Abstract
The objectives of this work were the analysis of the functional characteristics of circulating monocytes and T lymphocytes in patients with liver cirrhosis, and evaluation of the relationship with an increased exposure to antigens due to bacterial translocation. Forty patients with liver cirrhosis (20 with compensated cirrhosis and 20 with ascitic decompensation) and 20 healthy control subjects were studied. Bacterial translocation was evaluated by serum levels of lipopolysaccharide binding protein (LBP). Macrophage activation was studied by CD40 antigen expression. T lymphocytes were analysed for activation (CD25+, CD122+), effector function (CD8+CD45RO+CD57+), apoptosis (CD95+) and regulatory abilities, either by analysis of the membrane expression of co-stimulatory molecules CD80, CD86 and CD28, or by quantification of regulatory T cells CD4+CD25highforkhead box P3 (FoxP3). The percentage of activated monocytes and T lymphocytes in patients was increased significantly. The proportions of effector senescent cells and of those near to apoptosis were also significantly higher. With respect to these proportions, there were no significant differences between patients in function of the presence or absence of decompensation or in function of the increased or normal values of LBP. Conversely, those patients with elevated levels of LBP presented a significantly higher frequency of regulatory T cells than those with normal levels. In conclusion, patients with liver cirrhosis showed an intensive activation state with a higher percentage of cells committed to activation-induced death, even in non-advanced stages. It is possible that bacterial permeability and endotoxaemia contribute to the expansion of those lymphocyte populations implicated in the prevention of a more severe antigen-induced immunopathology.
Keywords: bacterial translocation, innate immunity, liver cirrhosis, lipoprotein-binding protein, proinflammatory cytokines, T lymphocytes
Introduction
Increased prevalence of bacterial infectious diseases has been observed in cirrhotic patients, attributed classically to immunosuppression [1,2]. Several immune system abnormalities have been associated with cirrhosis. A decrease in monocyte function, characterized by a deficiency in adherence and phagocytosis, has been detected [3,4]. Similarly, alterations in natural killer activity [5] and lectin-induced proliferation of T lymphocytes have both been described [6]. However, an increase of serum immunoglobulin concentration is present in patients with liver cirrhosis, and this is possibly indicative of an altered balance between T lymphocyte helper responses implicated in cytotoxicity [T helper type 1 (Th1)] and those implicated in the synthesis of specific antibodies (Th2) [7,8].
Conversely, advanced states of liver cirrhosis predispose patients to an increased antigenic load. A possible role has been attributed to the translocation of bacteria and endotoxins [lipopolysaccharide (LPS)] from the gut [9,10]. LPS increases the plasma levels of LPS binding protein (LBP), the principal plasma protein responsible for transporting LPS to immune effector cells [11]. It has been proposed that high serum LBP identifies a subset of cirrhotic patients with ascites characterized by the immune cells being activated to produce tumour necrosis factor (TNF)-α and other proinflammatory cytokines [12–15]. Moreover, raised levels of circulating LBP or proinflammatory cytokines have been implicated in the endothelial activation and haemodynamic derangement observed in cirrhosis [12,16].
To our knowledge, the plasma antigenic load and increased bacterial translocation have not been related previously to those immune abnormalities characteristic of an immunodepressed state in patients with liver cirrhosis. We theorized that a chronic antigenic stimulus will induce activation of monocytes (as detected by increased secretion of the monocyte receptor of LPS/LBP complex, CD14 and by the secretion of proinflammatory cytokines [17,18]) and activation of lymphocytes (as detected by the membrane expression of alpha and beta receptors of interleukin (IL)-2, CD25 and CD122, respectively [19]). Continuous exposure to antigens will be associated with an increase of ‘exhausted’ cytotoxic populations (CD8+CD45RO+CD57+) [20] and an increase of these that have evolved close to apoptosis (indicated by CD95 expression) [21]. To down-regulate the chronic activation, a modification of lymphocyte co-stimulatory molecules (monocyte CD80 and CD86 and lymphocyte CD28 molecules) [22] and an expansion of the population of suppressor or regulatory T lymphocytes [CD4+CD25highforkhead box P3 (FoxP3)+][23] would be expected. These modifications could explain the functional alterations in T and B lymphocytes described in liver cirrhosis. Thus, the present study evaluates the following factors in patients with liver cirrhosis: (i) the distribution of circulating monocytes and T lymphocytes, their state of activation and their membrane-associated functional characteristics (co-stimulatory molecules, naive/memory, regulatory, effector, apoptosis markers); and (ii) the correlations of these with an increased exposure to bacteria and antigens, as detected by plasma LBP concentrations.
Patients and methods
Patients
This prospective study was performed on 40 patients with liver cirrhosis, divided into two groups in function of the presence (20 patients) or the absence (20 patients) of portal hypertension, as evidenced by an ascitic decompensation, and 20 healthy control subjects.
