Abstract
Objectives
To determine the annualized rates of volumetric change of the hippocampus and temporal horn in cognitively normal elderly control subjects and individually matched patients with Alzheimer's disease (AD). To test the hypothesis that these rates were different .
Background
Cross-sectional studies consistently reveal cerebral atrophy in elderly non-demented subjects compared to healthy young adults, and greater atrophy in patients with AD relative to elderly controls. However, rates of atrophy are most accurately estimated by performing serial measurements in the same individuals.
Methods
Magnetic resonance imaging (MRI)-based volume measurements of the hippocampi and temporal horns were performed in 24 cognitively normal subjects ages 70–89 years who were individually matched with respect to gender and age with 24 patients with AD. Each subject underwent an MRI scanning protocol twice, separated by 12 months or more.
Results
The mean annualized rate of hippocampal volume loss among controls was −1.55% ± 1.38%/year and the temporal horns increased in volume by 6.15% ± 7.69%/year. These rates were significantly greater among AD patients: hippocampus −3.98% ± 1.92%/year, P <.001; temporal horn 14.16% ± 8.47%/year, P = .002.
Conclusion
A statistically significant yearly decline in hippocampal volume and increase in temporal horn volume was identified in elderly controls who represent typical aging individuals. These rates were approximately 2◻ times greater in patients with AD than in individually age and gender matched controls.
The ability of magnetic resonance imaging (MRI) to precisely depict in vivo neuroanatomy has lead investigators to employ this technique to evaluate brain morphometrics in aging and dementia [1–12]. Particular interest has arisen in MRI-based quantitative measures of the medial temporal lobe limbic structures because of the central role they play in memory function, and because these areas are involved first and most severely by the neurofibrillary pathology of Alzheimer's Disease (AD) [13]. While a number of pathologic and imaging studies have documented a decline in brain volume in typical aging, and an accelerated rate of volume loss in AD, with a few exceptions these studies have been cross-sectional in design. In order to directly assess the effects of aging on the medial temporal lobe, serial measurements in the same individuals are required. The goals of this project were: 1) to determine the annualized rate of volumetric change of the hippocampus and temporal horn in cognitively normal elderly control subjects who represent typical aging individuals, 2) to determine the annualized rates of volumetric change in a group of individually matched patients with AD and to test the hypothesis that this rate was different from that in controls, 3) to assess the influence of several clinical variables—age, gender, presence of hypertension, cardiac ischemic disease, diabetes, estrogen replacement, and apolipoprotein E (APOE) genotype—on these rates of volumetric change.
Materials And Methods
Recruitment and Characterization of Subjects
Forty-eight subjects are included in this report—24 AD patients and 24 controls who were individually matched with the patients for gender and age (± 4 years) (Table 1). Controls and cases ranged in age from 70 to 89 years. Patients with AD and the cognitively normal control subjects for this study were recruited from the Mayo Alzheimer's Disease Center /Alzheimer's Disease Patient Registry. Informed consent was obtained for participation from the subjects or an appropriate proxy.
Table 1.
Characterization of Subjects
| Controls (n=24) | Cases (n=24) | P Value3 | |
|---|---|---|---|
| A | Mean ± SD | Mean ± SD | |
| Age | 81.04 ± 3.78 years | 80.42 ± 4.02 years | NS |
| Education | 14.75 ± 2.51years | 13.21 ± 2.83 years | NS |
| MMSE | 28.79 ± 1.28 | 20.74 ± 4.60 | <.001 |
| DRS | 137.38 ± 4.69 | 108.48 ± 14.35 | <.001 |
| Interval Between MRI Studies | 1.96 ± 0.75 years | 1.89 ± 0.68 years | NS |
| B | Frequency (%) | Frequency (%) | |
|---|---|---|---|
| Hypertension | 13 (54) | 10 (42) | NS |
| Cardiac Ischemic Disease | 8 (33) | 10 (42) | NS |
| Diabetes | 2 (8) | 2 (8) | NS |
| Estrogen replacement1 | 7 (44) | 9 (56) | NS |
| APOE ∈42 | 6 (25) | 11 (46) | .042 |
16 patients in each group (case, control) were women
Number of subjects who were carriers of APOE genotypes known to increase risk of AD (3/4 and 4/4). Patients who were ∈2/4 (n = 3) are not included in the counts listed.
