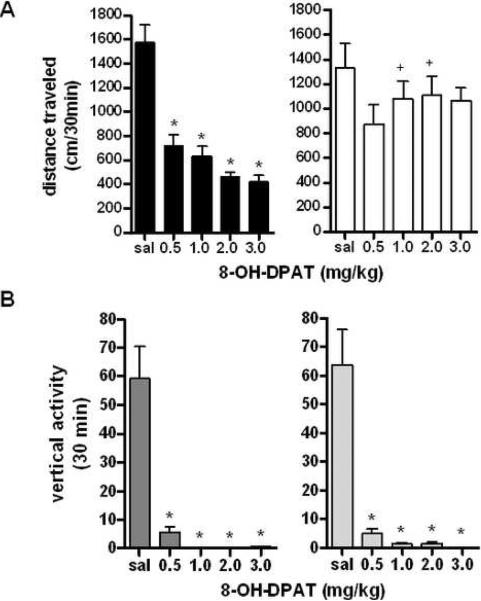
It has been observed that the 5-HT1A agonist 8-OH-DPAT decreases locomotor activity in mice (14), but effects with regard to sex differences have yet to be explored. Following two hours of habituation, animals were given either saline or 8-OH-DPAT (0.5–3.0 mg/kg) (5). There was a significant effect of sex (F1,115=25.92, p<0.01) over the first 30 minutes following drug administration, with a significant interaction between sex and drug dose (F4,115=2.495, p<0.05 analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis). For a full time course of drug effects at a representative dose, see Supplemental Figure 5A. Interestingly, subsequent analysis revealed that only male mice showed a response to drug, while females showed no significant effects at any dose tested (males: F4,50=24.55, p<0.001, females: F4,67=0.9706, p=0.43, Figure 4A, analyzed by one-way ANOVA for each sex relative to saline control).
Figure 4. Locomotor Responses to the 5-HT1A Agonist 8-OH-DPAT.
A: Effects of 5-HT1A receptor agonist 8-OH-DPAT on locomotor activity in male and female mice. *p<0.0001 effect of 8-OH-DPAT dose, +p<0.05 effect of sex at 1.0 mg/kg 8-OH-DPAT, +p<0.001 effect of sex at 2.0 mg/kg 8-OH-DPAT (n=9 males, n=17 females). B: Effects of 8-OH-DPAT on vertical rearings for 30 min following drug injection. p<0.001 effect of drug, p<0.001 effect of sex, no interaction. C: Effects of 8-OH-DPAT on vertical rearing for 30 min following drug injection. *p<0.001 effect of 8-OH-DPAT dose (n=9 males, n=17 females).
8-OH-DPAT reduces vertical activity in male and female mice
With regard to vertical activity following injection of 5-HT1A agonist 8-OH-DPAT, no effect of sex (F1,79=0.05198, p=0.82 analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis), and no interaction between sex and 8-OH-DPAT dose was detected (F1.108=0.03268, p=0.9979), although there was a significant overall effect of 8-OH-DPAT across all doses (F4,108=26.20, p<0.001, Figure 4B). For a full time course of drug effects on vertical activity at a representative dose, see Supplemental Figure 5B. In analyses for effects of drug dose on gender, both sexes showed significant decreases during the first 30 minutes in the number of vertical rears at all doses of drug, confirming the results of the two-way ANOVA. Overall, it appears that 8-OH-DPAT decreases vertical rearing behavior in both male and female mice, although females may be less sensitive to the effect of this 5-HT1A agonist on locomotor activity.
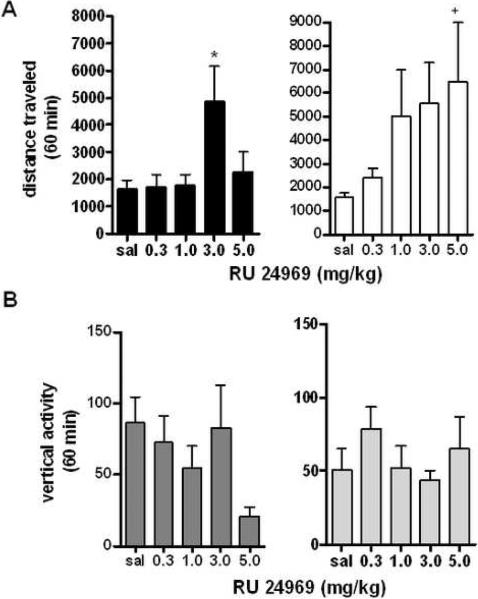
The 5-HT1B agonist RU 24969 increases locomotor activity in male and female mice
5-HT1B agonists have been shown to potentiate stimulant-induced locomotor activity when administered systemically (13), but the effects on vertical activity and the possible effect of sex have not been determined. Following habituation animals were given either saline or RU 24969 (0.3–5.0 mg/kg) (41). For a representative graph of locomotor effects of RU 24969 on male and female mice, see Supplemental Figure 6A. Across all mice, there was a significant increase in locomotor activity in response to RU 24969 (F4,78=2.706, p<0.05), but there was also a significant difference between sexes (F1,78=4.604, p<0.05 analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis). There was no significant interaction between sex and dose of RU 24969 (F4,78=1.019, p=0.4029). While both sexes showed significant increases in locomotor activity in response to drug administration (males: F4,43=5.380, p<0.01, females: F4,43=2.619, p<0.05, Figure 5A, analyzed by one-way ANOVA for each sex relative to saline control), males showed a decrease in locomotor responding at the highest dose compared to females, pointing to a possible difference between sexes in responses to RU 24969.
