In electrically excitable cells, voltage-gated calcium channels (VGCCs) serve a critical signaling role by translating changes in membrane potential to calcium ion influx. The resulting intracellular calcium signals are perfectly tuned to cellular needs in terms of amplitude, spatial distribution, and timing. This tuning is accomplished by a structural diversity of these channels and close functional cross talk with other signaling pathways. Structural diversity is achieved by the combination of pore-forming α1 subunit isoforms with modulatory accessory subunits. To form the Cav1 and Cav2 VGCC families in neurons, seven α1 subunit isoforms each combine with one of four different β and one of several α2-δ subunits in hetero-oligomeric complexes (see Catterall et al., 2005 for nomenclature and subunit isoforms). The functional repertoire is further enlarged through alternative splicing (especially of α1 and β subunits) and the differential susceptibility of channel subtypes to modulation by second messenger pathways (Catterall et al., 2005).
Several mechanisms underlie the modulation of neuronal N-type (Cav2.2) and P/Q-type (Cav2.1) VGCCs by neurotransmitters and hormones, and for some of them, the molecular details have been revealed. Activation of G protein–coupled receptors (GPCRs) at the cell surface can affect channel function either through fast, membrane-delimited mechanisms or more slowly via downstream second messengers (Dolphin, 2003; Tedford and Zamponi, 2006; Ikeda and Dunlap, 2007; Lipscombe and Raingo, 2007). Agonist binding to GPCRs can elicit a pertussis toxin–sensitive inhibition of P/Q- and N-type channels that develops and recovers in less than one second, and involves the rapid binding and unbinding of Gβγ subunits to well-defined regions of the pore-forming α1 subunits (Dolphin, 2003; Tedford and Zamponi, 2006). This form of inhibition is voltage dependent and relieved by strong depolarizations (Ikeda and Dunlap, 2007). As a consequence, inhibition is pronounced at slow firing rates but weaker in rapidly firing neurons. Many different GPCRs, such as muscarinic acetylcholine receptors (mAchRs) and opioid, dopamine, and somatostatin receptors, inhibit calcium currents through this mechanism, which is thought to play a major role in neurotransmitter-mediated control of neurotransmitter release.
Voltage-independent forms of inhibition after GPCR activation also exist. One mechanism involves channel internalization. In dorsal root ganglion (DRG) neurons, internalization of N-type VGCCs is induced either by prolonged activation of ORL1 receptors (Altier et al., 2006; Lipscombe and Raingo, 2006) or after brief activation of GABAB receptors (Tombler et al. 2006). Another form of voltage-independent inhibition of N-type channels by Gi/Go-coupled GPCRs in DRG neurons requires protein tyrosine kinase phosphorylation in a C-terminal domain (Lipscombe and Raingo, 2007; Raingo et al., 2007). Interestingly, tyrosine phosphorylation occurs within an alternatively spliced region; thus, this type of modulation is only present in exon37a-containing channels that are enriched in nociceptive DRG neurons (Bell et al., 2004). The resulting higher sensitivity of this splice variant to inhibition by neurotransmitters could provide an important mechanism for down-regulation of N-type channel-mediated pain transmission during periods of pain-induced rapid neuronal firing (Raingo et al., 2007).
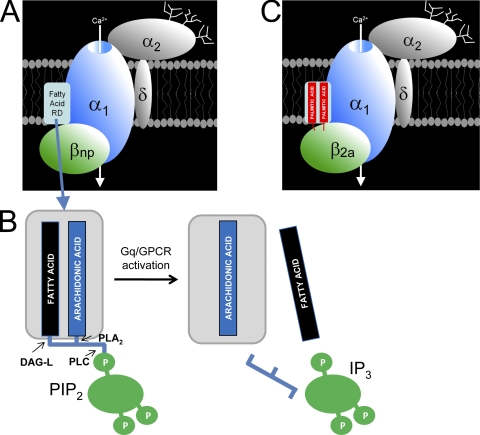
GPCRs that signal through Gq-like G proteins, such as M1-mAchRs and NK1 receptors, can exploit yet another form of voltage-independent inhibition of N- and L-type current in sympathetic cervical ganglion (SCGs) and cortical neurons. This form of inhibition is sensitive to intracellular calcium buffering, and both onset and recovery are slow (seconds to minutes). It has therefore been referred to as the “slow pathway” (Suh and Hille, 2005; Michailidis et al., 2007). This pathway has attracted special attention because lipids were implicated as effector molecules, thus representing another example of lipid modulation of ion channels and transporters (Wu et al., 2002; Gamper et al., 2004; Suh and Hille, 2005). Although the exact mechanism remains controversial, two distinct hypotheses are the main competitors for resolution: a phosphatidylinositol 4,5-bisphosphate (PIP2) depletion and an arachidonic acid (ArA) generation hypothesis. The former implies that N-type (and P/Q-type) channel activity is stabilized by PIP2, most likely through binding to a fatty acid/lipid regulatory site near the channel (Fig. 1 A). Inhibition would occur upon PLC-induced breakdown with membrane depletion, and dissociation of PIP2 from the channel (Wu et al., 2002; Gamper et al., 2004; Delmas et al., 2005). This is an attractive hypothesis because the type of modulation closely resembles the inhibition of M-current (KCNQ2/3) by M1 mACHR, a major regulator of neuronal excitability (Suh et al. 2006).