Inclusion criteria were: age between 18 and 80 years, histological or clinical diagnosis of cirrhosis, no evidence of metabolic, toxic or autoimmune liver disease and at least 1 year of alcohol abstinence. Exclusion criteria were the following: (i) clinical evidence of infection, including spontaneous bacterial peritonitis; (ii) neoplasia, including hepatocellular carcinoma; (iii) clinical, analytical or histological evidence of alcoholic hepatitis; (iv) treatments which could have modified the determination of cytokines or adhesion molecules (pentoxyfilline, steroidal or non-steroidal anti-inflammatory or immunosuppressive drugs); (v) gastrointestinal bleeding or shock; (vi) red blood cell or plasma transfusion in the month prior to inclusion in the study; (vii) antibiotic treatment, including prophylaxis of spontaneous bacterial peritonitis, during the month before admission; and (viii) lastly, heart failure (left ventricular ejection function < 50%), respiratory insufficiency (PaO2 < 60 mm Hg) and chronic renal failure (serum creatinine concentration > 1·5 mg/dl) were excluded. These were excluded in order to rule out the possible interference of contamination or reactions between these elements and the inflammatory and immune system measurements.
Diagnosis of cirrhosis was established according histological criteria when liver biopsy was performed [24], or by the combination of clinical, biochemical and ultrasound imaging data (presence of irregular margins on ultrasound, portal hypertension with laboratory evidence of chronic liver disease) consistent with such a diagnosis [25]. Patients were grouped according to Child–Pugh classification [26] and model for end-stage liver disease (MELD) score [27].
Presence of ascites was assessed by ultrasonography. Bacterial infection was ruled out by clinical history, physical examination, differential and total white blood cell count, analysis and culture of urine, thorax X-ray and by the culture and white blood cell count of ascetic fluid in patients with ascites.
The control population was comprised of healthy subjects age- and sex-matched with the patients. The absence of evidence of infection was confirmed by anamnesis, physical exploration and laboratory tests (absence of increase in leucocyte count, normal values of globular sedimentation rate and C-reactive protein and normal urine elemental analysis).
Informed consent was obtained from patients and controls. The protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional human research committee.
Serum levels of LBP and inflammatory markers
Blood samples, collected in sterile Vacutainer tubes (Becton-Dickinson Vacutainer System, Meylan Cedex, France), were centrifuged (150 g for 15 min at 4°C) and serum was stored at −80°C in pyrogen-free polyethylene tubes (Biofreeze, Costar, Acton, MA, USA) until LBP, cytokines and soluble cytokine receptors were assayed.
Bacterial translocation was evaluated by serum levels of LBP. LBP was measured by immunometric sandwich assay with a chemiluminescent substrate using an automated analysis system (Immulite LBP, DPC, Los Angeles, CA, USA).
Monocyte activation was evaluated by the monocyte expression of CD40 (see later) and by the secretion of soluble CD14 receptor (sCD14) and proinflammatory cytokines (TNF-α and IL-6). Because of the short life of TNF-α, soluble receptor of TNF (sTNF-RI) of 55 kDa, whose half-life is longer, was analysed; sTNF-RI is shed from the cell surface of polymorphonuclear cells and monocytes in response to the same inflammatory agents as those that are known to induce TNF-α; their concentrations are correlated with those of TNF-α[28].
Serum levels of sCD14, sTNF-RI and IL-6 were assayed with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer's instructions, with the following detection limits (lowest positive standard): sCD14, 0·4 ng/ml; IL-6, 30 pg/ml; sTNF-RI, 0·4 ng/ml.
Cell separation and surface immunofluorescence
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized venous blood by Ficoll-Hypaque (Lymphprep Nyegaard, Oslo, Norway) density gradient centrifugation. After counting, the cells were resuspended (1 × 106 cells/ml) in RPMI-1640 medium.
Proportions of monocyte and T cell subpopulations were determined in PBMC by three-colour flow cytometry in a fluorescence activated cell sorter (FACScalibur) cytometer using Cell Quest and Paint-A-Gate software (Becton-Dickinson, San Jose, CA, USA). PBMC were incubated with combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- and peridinin chlorophyll protein (PerCP)-labelled monoclonal antibodies. The monoclonal antibodies were: markers of monocyte activation: CD14+CD40+; markers of co-stimulation: monocyte (CD14+ cells) expression of CD80 and CD86 antigens; lymphocyte (CD3+ cells) expression of CD28 antigen; CD3− subpopulation CD4+ and CD8+; lymphocyte (CD4+ and CD8+ cells) activation antigens (alpha and beta receptors of IL-2, CD25 and CD122, respectively); regulatory T cells (CD4+CD25high intracellular FoxP3+); markers of memory lymphocytes (CD4+CD45RO+ and CD8+CD45RO+); ‘exhausted or senescent’ cytotoxic cells (CD8+CD45RO+CD57+) and markers of apoptosis (CD4+CD45RO+CD95+ and CD8+CD45RO+CD95+) (Fig. 1)
Fig. 1.