Figure 5. Locomotor Responses to the 5-HT1B Agonist RU 24969.
A: Effect of 5-HT1B agonist RU 24969 on locomotor activity from 40–70 minutes post administration in male and female mice. *p<0.0001 effect of RU 24969 at 3.0 mg/kg, +p<0.05 effect of sex at 5.0 mg/kg (n=9 per group). B: Effect of RU 24969 on vertical rearing from 40–70 minutes post administration. +p<0.05 effect of sex at 5.0 mg/kg, +p<0.01 effect of sex at 1.0 mg/kg (n=9 per group).
5-HT1B agonist RU 24969 produces no effects on vertical activity
While male and female mice showed consistent increases in locomotor activity, vertical activity appeared completely unaffected by dose of the 5-HT1B agonist (F4,76=1.060, p=0.3824), and with no effect of sex present (F1,76=0.2187, p=0.6414, Figure 5B, for a representative time course vertical effects of RU 249696, see Supplemental Figure 6B). Overall, it appears that the 5-HT1B agonist RU 24969 has a significant activating effect on locomotor activity in mice, and that females appear to be more sensitive to these effects.
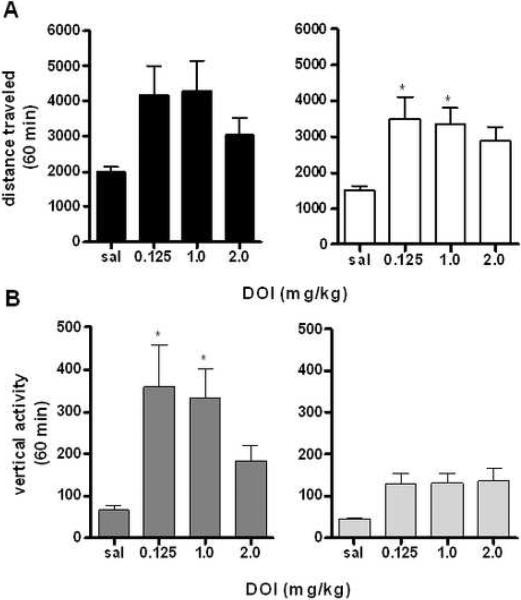
The 5-HT2A/2C agonist DOI increases locomotor activity in females
In previous studies, the 5-HT2A/2C agonist DOI alone did not change locomotor activity, although it has been found to potentiate the locomotor effects of psychostimulants such as cocaine (20). However, DOI has been known to exhibit anxiolytic effects, and it is possible that further studies with locomotor activity and vertical activity could reveal differences due to sex (42). Following two hours of habituation to the test chamber, animals were given either saline or DOI (0.125 –2.0 mg/kg) (31;42). Although no significant effect of sex (F1,63=1.192, p=0.2790) or interaction between sex and dose of drug (F3,63=0.03913, p=0.7596) was observed in initial analyses (for a time course of locomotor effects of DOI at a representative dose, see Supplemental Figure 7A), further analyses revealed that females showed a significant locomotor effect of DOI (F3,34=4.532, p<0.01 by one-way ANOVA for female responses to drug dose). Thus, while both male and female mice showed overall effects of drug on locomotor activity (F3,63=7.131, p<0.001) only females showed significant increases in activity at specific doses (0.125 and 1.0 mg/kg analyzed by One-way ANOVA, F3,35=4.532, p<0.01, Figure 6A).
Figure 6. Locomotor Responses to the 5-HT2A/2C Agonist DOI.
A: Effects of 5-HT2A/2C receptor agonist DOI on locomotor activity in male and female mice. *p<0.01 effect of RU 249696 dose, *p<0.001 effect of RU 24969 dose (n=9 per group). B: Effects of DOI on vertical rearing for 60 min following drug administration. *p<0.05 effect of RU 24969 dose (n=9 per group).
DOI increases vertical activity in males
In contrast to the results with locomotor activity, there was a marked difference between sex in vertical activity responses to the 5-HT2A/2C agonist DOI (F1,63=13.26, p<0.001), although all animals showed a significant response to drug (F3,63=6.342, p<.001). The sexes were subsequently separated to examine effects of DOI by dose. While both male and female mice showed overall effects of DOI on vertical activity (males: F3,35=4.625, p<0.01, females: F3,35=3.407, p<0.05, analyzed by one-way ANOVA for each sex relative to saline control, Figure 6B), only male mice showed significant effects of drug at specific doses (0.125 and 1.0 mg/kg, for a time course of vertical effects on male and female mice at 1.0 mg/kg, see Supplemental Figure 7B). Thus it appears that male and female mice show significant differences in their locomotor and vertical responses to DOI, although both sexes show a significant response to the 5-HT2A/2C agonist.