Rank sum test of difference between case and controls for continuous variables, Chisquare for dichotomous variables.
Control subjects were recruited from the pool of patients coming to Mayo primary care physicians for a general medical examination. The criteria for cognitively normal controls were 1) no active neurologic or psychiatric disorders and 2) some had ongoing medical problems, however the illnesses or their treatments did not interfere with cognitive function. A control subject was identified for each AD patient and matched by gender, and age ±4 years.
The diagnosis of AD was made according to the NINCDS/ADRDA criteria [14, 15]. The severity of AD was classified on the basis of the clinical dementia rating (CDR) score [16]. Eleven patients had very mild disease (CDR = 0.5); 11 patients had mild disease (CDR = 1); and 2 patients had moderate disease severity (CDR = 2). Cases and controls were well matched on education, and by virtue of the study design on age and gender as well(Table 1). The number of men in both the case and control groups was 8.
APOE genotyping was performed in all subjects. DNA was extracted from peripheral leukocytes and amplified by polymerase chain reaction [17]. Polymerase chain reaction products were digested with Hhal and the fragments were separated by electrophoresis on an 8% polyacrylamide non-denaturing gel. The gel was then treated with ethidium bromide for 30 minutes, and DNA fragments were visualized by UV illumination.
The presence or absence of three vascular risk factors—hypertension, ischemic cardiac disease, diabetes—was assessed by review of the medical records. Subjects were recorded as positive for hypertension, if hypertension or its treatment was identified at any point in time in the medical record. The same criteria were applied to the diagnosis of diabetes. Subjects were considered to have coronary ischemic disease if any of the following diagnoses were identified: angina pectorus, myocardial infarction, coronary bypass surgery, or coronary angioplasty.
The presence or absence of estrogen replacement therapy was documented in all female subjects. The age of menopause was established in each subject and subsequent estrogen replacement therapy was recorded as either present or absent through review of the medical records.
All subjects underwent a MRI examination protocol of the brain within 4 months of their initial clinical assessment. An identical MRI study was repeated 12 or more months after the initial MRI in all subjects, and this was linked with a second clinical assessment. Potential subjects were excluded if either of the MRI studies were of unacceptable diagnostic quality, demonstrated a focal structural abnormality, or if their clinical status changed between the serial MRI examinations (e.g. a control who developed cognitive impairment).
Imaging Methods
All subjects were imaged at 1.5T (G.E. Signa, Milwaukee, WI) using a standardized imaging protocol. A T1-weighted sagittal set of spin echo images was used to measure total intracranial volume. A 3D volumetric spoiled gradient echo sequence with TR = 27 msec, TE = 9 msec, 124 contiguous partitions, 1.6 mm slice thickness, a 22 × 16.5 cm field of view, 192 views, and 45° flip angle was used to measure the volumes of the hippocampus and temporal horn.
All image processing steps (including boundary tracing) in every subject were performed by the same trained research assistant who was blinded to all clinical information (age, gender and clinical status). The date of each MRI scan was also masked in the image file so that the image processing was done without knowledge of the chronologic ordering of the scans in each pair. This ensured rigorous quality control, completely unbiased data generation, and uniformity in the subjective aspects of image processing across all the subjects in this study.