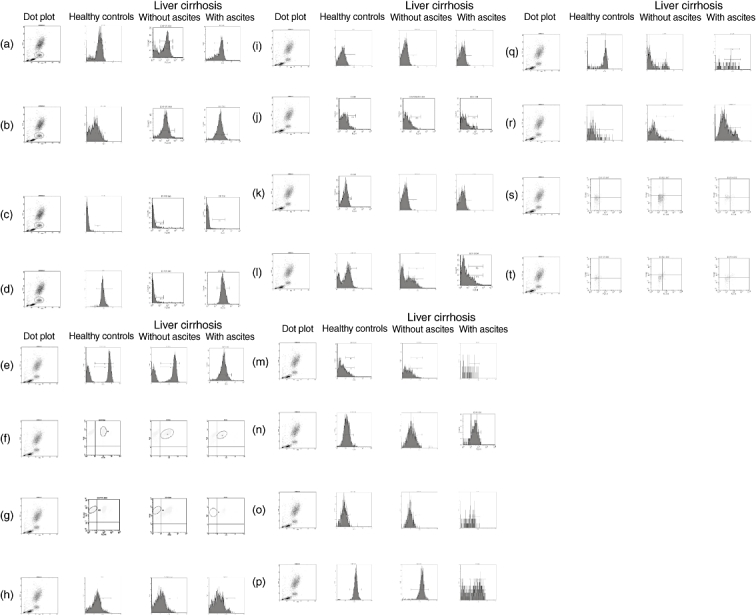
Representative dot blots of the fluorescence activated cell sorter (FACS) data of all the different antibodies used in healthy controls and patients with liver cirrhosis, with or without ascites. (a) CD14+ cells (referred to total leucocytes). (b) CD14+CD40+ cells. (c) CD14+CD80+ cells. (d) CD14+CD86+ cells. (e) CD3+ cells (referred to total lymphocytes). (f) CD3+CD4+ cells. (g) CD3+CD8+ cells. (h) CD4+CD25+ cells. (i) CD4+CD122+ cells. (j) CD8+CD25+ cells. (k) CD8+CD122+ cells. (l) CD4+CD45RO+ cells. (m) CD8+CD45RO+ cells. (n) CD4+CD45RO+CD95+ cells. (o) CD8+CD45RO+CD95+ cells. (p) CD4+CD28+ cells. (q) CD8+CD28+ cells. (r) CD8+CD57+ cells. (s) CD4+CD25+forkhead box P3 (FoxP3)+ negative control. (t) CD4+CD25+FoxP3+ cells.
Absolute counts for monocytes and lymphocytes were calculated by multiplying their percentage among PBMC by their blood Coulter count.
Statistical analysis
Continuous variables are expressed as median [interquartile range (IQR)] and categorical variables as number (percentage). Comparisons between categorical variables were made by the χ2 test or Fisher's test. For continuous variables Kruskal–Wallis one-way analysis of variance (ANOVA) was used.
Associations between continuous variables were established through linear regression analysis. The level of statistical significance was set at P < 0·05. The statistical analysis was carried out using the SPSS version 15 statistical software package (SPSS Inc., Chicago, IL, USA).
Results
Clinical and inflammatory-related markers of the cirrhotic patients
Table 1 shows the clinical and routine analytical features of the patients included in the study. In more than half the patients, liver cirrhosis was secondary to chronic hepatitis C virus infection, whereas alcohol consumption was the second cause of cirrhosis (nine cases, 23%); in the rest of the patients (nine cases, 23%), both alcohol and hepatitis C virus were considered the cause of cirrhosis. As expected, liver and/or renal function indices (Child–Pugh, MELD scores or creatinine concentration) were significantly higher in patients with ascites.
Table 1.
Clinical and elemental analytical characteristics in cirrhotic patients and healthy controls
| Healthy controls (n = 20) | Cirrhotic patients without ascites (n = 20) | Differences: healthy controls versus compensated cirrhosis (P) | Cirrhotic patients with ascites (n = 20) | Differences: healthy controls versus decompensated cirrhosis (P) | Differences: compensated versus decompensated cirrhosis (P) | |
|---|---|---|---|---|---|---|
| Age (years) | 60(45–66) | 63(54–70) | n.s. | 60(49–68) | n.s. | n.s. |
| Male sex(n, %) | 9(45·0) | 9(45·0) | n.s. | 9(45·0) | n.s. | n.s. |
| Patients with VHC-related cirrhosis(n, %) | 11(55·0) | 11(55·0) | n.s. | |||
| Child–Pugh score(points) | 5(5–6) | 9(8–10) | < 0·001 | |||
| MELD score(points) | 6(6–8) | 13(8–17) | < 0·001 | |||
| Serum ALT(UI/l) | 18(15–23) | 75(46–105) | < 0·001 | 59(13–76) | < 0·001 | n.s. |
| Serum creatinine(mg/dl) | 0·8(0·7–0·9) | 0·8(0·6–0·9) | n.s. | 1·1(0·9–1·4) | 0·010 | 0·013 |
| Leucocytes(1000× cells/mm3) | 6·6(5·4–7·1) | 5·2(3·9–6·4) | n.s. | 4·6(2·5–6·4) | n.s. | n.s. |
| Percentage of lymphocytes(% of total leucocytes) | 33(26–36) | 36(26–46) | n.s. | 16(12–23) | < 0·001 | < 0·001 |
| Percentage of monocytes(% of total leucocytes) | 7(6–8) | 9(7–10) | 0·004 | 11(9–15) | < 0·001 | 0·002 |
Continuous variables are expressed as median (interquartile range) and categorical variables as number (percentage). ALT, alanine aminotransferase; MELD, model for end-stage liver disease; n.s., not significant; VHC, virus hepatitis C.
Serum concentrations of LBP, sCD14, sTNF-RI and IL-6 were significantly higher in cirrhotic patients with and without ascites, compared with healthy controls. LBP, sTNF-RI and IL-6, but not sCD14, levels were significantly higher in patients with ascites compared with those without ascites (Table 2).
Table 2.