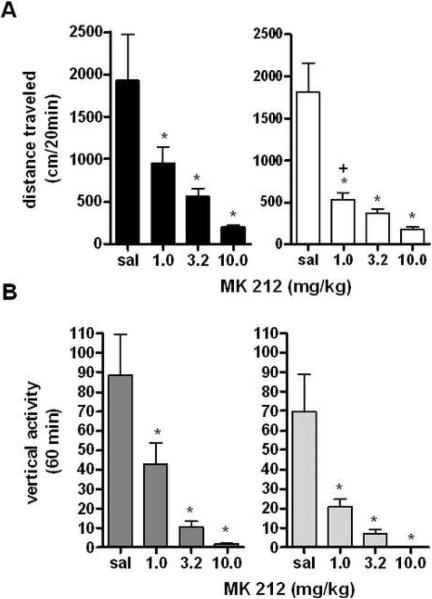
5-HT2C/2B agonist MK 212 decreases locomotor activity in male and female mice
Following two hours of habituation to the testing conditions, animals were given either saline or the 5-HT2C/2B agonist MK 212 (1.0–10.0 mg/kg) (50). Previous studies have shown that preferential activation of the 5-HT2C receptor produces significant decreases in locomotor activity (33). Our studies found similar results, with the 5-HT2C/2B agonist MK 212 producing drastic decreases in locomotor activity in all animals tested (F1,88=18.30, p<0.001), although no differences between the sexes in locomotor response was observed (F1,88=1.236, p=0.2694, Figure 7A). Separated by sex, male and female mice showed significant effects of MK 212 at all doses tested (males: F3,40=10.65, p<0.001, females: F3,40=28.40, p<0.001, analyzed by one-way ANOVA for each sex relative to saline control, for a time course of locomotor activity following a representative dose of MK 212, see Supplemental Figure 8A).
Figure 7. Locomotor Responses to the 5-HT2C/2B Agonist MK 212.
A: Effects of 5-HT2C/2B receptor agonist MK 212 on locomotor activity in male and female mice. *p<0.05 effect of 1.0 mg/kg MK 212, *p<0.001 effect of MK 212 dose (n=12 per group). B: Effects of MK 212 on vertical rearing for 20 min following drug administration. *p<0.05 effect of 1.0 mg/kg MK 212, *p<0.0001 effect of MK 212 dose (n=12 per group).
MK 212 decreases vertical activity in male and female mice
MK 212 appears to exert similar effects in both locomotor and vertical activity, with significant effects of drug on vertical rearing (F3,88=20.29, p<0.001). No differences between the sexes were observed (F1,88=0.2181, p=0.1433 analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis). For a time course of the effects of MK 212 on vertical activity, see Supplemental Figure 8B. When separated by sex, male and female mice both showed significant decreases in vertical activity at all doses of MK 212, confirming the results of the two-way ANOVA, matching results seen in the original two-way analysis. These results complement the work of earlier studies in the literature showing significant locomotor attenuating effects of 5-HT2C agonists in mice.
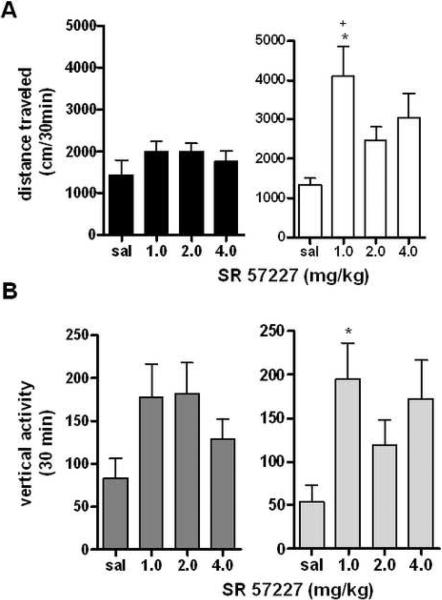
The 5-HT3 agonist SR 57227 increases locomotor activity in female mice
Very few studies have been performed examining the effects of 5-HT3 agonists on mouse locomotor behavior, most involving the induction of movement in paraplegic mice (21), with no examination of possible interactions with dopamine-related behaviors. Thus, a thorough examination of the locomotor effects of SR 57227 on male and female mice could provide further insight into the behavioral relevance of this receptor. Following two hours of habituation, animals were given either saline or SR 57227 (1.0–4.0 mg/kg) (23). The 5-HT3 agonist SR 57227 had a significant effect on locomotor activity when mice were grouped (F3,82=4.875, p<0.01), but a significant effect of sex was also detected (F1,82=9.225, p<0.01). Indeed, when sexs were separated and subjected to further analysis, it appeared that SR 57227 produced no locomotor effects in male mice (F3,43=0.9630, p=0.4196, analyzed by one-way ANOVA for each sex relative to saline control), but produced significant increases in locomotor activity in female mice (F3,45=4.411, p<0.01, effect of sex F1,82=9.225, p<0.01 analyzed by two-way repeated measures ANOVA with corrected Bonferroni post-hoc analysis). In female mice, this effect was particularly strong at the lowest dose of 1.0 mg/kg (p<0.05, Figure 8A), although increased activity as an effect of drug was evident at all doses (for a time course of locomotor effects at a selected dose of SR 57227, see Supplemental Figure 9A).
