Abstract
Purpose
To evaluate the relationship between human papillomavirus (HPV) status and known prognostic makers for head and neck cancers including tumor hypoxia, epidermal growth factor receptor (EGFR) expression and intratumoral T-cell levels and to determine the prognostic impact of these markers by HPV status.
Methods and Materials
HPV status in 82 evaluable head and neck squamous cell carcinomas patients was determined by pyrosequencing and related to p16INK4a staining and treatment outcomes. It was correlated with tumor hypoxia (tumor pO2 and carbonic anhydrase [CAIX] staining), EGFR status, and intratumoral lymphocyte expression (CD3 staining).
Results
Forty-four percent of evaluable tumors had strong HPV signal by pyrosequencing. There was a significant relationship between strong HPV signal and p16INK4a staining as well as oropharynx location. The strong HPV signal group fared significantly better than others, both in time to progression (TTP, p = 0.008) and overall survival (OS, p = 0.004) for all patients and for the oropharyngeal subset. Positive p16INK4a staining was associated with better TTP (p = 0.014) and OS (p = 0.00002). There was no relationship between HPV status and tumor pO2 or CAIX staining. However, HPV status correlated inversely with EGFR reactivity (p = 0.0006) and directly with CD3(+) T-lymphocyte level (p = 0.03). Whereas CAIX and EGFR overexpression were negative prognostic factors regardless of HPV status, CD3(+) T-cell levels was prognostic only in HPV(−) tumors.
Conclusion
HPV status was a prognostic factor for progression and survival. It correlated inversely with EGFR expression and directly with T-cell infiltration. The prognostic effect of CAIX and EGFR expression was not influenced by HPV status, whereas intratumoral T-cell levels was significant only for HPV(−) tumors.
Keywords: HPV, Head and neck cancer, p16INK4a, EGFR, Hypoxia, CD3
Introduction
Head and neck squamous cell carcinomas (HNSCC) are heterogeneous and traditionally associated with tobacco and alcohol use (1). Recently, human papillomavirus (HPV) has been implicated in the development of certain HNSCC, specifically oropharyngeal carcinomas (OP) (2–4). HPV positive [HPV(+)] tumors differ from HPV negative [HPV(−)] tumors in many aspects including histologic appearance, differentiation, risk factors and prognosis (5).
At the molecular level, several differences are linked to HPV status. HPV integration leads to increased expression of the E6 and E7 oncogenes, which neutralize the p53 and retinoblastoma (Rb) tumor suppressor pathways, causing perturbation in key cell-cycle proteins (6, 7). One protein is p16INK4A, which acts by binding to cell-cycle regulators. Loss of p16INK4A is associated with poor prognosis in HNSCC and the converse is true for its overexpression (8, 9). In tobacco-related HNSCC, p16INK4a is often absent because of homologous deletion or promoter hypermethylation. However, in HPV(+) HNSCC tumors, p16INK4a is over-expressed because of Rb loss and is subsequently used as a surrogate marker for HPV.
The improved apoptotic response to chemoradiation due to less p53 mutations and functional p16INK4a may explain the improved outcomes for HPV(+) tumors. However, these mechanisms alone cannot explain the better prognosis when these tumors were treated with surgery alone (10). Other hypotheses for improved outcomes in HPV(+) tumors include the lack of field cancerization and enhanced immune surveillance (5). Although several molecular markers have been studied for prognostication in HNSCC (11–15), few have been validated in large prospective studies. Certain validated factors include tumor hypoxia and epidermal growth factor receptor (EGFR) status (14, 15). The relationship between HPV infection and these factors are unclear. Therefore, in this study, we assessed HPV presence in HNSCC using high throughput pyrosequencing and p16INK4a staining, evaluated the prognostic significance of HPV and p16INK4a in these tumors, explored the relationship between HPV and tumor hypoxia (by tumor pO2 and tumor expression of carbonic anhydrase IX [CAIX], a hypoxia-induced protein), EGFR expression, and intratumoral T-cell levels (by staining for CD3, a pan-T-cell marker) and determined the prognostic impact of these three biomarkers in relation to HPV status.
Methods and Materials
Patients
Criteria for patient participation included (1) newly diagnosed HNSCC, (2) available tissue block, and (3) willingness to sign an informed consent. All tumors were staged using the 2002 American Joint Committee on Cancer staging system (16).
DNA extraction
Four-micron-thick hematoxylin-and-eosin (H&E) sections were first examined by a pathologist (C.K.) for tumor. Eighty-nine of 99 cases (90%) had 50% or more tumor in the 4-micron-thick H&E sections examined. The remaining 10/99 cases (10%) had at least 25% tumor. Three to four 10-micron-thick scrolls were then cut from each block and used for DNA extraction. DNA was isolated from paraffin-embedded tumors using Ambion's RecoverAll Total Nucleic Acid Isolation kit (Applied Biosystem, Austin, TX) as instructed. The eluted DNA concentration was determined using a NanoDrop ND-1000 Spectrophotometer (Thermoscientific, Pittsburgh, PA), and samples were stored at −20°C.
HPV PCR/Pyrosequencing
Polymerase chain reaction (PCR) for the L1 region of HPV was performed using the forward primer GP5+ (5′-TTTGTTACTGTTG TTGATACTAC-3′) and the biotinylated reverse primer GP6+ (5′-GAAAAATAAACTGTAAATCATATTC-3′) as described (17). β-globulin was used as an internal amplification control to ensure the DNA integrity. Seventeen of 99 samples were inevaluable for HPV analysis: 4 with negative and 13 with weak β-globulin amplification signals, yielding 82 samples for pyrosequencing analysis (17). The quantitative gel-based exACTGGene 50-bp Mini DNA Ladder (Fisher Scientific, Pittsburgh, PA) was used to measure DNA concentration of HPV-fragment amplicons. Amplicon concentration <100 ng/10 μL PCR was scored as “weak” and ≥100 ng/10 μL PCR as “strong.” The amplicons were thereafter pyrosequenced as described subsequently.