Serum concentrations of lipopolysaccharide binding protein (LBP), soluble CD14, soluble tumour necrosis factor (TNF) receptor 55 kDa and interleukin (IL)-6 in healthy controls and patients with compensated and decompensated cirrhosis
| Healthy controls (n = 20) | Cirrhotic patients without ascites (n = 20) | Differences: healthy controls versus compensated cirrhosis (P) | Cirrhotic patients with ascites (n = 20) | Differences: healthy controls versus decompensated cirrhosis (P) | Differences: compensated versus decompensated cirrhosis (P) | |
|---|---|---|---|---|---|---|
| LBP | 3·4 | 7·7 | < 0·001 | 28·2 | < 0·001 | < 0·001 |
| (µg/ml) | (2·7–4·2) | (5·7–9·1) | (10·7–40·6) | |||
| sCD14 | 2960 | 8455 | < 0·001 | 10790 | < 0·001 | n.s. |
| (ng/ml) | (2354–3715) | (4581–10579) | (7590–12778) | |||
| sTNF-RI | 144 | 232 | < 0·001 | 527 | < 0·001 | 0·008 |
| (pg/ml) | (108–177) | (143–259) | (234–1406) | |||
| IL-6 | 0 | 5 | < 0·001 | 26 | < 0·001 | < 0·001 |
| (pg/ml) | (0–2) | (2–7) | (14–58) |
Variables are expressed as median (interquartile range). n.s., not significant.
Peripheral blood monocytes (CD14+ cells) are markedly increased in number and are spontaneously activated in cirrhotic patients
Both the percentage of monocytes (CD14+ cells) (Table 1) and their absolute number [cirrhotic patients, 405 (300–600) versus healthy controls, 338 (264–380) cells/mm3, P = 0·038] were significantly higher in patients with liver cirrhosis compared with controls. In cirrhotic individuals, the proportion of activation markers in these cells (CD14+CD40+ cells) was significantly higher than in the healthy controls, while the differences between ascitic and non-ascitic patients in this respect were not significant (Fig. 2).
Fig. 2.
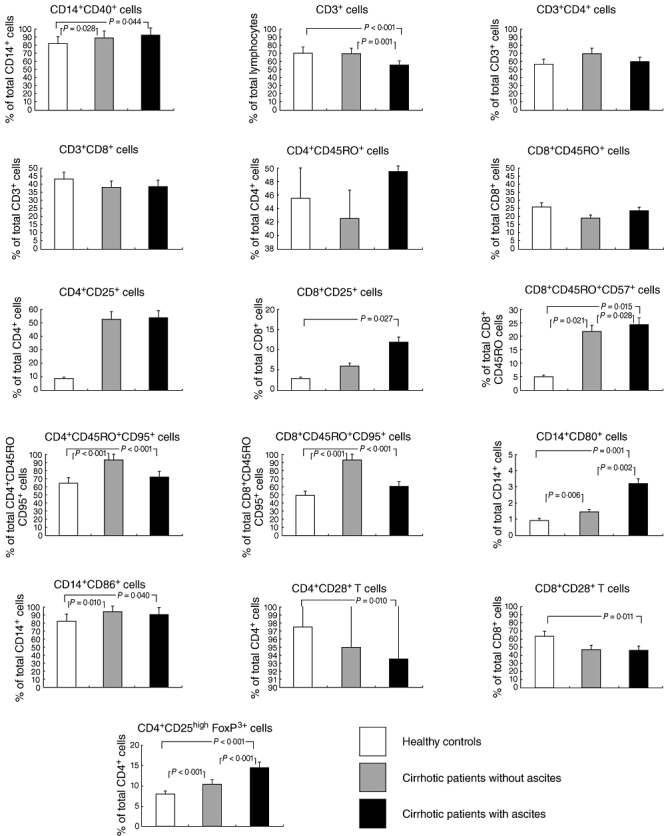
Monocyte and T lymphocytes expression of molecules implicated in lymphocyte co-stimulation and regulatory T cells in healthy controls and patients with compensated and decompensated cirrhosis.
Cirrhotic patients show T cell lymphopenia and redistribution of T cell subpopulations
Because of the significant differences in the absolute number of lymphocytes between patients with cirrhosis and controls [cirrhotic patients, 1100 (608–1810) versus healthy controls, 2030 (1680–2340) cells/mm3, P < 0·001], values of lymphocyte subpopulations will be expressed as percentages, and not as their absolute numbers. The total number of peripheral blood T cells (CD3+ cells) was reduced significantly in cirrhotic patients with ascites [cirrhotic patients, 712 (261–1200) versus healthy controls, 1407 (1093–1699) cells/mm3, P < 0·001].
The percentage of CD4+ and CD8+ T cells, referred to as total CD3+ T cells, were similar in patients both with and without ascites and in controls. The percentages of memory (CD45RO+) CD4 and CD8 T cells were similar in the various groups.
However, in patients with cirrhosis, the population of activated CD4+ T cells (demonstrated by the membrane expression of IL-2 receptors, CD25 and CD122 antigens) was significantly higher.
The percentage of the CD8+ T cells with markers of senescence (CD8+CD45RO+CD57+ cells) was also increased significantly in patients with cirrhosis in comparison with healthy controls.
Finally, a significantly higher proportion of memory CD4 and CD8 populations expressing apoptosis markers (CD95+) was detected in patients with cirrhosis compared with healthy controls. However, no significant differences were detected between patients with and without ascites with regard to the markers mentioned above.