Figure 8. Locomotor Responses to the 5-HT3 Agonist SR 57227.
A: Effects of 5-HT3 receptor agonist SR 57227 on locomotor activity in male and female mice. *p<0.05 effect of 1.0 mg/kg SR 57227, +p<0.01 effect of sex at 1.0 mg/kg SR 57227 (n=11 per group). B: Effects of SR 57227 on vertical rearing for 60 min following drug administration. No effect of SR 57227 or sex was observed (n=11 per group).
The 5-HT3 agonist SR 57227 increases vertical activity in female mice only
The effects of SR 57227 were less pronounced in the vertical plane, and although there was an significant overall effect of drug (F3,84=4.509, p<0.01), no significant overall different in sex was detected (F1,84=0.1048, p=0.7470), Figure 8B). However, when animals were separated by sex to look at individual doses of drug, SR 57227 did not show significant effects on vertical activity in males (males: F3,44=2.210, p=0.1014, analyzed by one-way ANOVA for each sex relative to saline control). In contrast, females showed significant overall effects of locomotor activity, with the strongest effect at the lowest dose (females: F3,45=3.091, p<0.05, Figure 8B), which suggests effects of 5-HT3 agonists on locomotor and vertical activity are weak at best, as intersex differences cannot be effectively drawn when differences are apparent only at the lowest dose tested (for a time course of the effects of SR 57227 on vertical activity at a representative dose, see Supplemental Figure 9B).
Discussion
5-HT receptors have strong modulatory actions on the DA system, influencing DA neuron firing rates, presynaptic DA release, and postsynaptic DA-responsive cells (1). In the present studies we documented the effects of systemic administration of various 5-HT direct and indirect agonists on locomotor activity and vertical rearing, two behaviors which are known to be DA-related (15;16). We investigated the effects of two SSRIs, fluoxetine, and the more selective citalopram, as well as receptor agonists for five receptors known to interact with the DA system: the 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C and 5-HT3 receptors. Our experiments showed that each receptor agonist can be distinguished by its locomotor profile, and that significant sex differences exist for many of the subtype-specific drugs tested.
There is a large literature on sex differences in responsiveness to direct and indirect DA receptor agonists in rodents. For example, females exhibit more intense locomotor activation in response to most stimulants, although males show greater DA responses to cocaine (22), and males are known to be more sensitive to the behavioral effects of D2 agonists (47). Since 5-HT agonists are modulators of dopaminergic activity, underlying sex differences in the DA system could play an important role in the effects of 5-HT receptor agonists on locomotor activity in males and females.
The SSRI fluoxetine dose-responsively decreased activity in the present studies, in contrast to previous reports showing that fluoxetine increases locomotor activity in adult CD1 and NMRI mice (18;8). Our studies also found dose-dependent locomotor decreases. However, it should be noted that in the previous studies showing increases in activity, animals were exposed to a novel environment following drug administration (18), an important difference from the present study. Also, it should be noted that there are differential effects of fluoxetine between strain of mice, as well as between mice and rats. Fluoxetine is known to decrease locomotor activity in young (49) and adult rats (32). Additionally, our study used a specific strain of mouse, C57BL/6J, a strain noted for its insensitivity to antidepressants such as fluoxetine in the forced swim test. The neurochemical effects of fluoxetine which could produce attenuation of locomotor activity are complex because it is an uptake inhibitor, elevating extracellular 5-HT levels and causing activation of potentially diverse receptors in various brain regions at different doses. Also, in addition to being an inhibitor of the 5-HT transporter, fluoxetine is an agonist at 5-HT2C receptors (38) which are known to decrease locomotor activity (33), and which could have produced some of the effects seen in the current study.