Pyrosequencing was performed by immobilizing the biotinylated PCR products onto streptavidin-coated High Performance Sepharose beads (Amersham Biosciences/GE Healthcare Biosciences Corp., Piscataway, NJ). The beads were subjected to the following: washing with 70% ethanol, denaturing DNA with 0.2 M sodium hydroxide and washing with TE-buffer to remove nonimmobilized complementary strands. The beads were incubated in 12 uL annealing buffer containing 0.4 pmol sequencing primer at 95°C for 2 min, 50°C for 5 min, and 25°C for 5 min (17).
For each sample, four reactions were prepared, one for each primer pool. GP5+ is a general primer designed to provide sequence signals for any HPV type. Three multiple sequencing primer pools (MSP), each containing four type-specific primers, were also used. MSP1 contains primers specific to HPV16, 31, 59, and 39; MSP2 for HPV18, 33, 52, and 56; and MSP3 for HPV35, 45, 51, and 58. These pools were designed to target the most common high-risk HPV types.
Following primer hybridization, samples were sequenced using the PSQ HS96A System (Biotage, Charlottesville, VA) as described (17). Ten cycles of A-C-G-T were dispensed for each assay. Nucleotide incorporation events were determined by detection of photons generated upon pyrophosphate release during polymerase extension. Sample type was determined by comparing sequence signals for each primer pool to known data.
Tissue array generation and immunohistochemical staining
The tissue microarray (TMA) was constructed as previously described (18). Immunoperoxidase stains for p16INK4a (clone E6H4, Dako, Carpinteria, CA), EGFR (31G7 mouse monoclonal, Zymed, South San Francisco, CA), CD3 (rabbit monoclonal, Cell Marque, Rocklin, CA), and CAIX (M75 mouse monoclonal, Bayer, Pittsburgh, PA) were performed on 4-μM-thick sections of the TMA. CD3 was also performed on corresponding 4-μM-thick whole tumor sections.
The TMA and whole sections were interpreted by a pathologist (CSK), who was blinded to the clinical data, and scored for EGFR and CAIX staining as follows: 0 as negative, 1 as uninterpretable (when the core was either missing or heavily folded, making it impossible to interpret), 2 as weak and 3 as strong staining. For EGFR, scoring was based on membrane staining; the percentage of positive cell staining was also scored as follows: 1 for 0%–10% positive, 2 for 11%–50%, 3 for 51%–80%, and 4 for 81%–100%. For CAIX, scoring was performed separately for cytoplasmic and membrane staining but interpreted as strong if either was scored strongly positive. For p16INK4a, weak cytoplasmic staining in <5% of the cells was interpreted as negative, focal strong nuclear and/or cytoplasmic staining in 5%–80% of the cells as focally positive, and diffuse strong staining in >80% of the cells as diffusely positive. For the purpose of multivariate analysis, both focally and diffusely positive tumors were considered positive. Previous studies on cervical dysplasia have shown that diffusely strong p16INK4a reactivity correlated with high-risk HPV, whereas focally strong reactivity correlated with both high and low-risk HPV (19–21).
Tumor pO2 measurement
All measurements were performed using a computerized histograph (Sigma Eppendorf PO2 Histograph, Hamburg, Germany) as described (22).
Statistical analysis
Statistical analyses were performed using the R version 2.5.l (23). The Fisher exact test was used to correlate HPV PCR status to different parameters. Time to progression (TTP), disease-specific survival (DSS), and overall survival (OS) (24). Log-rank statistics were used to compare survival curves (25). Variables evaluated on univariate model were age, sex, smoking status, hemoglobin, N-classification, HPV status, p16INK4a staining, and treatment. Only those that achieved a p value <0.05 were included in a Cox proportional hazard model for multivariate analysis (25).
Results
HPV status and typing
Tissues from 99 patients including 61 with OP primary and 38 with HNSCC from other primary sites (13 oral cavity, 8 larynx, 11 hypopharynx, 3 nasopharynx, 2 paranasal sinuses, and 1 unknown primary) were tested for the presence of HPV using pyrosequencing. Eighty-two of 99 patients had evaluable HPV pyrograms and formed the final cohort of this study. Of these 82 patients, 26 had undetectable PCR signal, 20 had weak signal defined as PCR amplicon concentration <100 ng/10 μL, and 36 had strong signals defined as PCR amplicon concentration ≥100 ng/10 μL (Fig. 1A). The classification of weak and strong signal was further validated in a blinded fashion using quantitative real-time PCR and normalized against β-globulin in 12 randomly selected samples. As shown by the box-plot in Fig. 1B, all strong samples has a normalized ratio >1.5, whereas all but one of the weak samples had normalized ratio <1; the difference between the two groups was highly statistically significant (p < 0.0001), confirming the validity of the classification. Twelve HPV subtypes were simultaneously evaluated; infection by a single type was noted in 51 patients and mixed infection in 5 patients (1 with 16/18, 1 with 16/33, and 3 with multiple strains). Fifty of 51 patients with single infection harbored HPV16.
Fig. 1.