The 3D MRI data for both MRI scans were interpolated in the slice select dimension to give cubic voxels [18]. An automated image registration program was employed to coregister the 3D image data set of the first scan to that of the second scan. This program was developed in-house and was based on the principle of minimization of inter scan signal intensity differences across all voxels. The image data of both scan 1 and scan 2 were then interpolated in plane to the equivalent of a 512 × 512 matrix and magnified times two. The voxel size of the fully processed image data was 0.316mm3. The images of the whole brain were then subvolumed to include the temporal lobes. An intensity inhomogeneity correction algorithm developed in-house was then applied to both MRI scans. After the boundaries of the hippocampi and temporal horns had been delineated on each anatomic slice, the number of voxels in each structure was calculated automatically with a summing region of interest function. These were multiplied by voxel volume to give a numeric value in mm3.
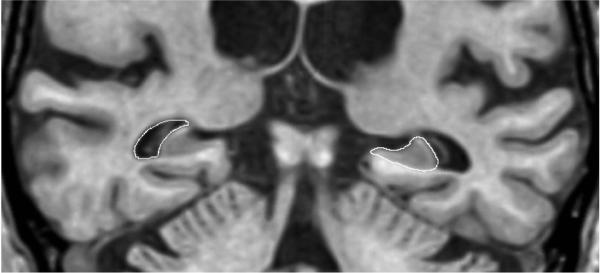
The borders of the right and left hippocampi were manually traced with a mouse driven cursor for each slice sequentially from posterior to anterior [18]. Inplane hippocampal anatomic boundaries were defined to include the CA1-CA4 sectors of the hippocampus proper, the dentate gyrus, and subiculum(Figure 1). The posterior boundary of the hippocampus was determined by the oblique coronal anatomic section on which the crura of the fornices were identified in full profile. Thus, essentially the entire hippocampus from tail through head was included in these measurements. The entire hippocampal tracing process takes approximately 2 hours per patient. Subdivision of the hippocampus along its antero-posterior axis into three segments labeled head, body, and tail was accomplished as follows: the hippocampal head was defined to encompass those imaging slices extending from the intralimbic gyrus forward to the anterior termination of the hippocampal formation. The posterior margin of the hippocampal head was labeled imaging slice x, and the volume of the hippocampal tail was determined by summing the area of the hippocampus on successive slices beginning from the forniceal crura to slice . The volume of the body consisted of the sum of areas on successive slices beginning with slice and extending to slice x-1. This method allowed for assignment of fractional slice areas in the event of an odd number of slices posterior to the hippocampal head.
Figure 1. Structure Boundaries.
Boundaries of the hippocampus indicated on the patient's left and the temporal horn on the right
A region growing autotrace algorithm was employed to define the boundaries of the temporal horns bilaterally(Figure 1). The signal intensities of temporal lobe white matter adjacent to the temporal horn, as well as CSF in the temporal horn were sampled in multiple places. The signal intensity threshold employed to define the temporal horn boundary was half of the maximum temporal lobe parenchymal signal intensity above background, where background was defined as CSF signal intensity[19]. The posterior extent of the temporal horn was defined as the same imaging slice used to demarcate the posterior boundary of the hippocampal formation. The anterior boundary of the temporal horn was determined by its full anterior anatomic extent.
Statistical Analysis
The primary endpoint in these analyses was the annualized percent change in hippocampal and temporal horn volume. This was computed as the volume in mm3 of scan 2 minus that of scan 1 divided by structure volume on the scan 1, divided by the duration between the two scans (in years). To compare the annualized percent change between AD cases and controls, the rank sum test was employed. Stepwise regression (stepping up) was employed to identify explanatory variables which might be associated with the volumetric endpoints. The regression analyses were performed separately for each group and also with the groups combined using group as a variable. After identifying significant main effects we looked for two-way interactions. The variables included in the regression analysis were age, gender, presence of hypertension, cardiac ischemic disease, diabetes, estrogen replacement, apolipoprotein E genotype, and duration of clinical followup. Differences in the annualized rate of change between sides (right vs left) and between hippocampal segments (head, body, tail) were assessed with paired t-tests.