In contrast to fluoxetine, the more selective SSRI citalopram produced decreases in locomotor and vertical activity only at the highest dose used (20.0 mg/kg, i.p.). Given that citalopram is an indirect agonist, it is possible that increases in extracellular 5-HT produced by this drug will activate diverse subtypes of receptors at different doses, producing mixed effects on activity. In previous studies of habituated rats and mice, citalopram had no effects on locomotor activity, although increases in activity have been shown in unhabituated mice (8). In general, SSRIs have no stimulant properties on their own and do not promote self-administration (51), but acute SSRI administration potentiates the locomotor-activating effects of psychostimulants such as cocaine and amphetamine (9). However, in our hands, acute SSRI administration alone had attenuating effects on locomotor activity. It should be noted, however, that female mice appeared to show a significant locomotor stimulating effect of citalopram at the two lowest doses, although female mice also showed significant locomotor attenuation at the highest dose tested. This augmentation in locomotor activity is surprising, especially considering the significant locomotor attenuating effects of the similar SSRI fluoxetine. However, fluoxetine and citalopram do have significant differences, as fluoxetine is known to be less selective, and in particular, to have activity at the 5-HT2C receptor, which could account for some of the locomotor attenuating effects (32). Additionally, it is possible that the effects seen with citalopram could be due to the extremely low activity exhibited by the female mice in response to saline. However, these mice are the only appropriate control for these locomotor responses. Additionally, because the differences measured are in behavior following drug administration, differences in absorption and distribution of drug in male and female mice should not be discounted.
The 5-HT1A receptor is one of the most-studied 5-HT receptors with regard to sex differences. It appears that, although there are no differences in 5-HT1A receptor binding levels (53), female rats have increased sensitivity to the anxiolytic effects of 5-HT1A agonists (6). The 5-HT1A agonist 8-OH-DPAT has also been shown to decrease locomotor activity in mice (14), and in the present studies, 8-OH-DPAT significantly attenuated locomotor activity in male mice at all doses tested and attenuated vertical rearing in both male and female mice. In the present studies, female mice were found to be less sensitive to the locomotor attenuating effects of 8-OH-DPAT, and both sexes were affected equally with regard to vertical activity. Our data thus imply that 5-HT1A activation results in locomotor attenuation in male and female mice, although females are less susceptible to the locomotor-attenuating effects. It should be noted that 8-OH-DPAT is also an agonist at 5-HT7 receptors (2), and while this may have some effect on locomotor activity, 5-HT7 receptors are located in the thalamus, hypothalamus, and amygdala, and thus may not have direct effects on dopamine system interactions resulting in changes in locomotor activity (24). While the effects of 8-OH-DPAT at 5-HT7 receptors cannot be ignored and may play a role in the effects, this mechanism seems less likely to directly affect locomotor activity than the actions of 8-OH-DPAT at 5-HT1A. The 5-HT1B receptor has been relatively well-characterized with regard to locomotor activity, although sex differences have not been extensively explored. It is known that the 5-HT1B agonist RU 24969 enhances locomotor activity (13), and our results are consistent with this, showing that systemic RU 24969 potentiates locomotor activity in both sexes with no effect on vertical activity, although females appear to be more sensitive to the locomotor stimulating effects. It is hypothesized that activation of 5-HT1B receptors on GABA neurons in the VTA produces inhibition of a tonic inhibitory influence on DA neurons, resulting in increased DA neuron firing to regions such as the NAc (40), where increased DA levels lead to locomotor activation (39). Although RU 24969 is known to also activate 5-HT1A receptors, these effects are generally opposite to those seen with 5-HT1B agonists, resulting in substantial decreases in locomotor activity. The significant increase in locomotor activity in response to RU 24969 in male and female mice thus seem to indicate the effect of the 5-HT1B agonist, although the effects of RU 24969 at 5-HT1A receptors should not be discounted. Thus, it appears that 5-HT1B receptors have activating effects on the DA system and lead to enhanced locomotion both in isolation (present results, 11) and in combination with stimulants (7;44).
Sex differences in 5-HT2A receptors have been documented. For example, juvenile female rats have higher 5-HT2A receptor binding levels than their male counterparts (53). In previous studies, 5-HT2A stimulation has been shown to increase DA cell firing in the VTA (43). In this study, administration of the 5-HT2A/2C agonist DOI increased locomotor activity significantly above baseline in all animals, although only female mice showed significant effects at specific doses. Differences between the sexes were also marked in measures of vertical activity, where males showed significant increases in vertical rearing in response to intermediate doses of DOI. It is interesting that the effects of 5-HT2A agonists should reveal such strong sex differences between locomotor and vertical activity measures, and further studies may reveal whether this change is due to differences in receptor densities.
Because DOI is an agonist at both 5-HT2A and 5-HT2C receptors, we also looked at the effects of the 5-HT2C/2B agonist MK 212, which activates 5-HT2C receptors preferentially (37). 5-HT2C receptor activation has been shown to decrease locomotor activity (33), and our findings agree with previous studies in the literature. MK 212 decreased spontaneous locomotor activity at all doses tested in both male and female mice, with no differences between sexes at any dose. Previous literature found that males are more sensitive to 5-HT2C stimulation (3), and it is possible that doses used in this study were not low enough to show such differences. Interestingly, the locomotor profile of MK 212 appeared similar to that of the SSRI fluoxetine, indicating that the locomotor attenuating effects of fluoxetine may be partially due to stimulation of the 5-HT2C receptor.