Figure 1.
Mechanisms involved in lipid modulation of voltage-gated N- and L-type calcium channels by the slow pathway. The subunit structure of high threshold neuronal calcium channel complexes is illustrated together with a putative fatty acid regulatory domain (RD) that can also bind the palmitic acid side chains present in the N terminus of β2a subunits. For details see text. PLC, phospholipase C; PLA2, phospholipase A2; DAG-L, diacylglycerol lipase; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol trisphosphate; βnp, nonpalmitoylated β subunit.
A series of reports from the Rittenhouse laboratory provides support for an alternative view, an “ArA generation” hypothesis. This hypothesis predicts that whereas M-current inhibition requires only PIP2 breakdown, L- and N-current inhibition in SCGs may depend on additional signaling events downstream of PLC/PIP2 (Liu and Rittenhouse, 2003; Liu et al., 2006). They found that the M1 mAchR signaling cascade for N-type (and L-type) current modulation in SCG neurons not only involved activation of Gq, but also complete PIP2 breakdown by PLC, PLA2, and diacylglycerol lipase (Fig. 1 B) (Liu and Rittenhouse, 2003; Liu et al., 2006, 2008). ArA was the most likely downstream effector because different measures reducing ArA availability (PLA2 gene knockout, PLA2 pharmacological inhibition, and ArA scavenging by BSA) attenuated modulation by receptor activation. Moreover, exposure of cells to ArA largely mimicked the typical characteristics of M1 mAChR modulation of native currents in SCG neurons. Although these results favor the “ArA generation” over the “PIP2 depletion” hypothesis, these two are not mutually exclusive, as discussed below.
ArA treatment also mimicked another typical feature of M1-mAChR–mediated current modulation in SCG neurons. Depolarization of cells to a wide range of different potentials revealed stimulation of current at more negative (below 0 mV) and inhibition at more positive (above 0 mV) test potentials. It was this dual response that finally provided an important hint to the molecular mechanism of this pathway.
The Rittenhouse laboratory presents two papers (see Heneghan et al. and Mitra-Ganguli et al. in this issue) describing novel findings shedding more light on how N-type channel function is modified by lipids. The key observations were made in a heterologous expression system. When Cav2.2 α1 subunits were expressed with β3 (or β1 or β4) subunits, the inhibitory effect at positive potentials of M1-mAChR or NK1 receptor activation or of externally applied ArA was nicely reproduced, but the stimulatory component at negative potentials was absent. Moreover, the currents showed much faster voltage-dependent inactivation during depolarizations than N-type currents in SCGs. This directed attention to β2a subunits, one of the many β2 subunit splice variants expressed in neurons, well known for its unique capacity to induce slow inactivation of VGCCs. Like all the other β subunits, it binds to a conserved motif (termed AID) within the cytoplasmic loop between the first transmembrane helix of repeat I and the last transmembrane helix of repeat II of the α1 subunit. The former (IS6) is part of the inner portion of the ion-conducting pathway and participates in channel inactivation. Recent elegant work from the Perez-Reyes (Vitko et al., 2008) and Dan Minor (Findeisen and Minor, 2009) laboratories revealed that the modulatory effects of β subunits are transmitted to the pore by the rigid linker between AID and IS6. Unlike all other known β splice variants, β2a is palmitoylated at two N-terminal cysteins (Chien et al., 1996). These fatty acids provide a membrane anchor for the N terminus and must restrict conformational flexibility of β2a (Van Petegem et al., 2004). This restriction may in turn impair the flexibility of IS6 movements and thereby stabilize slow inactivation gating. Surprisingly, when Heneghan et al. (2009) coexpressed β2a subunits, they not only observed the expected slowing of inactivation of recombinant N-type currents, but also found that receptor activation or ArA treatment caused channel stimulation rather than inhibition, as observed for the other βs. But how could different β subunits stabilize opposite modulatory actions?