Reproducibility
In order to assess the reproducibility of the method, 10 young adult volunteers underwent the MRI imaging protocol described above on two separate occasions, separated by 2–4 weeks. The volumes of the hippocampi and temporal horns in both scans of each of the volunteers were then measured as described above. Test-retest measurement reproducibility in 10 young adult volunteers was defined in terms of the coefficient of variation.
Results
Reproducibility
For the sum of the right and left hippocampus the median coefficient of variation was 0.28% (range 0.02% to 0.70%). For the temporal horn the median coefficient of variation was 0.95% (range 0.08% to 3.36%).
Controls
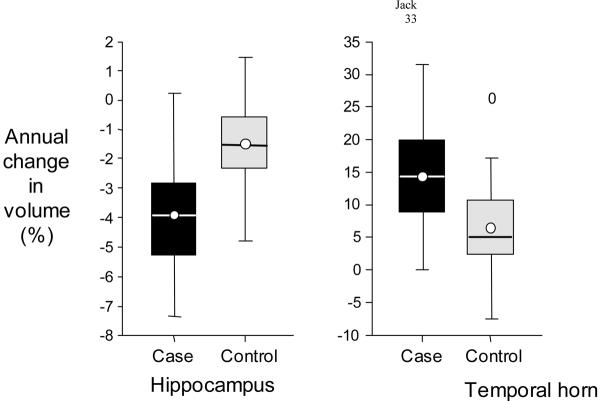
The average annualized rate of hippocampal volume loss among controls was 75 mm3 ± 60 mm3/year or −1.55% ± 1.38%/year. The negative sign in Table 2 indicates a decline in volume from scan 1 to scan 2. The temporal horns increased in volume by 167 mm3 ± 200 mm3/year, or 6.15% ± 7.69%. Hippocampal volume measurements increased between the first and second MRI studies in 1 control subject, and temporal horn volumes decreased in 4 control subjects(Figure 2).
Table 2.
Annual Percent Volumetric Change
| Controls (n=24) Mean ± SD | Cases (n=24) Mean ± SD | P-value* | |
|---|---|---|---|
| Hippocampi | −1.55 ± 1.38 | −3.98 ± 1.92 | <.001 |
| Temporal Horn | 6.15 ± 7.69 | 14.16 ± 8.47 | .002 |
Rank sum test of difference between cases and controls
Figure 2. Annual Percent Volumetric Change.
Box plots illustrating the annual percent volumetric change of the hippocampus and temporal horn separately for cases and controls. For each group the horizontal line in the box represents the median value, the box represents the 25th and 75th percentiles, the circle represents the mean, and the vertical line represents the range of values in each group. The 0 in the temporal horn column of controls indicates the value of a lone outlier.
The annual percent volume loss was significantly greater for the hippocampal head than for either the hippocampal body (P=.001) or tail (P=.014). The rates of hippocampal and temporal volume change were not different between the right and left side, nor were they associated with the interval between the 2 MRI studies. None of the clinical variables analyzed-age, gender, APOE genotype, estrogen replacement, hypertension, cardiac ischemic disease or diabetes were significantly associated with the rate of hippocampal or temporal horn volume change.
AD Cases
The mean annualized rate of hippocampal volume loss among AD cases was 150 mm3 ± 73 mm3/year or −3.98% ± 1.92%/year (Table 2). The mean annualized increase in temporal horn volume among AD cases was 660 mm3 ± 439 mm3 or 14.16% ± 8.47%. Measured hippocampal volumes decreased over time in all but 1 AD case and temporal horn volumes increased in all AD cases(Figure 2). The annualized rates of volume change for the hippocampus and temporal horn were not associated with the interval between MRI scans, position within the hippocampus (head, body, tail), or side (right, left). None of the clinical variables analyzed—age, gender, APOE genotype, estrogen replacement, hypertension, cardiac ischemic disease, or diabetes were significantly associated with the annualized rate of hippocampal or temporal horn volume change. No difference in the annualized percent change in hippocampal or temporal horn volume was present between AD patients with very mild (CDR = 0.5) vs mild (CDR = 1.0) disease severity.