Co-stimulatory antigens and regulatory populations in patients with liver cirrhosis
In patients with liver cirrhosis, mainly those with ascites, the percentage of monocytes expressing both CD86 and CD80 co-stimulatory molecules was significantly higher than that observed in healthy controls. Moreover, the proportion of T lymphocytes expressing CD28 was significantly lower in those patients with cirrhosis and ascites (Fig. 2).
An analysis was made of the proportion of T cells implicated in regulatory suppressor functions and characterized by the high intensity of membrane expression of CD25 antigen, and by the intracellular expression of FoxP3 by CD4+ T cells [23]. The percentage of these regulatory cells was significantly higher in patients with liver cirrhosis. Moreover, the proportion of regulatory T cells was significantly higher in patients with ascites than in those without ascites (Fig. 2).
Higher intestinal antigen exposure does not explain completely the modifications of monocyte and lymphocyte subpopulations
To ascertain the possible pathogenesis of monocyte and T lymphocyte modification, patients were grouped in function of the aetiology of the cirrhosis. No significant differences were detected between patients with viral- or alcohol-induced cirrhosis in respect of the markers analysed previously (data not shown).
Secondly, patients were divided in function of the serum values of LBP, as indicative of increased intestinal permeability and bacterial translocation. Serum LBP was above the healthy control threshold level (< 9·62 µg/ml) in the 20 (100%) patients with ascites and in five (25%) of those without ascites. Patients with elevated LBP values presented a significantly higher serum concentration of sTNF-RI [550 (248–1523) versus 232 (152–253) pg/ml, P = 0·003] and IL-6 [26 (7–61) versus 5 (2–13) pg/ml, P = 0·004], as well as an increased proportion of regulatory T cells [14 (11–15) versus 10 (10–11)%, P = 0·009], in comparison with those with normal LBP values. The rest of the monocyte and T lymphocyte subpopulations showed similar values in both groups (Fig. 3). A significant correlation was observed between LBP concentrations and percentages of regulatory T cells (Fig. 4).
Fig. 3.
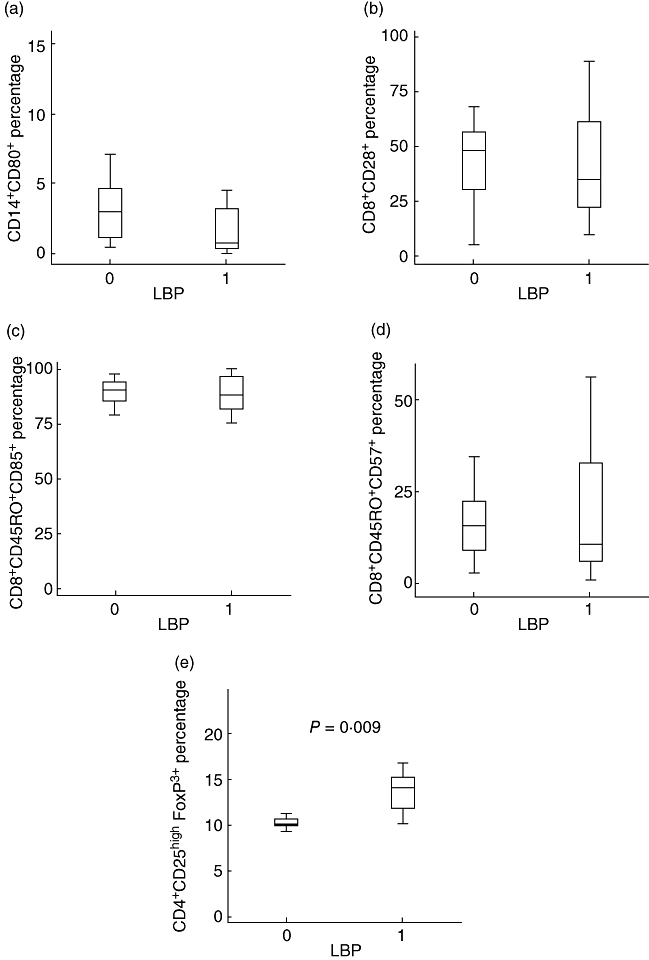
Percentages of monocyte and T lymphocyte subpopulations distributed according the values of lipopolysaccharide binding protein [normal (0): < 9·62 mg/ml; increased (1): > 9·62 mg/ml]. (a) CD14+CD80+ cells (% of total CD14+ cells). (b) CD8+ CD28+ T cells (% of total CD8+ cells). (c) CD8+CD45RO+CD95+ cells (% of total CD8+CD45RO+ cells). (d) CD8+CD45RO+CD57+ cells (% of total CD8+CD45RO+ cells). (e) CD4+CD25high forkhead box P3+ cells (% of total CD4+ cells).
Fig. 4.
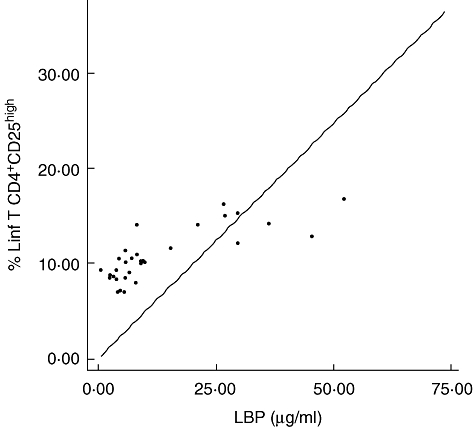
Correlation between lipopolysaccharide binding protein concentration and percentage of T regulatory lymphocytes (CD4+CD25+) in patients with liver cirrhosis (r = 0·787, P < 0·001).