From earlier work it was known that current enhancement by ArA occurs from the extracellular side and inhibition from inside (Barrett et al., 2001; Liu et al., 2001). Therefore, Rittenhouse and colleagues reasoned that the differential effects of the cytoplasmic β subunits must result from interaction with the intracellular inhibitory site. The simplest explanation was that β2a is able to somehow block intracellular inhibition by ArA, thereby unmasking stimulation, whereas β1, β3, and β4 subunits are unable to do so and allow ArA to decrease channel currents. If this explanation is true, the palmitoylation of β2a was the logical molecular explanation for this difference between β subunits. Accordingly, coexpression of a palmitoylation-deficient β2a prevented the stimulatory effects of receptor activation and ArA exposure. Therefore, it was reasonable to hypothesize that the anchoring palmitic acid side chains interfere with ArA binding at a channel site located within the reach of the β2α N terminus (Fig. 1 C). The addition of free palmitic acid also attenuated inhibition. Although supportive, this finding could hardly be taken as strong molecular evidence for the proposed mechanism. However, they correctly interpreted another (at first sight disappointing) experimental observation. When they introduced the palmitoylated N terminus of β2a into β1, the resulting chimera more or less reproduced the β2a behavior on modulation. However, a similar chimeric construct with β3 did not block the inhibitory component effectively. As the docking of all β subunits to α1 subunits via AID should occur in a similar manner, the most likely explanation was that differences in β subunit size and geometry prevented the precise placement of the palmitic acid side chains in the putative ArA binding pocket by the β3 chimera. To support this hypothesis, they used a more defined approach to disturb the position of the palmitoylated β2a N terminus. They exploited previously characterized Cav2.2 α1 subunit constructs in which the orientation of β subunits relative to α1 is altered by deleting one or two residues of the putative β sheet in the IS6-AID linker (Vitko et al., 2008). In accordance with their predictions, a minor (only one residue deleted) disturbance of β subunit orientation destabilized the β2a-mediated block of inhibition, whereas a two-residue deletion completely relieved inhibition and resulted in the expected inhibitory modulation similar to nonpalmitoylated βs.
These findings draw a completely new picture of the role of β2a subunit palmitolyation. Clearly, it not only serves as a simple membrane anchor like in many other proteins, but it also must be considered as a steric inhibitor of an internal fatty acid regulatory domain for ArA, close to the channel (Fig. 1 C). Overall, this mechanism allows VGCCs to exploit β subunit diversity not only to obtain different gating properties, but also to select between stimulation or inhibition by Gq-coupled GPCRs. This versatile modulation of VGCCs depends on the relative abundance of β2a in relation to other nonpalmitoylated β2 splice variants and β subunit isoforms. These ideas and results should prompt further studies to quantify the subcellular distribution of individual β subunits in neurons and to test for dynamic changes of their expression under different physiological and pathophysiological conditions. Because palmitoylation is a reversible process and as such has been reported to modulate chromaffin cell calcium channel function (Hurley et al., 2000), the possibility of neuronal calcium channel fine-tuning by dynamic palmitoylation of β2a will need further attention.
An important final question remains: How do the present findings affect the controversy between the “PIP2 breakdown” and “ArA generation” hypotheses? They clearly uphold the latter by providing a plausible molecular mechanism supporting it. However, previous experimental evidence arguing against a role for PLA2 and ArA (Wu et al., 2002; Gamper et al., 2004) remains valid. Unfortunately, some differences in experimental design between these latter studies (use of the oocyte expression system instead of HEK cells, no use of β2a in heterologous system, and frog instead of mammalian sympathetic neurons) and those from the Rittenhouse laboratory (use of barium instead of physiological calcium as charge carrier) exist and complicate direct comparisons. Nevertheless, a unifying hypothesis can be proposed that could serve as a basis for further experiments. Most PIP2 contains ArA in the sn2 position and another fatty acid (mostly stearic acid) in the sn1 position (Fig. 1 B). Release of ArA is catalyzed by PLA2, whereas release in sn1 position is catalyzed by diacylglycerol lipases. The Rittenhouse group has found that both lipases in addition to PLC (which removes the negatively charged IP3) are required for M1-mAChR–induced inhibition of N- and L-type channel activity through the slow pathway (Liu et al., 2008). This suggests that PIP2 stabilizes the channel and does so by occupying the fatty acid regulatory domain with its fatty acid side chains. Tethering to the inositol and glycerol backbones may stabilize a conformation preventing inhibition and stabilizing channel function. However, once all three enzymes have disassembled the PIP2 molecule (Fig. 1 B), the fatty acid regulatory domain becomes available for inhibition by ArA released from PIP2 and perhaps other sources. Stable occupation of the site by palmitic acid anchored to β2a could prevent any inhibitory effects by released ArA. Promoting PIP2 formation would stabilize channel function by counteracting ArA inhibition, whereas external ArA addition would counteract stabilization by PIP2. No information exists regarding a possible interaction between PIP2 and the palmitic acid side chains of β2a.