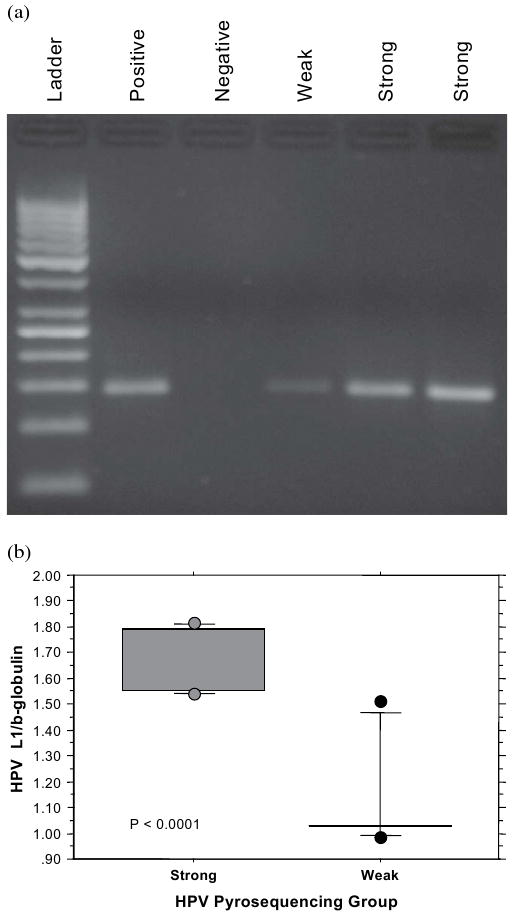
(a) Representative agarose gel stained with ethidium bromide for fragment size determination (at expected size of ∼185 base pairs) and polymerase chain reaction (PCR) amplification yield for the L1 region of human papillomavirus (HPV) genome. The gel shows PCR results for samples with strong, weak, and negative signal intensity based on measured amplicon concentration (undectable level as negative, <100 ng/10 μL as weak and ≥100 ng/10 μL strong). (b) Box and whisker plot, showing the quantitative real-time PCR results for HPV L1 signals normalized against β-globulin in 12 randomly selected samples: 6 from the HPV pyrosequencing strong group and 6 from the HPV pyrosequencing weak group.
Relationship between HPV status, p16INK4a staining, and clinical parameters
Table 1 shows the relationship between p16INK4a expression and HPV status by pyrosequencing. p16INK4a expression strongly correlated with HPV status; specifically most tumors with strong HPV signal showed diffuse-strong staining for p16INK4a, and those with negative or weak HPV signal were predominantly p16INK4a negative. Of the six tumors that displayed strong HPV pyrosequencing signal but negative p16INK4a staining, only one patient fit the HPV-related tumor profile as a nonsmoker with an oropharyngeal carcinoma. The other five patients were all heavy smokers with 30–60 pack-year of cigarette use, and their tumors were located in the oropharynx in 3 patients, hypopharynx in 1 patient, and oral cavity in 1 patient.
Table 1.
Relationship between HPV pyrosequencing status and p16INK4a staining intensity*
| p16INK4a staining intensity | HPV pyrosequencing status | Strong | Total | |
|---|---|---|---|---|
| Negative | Weak | |||
| Negative | 20 (24.4%) | 16 (19.5%) | 6 (7.3%) | 42 (51.2%) |
| Focally strong | 5 (6.1%) | 3 (3.7%) | 6 (7.3%) | 14 (17.1%) |
| Diffusely strong | 1 (1.2%) | 1 (1.2%) | 24 (29.3%) | 26 (31.7%) |
| Total | 26 (31.7%) | 20 (24.4%) | 36 (43.9%) | 82 (100%) |
Abbreviation: HPV = human papillomavirus.
p <0.0001, χ2 test.
We also evaluated the relationship between HPV status by pyrosequencing and tumor site. Similar to prior reports, 92% of strong HPV tumors were located in the oropharynx. However, only 35% of the tumors with weak HPV signal were located in the oropharynx, a frequency that was identical to that of the HPV(−) group. Because of the similarities between the HPV(−) and weak groups in p16INK4a staining and primary tumor site, we combined these two groups together when analyzing the relationship between HPV status and various clinical factors (Table 2). In general, patients with strong HPV signal were younger, had more advanced nodal stages, and had higher hemoglobin levels. With respect to treatment strategy, patients were classified as receiving either definitive nonsurgical therapy (64 patients) with either radiation alone (3 patients) or chemoradiation (61 patients) or definitive surgical therapy (18 patients) without any adjuvant therapy (2 patients), with adjuvant radiation (9 patients), or with adjuvant chemoradiation (7 patients) for high-risk pathologic features such as extracapsular nodal extension or involved surgical margins. Because of our institutional preference of treating most oropharyngeal carcinomas with nonsurgical therapy, the majority of patients with strong HPV signal (33 of 36) received nonsurgical treatment, whereas only three patients were treated with primary surgery for tonsillar carcinoma.
Table 2.
Relationship between HPV pyrosequencing status and clinical parameters
| Parameter | HPV Neg/wk (n = 46) | HPV Strong (n = 36) | p value |
|---|---|---|---|
| Age | |||
| Mean (±SE) | 61 (± 2.1) | 55 (± 1.6) | 0.03 |
| <60 | 20 (24.4%) | 26 (31.7%) | 0.01 |
| ≥60 | 26 (31.7%) | 10 (12.2%) | |
| Gender | |||
| Male | 36 (43.9%) | 33 (40.2%) | 0.13 |
| Female | 10 (12.2%) | 3 (3.7%) | |
| Site | |||
| Oropharynx | 16 (19.5%) | 33 (40.2%) | <0.0001 |
| Oral cavity | 12 (14.6%) | 1 (1.2%) | |
| Larynx | 6 (7.3%) | 1 (1.2%) | |
| Hypopharynx | 6 (7.3%) | 1 (1.2%) | |
| Nasopharynx | 3 (3.7%) | 0 (1.2%) | |
| Other* | 3 (3.7%) | 0 | |
| Cigarette pack-year | |||
| Mean (±SE) | 40.0 (± 5.5) | 28.3 (± 4.5) | 0.12 |
| Differentiation | |||
| Well | 8 (9.8%) | 4 (4.9%) | 0.52 |
| Moderate | 29 (35.4%) | 26 (31.7%) | |
| Poor | 9 (11.0%) | 6 (7.3%) | |
| T-classification | |||
| 0–2 | 20 (24.4%) | 22 (26.8%) | 0.12 |
| 3–4 | 26 (31.7%) | 14 (17.1%) | |
| N-classification | |||
| 0–1 | 10 (12.2%) | 1 (1.2%) | 0.008 |
| 2 | 23 (28.0%) | 29 (35.4%) | |
| 3 | 13 (15.9%) | 6 (7.3%) | |
| Stage | |||
| II–III | 8 (9.8%) | 1 (1.2%) | 0.07 |
| IV | 38 (46.3%) | 35 (42.7%) | |
| Treatment | |||
| Nonsurgical therapy† | 31 (37.8%) | 33 (40.2%) | 0.01 |
| Surgical therapy‡ | 15 (18.3%) | 3 (3.7%) | |
| Hemoglobin | |||
| Mean (±SE) | 13.5 (±0.28) | 14.3 (±0.23) | 0.06 |
| ≤12 g/dL | 13 (15.9%) | 3 (3.7%) | 0.05 |
| >12 g/dL | 33 (40.2%) | 31 (37.8%) | |
| Unknown | 0 | 2 (2.4%) |
Abbreviations: CRT = chemoradiation; HPV = human papillomavirus; Neg = negative; OP = Oropharynx; RT = radiation; S = surgery; SE = standard error; Wk = weak.