Discussion
The present study shows the intensive derangement of the monocyte and T cell compartments of the immune system in patients with liver cirrhosis, some of them associated with biological markers of exposure to bacterial products of probable gut origin.
The finding of a marked increase in the number and activation of blood monocytes in these patients has been demonstrated previously in patients with alcoholic cirrhosis [6,12]. Furthermore, monocytes of cirrhotic patients exhibited a markedly increased capacity for spontaneous and LPS-stimulated proinflammatory cytokine expression [15,29]. Interestingly, although sTNF-RI and IL-6 levels were elevated in patients with compensated cirrhosis, they were increased significantly in patients with ascites; higher values were detected predominantly in patients with high endotoxaemia, as measured by the serum levels of LBP. Consequently, we attribute a significant role to the consequences of intestinal permeability and bacterial translocation in the pathogenesis of innate immunity alterations observed in patients with cirrhosis. However, other factors must also be operative because, in those patients with compensated cirrhosis and/or normal LBP, the proportion of activated monocytes or the serum levels of proinflammatory cytokines was higher in comparison with healthy controls, although lower than in patients without ascites and/or elevated LBP.
T lymphocyte activation was also detected in these patients. In both compensated and decompensated cirrhosis, the proportion of CD4+ T cells expressing CD25 and CD122 antigens was increased significantly. Moreover, as expected in a situation of a prolonged antigen stimulus, the proportion of the memory CD4+ and CD8+ T cells expressing apoptosis markers was increased [30]. After initiation of the immune response, lymphocytes proliferate and differentiate into effector cells. This process needs to generate sufficient numbers of effector T cells specific for the pathogen. Following the immune response, activated lymphocytes have to be eliminated in order to maintain lymphocyte homeostasis. In this context, activation-induced cell death (AICD) describes the induction of cell death in already activated T cells by restimulation of their T cell receptors (TCR). It is believed commonly that AICD involves the engagement of death receptors such as CD95 [31,32]. Our findings support the existence of an increased proportion of T lymphocytes with these characteristics in patients with liver cirrhosis.
However, it is probably of more interest that a subset of CD8+ T lymphocytes, with characteristics of previously activated cells, expressed the CD57+ antigen in significantly higher proportions. Expression of the CD57 receptor on CD8+CD45RO+ T cells can be considered a marker of cytotoxic effector T cells that are increased in vivo after antigenic activation. The persistence of the antigen will lead to loss of co-stimulatory molecules, telomere shortening and defective IL-2 production, changes that define the state of replicative senescence in T lymphocytes [32,33]. In short, these effector cells are close to being senescent [20].
The complete picture is that of a prolonged activation of T lymphocytes, with a significant number of effector cells implicated in the antigenic response and with a higher probability of activation-induced death. It is recognized that these cells are unable to proliferate after lectin or new antigenic load [34]. Thus, it is possible to speculate that immunosuppression, associated classically with liver cirrhosis, could be secondary to an exhausted state of the adaptative immunity.
However, other data from this study reveal the existence of modifications in monocyte and T lymphocyte populations to adapt to continuous activation. Thus, a situation compatible with an antigenic tolerance is that of an increase of monocyte expression of CD80 and a decrease of T lymphocyte CD28. Lymphocyte activation requires antigen recognition and additional co-stimulatory signals produced, among others, by coupling the T lymphocyte membrane antigen CD28 with the monocyte antigen CD86. Chronic antigenic stimulus induces an increased expression of CD86 in the monocyte, as well as the expression of an alternative molecule CD80, and in the T lymphocyte the expression of CD152 [cytotoxic T lymphocyte antigen (CTLA-4)] is induced. The union of CD80 and CD152 induces a negative signal sent to the T lymphocyte and, thus, a tolerance state [21,22]. Expression of CTLA-4 (CD152) was not analysed because internalization of this molecule is rapid and its determination produces erroneous results [35]. However, it is accepted that the reduced expression of CD28 is linked systematically to an increased expression of CD152 [21,22]. There is long-standing evidence that LPS leads to activation of the signalling pathways that induce increased expression of the CD80 co-stimulatory molecule on the surface of the monocytes [36,37]. Although significant differences were not found between patients with increased and normal LBP levels, an increase of CD80 expression was detected in those with elevated LBP concentration, as well as a non-significant reduction of CD28 expression; this pattern is consistent with a continuous antigenic signalling and a decrease of the induced activation.
