Abstract
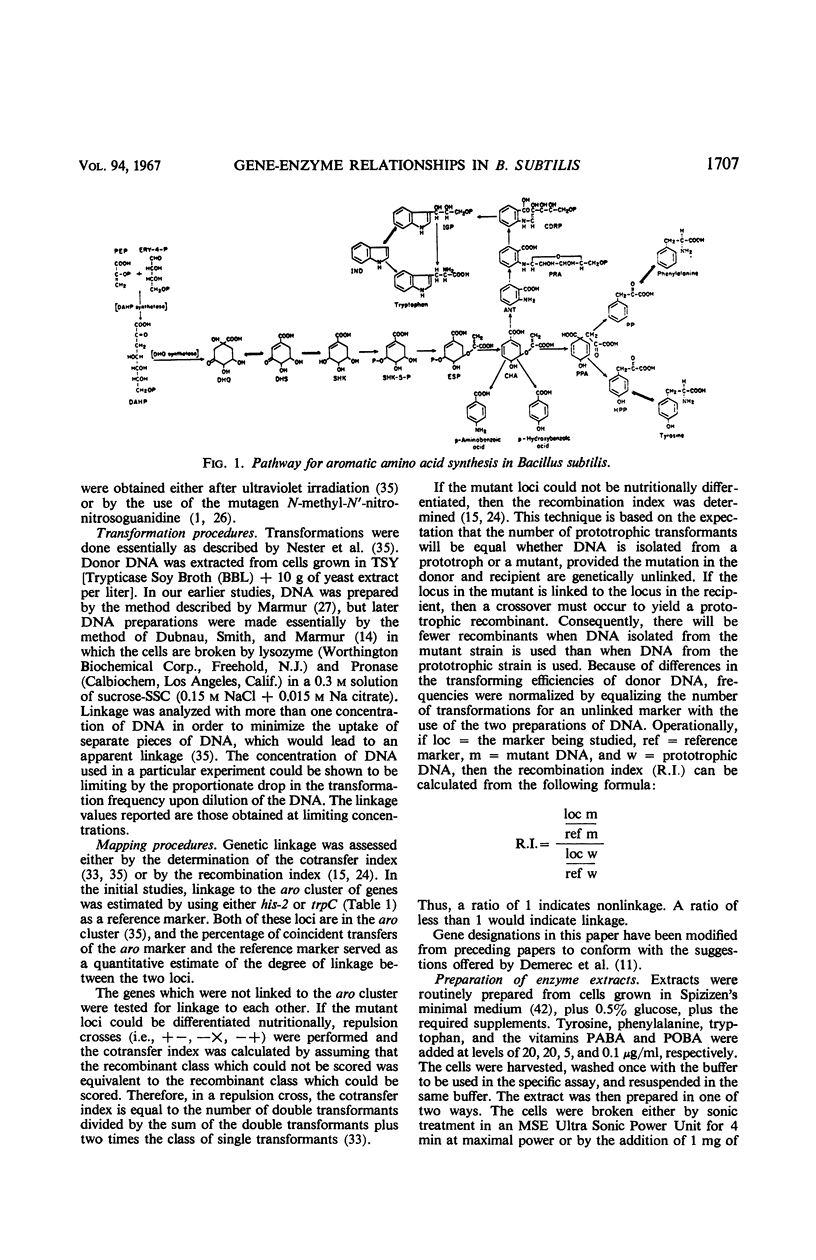
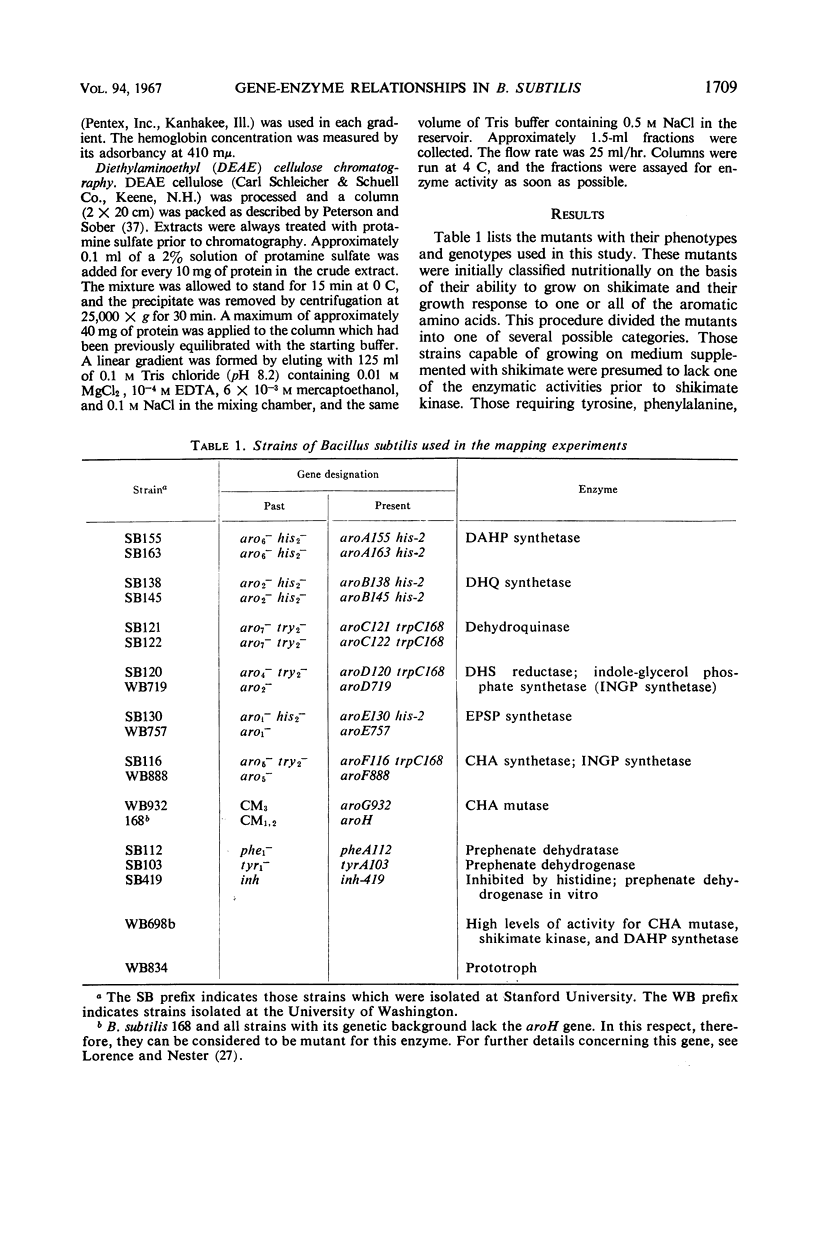
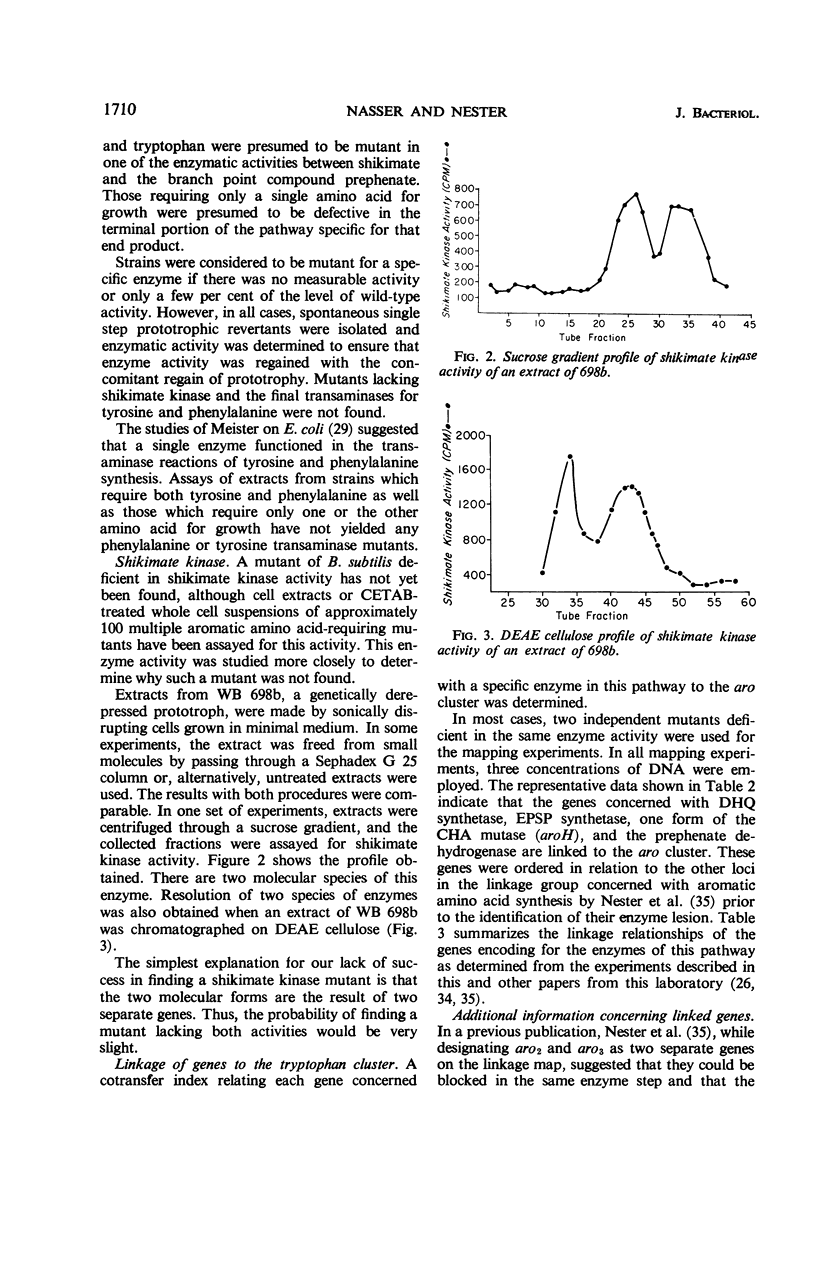
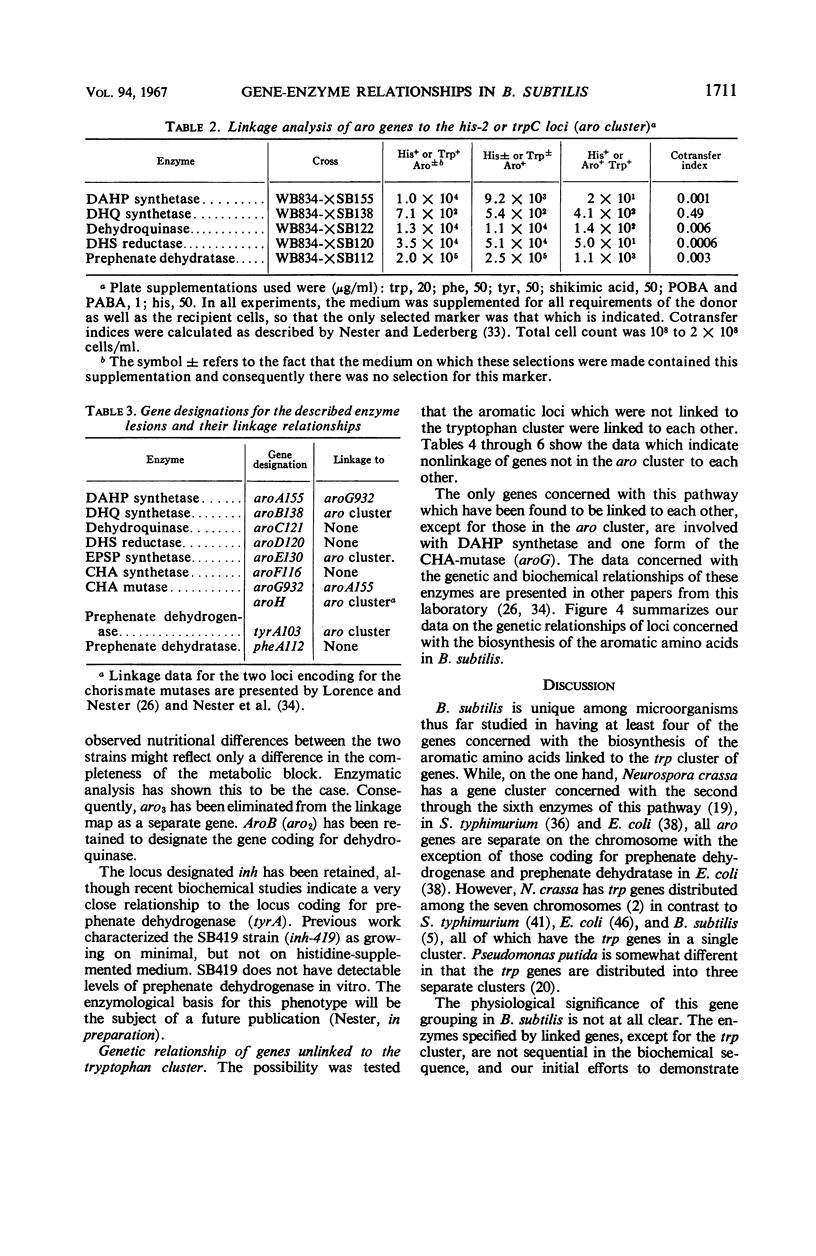
Single step mutants of Bacillus subtilis which required either one or all of the aromatic amino acids for growth were isolated. The relevant gene defect was determined for each mutant by enzyme assays in vitro. A mutant deficient in each enzyme step of aromatic amino acid biosynthesis was found with the exceptions of the shikimate kinase and the phenylalanine and tyrosine transaminases. Representative mutants carrying the defective genes were mapped by deoxyribonucleic acid mediated transformation by reference to the aromatic amino acid gene (aro) cluster and, alternately, to any of the other unlinked aro genes. The genes coding for dehydroquinate synthetase, 3-enol pyruvylshikimate 5-phosphate synthetase, one form of chorismate mutase, and prephenate dehydrogenase are linked to the aro cluster. Except for the previously identified linkage between the genes of 3-deoxy-d-arabino heptulosonic acid 7-phosphate synthetase and one species of chorismate mutase, the other genes involved in this pathway are neither linked to the aro cluster nor to each other.