Abstract
The vitamin E and donepezil trial for the treatment of amnestic mild cognitive impairment (MCI) was conducted at 69 centers in North America; 24 centers participated in an MRI sub study. The objective of this study was to evaluate the effect of treatment on MRI atrophy rates; and validate rate measures from serial MRI as indicators of disease progression in multi center therapeutic trials for MCI. Annual percent change (APC) from baseline to follow-up was measured for hippocampus, entorhinal cortex, whole brain, and ventricle in the 131 subjects who remained in the treatment study and completed technically satisfactory baseline and follow-up scans. Although a non-significant trend toward slowing of hippocampal atrophy rates was seen in APOE ∈4 carriers treated with donepezil; no treatment effect was confirmed for any MRI measure in either treatment group. For each of the four brain atrophy rate measures, APCs were greater in subjects who converted to AD than non-converters, and were greater in APOE ∈4 carriers than non-carriers. MRI APCs and changes in cognitive test performance were uniformly correlated in the expected direction (all p < 0.000). Results of this study support the feasibility of using MRI as an outcome measure of disease progression in multi center therapeutic trials for MCI.
Keywords: dementia, Alzheimer's disease, mild cognitive impairment, clinical trials, therapeutic trials, MRI, magnetic resonance imaging, serial MRI, longitudinal imaging, brain atrophy, brain atrophy rates
1.0 Introduction
The vitamin E and donepezil clinical trial for the treatment of mild cognitive impairment (MCI) was conducted in 769 subjects at 69 centers in North America (Petersen et al., 2005). The study was designed to determine whether the antioxidant vitamin E or donepezil, the only cholinesterase inhibitor available at the time the study was designed, would be effective at delaying the clinical progression of subjects with the amnestic form of MCI to the clinical diagnosis of Alzheimer's disease (AD). While vitamin E had no beneficial effect, donepezil reduced the risk of progression from amnestic MCI to clinically possible or probable AD for up to 12 months. This treatment effect was extended to 24 months in carriers of the apolipoprotein E ∈4 (APOE e4) allele. Twenty-four of the recruiting sites voluntarily participated in a Magnetic Resonance Imaging (MRI) sub study which was grafted onto the existing clinical therapeutic study design. The purpose of the MRI sub study was to determine if a therapeutic effect of either vitamin E or donepezil could be detected on rates of brain atrophy from serial MRI studies. Four different brain atrophy brain measures were assessed; the hippocampus, entorhinal cortex (ERC), whole brain, and ventricular volume. Each of these MRI measurements has figured prominently in anatomic MRI based studies of MCI, AD, and normal aging (Jack et al., 1992; Fox et al., 1996; Jack et al., 1997; Fox et al., 1999; Killiany 2000; Dickerson et al., 2001; Killiany et al., 2002; Du et al., 2003). A primary objective of this analysis was to test the hypothesis that vitamin E or donepezil slowed the rate of brain atrophy relative to placebo in subjects with amnestic MCI (Petersen 1995; Petersen et al., 1999). An additional objective was to validate the utility of MRI brain atrophy rate measures as indicators of disease progression in a multi-site therapeutic MCI setting. To accomplish this we correlated MRI atrophy rate measures with three established independent measures of disease risk and/or disease progression. We tested the hypothesis that atrophy rates would be greater in individuals who converted from MCI to AD than in individuals who did not convert; that atrophy rates would be greater in individuals who were APOE ∈4 carriers than in non-carriers; and that MRI atrophy rates would correlate with concurrent change on widely used tests of cognitive performance (Clinical Dementia Rating (CDR) (Berg 1988), Mini-Mental State Exam (MMSE) (Folstein et al., 1975), and ADAS-cog).