The effector mechanisms used by the host to control infection must be controlled tightly in order to prevent excessive tissue damage and the development of autoimmunity. There has been considerable interest in the CD4+CD25high T cell subset, which is believed to be critical in maintaining host immune homeostasis [23,38]. This subset can be derived normally from the thymus prior to activation, or it can develop from conventional CD4+CD25− T cells in the periphery during activation in vivo or in vitro[39]. The CD4+CD25high regulatory T cells are characterized by the constitutive expression of several activation markers, including FoxP3, a key molecule for their development and function [23]. Regulatory T cells are able to inhibit both proliferation and cytokine production of CD4+ and CD8+ T cells [40]. The CD4+CD25+ regulatory T cells play a pivotal role in controlling autoimmune diseases, allergies and graft rejection. However, they are also involved in parasite persistence, inhibition of anti-tumour immunity and viral clearance [41,42]. In our study, an increased population of regulatory T cells was detected in patients with either viral or ethanol-induced cirrhosis. Furthermore, frequency of regulatory T cells was correlated with an increased antigenic charge, as measured by LBP. This probably implies that CD4+ regulatory T cells can prevent antigen-induced immunopathology, but may also increase the antigenic load and prolong pathogen persistence by suppressing protective immune responses.
Putative signals implicated in the monocyte and lymphocyte activation are the following: (i) first, bacterial translocation and endotoxaemia are known causes of monocyte activation [12,14,43]. In fact, this was our original hypothesis. Patients with increased LBP, a validated marker of endotoxaemia [11–14], present elevated serum levels of monocyte-derived cytokines or a higher percentage of regulatory T cells. It could be speculated that intestinal decontamination, for example with quinolones, could modify these altered factors. The presence of an increased LBP, however, does not explain completely the findings mentioned, because percentages of activated monocytes or T lymphocytes or senescent CD8+ or pre-apoptotic CD4+ and CD8+ T cells were increased in both patients with and without elevated LBP. Thus, other monocyte and lymphocyte activation signals are suggested. (ii) Secondly, aetiological factors of the liver disease. Both hepatitis C virus (HCV) and neo-antigens generated by the action of alcohol on the liver membranes have been reported as potent stimuli of the innate and acquired immunity [44,45]. No differences between these two factors were detected in patients analysed in our study. (iii) Thirdly, the inflammatory activity itself in the liver is implicated in the necrosis and fibrosis of this organ. In liver cirrhosis, both antigen-specific and non-specific mechanisms work together to induce an immune-mediated injury. It has been considered that repeated cycles of inflammation and damage sustain a continuing recruitment of effector leucocytes within the liver and amplify effector responses mediated by T cells, macrophages, natural killer or neutrophils [46,47]. Moreover, in recent years much attention has been devoted to the ability of cytokines and regulatory T cells to control the proliferation of memory T cells in the absence of antigen stimulation [48]. It is highly probable that the notable inflammatory activity generated in the liver could be partially responsible for the increased proportion of activated monocytes or T lymphocytes, senescent CD8+ and pre-apoptotic CD4+ and CD8+ T cells.
In conclusion, we have demonstrated profound alterations in the peripheral blood monocyte and T cell compartments of cirrhotic patients. These alterations are consistent with a state of monocyte and T lymphocyte activation, with the presence of an increased population of both CD4+ and CD8+ T cells committed to apoptosis, and with an increased population of effector CD8+ T cells with characteristics of senescent cells. Modulation of this activation state can be attributed to the described changes in the expression of co-stimulatory molecules required for the activation of T lymphocytes, as well as to the increased population of regulatory T lymphocytes. These findings at the cell level may contribute to understanding liver cirrhosis as an inflammatory process with broad cellular roots.
Acknowledgments
This work was supported by grants from the Spanish Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo (FIS 05/1440 and FIS 08/0869).
Disclosure
None.
References
- 1.Navasa M, Rimola A, Rodes J. Bacterial infection in liver disease. Semin Liver Dis. 1997;17:323–33. doi: 10.1055/s-2007-1007209. [DOI] [PubMed] [Google Scholar]
- 2.Christou L, Pappas G, Falagas ME. Bacterial infection related morbidity and mortality in cirrosis. Am J Gastroenterol. 2007;102:1510–17. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 3.Gomez F, Ruiz P, Schreiber AD. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. N Engl J Med. 1994;331:1122–8. doi: 10.1056/NEJM199410273311704. [DOI] [PubMed] [Google Scholar]
- 4.Bolognesi M, Merkel C, Bianco S, et al. Clinical significance of the evaluation of hepatic reticulo-endothelial removal capacity in patients with cirrhosis. Hepatology. 1994;19:628–34. doi: 10.1002/hep.1840190313. [DOI] [PubMed] [Google Scholar]
- 5.Laso FJ, Madruga JI, Girón JA, et al. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25:1096–100. doi: 10.1002/hep.510250508. [DOI] [PubMed] [Google Scholar]
- 6.Girón JA, Alvarez-Mon M, Menéndez-Caro JL, et al. T Lymphocytes from alcoholic cirrhotic patients show normal interleukin 2 production but a defective proliferative response to polyclonal mitogens. Am J Gastroenterol. 1994;89:767–73. [PubMed] [Google Scholar]
- 7.Girón JA, Alvárez-Mon M, Menéndez-Caro JL, et al. Increased spontaneous and lymphokine-conditioned immunoglobulin A and G synthesis by B cells from alcoholic cirrhotic patients. Hepatology. 1992;16:664–70. doi: 10.1002/hep.1840160309. [DOI] [PubMed] [Google Scholar]