The ionotropic 5-HT3 receptor remains little studied with regard to sex differences. 5-HT3 agonists increase somatodendritic release of DA in the VTA (12), while antagonists appear to reduce the number of spontaneously active DA cells (35). In this study, the 5-HT3 agonist SR 57227 appeared to have little effect on locomotor activity, increasing activity only in female animals at the lowest dose used. In contrast, SR 57227 significantly elevated vertical activity in both males and females, although no sex difference was observed. This is in accordance with literature on SR 57227, indicating that 5-HT3 agonists have little effect on locomotor activity when administered alone (52), (23).
Although we have attributed the behavioral and sex-specific effects of 5-HT system and receptor agonists to their activity at 5-HT receptors, differences in the absorption, distribution, and metabolism of these drugs cannot be discounted. Unfortunately, few studies have examined the pharmacokinetics of these drugs in mice, and to the best of the authors' knowledge, no studies have been done on potential sex differences in metabolism in male and female mice. Studies that have been performed with fluoxetine have assumed no difference between male and female mice (27;28). Studies on the distribution and metabolism of citalopram and fluoxetine in humans have found no differences have been found due to sex (26), although strong effects of age are evident.
In addition, it is best to be careful when making the assumption that the locomotor effects seen in response to 5-HT receptor agonists mediate these effects through interactions with the DA system. Although changes in DA in the area of the striatum and NAc are strongly correlated with changes in locomotor activity (29;15;10), there is also evidence that increases in locomotor activity are not necessarily dependent on increases in DA (36). Thus, it is possible that the effects of the 5-HT receptor agonists tested in this study could be exerting their locomotor effects via both direct and indirect means, either through interactions with the dopaminergic system, or as locomotor agonists in their own right. Unfortunately, the present study cannot address this point, and future studies will be needed to separate the direct and indirect effects of serotonergic agonists.
Taken together, our data indicate that the effects of many 5-HT receptors within the DA system can be detected using a simple locomotor behavior paradigm, and that these effects are characteristic of agonists of specific receptor subtypes. SSRIs and specific 5-HT receptor agonists all exhibited sex differences in their locomotor and vertical activity profiles, indicating that DA/5-HT interactions may differ extensively between sexes. These data could provide an effective measure of differences between male and female mice with regard to DA/5-HT interactions. Given that both the DA and 5-HT systems are intimately involved with many psychiatric disturbances, such as anxiety, depression, and drug abuse, differences in D/-5-HT interactions between males and females could provide insight into sex difference in these disorders, as well as aid in determining possible sex differences in responses to drugs targeting these receptors in humans. Thus, a convenient behavioral screen for differences in locomotor responses to 5-HT agonists could reveal differences in receptor responses in DA/5-HT interactions. It is hoped that these studies will shed further light on the behavioral effects of DA/5-HT interactions, as well as differences between the sexes, providing a useful template to study DA/5-HT interactions in the context of both normal and neurobiological disease states.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Dr. Mark Ferris and Dr. Rodrigo España for their assistance with statistical analyses.
Abbreviations
- DA
Dopamine
- 5-HT
serotonin
- SSRI
selective serotonin reuptake inhibitor
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tertraline
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2 amino propane
- RU 24969
5-methoxy-n1N-dimethyltryptamine and 5-methoxy-3(1,2,3,6,-tetrahydro-4-pyrindinyl)-1H-indole
- MK 212
6-Chloro-2-(1-piperazinyl)pyrazine hydrochloride
- SR 57227
1-(6-Chloro-2-pyridinyl)-4-piperidinamine hydrochloride
- fluoxetine
(±)-N-Methyl-γ-[4-(trifluoromethyl)phenoxy]benzenepropanamine hydrochloride
- citalopram
1-[3-(Dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancarbonitrile hydrobromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol.Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvidsson LE, Hacksell U, Nilsson JL, Hjorth S, Carlsson A, Lindberg P, Sanchez D, Wikstrom H. 8-Hydroxy-2-(di-n-propylamino)tetralin, a new centrally acting 5-hydroxytryptamine receptor agonist. J.Med.Chem. 1981;24:921–923. doi: 10.1021/jm00140a002. [DOI] [PubMed] [Google Scholar]
- 3.Bagdy G. Serotonin, anxiety, and stress hormones. Focus on 5-HT receptor subtypes, species and gender differences. Ann.N.Y.Acad.Sci. 1998;851:357–363. doi: 10.1111/j.1749-6632.1998.tb09009.x. [DOI] [PubMed] [Google Scholar]