Others included two paranasal sinus and one unknown squamous cell carcinomas.
Primary nonsurgical therapy included radiation alone (3 patients) and chemoradiation (61 patients).
Primary surgical therapy included surgery alone (2 patients), surgery + postoperative radiation (9 patients), and surgery + postoperative chemoradiation (7 patients)
Association between HPV, p16INK4a, and treatment outcomes
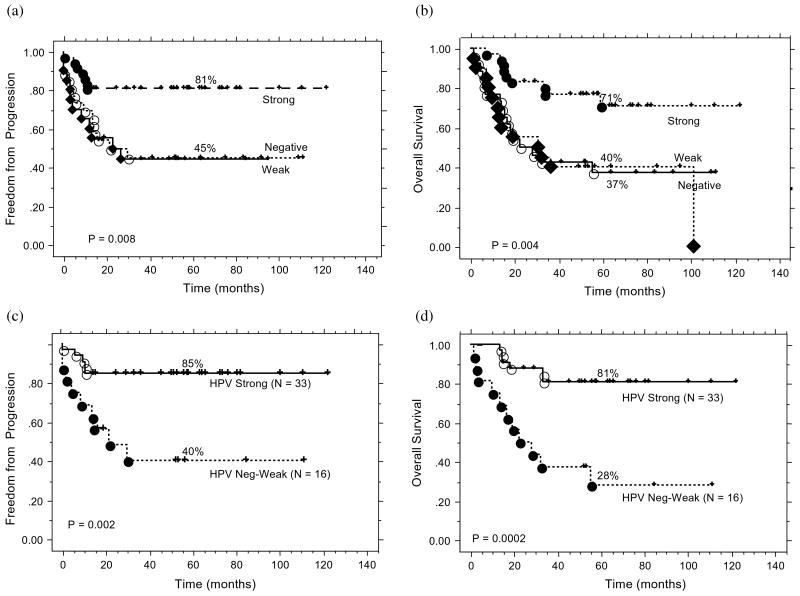
Figure 2 shows the TTP (2A, p = 0.008) and OS (2B, p = 0.004) by HPV status by pyrosequencing. Patients with strong HPV signal fared significantly better than those with weak or negative signal, confirming our hypothesis that the latter two were biologically similar. An analogous pattern was noted for DSS by HPV status; the 5-year DSS was 89% for strong, 49% for weak and 42% for negative HPV signals (p = 0.0005). Subsequently, the weak/negative HPV cohorts were grouped together. Pattern of failure study suggested that patients whose tumor yielded strong HPV signal had better local control, regional control, and freedom from distant metastasis than the rest (Table 3). We also analyzed treatment outcomes by HPV status within the 49 patients with OP tumors and evaluable HPV programs. As shown in Fig. 2C and 2D, patients with OP tumors harboring strong HPV signal had significantly better TTP (Fig. 2C) and overall survival (Fig. 2D) compared with those with OP tumors harboring weak or negative signal. Because of the small number of patients and events, multivariate analysis was not performed for this patient subgroup.
Fig. 2.
(a) Time to progression by human papillomavirus (HPV) pyrosequencing status for all patients. (b) Overall survival by HPV pyrosequencing status for all patients. (c) Time to progression by HPV pyrosequencing status for the subset of patients with oropharyngeal carcinoma (n = 49). (d) Overall survival by HPV pyrosequencing status for the subset of patients with oropharyngeal carcinoma (n = 49)
Table 3.
Pattern of failure by HPV signal intensity
| HPV Status | p value | χ2 | ||
|---|---|---|---|---|
| Strong (n = 36) |
Negative-weak (n = 46) |
|||
| % 5-year local control | 94.4 ± 3.9 | 66.4 ± 7.5 | 0.004 | 8.3 |
| % 5-year regional control | 88.7 ± 5.3 | 67.1 ± 7.0 | 0.012 | 6.3 |
| % 5-year freedom from distant metastasis | 94.4 ± 3.8 | 73.6 ± 7.8 | 0.032 | 4.6 |
Abbreviation: HPV = human papillomavirus.