- 8.Menéndez JL, Alvarez-Mon M, Girón JA, et al. IgM B cell differentiation production by T lymphocytes from patients with primary biliary cirrhosis. J Hepatol. 1994;20:446–53. doi: 10.1016/s0168-8278(05)80488-x. [DOI] [PubMed] [Google Scholar]
- 9.Wiest R, Das S, Cadelina G, García-Tsao G, Milstien S, Groszman RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–33. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zapater P, Francés R, González-Navajas JM, et al. Serum and ascitic fluid bacterial DNA: a new independent prognostic factor in non-infected patients with cirrhosis. Hepatology. 2008;48:1924–31. doi: 10.1002/hep.22564. [DOI] [PubMed] [Google Scholar]
- 11.Schumann RR, Zweigner J. A novel acute-phase marker: lipopolysaccharide binding protein (LBP) Clin Chem Lab Med. 1999;37:271–4. doi: 10.1515/CCLM.1999.047. [DOI] [PubMed] [Google Scholar]
- 12.Albillos A, de la Hera A, González M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–17. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 13.Genesca J, Marti R, Rojo F, et al. Increased tumor necrosis factor-α production in mesenteric lymph nodes of cirrhotic patients with ascites. Gut. 2003;52:1054–9. doi: 10.1136/gut.52.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girón-González JA, Martínez-Sierra C, Rodriguez-Ramos C, et al. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004;24:437–45. doi: 10.1111/j.1478-3231.2004.0951.x. [DOI] [PubMed] [Google Scholar]
- 15.Albillos A, de la Hera A, Reyes E, et al. Tumour necrosis factor-alpha expression by altered monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin. J Hepatol. 2004;40:624–31. doi: 10.1016/j.jhep.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Albillos A, de la Hera A, Alvarez-Mon M. Serum lipopolysaccharide binding-protein prediction of severe bacterial infection in cirrhotic patients with ascites. Lancet. 2004;363:1608–10. doi: 10.1016/S0140-6736(04)16206-5. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins PA, Fraser JD, Pridmore AC, Russell HH, Read RC, Sriskandan S. Superantigen recognition by HLA class II on monocytes up-regulates Toll-like receptor 4 and enhances proinflammatory responses to endotoxin. Blood. 2005;105:3655–62. doi: 10.1182/blood-2004-07-2523. [DOI] [PubMed] [Google Scholar]
- 18.Sriskandan S, Altmann D. The immunology of sepsis. J Pathol. 2008;214:211–23. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- 19.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 21.Bernard A, Lamy LA, Alberti I. The two-signal model of T-cell activation after 30 years. Transplantation. 2002;73:S31–S35. doi: 10.1097/00007890-200201151-00011. [DOI] [PubMed] [Google Scholar]
- 22.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for co-stimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FoxP3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 24.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 25.Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med. 2002;21:1023–32. doi: 10.7863/jum.2002.21.9.1023. [DOI] [PubMed] [Google Scholar]
- 26.Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 27.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 28.Porteu F, Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990;172:599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deviere J, Content J, Denys C, et al. Excessive in vitro bacterial lipopolysaccharide-induced production on monokines in cirrhosis. Hepatology. 1990;11:628–34. doi: 10.1002/hep.1840110416. [DOI] [PubMed] [Google Scholar]
- 30.Lavrik I, Golks A, Krammer PH. Death receptor signalling. J Cell Sci. 2005;118:265–7. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 31.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 32.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66:52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Marinova E, Han S, Zheng B. Human germinal center T cells are unique T cells with high propensity for apoptosis induction. Int Immunol. 2006;18:1337–45. doi: 10.1093/intimm/dxl066. [DOI] [PubMed] [Google Scholar]
- 34.Tarazona R, De la Rosa O, Alonso C, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 35.Brunner-Weinzierl MC, Hoff H, Burmester GR. Multiple functions for CD28 and cytotoxic T lymphocyte antigen-4 during different phases of T cell responses: implications for arthritis and autoimmune diseases. Arthritis Res Ther. 2004;6:45–54. doi: 10.1186/ar1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattern T, Flad HD, Brade L, Rietschel ET, Ulmer AJ. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160:3412–18. [PubMed] [Google Scholar]
- 37.Medzhitov R, Janeway C. Innate immunity. N Engl J Med. 2000;343:338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 38.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 39.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells downregulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Stassen M, Schmitt E, Jonuleit H. Human CD4+CD25+ regulatory T cells and infectious tolerance. Transplantation. 2004;77(Suppl.):S23–5. doi: 10.1097/00007890-200401151-00009. [DOI] [PubMed] [Google Scholar]
- 41.Cabrera R, Tu Z, Xu Y, et al. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 42.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 43.Roth P, Bartocci A, Stanley ER. Lipopolysaccharide induces synthesis of mouse colony-stimulating factor-1 in vivo. J Immunol. 1997;158:3874–80. [PubMed] [Google Scholar]
- 44.Kanto T, Hayashi N. Immunopathogenesis of hepatitis C virus infection: multifaceted strategies subverting innate and adaptative immunity. Intern Med. 2006;45:183–91. doi: 10.2169/internalmedicine.45.1530. [DOI] [PubMed] [Google Scholar]
- 45.Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–54. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 46.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 47.Eksteen B, Afford SC, Wigmore SJ, Holt AP, Adams DH. Immune-mediated liver injury. Semin Liver Dis. 2007;27:351–66. doi: 10.1055/s-2007-991512. [DOI] [PubMed] [Google Scholar]
- 48.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J Exp Med. 1994;180:1159–64. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]