Comparison of Cases and Controls
No differences in the prevalence of any of the three vascular risk factors—hypertension, cardiac ischemic disease, or diabetes— nor in the prevalence or duration of estrogen replacement was identified between the case and control groups(Table 1). Six controls and 11 cases had APOE genotypes (∈3/4 or 4/4) which are known to increase the risk of AD. The difference was significant (p = 0.042). The rates of hippocampal volume loss,P < .001, and temporal horn enlargement, P = .002, were significantly greater in AD cases than in controls (Table 2).
The initial (at scan 1) hippocampal and temporal horn volumes for cases and controls are found in Table 3. Values are reported in absolute terms (in units of mm3) and have also been normalized for intersubject variation in head size by dividing by total intracranial volume. All volumes were significantly different between controls and cases, P<.001.
Table 3.
Initial Hippocampal and Temporal Horn Volumes***
| Controls ( n=24) Mean ± SD | Cases (n = 24) Mean ± SD | |
|---|---|---|
| Hippocampi* | 5078.28 ± 766.17 | 3945.02 ± 945.19 |
| Hippocampi/TIV** | 3.61 ± 0.45 | 2.88 ± 0.64 |
| Temporal Horn* | 2779.77 ± 1372.01 | 4564.24 ± 1697.37 |
| Temporal Horn/TIV** | 1.94 ± 0.86 | 3.38 ± 1.35 |
values in units of mm3
structure volume normalized by dividing by total intracranial volume (TIV) in units of mm3/cm3 × 103
difference between volumes of cases and controls significant for both anatomic strucutres at P<.001, rank sum test, with and without normalization.
Discussion
Reproducibility
In performing a study which involves serial MRI scans on the same individuals a potential source of test-retest measurement variability is variation due to changes in the MRI scanning procedure itself. In addition to the subjective aspects of image tracing, it is possible that instrument drift of the MRI scanner or differences in subjects' head position may introduce scan to scan variation that would effect serial volume measures. For that reason we assessed the stability of MRI measures in a serial fashion in healthy young volunteers in whom no substantial biologic change in hippocampal or temporal horn volumes would be expected over the course of a 2–4 week period. The median test-retest coefficients of variation were quite small for both the hippocampi (0.28%) and temporal horns (0.95%) indicating excellent stability of serial measurements of these structures.
In our group of 48 cases and controls, hippocampal volume measurements increased by a small amount between the 2 MRI studies in one control (by 1.41%) and one AD case (by 0.19%)(Figure 2). These are clearly examples of measurement imprecision, but are reasonably close to the expected test-retest variability found in the reproducibility study. The small decrease in temporal horn volume measured in 4 controls may represent measurement imprecision or may reflect true changes in ventricular size due to changes over time in medications, nutritional, or hydration status.
Typical Aging
Studies on aging often identify two subgroups—typical aging and successful aging. Typical aging individuals are those who may have non-dementing illnesses which increase in prevalence with advancing age. Of particular interest are several vascular risk factors, most notably hypertension, which increase in prevalence with advancing age and also are associated with impaired cognition in elderly individuals[20, 21]. Successful aging on the other hand refers to individuals who remain free of these co-morbidities. Most imaging studies have used subjects who fall into the typical aging category as “normal” controls. Whether “normal” aging should be defined as typical aging or successful aging is a contentious topic. The control subjects in this study were not selected on the basis of the presence or absence of vascular risk factors. This control group should therefore be fairly representative of the general elderly population.
Controls
The effect of aging on brain morphology has been studied for over a century. Initial investigations involved the examination of autopsy material. In general, a loss of brain weight with age has been found. Some studies [22, 23], indicate that brain weight is stable from roughly age 20–60, after which an age related decline is seen. Other autopsy studies [24, 25] found a linear decline with advancing age beginning about at age 20.