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., MARTIN R. G. BIOCHEMICAL ASPECTS OF GENETICS: THE OPERON. Annu Rev Biochem. 1964;33:235–258. doi: 10.1146/annurev.bi.33.070164.001315. [DOI] [PubMed] [Google Scholar]
- AMES B. N., MARTIN R. G., GARRY B. J. The first step of histidine biosynthesis. J Biol Chem. 1961 Jul;236:2019–2026. [PubMed] [Google Scholar]
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D. Intermediates in amino acid biosynthesis. Adv Enzymol Relat Subj Biochem. 1955;16:247–312. doi: 10.1002/9780470122617.ch5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Aromatic biosynthesis. VII. Accumulation of two derivatives of shikimic acid by bacterial mutants. J Bacteriol. 1953 Aug;66(2):129–136. doi: 10.1128/jb.66.2.129-136.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M. CLUSTERING OF FUNCTIONALLY RELATED GENES IN SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1964 Jun;51:1057–1060. doi: 10.1073/pnas.51.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A., Wegman J. An enzyme aggregate in the tryptophan pathway of Neurospora crassa. Proc Natl Acad Sci U S A. 1965 Jul;54(1):241–247. doi: 10.1073/pnas.54.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Control of enzyme steps during the bacterial cell cycle. Nature. 1965 Mar 13;205(976):1084–1086. doi: 10.1038/2051084a0. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Smith I., Marmur J. Gene conservation in Bacillus species. II. The location of genes concerned with the synthesis of ribosomal components and soluble RNA. Proc Natl Acad Sci U S A. 1965 Sep;54(3):724–730. doi: 10.1073/pnas.54.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRATI-ELIZUR E., SRINIVASAN P. R., ZAMENHOF S. Genetic analysis, by means of transformation, of histidine linkage groups in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Jan 15;47:56–63. doi: 10.1073/pnas.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON M. I., GIBSON F. A new intermediate in aromatic biosynthesis. Biochim Biophys Acta. 1962 Nov 19;65:160–163. doi: 10.1016/0006-3002(62)90166-x. [DOI] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- JENSEN R. A., NESTER E. W. THE