- 4.Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- 5.Bert B, Fink H, Hortnagl H, Veh RW, Davies B, Theuring F, Kusserow H. Mice over-expressing the 5-HT(1A) receptor in cortex and dentate gyrus display exaggerated locomotor and hypothermic response to 8-OH-DPAT. Behav.Brain Res. 2006;167:328–341. doi: 10.1016/j.bbr.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard DC, Shepherd JK, Rodgers RJ, Blanchard RJ. Evidence for differential effects of 8-OH-DPAT on male and female rats in the Anxiety/Defense Test Battery. Psychopharmacology (Berl) 1992;106:531–539. doi: 10.1007/BF02244826. [DOI] [PubMed] [Google Scholar]
- 7.Borycz J, Zapata A, Quiroz C, Volkow ND, Ferre S. 5-HT 1B receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008;33:619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- 8.Brocco M, Dekeyne A, Veiga S, Girardon S, Millan MJ. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake. A pharmacological characterization of diverse classes of antidepressant agents. Pharmacol.Biochem.Behav. 2002;71:667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- 9.Bubar MJ, McMahon LR, De Deurwaerdere P, Spampinato U, Cunningham KA. Selective serotonin reuptake inhibitors enhance cocaine-induced locomotor activity and dopamine release in the nucleus accumbens. Neuropharmacology. 2003;44:342–353. doi: 10.1016/s0028-3908(02)00381-7. [DOI] [PubMed] [Google Scholar]
- 10.Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci.Lett. 2000;281:9–12. doi: 10.1016/s0304-3940(00)00813-2. [DOI] [PubMed] [Google Scholar]
- 11.Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav.Brain Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 12.Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- 13.Chaouloff F, Courvoisier H, Moisan MP, Mormede P. GR 127935 reduces basal locomotor activity and prevents RU 24969-, but not D-amphetamine-induced hyperlocomotion, in the Wistar-Kyoto hyperactive (WKHA) rat. Psychopharmacology (Berl) 1999;141:326–331. doi: 10.1007/s002130050841. [DOI] [PubMed] [Google Scholar]
- 14.Chojnacka-Wojcik E. Involvement of dopamine autoreceptors in the hypoactivity induced by 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in mice. Pol.J.Pharmacol.Pharm. 1992;44:135–146. [PubMed] [Google Scholar]
- 15.Christie JE, Crow TJ. Possible role of dopamine-containing neurones in the behavioural effects of cocaine. Br.J.Pharmacol. 1971;42:643P–645P. [PMC free article] [PubMed] [Google Scholar]
- 16.Costall B, Eniojukan JF, Naylor RJ. Spontaneous climbing behaviour of mice, its measurement and dopaminergic involvement. Eur.J.Pharmacol. 1982;85:125–132. doi: 10.1016/0014-2999(82)90457-5. [DOI] [PubMed] [Google Scholar]
- 17.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 18.de Angelis L. Experimental anxiety and antidepressant drugs: the effects of moclobemide, a selective reversible MAO-A inhibitor, fluoxetine and imipramine in mice. Naunyn Schmiedebergs Arch.Pharmacol. 1996;354:379–383. doi: 10.1007/BF00171072. [DOI] [PubMed] [Google Scholar]
- 19.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 20.Filip M, Bubar MJ, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J.Pharmacol.Exp.Ther. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841. [DOI] [PubMed] [Google Scholar]
- 21.Geyer MA. Serotonergic functions in arousal and motor activity. Behav.Brain Res. 1996;73:31–35. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Griffin WC, III, Middaugh LD. The influence of sex on extracellular dopamine and locomotor activity in C57BL/6J mice before and after acute cocaine challenge. Synapse. 2006;59:74–81. doi: 10.1002/syn.20218. [DOI] [PubMed] [Google Scholar]
- 23.Guertin PA, Steuer I. Ionotropic 5-HT3 receptor agonist-induced motor responses in the hindlimbs of paraplegic mice. J.Neurophysiol. 2005;94:3397–3405. doi: 10.1152/jn.00587.2005. [DOI] [PubMed] [Google Scholar]
- 24.Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br.J.Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- 26.Hildebrandt MG, Steyerberg EW, Stage KB, Passchier J, Kragh-Soerensen P. Are gender differences important for the clinical effects of antidepressants? Am.J.Psychiatry. 2003;160:1643–1650. doi: 10.1176/appi.ajp.160.9.1643. [DOI] [PubMed] [Google Scholar]
- 27.Holladay JW, Dewey MJ, Yoo SD. Quantification of fluoxetine and norfluoxetine serum levels by reversed-phase high-performance liquid chromatography with ultraviolet detection. J.Chromatogr.B Biomed.Sci.Appl. 1997;704:259–263. doi: 10.1016/s0378-4347(97)00470-2. [DOI] [PubMed] [Google Scholar]
- 28.Holladay JW, Dewey MJ, Yoo SD. Pharmacokinetics and antidepressant activity of fluoxetine in transgenic mice with elevated serum alpha-1-acid glycoprotein levels. Drug Metab Dispos. 1998;26:20–24. [PubMed] [Google Scholar]