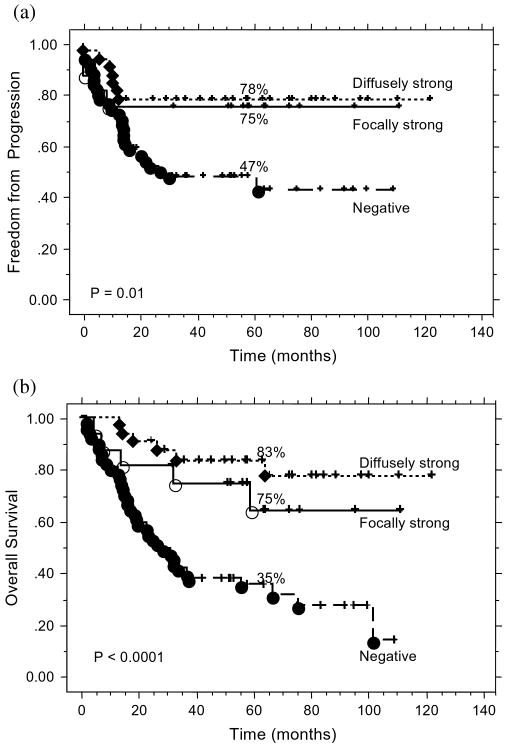
Figure 3A and 3B show TTP and OS by p16INK4a status. Patients with positive p16INK4a staining, regardless of staining pattern, did significantly better than those with negative staining. A similar pattern was also noted for DSS by p16INK4a status (p = 0.002, data not shown). Subsequently, all p16INK4a positive (focal or diffuse strong) tumors were combined together for further analysis.
Fig. 3.
(a) Time to progression by p16INK4a status for all patients. (b) Overall survival by p16INK4a status for all patients.
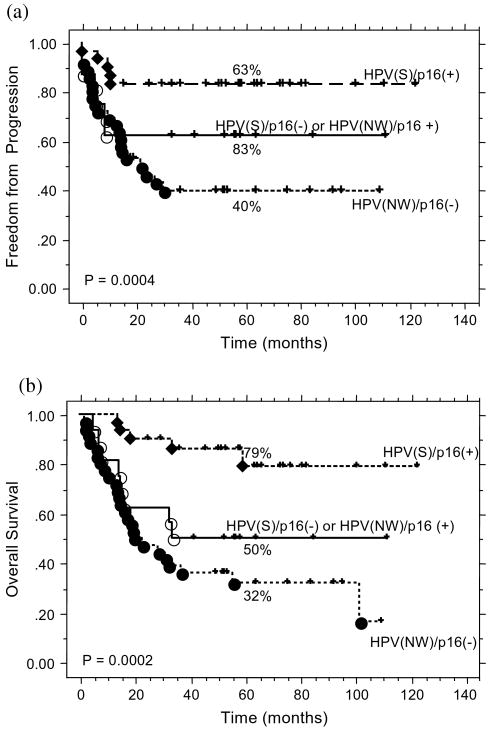
Given the strong association between HPV and p16INK4a, we grouped the patients on the basis of these markers' status and scored the combinations as follows: 0 for neither being strongly positive [HPV(negative-weak)/p16INK4a(−)], 1 for either marker being strongly positive [HPV(strong)/p16INK4a(−) or HPV(negative-weak)/p16INK4a(+)], and 2 for both being strongly positive [HPV(strong)/p16INK4a(+)]. Figure 4 shows TTP (4A) and OS (4B) for the three groups. Patients with both markers strongly positive fared the best, and those with both markers being either weak or negative the worst. We then considered this new HPV/p16INK4a classification as a categorical variable on multivariate analysis, adjusting for potential clinical prognostic factors that achieved a p value <0.05 on univariate analyses. Patients with both markers strongly positive fared significantly better than those with both negative on multivariate analysis (Table 4).
Fig. 4.
(a) Time to progression by human papillomavirus (HPV) and p16INK4a status combined for all patients. (b) Overall survival by HPV and p16INK4a status combined for all patients. HPV(S) = strong HPV signal; HPV(NW) = negative or weak HPV signal.
Table 4.
Results of univariate and multivariate analyses
| Parameter | TTP | p value | DSS | p value | OS | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| Univariate p value |
Multivariate HR (95% CI) |
Univariate p value |
Multivariate HR (95% CI) |
Univariate p value |
Multivariate HR (95% CI) |
||||
| Age (cont, favor younger age) | 0.16 | NI | NI | 0.06 | NI | NI | 0.017 | NI* | NI* |
| Sex (F vs. M) | 0.31 | NI | NI | 0.25 | NI | NI | 0.76 | NI | NI |
| Smoking status (cont, favor less PY) | 0.35 | NI | NI | 0.2 | NI | NI | 0.0031 | 1.01 (1.00–1.02) | 0.014 |
| HPV/p16INK4A score | |||||||||
| (1)HPV(NW)/p16INK4a(−) | — | — | — | — | |||||
| (2)HPV(S)/p16INK4a(−) or HPV(NW)/p16INK4a(+) | 0.2 | 0.55 (0.22—1.37) | .21 | 0.12 | 0.42 (0.16–1.12) | 0.08 | 0.24 | 0.66 (0.29–1.51) | 0.33 |
| (3)HPV(S)/p16INK4a(+) Favor group 2 and 3 | 0.001 | 0.23 (0.08–0.69) | 0.008 | 0.0007 | 0.11 (0.03–0.50) | 0.004 | 0.0002 | 0.20 (0.07–0.61) | 0.004 |
| N-Classification (N0–1 vs. N2–3) | 0.62 | NI | NI | 0.73 | NI | NI | 0.43 | NI | NI |
| Hb (Cont, g/dL, favor higher level) | 0.02 | 0.86 (0.69–1.06) | 0.15 | 0.005 | 0.82 (0.66–1.02) | 0.073 | 0.0015 | 0.79 (0.66–0.96) | 0.017 |
| Treatment (CRT vs. S+RT, favor CRT) | 0.007 | 2.31 (1.09–4.88) | 0.028 | 0.002 | 2.64 (1.20–5.84) | 0.016 | 0.032 | 2.42 (1.11–5.28) | 0.027 |
Abbreviations: CI = confidence interval; Cont = on a continuum; CRT = chemoradiation; DSS = disease-specific survival; F = female; Hb = hemoglobin; HPV = human papillomavirus; HR = hazard ratio; M = male; NI = not included; OS = overall survival; PY = pack year of cigarette use; RT = radiation; S = surgery; TTP = time to progression.