REGULATORY SIGNIFICANCE OF INTERMEDIARY METABOLITES: CONTROL OF AROMATIC ACID BIOSYNTHESIS BY FEEDBACK INHIBITION IN BACILLUS SUBTILIS. J Mol Biol. 1965 Jun;12:468–481. doi: 10.1016/s0022-2836(65)80270-4. [DOI] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lederberg J. SIBLING RECOMBINANTS IN ZYGOTE PEDIGREES OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1957 Dec 15;43(12):1060–1065. doi: 10.1073/pnas.43.12.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MEISTER A. Transamination. Adv Enzymol Relat Subj Biochem. 1955;16:185–246. doi: 10.1002/9780470122617.ch4. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- MORGAN P. N., GIBSON M. I., GIBSON F. THE CONVERSION OF SHIKIMIC ACID INTO CERTAIN AROMATIC COMPOUNDS BY CELL-FREE EXTRACTS OF AEROBACTER AEROGENES AND ESCHERICHIA COLI. Biochem J. 1963 Nov;89:229–239. doi: 10.1042/bj0890229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Jensen R. A. Control of aromatic acid biosynthesis in Bacillus subtilis: sequenial feedback inhibition. J Bacteriol. 1966 Apr;91(4):1594–1598. doi: 10.1128/jb.91.4.1594-1598.