2.0 Methods
2.1 Participants
Participants in this MRI study represent a subset of those participating in the larger primary therapeutic trial. The details of study rationale, design and subject characteristics for the parent study have been previously described (Petersen et al., 2005). Briefly, the parent treatment study was conducted in 769 participants from 69 Alzheimer's Disease Cooperative Center (ADCS) sites in North America. For inclusion, subjects needed to meet criteria for amnestic MCI (Petersen 1995; Petersen et al., 1999) of a degenerative nature (insidious onset, gradual progression), have a memory complaint, a Logical Memory delayed recall score 1.5 SD or more below the education adjusted norm, be 55 to 90 years of age inclusive, have a Clinical Dementia Rating (CDR) (Berg 1988) of 0.5 and a Mini-Mental State Exam (MMSE) (Folstein et al., 1975) between 24 and 30 inclusive. The study was a randomized, double-blind, placebo-controlled, parallel-group study of vitamin E and donepezil. Participants were randomized to: 1) vitamin E (2000 IU) and placebo donepezil plus a multivitamin daily, 2) donepezil (10 mg) and placebo vitamin E plus a multivitamin daily, or 3) placebo vitamin E and placebo donepezil plus a multivitamin daily. The study was designed by the ADCS MCI protocol committee, and executed and analyzed by the ADCS. Informed consent was obtained from all subjects.
The primary endpoint was time to the development of possible or probable AD according to NINCDS-ADRDA criteria as determined by the examining physician at the site and verified by a central review committee (McKhann et al., 1984). Individuals who converted to AD at an interim point were offered the choice of completing the full 36 month study on open label donepezil treatment. A number of secondary endpoints, primarily cognitive assessment instruments, were also evaluated in the main treatment study. Correlation between MRI annual percent change (APC) and annualized change in four of these instruments was assessed here. These were the MMSE, CDR, ADAS-cog 11 item scale and ADAS-cog 13 item scale.
Apolipoprotein E (APOE) carrier (∈2/4, ∈3/4, or ∈4/4) status or non-carrier (∈2/2, ∈2/3, or ∈3/3) status was determined for all subjects. One hundred ninety four subjects at 24 sites participating in the MRI sub study received a research brain MRI examination at entry to the main treatment study (Grundman et al., 2004). These 194 individuals were selected for their willingness to have a research MRI, standard MRI safety screening criteria, the willingness of the site principal investigator to participate in the MRI sub study, and the availability of suitable MRI equipment on site. No other criteria were used to select these subjects.
2.2 MRI methods
All scanning was performed at 1.5T. At each site, scanning for all subjects was performed on the same MRI system. However, scanners from different manufacturers as well as various hardware/software platforms within each manufacturer's product line were included in the study. Consequently, the precise hardware and software configuration of scanners at different sites varied. Of the 24 scanners that contributed images to the study, 14 were manufactured by General Electric, nine Siemens, and one Philips. The individual imaging sequences in the examination protocol were:
A sagittal T1-weighted scan with contiguous 5-mm slices.
A 3D volumetric spoiled gradient recalled echo (SPGR or equivalent) scan obtained in the coronal plane with minimum full echo-time, minimum repetition time, 124 partitions, 25 degree flip angle, and 1.6 mm partition thickness. The range in repetition time was 9.7ms to 33 ms; and the range in echo time was 3 to 11 ms.
A T2-weighted scan was acquired in order to identify abnormalities of potential clinical significance.
MRI data were transmitted by optic disc or tape from participating sites to a central data analysis center at the Mayo Clinic in Rochester, Minnesota. Imaging data were checked for compliance with the prescribed imaging protocol, checked for quality, cataloged, and analyzed quantitatively. Cataloged images were crosschecked with case-report forms forwarded from participating clinical sites to the ADCS coordinating center in San Diego. After the images were analyzed, the data were merged with clinical data on each subject residing in the ADCS central database.
2.3 Quality control measures
Each site went through an initial qualifying phase prior to scanning patients for the trial. Sites were required to perform the entire imaging protocol, film the examination, and send the films to the central analysis site at the Mayo Clinic. The imaging sequence parameters were checked to insure that each participating site could execute the specified imaging protocol correctly.
Because the MRI sub study was added late in the study planning process, it was not possible to incorporate methods for MRI quality control beyond simple inspection of incoming image files. Images were inspected by research technologists at the MRI analysis center at Mayo Clinic for quality and usability in the different MRI image processing routines. Hippocampus and ERC measures were obtained by manual tracing while whole brain and ventricular atrophy rate measures employ a highly automated routine (boundary shift integral or BSI, described below). The human eye can “read through” many artifacts that irretrievably corrupt automated image analysis algorithms. Scans were rejected only if artifacts precluded making the measurements. Common sense was used in making these decisions. For example, if the field-of-view was mis positioned and the patient's face aliased into the back of the brain, that scan was excluded from BSI whole brain, but not from hippocampal or ERC measures. The number of cases discarded from the whole brain analysis was greater than the ventricle because some types of artifact, such as streaking in the phase encode direction due to pulsatile arterial flow in the circle of Willis, affect the basal portions of the brain but not the ventricular margins.