Age not included because it highly correlated with pack-year of cigarette use.
Relationship between HPV status, tumor hypoxia, EGFR expression, and T cell level
To investigate the possible reason why HPV(+) tumors fared better than others, we determined the relationship between HPV pyrosequencing status and tumor hypoxia, EGFR expression, and T-cell tumoral infiltration, three factors that may affect outcomes. Table 5 summarizes the results. There was no significant association between HPV status and tumor hypoxia as detected by either pO2 measurements or immunohistochemical (IHC) staining for CAIX. In contrast, there was a strong association between HPV status and EGFR staining intensity (p = 0.0006), as well as % of EGFR(+) tumor cells (p = 0.0002). Most tumors with strong HPV signal were EGFR negative and vice versa. Similarly, HPV status significantly correlated with the level of intratumoral T lymphocytes (by IHC for CD3) (p = 0.03). Tumors with strong HPV signal exhibited extensive CD3 reactivity.
Table 5.
Relationship between HPV pyrosequencing signal intensity and other molecular markers
| Parameters | All (%) | HPV negative-weak | HPV strong | p value |
|---|---|---|---|---|
| Median tumor pO2 (mm Hg) | ||||
| Mean value | 14.0 | 13.3 | 15.5 | 0.43 |
| CAIX | ||||
| Negative | 38 (46.3%) | 19 (23.3%) | 19 (23.2%) | 0.44 |
| Weak | 30 (36.6%) | 18 (22.0%) | 12 (14.6%) | |
| Strong | 13 (15.8%) | 9 (11.0%) | 4 (4.9%) | |
| Inevaluable | 1 (1.2%) | 0 | 1 (1.2%) | |
| EGFR staining intensity | ||||
| Negative | 45 (54.9%) | 17 (20.7%) | 28 (34.1%) | 0.0006 |
| Weak | 3 (3.7%) | 1 (1.2%) | 2 (2.4%) | |
| Strong | 29 (35.4%) | 24 (29.3%) | 5 (6.1%) | |
| Inevaluable | 5 (6.1%) | 4 (4.9%) | 1 (1.2%) | |
| % cells staining positive for EGFR | ||||
| 0%–10% | 47 (57.3%) | 17 (20.7%) | 30 (36.6%) | 0.0002 |
| 11%–80% | 16 (19.5%) | 12 (14.6%) | 4 (4.9%) | |
| 81%–100% | 14 (17.0%) | 13 (15.8%) | 1 (1.2%) | |
| Inevaluable | 5 (6.1%) | 4 (4.9%) | 1 (1.2%) | |
| CD3 staining intensity | ||||
| Negative | 4 (4.9%) | 3 (3.7%) | 1 (1.2%) | 0.03 |
| Weak | 35 (42.7%) | 24 (29.3%) | 11 (13.4%) | |
| Strong | 40 (48.9%) | 16 (19.5%) | 24 (29.3%) | |
| Inevaluable | 3 (3.7%) | 3 (3.7%) | 0 |
Abbreviation: HPV = human papillomavirus.
To determine whether the prognostic impact of CAIX, EGFR, and CD3 is influenced by HPV status, we evaluated the effect of these markers on OS in these patients, stratified by HPV status. Table 6 summarizes the results. Patients with tumors stained negative or weak for these markers were grouped together because they had similar 5-year survival rates. In general, within each HPV category, patients with strong CAIX staining had relatively lower survival compared with those with negative or weak staining; however, the number of patients with strong CAIX staining for each subgroup was too small to reach statistical significance. Similar observation was noted for EGFR, yet there were only five patients in the HPV strong group with intense EGFR staining. With regard to CD3 staining, its impact was mainly confined to the HPV negative/weak group with significantly better survival noted for patients with diffusely strong lymphocyte infiltration. There was no obvious survival difference by CD3 status noted for the HPV-strong patients.
Table 6.
Five-year overall survival by different biomarker categories, stratified by HPV status
| Marker | Staining intensity | All patients | p value | HPV neg-weak | p value | HPV strong | p value |
|---|---|---|---|---|---|---|---|
| CAIX | Neg-weak | 61% (n = 68) | 0.007 | 42% (n = 37) | 0.09 | 79% (n = 31) | 0.06 |
| Strong | 31% (n = 13) | 22% (n = 9) | 50% (n = 4) | ||||
| EGFR | Neg-weak | 72% (n = 48) | <0.0001 | 52% (n = 18) | 0.03 | 83% (n = 30) | 0.05 |
| Strong | 19% (n = 29) | 17% (n = 24) | 30% (n = 5) | ||||
| CD3 | Neg-weak | 34% (n = 39) | 0.005 | 22% (n = 27) | 0.04 | 64% (n = 12) | 0.97 |
| Strong | 70% (n = 40) | 60% (n = 16) | 75% (n = 24) |
Abbreviations: HPV = human papillomavirus; neg = negative.
Discussion
Since the implication of HPV in HNSCC development was reported, several studies have found better prognosis for HPV(+) tumors (2, 10, 26–28). The first study (2) reported that HPV status was an independent prognostic factor for DSS. Other studies reported similar findings in patients treated with either primary surgical or nonsurgical therapies (10, 26–28). A meta-analysis confirmed that HPV(+) oropharyngeal cancer patients had a 28% lower risk of death than their negative counterparts (29). HPV status was also associated with a better response to induction chemotherapy in two recent studies (30, 31). The reasons for improved survival are unclear but have been attributed to several factors as discussed earlier. Our study suggests an additional potential mechanism, involving lower EGFR expression for HPV(+) tumors.