Imaging studies, both MRI and CT, have generally found a loss of global hemispheric parenchymal volume, and an increase in CSF volume with advancing age in cognitively normal subjects[10, 26–29]. Results from recent studies assessing the impact of aging on hippocampal and other temporal lobe structures have been variable. Some have found age-related declines in volume in normal elderly individuals [4, 30, 31] while others have not [3, 32]. Variability in the results from different centers are likely due to differences in i) the age range of the study population, ii) the definition of “normal” aging, and iii) the MRI methods and neuroanatomic boundaries employed for measuring structure volume.
Most morphometric studies on aging have been cross-sectional in design. In contrast, our data represents a true longitudinal sample; the initial scan served as the reference point and each subject acted as his/her own control. Therefore, we can be confident that bias was not introduced by such factors as different environmental or socioeconomic conditions experienced by successive age groups [2]. The rates of volumetric change in the hippocampus (−1.55%/year), and the temporal horn (6.15%/year) that we found, seem large in comparison to those reported in prior cross-sectional studies. For example, Miller et al [23] calculated a loss of brain weight of 2%/decade in autopsied individuals over 55 years of age. Coffey et al [30] estimated a rate of volume loss of the amygdala/hippocampus of 0.3%/year. In addition to their cross-sectional nature, another difference between these studies and ours, is that the control subjects that we studied were older (range 70–89 years). In a true longitudinal MRI study of the “oldest old” (mean age 86.8 years), Kaye et al [33] found an annual decline in hippocampal volume of −2.09% in cognitively normal controls; this is similar to the rate we found of −1.55%/year in slightly younger controls (mean 81.0 years).
In control subjects, the segment of the hippocampus with the greatest annual percent volume loss was the head. This regional variation in the age-related hippocampal volume loss was hypothesized on the basis of a prior cross-sectional study [34]. Results of this longitudinal study confirm differential sensitivity of various portions of the hippocampus to age-related volume loss in typical aging.
We found no difference in the rates of volumetric change between men and women. In contrast, Gur et al [29] in a cross-sectional study, found an effect of gender on the rates of regional hemispheric atrophy.
The absence of an association between age and the rates of volumetric change or the interval between scans suggests a linear decline in volume with age. However, the age range evaluated in this study was fairly narrow—70 to 89 years—and the sample size was modest.
We found no evidence to indicate that the rates of hippocampal and temporal horn volumetric change in controls were related to several other clinical variables—estrogen replacement, APOE genotype, presence of hypertension, ischemic cardiac disease, or diabetes. It is possible however that the absence of association was due to the modest number of subjects. In contrast some authors have found an association between hemispheric atrophy/ventricular enlargement and hypertension[35, 36].
AD Cases
The temporal horns increased in volume in all AD cases and the hippocampi declined in volume in all but one case(Fig. 2). The rate of hippocampal atrophy was not different between the right and left sides or among the hippocampal segments (head, body, tail). We found no association between age and the rates of volume change which suggests that AD is associated with a linear rate of medial temporal lobe atrophy although the same limitations mentioned above for controls apply to the AD cases. None of the vascular risk factors—hypertension, ischemic cardiac disease, or diabetes— were associated with the rates of volume change, which as indicated above for controls, could be a function of small sample size. The rates of atrophy were not associated with APOE genotype.
The annualized rate of hippocampal volume loss in our group of AD patients, 3.98%/yr ±1.92%, was smaller than reported by Jobst et al [37] which was 15%/yr. There were several methodological differences between the 2 studies. Jobst et al [37] used CT, the images were oriented in a tilted axial plane, and a single linear measurement of the minimum thickness of each medial temporal lobe was made. Using MRI-based hippocampal volume measurements, Fox et al [38] found 1.4% to 8.6% annual volume loss in three symptomatic family members of an autosomal dominant pedigree. These were individuals in their 40's with an amyloid precursor protein mutation, nonetheless the rates of hippocampal atrophy are fairly similar to those observed in our group of older patients with sporadic AD.