2.4 Image processing
Hippocampal and ERC measurements were performed by manual tracing after completion of several image pre-processing steps (Figure 1). The 3D image data set of the follow up scan was spatially aligned to the baseline scan using 6 degrees-of-freedom registration and normalized mutual information for data resampling (Studholme et al., 1998). Both scans were then interpolated in-plane to the equivalent of a 512 × 512 matrix and magnified times two. An intensity inhomogeneity correction algorithm developed in-house was then applied to both MR scans (Jack et al., 1998). Tracing of the paired scans for each subject was performed side-by-side. The research technicians who performed the tracing were blinded to all clinical information including the date of each MR scan so that the analysis was done without knowledge of the chronological ordering of the scans in each scan pair. After hippocampal and ERC boundaries had been delineated on each anatomic slice, the number of voxels was calculated automatically with a summing region of interest function. These were multiplied by voxel volume to give a numeric value in mm3. Anatomic criteria used to define the boundaries of the hippocampus and ERC have been published (Jack et al., 1989; Jack et al., 2004)
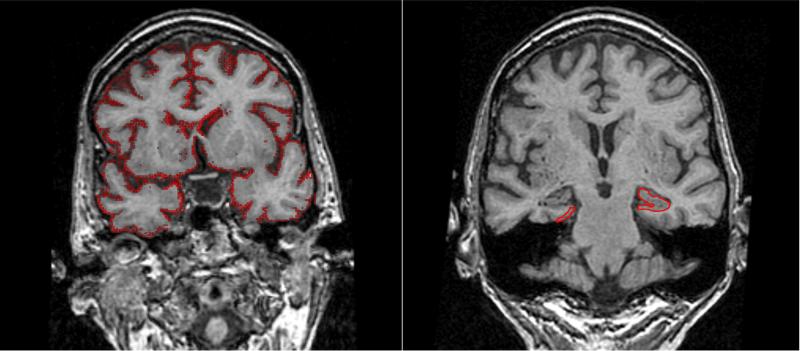
Figure 1. Volume Measures.
Image on the readers right illustrates tracing of the hippocampal and entorhinal cortex outlines. The image on the left illustrates computation of volume change with the boundary shift integral. Red demarcates pixels at the brain- CSF boundary where volume was lost from the baseline to follow up scan. The ventricular BSI is calculated by creating a hand drawn mask of the ventricle.
Rates of change of the whole brain and ventricle were made using the boundary shift integral algorithm (BSI) (Figure 1) (Fox et al., 1997; Freeborough et al., 1997; Gunter et al., 2003). Inputs for this algorithm are two or more 3D volumetric MRI scans obtained at different times. The algorithm begins with a series of data preprocessing steps including extracting the brain from the overlying skull and scalp, creating binary masks of brain and ventricle, correcting intensity non-uniformity, spatial alignment of the serial image volumes, linear scaling correction, and global intensity normalization matching the cerebro-spinal fluid (CSF) and white matter intensities. The pre-processed brain volumes are then entered into a module that computes change in brain and ventricular volume from baseline to follow-up MRI volumes(s) on the basis of intensity differences between the two scans at the brain-CSF boundary. The whole brain atrophy rate reflects shrinkage of the brain on follow up scan(s) relative to baseline - from out to in at the cortical surface and from in to out at the ventricular surface. The ventricular atrophy rate is derived by creating a binary mask for each subject that selectively extracts ventricular change. The binary mask is an approximate area overlaying the ventricles within which the BSI was measured.
2.5 Statistical methods
MRI change rates are reported in units of annualized percent change (APC). If V1 = volume at baseline and V2 = volume at a later time (either at conversion or month 36), then APC = (V2 - V1)/V1* (inter-scan interval in years). Other methods of reporting volume change over time are possible; however, the APC is both intuitive and consistent with data reporting in prior publications in this field (Fox et al., 1999; Jack et al., 2000; Du et al., 2003). In individuals with a conversion scan the scans used to perform this calculation were baseline and conversion scan (conversion could occur at the month 36 visit or at an interim visit). In stable individuals with no conversion scan the two scans used to perform this calculation were baseline and month 36 scans.