Some studies have evaluated the relationship between EGFR and HPV in HNSCC. Two studies found no EGFR mutation and one reported less EGFR gene amplification for HPV(+) compared with HPV(−) tumors (6% vs. 43%) (32–34). However, the relationship between HPV and EGFR protein expression is controversial. Almadori et al. (33, 35), using a radioligand receptor assay, noted that HPV(+) laryngeal cancers had higher EGFR levels than HPV(−) tumors. Perrone et al. observed no difference in EGFR IHC expression by HPV status despite more EGFR gene amplification in HPV(−) tumors; however, most tumors in their study had high EGFR protein expression (33). In contrast, Reimers et al. (36) reported that p16INK4a(+) oropharyngeal carcinomas had lower EGFR expression than p16INK4a (−) tumors. Kumar et al. (37) reported in a brief communication that EGFR intensity was inversely related to HPV titer in 42 OP patients, but they did not provide details of this inverse correlation. In an expanded study of 66 OP patients, they confirmed this observation and found that the combination of EGFR and HPV was useful in stratifying DSS in these patients (38). These findings are highly consistent with our data, showing an inverse relationship between EGFR expression and HPV status both in intensity and percent positive cell staining. This finding suggests that improved prognosis for HPV(+) tumors may be partially due to a larger proportion of these tumors having negative or low EGFR expression. The reason HPV(+) tumors have less EGFR expression is unknown but could be because of the lesser EGFR amplification in these tumors. However, within the HPV(+) group, EGFR staining intensity continued to be prognostic because survival of the five patients with strong EGFR staining was significantly worse than those with negative or weak staining. These data are consistent with prior reports (36, 38) and suggested that aggressive therapy, including EGFR targeting, should still be considered in a small subset of tumors with high HPV and EGFR expression.
Patients with HNSCC have cell-mediated immune defects (39, 40). Low levels of circulating natural killer cells are associated with poorer survival (41). Here we evaluated the intratumoral T-cell levels in relation to HPV status. We noted a larger proportion of HPV(+) tumors with diffusely strong CD3(+) T-cell infiltration and that CD3 status was prognostic only for HPV(−) but not HPV(+) tumors. This suggested that the impact of T-cell-mediated immunity for tumor control is more significant in HPV(−) than HPV(+) tumors. However, the limitations of using CD3 as a pan T-cell marker is the lack of information on the different T-cell populations and the functional significance of the T-cell infiltrate with regard to antitumor immunity. Direct comparison of the global and intratumoral immune function between HPV(+) and (−) tumors is necessary to confirm these findings.
We found no obvious relationship between HPV status and either tumor pO2 measurements or CAIX staining, suggesting that HPV infection does not influence tumor hypoxia. In addition, the CAIX expression intensity had a similar prognostic effect on OS, regardless of HPV status. To our knowledge, this is the largest study evaluating the association between HPV and tumor oxygenation. One caveat in studying tumor hypoxia is the lack of uniform agreement in its assessment. We and others have found a lack of association between tumor pO2 and IHC expression of known hypoxia-induced proteins such as CAIX (11, 42). To minimize the potential biases associated with each approach, we used both to assess hypoxia. Regardless of the approach used, we did not observe any association between HPV status and tumor hypoxia in this small cohort of patients. This needs to be validated in a larger patient group to rule out Type II error.
DNA sequencing is the most accepted standard for viral typing. However, traditional sequencing techniques can be challenging for HPV genotyping when specimens harbor multiple genotypes. A type-specific, multiple-sequencing-primers method, combined with pyrosequencing, was developed to address this issue (17, 43). This approach was as robust and accurate in detecting several HPV subtypes in mixed infections. We have previously employed this approach to characterize HPV genotypes in 20 cervical cancer cases (43). This is the first and the largest series in which pyrosequencing was used to genotype HPV in HNSCC from paraffin embedded tissues. It revealed that most HPV(+) tumors harbored HPV16 subtypes. Because this is a PCR-based approach, different signal intensities are detected for HPV(+) tumors. The distinction between weak vs. strong signal was defined a priori on the basis of PCR amplicon concentration and was further validated by quantitative RT PCR assay. Although it is possible that the weaker HPV signal is due to contamination, it is also possible that it represents a low-level, clinically irrelevant infection. Of interest, Worden et al. (30), using a combination of real-time PCR and mass spectroscopy for HPV detection, noted that HPV copy number correlated with induction chemotherapy response and DSS, with the best results observed in patients with the highest copy numbers (>65 copies/cell). With more sensitive HPV detection methods such as the PCR-based approach, a distinction needs to be made between clinical and analytic sensitivity (44). Therefore, HPV results by pyrosequencing are best interpreted in combination with a functional marker such as p16INK4a.
In summary, we found that the HPV/p16INK4a grouping, as used here, was an independent prognostic factor for relapse and survival in HNSCC. HPV(+) status had no direct correlation with hypoxia but had an inverse correlation with EGFR expression, which remained prognostic for both HPV groups. Intratumoral T-cell infiltration was more likely to be absent or weaker in HPV(−) tumors and was prognostic only in the HPV(−) subset. Drawbacks from this study include its retrospective nature, the mix of primary sites, the small sample size, and the heterogeneity of treatment employed. These preliminary findings need to be validated in larger and more homogeneous HNSCC patient cohorts.
Acknowledgments
This study was supported by the following grants from the National Institute of Health: 1 R01 CA118582-01 (QTL, CSK, HC and SK), PO1- CA67166 (QTL, AK), and HG000205 (JPE and NP) and by the Stanford Cancer Council Grant (CSK).
Footnotes
Presented at 2007 Annual AACR meeting, April 2008, San Diego, CA.