The investigations of Rittenhouse and colleagues further emphasize the role of β subunits as important targets for VGCC modulation. Ras-related RGK family of small GTP-binding proteins can bind to β subunits and thereby inhibit channel activity (Béguin et al., 2001). As another recent example, β subunit interaction with the presynaptic active zone protein RIM induces profound changes of voltage-dependent activation and inactivation gating in Cav2 VGCCs, thus supporting calcium influx for neurotransmitter release (Kiyonaka et al., 2007).
The novel findings described in this issue provide an important first step into the molecular understanding of the modulation of VGCCs by ArA and/or PIP2. Hopefully, further work will precisely localize these regulatory domains within one or more of the VGCC subunits. Elegant work on other ion channels, such as the functional interconversion between A-type and delayed rectifier K+ channels by PIP2 and ArA (Oliver et al., 2004), provides important motivation for such studies.
Acknowledgments
J. Striessnig is supported by the FWF (P20670).
Footnotes
Abbreviations used in this paper:
- ArA
- arachidonic acid
- DRG
- dorsal root ganglion
- GPCR
- G protein–coupled receptor
- mAchR
- muscarinic acetylcholine receptor
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- SCG
- sympathetic cervical ganglion
- VGCC
- voltage-gated calcium channel
References
- Altier C., Khosravani H., Evans R.M., Hameed S., Peloquin J.B., Vartian B.A., Chen L., Beedle A.M., Ferguson S.S., Mezghrani A., et al. 2006. ORL1 receptor-mediated internalization of N-type calcium channels. Nat. Neurosci. 9:31–40 10.1038/nn1605 [DOI] [PubMed] [Google Scholar]
- Barrett C.F., Liu L., Rittenhouse A.R. 2001. Arachidonic acid reversibly enhances N-type calcium current at an extracellular site. Am. J. Physiol. Cell Physiol. 280:C1306–C1318 [DOI] [PubMed] [Google Scholar]
- Béguin P., Nagashima K., Gonoi T., Shibasaki T., Takahashi K., Kashima Y., Ozaki N., Geering K., Iwanaga T., Seino S. 2001. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 411:701–706 10.1038/35079621 [DOI] [PubMed] [Google Scholar]
- Bell T.J., Thaler C., Castiglioni A.J., Helton T.D., Lipscombe D. 2004. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 41:127–138 10.1016/S0896-6273(03)00801-8 [DOI] [PubMed] [Google Scholar]
- Catterall W.A., Perez-Reyes E., Snutch T.P., Striessnig J. 2005. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57:411–425 10.1124/pr.57.4.5 [DOI] [PubMed] [Google Scholar]
- Chien A.J., Carr K.M., Shirokov R.E., Rios E., Hosey M.M. 1996. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J. Biol. Chem. 271:26465–26468 10.1074/jbc.271.43.26465 [DOI] [PubMed] [Google Scholar]
- Delmas P., Coste B., Gamper N., Shapiro M.S. 2005. Phosphoinositide lipid second messengers: new paradigms for calcium channel modulation. Neuron. 47:179–182 10.1016/j.neuron.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Dolphin A.C. 2003. G protein modulation of voltage-gated calcium channels. Pharmacol. Rev. 55:607–182 [DOI] [PubMed] [Google Scholar]
- Findeisen F., Minor D.L., Jr 2009. Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J. Gen. Physiol. 133:327–343 10.1085/jgp.200810143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N., Reznikov V., Yamada Y., Yang J., Shapiro M.S. 2004. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 24:10980–10992 10.1523/JNEUROSCI.3869-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan J.F., Mitra-Ganguli T., Stanish L.F., Liu L., Zhao R., Rittenhouse A.R. 2009. The Ca2+ channel β-subunit determines whether stimulation of Gq-coupled receptors enhances or inhibits N current. J. Gen. Physiol. 134:369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J.H., Cahill A.L., Currie K.P., Fox A.P. 2000. The role of dynamic palmitoylation in Ca2+ channel inactivation. Proc. Natl. Acad. Sci. USA. 97:9293–9298 10.1073/pnas.160589697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S.R., Dunlap K. 2007. Calcium channels diversify their signaling portfolio. Nat. Neurosci. 10:269–271 10.1038/nn0307-269 [DOI] [PubMed] [Google Scholar]