Wilcoxon rank sum tests were used to test for difference in unadjusted APC between groups formed on the basis of treatment received, conversion status, APOE ∈4 status, and change in cognitive test performance. To control for patient related variables in treatment analyses we constructed a linear regression model of MRI APC as a function of age, APOE ∈4 status, baseline MMSE, and treatment group. Interaction between treatment and APOE 4 status was also assessed. Because the primary treatment effect in main study was observed in APOE 4 carriers, a split group analysis was performed in APOE 4 carriers and also in non carriers. P values in this analysis were permuted to correct for the multiple comparisons associated with splitting the sample into two groups (APOE ∈4 carriers and non carriers) and testing for the effect of treatment in each group separately. As will be discussed, questions arose about a possible treatment effect on hippocampal volume in APOE ∈4 carriers treated with donepezil. A survival analysis (log rank test) was performed to assess the possibility of a reduction in the rate of clinical conversion from MCI to AD in subjects treated with donepezil vs. placebo. We considered adding center to analysis models to control for differences in scanner across subjects; however, there was not enough data at individual centers to evaluate a possible center effect on rates.
To control for age when testing for differences in MRI rates between converter and non-converter subjects, a linear regression model was constructed with MRI APC as a function of age and conversion status. To control for age when testing for differences in MRI rates between APOE ∈4 carrier and non carrier subjects, a linear regression model was constructed with MRI APC as a function of age and APOE ∈4 carrier status.
Testing for differences between subjects in this MRI study and those in the main treatment study was done with the Fisher Exact Test for categorical variables (e.g. gender) and Wilcoxon Rank Sum Test for continuous variables (e.g. age). Significance was set at p < 0.05 for all analyses.
3.0 Results
3.1 Study population
Each subject participating in the MRI portion of the parent treatment trial could have had up to three possible scans - baseline, month 36, an interim follow up scan obtained at the time of conversion or study withdrawal, or both month 36 and interim scans. All subjects must have had at least baseline and at least one follow up scan for inclusion in the MRI analysis reported here. 194 subjects had a baseline MRI study. Of these 52 dropped out of the study for reasons unrelated to MRI - e.g. died, lost to follow up, left the study due to medication side effects, etc. - leaving 142 with baseline plus one or more follow up MRI studies. Of these 142, 7 subjects converted to AD, but did not have a conversion scan for various reasons, leaving 135. Of these 135, 4 were discarded because the follow up MRI studies were not completed to protocol or were unusable due to artifact, leaving 131 subjects with appropriate baseline and follow up MRI studies. Demographic characteristics of these 131 subjects are listed along with those of the 769 subjects who participated in the main treatment study in Table 1. Although the data for the entire main treatment group is listed in the right hand column of Table 1, all statistical tests of difference between the MRI cohort and the main treatment group were performed with the MRI subjects removed from the main treatment cohort. The 131 subject MRI cohort did not differ from the main treatment study group with respect to age (p = 0.36), gender distribution (p = 0.29), baseline CDR sum of boxes score (p= 0.15), or baseline ADAS-Cog 11 score (p = 0.45). Baseline MMSE scores were higher (p= 0.04) and educational attainment slightly lower in MRI participants (p = 0.02).. The proportion of subjects who were APOE ∈4 carriers (p= 0.02) and the proportion who converted from MCI to AD (p = 0.01) were greater in the MRI cohort than in the main treatment study group. The MRI cohort did not differ from the main treatment study group in the proportions of subjects assigned to placebo, donepezil, or vitamin E (p = 0.49). Fifty –two of the 131 subjects in the MRI sub-study converted to AD during the study. Of these, 45 were APOE ∈4 carries. The mean time to conversion was 556 (SD 247) days from baseline in the APOE ∈4 carriers who converted, vs. 680 (SD330) days in the APOE ∈4 non carries who converted.
Table 1.