Conflict of interest: none.
References
- 1.Le QT, Giaccia AJ. Therapeutic exploitation of the physiological and molecular genetic alterations in head and neck cancer. Clin Cancer Res. 2003;9:4287–4295. [PubMed] [Google Scholar]
- 2.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 3.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storey A, Thomas M, Kalita A, et al. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 7.Wiest T, Schwarz E, Enders C, et al. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene. 2002;21:1510–1517. doi: 10.1038/sj.onc.1205214. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger PM, Yu Z, Haffty BG, et al. Prognostic significance of p16 protein levels in oropharyngeal squamous cell cancer. Clin Cancer Res. 2004;10:5684–5691. doi: 10.1158/1078-0432.CCR-04-0448. [DOI] [PubMed] [Google Scholar]
- 9.Bova RJ, Quinn DI, Nankervis JS, et al. Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin Cancer Res. 1999;5:2810–2819. [PubMed] [Google Scholar]
- 10.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 11.Le QT, Kong C, Lavori PW, et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2007;69:167–175. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 12.Le QT, Shi G, Cao H, et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J Clin Oncol. 2005;23:8932–8941. doi: 10.1200/JCO.2005.02.0206. [DOI] [PubMed] [Google Scholar]
- 13.Petrik D, Lavori PW, Cao H, et al. Plasma osteopontin is an independent prognostic marker for head and neck cancers. J Clin Oncol. 2006;24:5291–5297. doi: 10.1200/JCO.2006.06.8627. [DOI] [PubMed] [Google Scholar]
- 14.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 16.Sobin LH, Hermanek P, Hutter RV. TNM classification of malignant tumors. A comparison between the new (1987) and the old editions. Cancer. 1988;61:2310–2314. doi: 10.1002/1097-0142(19880601)61:11<2310::aid-cncr2820611127>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Gharizadeh B, Oggionni M, Zheng B, et al. Type-specific multiple sequencing primers: a novel strategy for reliable and rapid genotyping of human papillomaviruses by pyrosequencing technology. J Mol Diagn. 2005;7:198–205. doi: 10.1016/S1525-1578(10)60546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Shi G, Xia W, et al. Identification of hypoxia-regulated proteins in head and neck cancer by proteomic and tissue array profiling. Cancer Res. 2004;64:7302–7310. doi: 10.1158/0008-5472.CAN-04-0899. [DOI] [PubMed] [Google Scholar]
- 19.Sano T, Oyama T, Kashiwabara K, et al. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keating JT, Cviko A, Riethdorf S, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–891. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kong CS, Balzer BL, Troxell ML, et al. p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. Am J Surg Pathol. 2007;31:33–43. doi: 10.1097/01.pas.0000213347.65014.ee. [DOI] [PubMed] [Google Scholar]
- 22.Terris DJ, Dunphy EP. Oxygen tension measurements of head and neck cancers. Arch Otolaryngol Head Neck Surg. 1994;120:283–287. doi: 10.1001/archotol.1994.01880270031006. [DOI] [PubMed] [Google Scholar]
- 23.Team RDC. R: A language and environment for statistical computing. Version 2.5.1. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 24.Glanz SA, Slinker BK. Primer of applied regression analysis of variance. New York: McGraw-Hill; 1990. [Google Scholar]
- 25.Cox DR. Regression models and life tables. J Royal Stat Soc. 1972;34:187–229. [Google Scholar]
- 26.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 27.Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–304. [PubMed] [Google Scholar]
- 28.Li W, Thompson CH, O'Brien CJ, et al. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer. 2003;106:553–558. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 29.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int J Cancer. 2007;121:1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 30.Worden FP, Kumar B, Lee JS, et al. Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: Response and survival positively associated with HPV16 copy number. J Clin Oncol. 2008;26:3138–3146. doi: 10.1200/JCO.2007.12.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 33.Perrone F, Suardi S, Pastore E, et al. Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res. 2006;12:6643–6651. doi: 10.1158/1078-0432.CCR-06-1759. [DOI] [PubMed] [Google Scholar]
- 34.Na II, Kang HJ, Cho SY, et al. EGFR mutations and human papillomavirus in squamous cell carcinoma of tongue and tonsil. Eur J Cancer. 2007;43:520–526. doi: 10.1016/j.ejca.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Almadori G, Cadoni G, Cattani P, et al. Human papillomavirus infection and epidermal growth factor receptor expression in primary laryngeal squamous cell carcinoma. Clin Cancer Res. 2001;7:3988–3993. [PubMed] [Google Scholar]
- 36.Reimers N, Kasper HU, Weissenborn SJ, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 37.Kumar B, Cordell KG, Lee JS, et al. Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys. 2007;69:S109–S111. doi: 10.1016/j.ijrobp.2007.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadden JW. The immunopharmacology of head and neck cancer: An update. Int J Immunopharmacol. 1997;19:629–644. doi: 10.1016/s0192-0561(97)00063-5. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside TL. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 42.Mayer A, Hockel M, Vaupel P. Carbonic anhydrase IX expression and tumor oxygenation status do not correlate at the micro-regional level in locally advanced cancers of the uterine cervix. Clin Cancer Res. 2005;11:7220–7225. doi: 10.1158/1078-0432.CCR-05-0869. [DOI] [PubMed] [Google Scholar]
- 43.Akhras MS, Thiyagarajan S, Villablanca AC, et al. Pathogen-Mip assay: A multiplex pathogen detection assay. PLoS ONE. 2007;2:e223. doi: 10.1371/journal.pone.0000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snijders PJ, van den Brule AJ, Meijer CJ. The clinical relevance of human papillomavirus testing: Relationship between analytical and clinical sensitivity. J Pathol. 2003;201:1–6. doi: 10.1002/path.1433. [DOI] [PubMed] [Google Scholar]