- Kiyonaka S., Wakamori M., Miki T., Uriu Y., Nonaka M., Bito H., Beedle A.M., Mori E., Hara Y., De Waard M., et al. 2007. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat. Neurosci. 10:691–701 10.1038/nn1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscombe D., Raingo J. 2006. Internalizing channels: a mechanism to control pain? Nat. Neurosci. 9:8–10 10.1038/nn0106-8 [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Raingo J. 2007. Alternative splicing matters: N-type calcium channels in nociceptors. Channels (Austin). 1:225–227 [DOI] [PubMed] [Google Scholar]
- Liu L., Rittenhouse A.R. 2003. Arachidonic acid mediates muscarinic inhibition and enhancement of N-type Ca2+ current in sympathetic neurons. Proc. Natl. Acad. Sci. USA. 100:295–300 10.1073/pnas.0136826100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Barrett C.F., Rittenhouse A.R. 2001. Arachidonic acid both inhibits and enhances whole cell calcium currents in rat sympathetic neurons. Am. J. Physiol. Cell Physiol. 280:C1293–C1305 [DOI] [PubMed] [Google Scholar]
- Liu L., Zhao R., Bai Y., Stanish L.F., Evans J.E., Sanderson M.J., Bonventre J.V., Rittenhouse A.R. 2006. M1 muscarinic receptors inhibit L-type Ca2+ current and M-current by divergent signal transduction cascades. J. Neurosci. 26:11588–11598 10.1523/JNEUROSCI.2102-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Heneghan J.F., Michael G.J., Stanish L.F., Egertová M., Rittenhouse A.R. 2008. L- and N-current but not M-current inhibition by M1 muscarinic receptors requires DAG lipase activity. J. Cell. Physiol. 216:91–100 10.1002/jcp.21378 [DOI] [PubMed] [Google Scholar]
- Michailidis I.E., Zhang Y., Yang J. 2007. The lipid connection-regulation of voltage-gated Ca(2+) channels by phosphoinositides. Pflugers Arch. 455:147–155 10.1007/s00424-007-0272-9 [DOI] [PubMed] [Google Scholar]
- Mitra-Ganguli T., Vitko I., Perez-Reyes E., Rittenhouse A.R. 2009. Orientation of palmitoylated Cavβ2a relative to Cav2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation. J. Gen. Physiol. 134:385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D., Lien C.C., Soom M., Baukrowitz T., Jonas P., Fakler B. 2004. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 304:265–270 10.1126/science.1094113 [DOI] [PubMed] [Google Scholar]
- Raingo J., Castiglioni A.J., Lipscombe D. 2007. Alternative splicing controls G protein-dependent inhibition of N-type calcium channels in nociceptors. Nat. Neurosci. 10:285–292 10.1038/nn1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B.C., Hille B. 2005. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15:370–378 10.1016/j.conb.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Suh B.C., Inoue T., Meyer T., Hille B. 2006. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 314:1454–1457 10.1126/science.1131163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedford H.W., Zamponi G.W. 2006. Direct G protein modulation of Cav2 calcium channels. Pharmacol. Rev. 58:837–862 10.1124/pr.58.4.11 [DOI] [PubMed] [Google Scholar]
- Tombler E., Cabanilla N.J., Carman P., Permaul N., Hall J.J., Richman R.W., Lee J., Rodriguez J., Felsenfeld D.P., Hennigan R.F., Diversé-Pierluissi M.A. 2006. G protein-induced trafficking of voltage-dependent calcium channels. J. Biol. Chem. 281:1827–1839 10.1074/jbc.M508829200 [DOI] [PubMed] [Google Scholar]
- Van Petegem F., Clark K.A., Chatelain F.C., Minor D.L. 2004. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 429:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitko I., Shcheglovitov A., Baumgart J.P., Arias-Olguín I.I., Murbartián J., Arias J.M., Perez-Reyes E. 2008. Orientation of the calcium channel beta relative to the alpha(1)2.2 subunit is critical for its regulation of channel activity. PLoS One. 3:e3560 10.1371/journal.pone.0003560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Bauer C.S., Zhen X.G., Xie C., Yang J. 2002. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 419:947–952 10.1038/nature01118 [DOI] [PubMed] [Google Scholar]