- 29.Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- 30.Kelly PH. Unilateral 6-hydroxydopamine lesions of nigrostriatal or mesolimbic dopamine-containing terminals and the drug-induced rotation of rats. Brain Res. 1975;100:163–169. doi: 10.1016/0006-8993(75)90253-x. [DOI] [PubMed] [Google Scholar]
- 31.Liang J, Wang X, Lu Y, Liu R, Zhang Q, Sun H, Li L. Effects of antidepressants on the exploration, spontaneous motor activity and isolation-induced aggressiveness in mice. Beijing Da.Xue.Xue.Bao. 2003;35:54–60. [PubMed] [Google Scholar]
- 32.Maj J, Rogoz Z, Skuza G, Wedzony K. The synergistic effect of fluoxetine on the locomotor hyperactivity induced by MK-801, a non-competitive NMDA receptor antagonist. J.Neural Transm. 1996;103:131–146. doi: 10.1007/BF01292622. [DOI] [PubMed] [Google Scholar]
- 33.Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Kohler C, Delft AM. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J.Pharmacol.Exp.Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- 34.Mateo Y, Budygin EA, John CE, Jones SR. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc.Natl.Acad.Sci.U.S.A. 2004;101:372–377. doi: 10.1073/pnas.0207805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minabe Y, Ashby CR, Jr., Wang RY. The effect of acute and chronic LY 277359, a selective 5-HT3 receptor antagonist, on the number of spontaneously active midbrain dopamine neurons. Eur.J.Pharmacol. 1991;209:151–156. doi: 10.1016/0014-2999(91)90163-k. [DOI] [PubMed] [Google Scholar]
- 36.Murphy NP, Lam HA, Maidment NT. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J.Neurochem. 2001;79:626–635. doi: 10.1046/j.1471-4159.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- 37.Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav.Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- 38.Ni YG, Miledi R. Blockage of 5HT2C serotonin receptors by fluoxetine (Prozac) Proc.Natl.Acad.Sci.U.S.A. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Dell LE, Manzardo AM, Polis I, Stouffer DG, Parsons LH. Biphasic alterations in serotonin-1B (5-HT1B) receptor function during abstinence from extended cocaine self-administration. J.Neurochem. 2006;99:1363–1376. doi: 10.1111/j.1471-4159.2006.04163.x. [DOI] [PubMed] [Google Scholar]
- 40.O'Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J.Pharmacol.Exp.Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- 41.O'Neill MF, Fernandez AG, Palacios JM. GR 127935 blocks the locomotor and antidepressant-like effects of RU 24969 and the action of antidepressants in the mouse tail suspension test. Pharmacol.Biochem.Behav. 1996;53:535–539. doi: 10.1016/0091-3057(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 42.Onaivi ES, Bishop-Robinson C, Darmani NA, Sanders-Bush E. Behavioral effects of (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane, (DOI) in the elevated plus-maze test. Life Sci. 1995;57:2455–2466. doi: 10.1016/0024-3205(95)02242-9. [DOI] [PubMed] [Google Scholar]
- 43.Pessia M, Jiang ZG, North RA, Johnson SW. Actions of 5-hydroxytryptamine on ventral tegmental area neurons of the rat in vitro. Brain Res. 1994;654:324–330. doi: 10.1016/0006-8993(94)90495-2. [DOI] [PubMed] [Google Scholar]
- 44.Przegalinski E, Siwanowicz J, Nowak E, Papla I, Filip M. Role of 5-HT(1B) receptors in the sensitization to amphetamine in mice. Eur.J.Pharmacol. 2001;422:91–99. doi: 10.1016/s0014-2999(01)01079-2. [DOI] [PubMed] [Google Scholar]
- 45.Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;253:231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- 46.Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS.J. 2007;9:E1–10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol.Biochem.Behav. 2002;72:857–863. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 48.SMITH CB. EFFECTS OF D-AMPHETAMINE UPON BRAIN AMINE CONTENT AND LOCOMOTOR ACTIVITY OF MICE. J.Pharmacol.Exp.Ther. 1965;147:96–102. [PubMed] [Google Scholar]
- 49.Stanford JA, Currier TD, Gerhardt GA. Acute locomotor effects of fluoxetine, sertraline, and nomifensine in young versus aged Fischer 344 rats. Pharmacol.Biochem.Behav. 2002;71:325–332. doi: 10.1016/s0091-3057(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 50.Walker EA, Kohut SJ, Hass RW, Brown EK, Jr., Prabandham A, Lefever T. Selective and nonselective serotonin antagonists block the aversive stimulus properties of MK212 and m-chlorophenylpiperazine (mCPP) in mice. Neuropharmacology. 2005;49:1210–1219. doi: 10.1016/j.neuropharm.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Woods JH, Tessel RE. Fenfluramine: amphetamine congener that fails to maintain drug-taking behavior in the rhesus monkey. Science. 1974;185:1067–1069. doi: 10.1126/science.185.4156.1067. [DOI] [PubMed] [Google Scholar]
- 52.Yoo JH, Cho JH, Yu HS, Lee KW, Lee BH, Jeong SM, Nah SY, Kim HC, Lee SY, Jang CG. Involvement of 5-HT receptors in the development and expression of methamphetamine-induced behavioral sensitization: 5-HT receptor channel and binding study. J.Neurochem. 2006;99:976–988. doi: 10.1111/j.1471-4159.2006.04137.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.