Comparison of Cases and Controls
Both indices of the rate of medial temporal lobe atrophy (hippocampal volume loss and increased temporal horn volume) were approximately 2□ greater in AD patients than in individually age and gender matched controls. It seems reasonable to conclude that this is a direct manifestation of the progressive pathology of AD superimposed on that associated with typical aging. In addition, the hippocampi of AD patients were smaller (P<.001) and the temporal horns larger (P< .001) than those of matched controls at the initial baseline scan (Table 3). These results are in accord with several CT and MRI studies which demonstrated both cross-sectional and longitudinal differences in global hemispheric brain/CSF measurements between controls and AD patients [39, 40].
The prevalence of vascular risk factors was not significantly different between AD cases and controls, nor was the number of women treated with estrogen replacement or the treatment duration. Therefore the differing rates of atrophy in controls vs AD patients could not have been due to the influence of these potentially confounding variables. With regard to APOE genotype, our results are in agreement with those of Growdon et al [41, 42] who found that while the prevalence of APOE ∈4 was greater in AD patients than controls, the rate of clinical progression in patients with established AD was not different for ∈4 carriers vs ∈4 non-carriers. In our study, the proportion of AD cases with APOE ∈3/4 or 4/4 was significantly greater than that in controls, consistent with APOE ∈4 as a major risk factor for developing AD. However, we found no association between APOE genotype and the rates of volumetric change in AD cases.
Because the annualized rates of hippocampal and temporal horn volume change overlapped between controls and AD patients, our data would indicate that it is unlikely that these measures could serve as a useful stand alone diagnostic test for AD in individual patients(Figure 2). However, each MRI measurement is associated with an error term which is fairly large relative to the annualized volumetric change. As the time interval between serial MRI scans increases, the absolute volumetric difference between them will also increase and the magnitude of measurement error in proportion to measured difference will decrease. It is possible, therefore, that the rate of volume change over a larger time interval might better discriminate AD patients from controls because of improved precision in the difference measurement. The ability to measure with this technique the rate of volumetric change in areas of the brain which are selectively involved early by the pathology of AD might also be useful as a means of assessing the results of therapeutic intervention.
Acknowledgments
Brenda Maxwell - Typing
Ruth Cha - Statistical Analysis
Supported by NIH-NIA-AG11378; AG-08031; AG-06786; NINCDS-NS29059; The DANA Foundation; The Alzheimer's Association
References
- 1.Convit A, de Leon MJ, Golomb J, George AE, et al. Hippocampal atrophy in early Alzheimer's disease: anatomic specificity and validation. Psychiatric Quarterly. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- 2.DeCarli C, Kaye JA, Horwitz B, Rapoport SI. Critical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer type. Neurology. 1990;40:872–883. doi: 10.1212/wnl.40.6.872. [DOI] [PubMed] [Google Scholar]
- 3.DeCarli C, Murphy DGM, Gillette JA, et al. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR. 1994;15:689. [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr., Petersen RC, O'Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 5.Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41:51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- 6.de Leon MJ, George AE, Stylopoulos LA, et al. Early marker for Alzheimer's disease: the atrophic hippocampus. Lancet. 1989;2:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 7.Killiany RJ, Moss MB, Albert MS, et al. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Arch Neurol. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- 8.Laakso MP, Soininen H, Partanen K, Helkala E-L, Hartikainen P, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J of Neural Transmission. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- 9.Lehericy S, Baulac M, Chiras J, et al. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR. 1994;15:927–937. [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DGM, DeCarli C, Schapiro MB, et al. Age-related differences in volumes of subcortical muclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol. 1992;49:839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 11.Pearlson GD, Harris GJ, Powers RE, Barta PE, et al. Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer's disease. Arch Gen Psychiatry. 1992;49:402–408. doi: 10.1001/archpsyc.1992.01820050066012. [DOI] [PubMed] [Google Scholar]