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Demerec M., Eisenstark A. Genetic analysis of aromatic mutants of Salmonella typhimurium. Genetics. 1967 Jun;56(2):341–351. doi: 10.1093/genetics/56.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVERA A., Jr, SRINIVASAN P. R. THE ROLE OF 3-ENOLPYRUVYLSHIKIMATE 5-PHOSPHATE IN THE BIOSYNTHESIS OF ANTHRANILATE. Biochemistry. 1963 Sep-Oct;2:1063–1069. doi: 10.1021/bi00905a026. [DOI] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINSON D. B. The biosynthesis of aromatic compounds from D-glucose. Adv Carbohydr Chem. 1960;15:235–270. doi: 10.1016/s0096-5332(08)60189-7. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., ROTHSCHILD J., SPRINSON D. B. THE ENZYMIC CONVERSION OF 3-DEOXY-D-ARABINO-HEPTULOSONIC ACID 7-PHOSPHATE TO 5-DEHYDROQUINATE. J Biol Chem. 1963 Oct;238:3176–3182. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINS J. H., BARNES J. H. Effects of phencyclidine on the radiosensitivity of mice. Nature. 1962 Sep 22;195:1173–1175. [PubMed] [Google Scholar]
- YANIV H., GILVARG C. Aromatic biosynthesis. XIV. 5-Dehydroshikimic reductase. J Biol Chem. 1955 Apr;213(2):787–795. [PubMed] [Google Scholar]