- 12.Soininen HS, Partanen K, Pitkanen A, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 13.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition Washington, D C.: 1987. [Google Scholar]
- 16.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 17.Tsai MS, Tangalos EG, Petersen RC, et al. Apolipoprotein E: risk factor for Alzheimer's Disease. Am J Hum Genet. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- 18.Jack CR., Jr. MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35(Suppl 6):S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 19.Jack CR, Jr., Gehring D, Sharbrough F, et al. Temporal lobe volume measurement from MR images: accuracy and left-right asymmetry in normal individuals. JCAT. 1988;12(1):21–29. doi: 10.1097/00004728-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Elias MF, Wolf PA, D'Agostini RB, et al. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham study. Amerian Journal of Epidemiology. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 21.Launer LJ, Masaki K, Petrovitch H, et al. The association between midlife blood pressure levels and late-life cognitive function. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 22.Davis PJM, Wright EA. A new method for measuring cranial cavity volume and its application to the assessment of cerebral atrophy at autopsy. Neuropathology and Applied Neurobiology. 1977;3:341–358. [Google Scholar]
- 23.Miller AKH, Alston RL, Corsellis JAN. Variation with age in the volumes of grey and white matter in the cerebral hemispheres of man: measurements with an image analyser. Neuropathol Appl Neurobiol. 1980;6:119–132. doi: 10.1111/j.1365-2990.1980.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 24.Pakkenberg H, Voight F. Brain weight of the Danes. Acat Anatomaica. 1964;56:297–307. [Google Scholar]
- 25.Peress NS, Kane WC, M AS. Central nervous system findings in a tenth decade autopsy population. In: Ford DH, editor. Progress in Brain Research. Elsevier; Amsterdam: 1973. [DOI] [PubMed] [Google Scholar]
- 26.Pfefferbaum A, Mathalon DH, Sullivan EV, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 27.George AE, deLeon MJ, Rosenbloom S, et al. Ventricular volume and cognitive deficit: a computed tomographic study. Radiology. 1983;149:493–498. doi: 10.1148/radiology.149.2.6622694. [DOI] [PubMed] [Google Scholar]
- 28.Kaye JA, DeCarli C, Luxenberg JS, Rapoport SI. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed x-ray tomography. J Am Geriatr Soc. 1992;40:225–231. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- 29.Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl. Acad. Sci. 1991;88:2845–2849. doi: 10.1073/pnas.88.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffey CE, Wilkinson WE, Parashos IA, et al. Quantitative cerebral anatomy of the aging human brain: A cross-sectional study using magnetic resonance imaging. Neurology. 1992;42:527–536. doi: 10.1212/wnl.42.3.527. [DOI] [PubMed] [Google Scholar]
- 31.Blatter DD, Bigler ED, Gale SD, et al. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiology of Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- 33.Kaye JA, Swihart T, Howieson D, Dame A. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 34.Jack CR, Jr., Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeCarli C, Murphy DGM, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 36.Salerno JA, Murphy DGM, Horwitz B, et al. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension. 1992;20:340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- 37.Jobst KA, Smith AD, Szatmari M, et al. Rapidly progressing atrophy of medial temporal lobe in Alzheimer's disease. Lancet. 1994;343:829–30. doi: 10.1016/s0140-6736(94)92028-1. [DOI] [PubMed] [Google Scholar]
- 38.Fox MC, Warrington EK, Freeborough PA. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 39.DeCarli C, Haxby JV, Gillette JA, et al. Longitudinal changes in lateral ventricular volume in patients with dementia of the Alzheimer type. Neurology. 1993;42:2029–2036. doi: 10.1212/wnl.42.10.2029. [DOI] [PubMed] [Google Scholar]
- 40.Wippold FJ, Gado MH, Morris JC, et al. Senile dementia and healthy aging: a longitudinal CT study. Radiology. 1992;179:215–219. doi: 10.1148/radiology.179.1.2006279. [DOI] [PubMed] [Google Scholar]
- 41.Growdon J, Locasio J, Corkin S, et al. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer's disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]