Demographic Characteristics of MRI Cohort and Total Study Cohort
| Total treatment group (n=769) |
MRI cohort total (n=131) |
Placebo Total (N=54) |
Placebo APOE ∈4+ (n=36) |
Placebo APOE ∈4− (n=18) |
Vit E total + (n=40) |
Vit E APOE ∈4+ (n=25) |
Vit E APOE ∈4− (n=15) |
Donepezil total (N=37) |
Donepezil APOE ∈4+ (n=24) |
Donepezil APOE ∈4− (n=13) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 72.9 (7.3) | 72.6 (6.8) | 72.0 (7.0) | 72.4 (6.1) | 71.2 (8.7) | 73.3 (7.4) | 73.1 (6.3) | 73.8 (9.0) | 72.6 (5.8) | 72.6 (5.5) | 72.5 (6.6) |
| Female N (%) | 352 (45.8%) | 54 (41.2%) | 24 (44.4%) | 15 (41.7%) | 9 (50%) | 16 (40%) | 12 (48%) | 4 (26.7%) | 14 (37.8%) | 9 (37.5%) | 5 (38.5%) |
| Education years | 14.6 (3.1) | 15.2 (3.1) | 15.1 (3.1) | 14.9 (3.3) | 15.5 (2.7) | 15.9 (2.8) | 15.5 (2.6) | 16.5 (3) | 14.7 (3.3) | 14.6 (2.9) | 14.8 (4.1) |
| APOE ∈4 carrier N (%) | 424 (55.1%) | 85 (64.9%) | 36 (66.7%) | - | - | 25 (62.5%) | - | - | 24 (64.9%) | - | - |
| Number of converters | 212 (27.6%) | 52 (39.7%) | 23 (42.6%) | 21 (58.3%) | 2 (11.1%) | 14 (35%) | 12 (48%) | 2 (13.3%) | 15 (40.1%) | 12 (50%) | 3 (23.1%) |
| Baseline ADAS -Cog | 11.3 (4.4) | 10.9 (3.9) | 11 (4.2) | 12 (4.2) | 9.1 (3.4) | 10.7 (3.6) | 11.3 (4) | 9.7 (2.6) | 10.9 (3.8) | 11.3 (4.4) | 10 (2.4) |
| Baseline MMSE | 27.3 (1.8) | 27.6 (1.8) | 27.4 (1.9) | 27.3 (2) | 27.7 (1.9) | 27.9 (1.6) | 27.5 (1.5) | 28.7 (1.4) | 27.4 (1.9) | 27 (1.8) | 27.9 (1.9) |
| Baseline CDR sum of boxes | 1.8 (0.8) | 1.7 (0.8) | 1.8 (0.9) | 1.9 (0.8) | 1.4 (0.8) | 1.8 (0.8) | 1.8 (0.8) | 1.7 (0.9) | 1.6 (0.8) | 1.6 (0.7) | 1.7 (1) |
| Baseline hippocampal volume* | -- | 5043 (801) | 5084 (786) | 4916 (745) | 5419.8 (778) | 5049.8 (864) | 4923.1 (917) | 5261 (749) | 4976 (768) | 5062.8 (774) | 4815.8 (762) |
| Baseline entorhinal cortex volume* | -- | 118 (34) | 119 (39) | 113 (43) | 131 (39) | 119 (31) | 121 (33) | 116 (27) | 118 (31) | 119 (31) | 118 (32) |
| Inter-scan interval | -- | 29.7 (10.6) | 28.5 (11.5) | 25.5 (11.5) | 34.6 (8.9) | 30.8 (10.9) | 28 (10.3) | 35.5 (10.6) | 30.4 (9) | 29.2 (8.6) | 32.5 (9.7) |
Mean (standard deviation) or % where indicated
Hippocampal and entorhinal cortex volume equal the sum of the right plus left sides in mm3
3.2 MRI scans available for analysis
A total of 131 subjects had baseline and appropriate follow up MRI scans which were performed according to protocol. Focal artifact preventing accurate hippocampal and ERC measures in one subject, leaving 130 subjects with these measures. The presence of artifacts precluded analysis for ventricular volume in 21 subjects and whole brain volume in 27 subjects, leaving 109 subjects with ventricular and 103 subjects with whole brain APC measures.
3.3 Effect of treatment on MRI atrophy rates
APC for each of the four MRI volume measures by treatment group are found in Table 2. These values are unadjusted for age, MMSE, baseline MRI volume, or APOE ∈4 status. Wilcoxon tests were performed testing for differences in MRI APC between placebo and vitamin E, and between placebo and donepezil for each of the four MRI rate volume measures. No significant differences were found in rates by treatment in the groups that included both APOE ∈4 carriers and non carriers. We next tested the hypothesis that treatment would affect rates of atrophy after controlling for key patient related variables. A linear regression model of MRI APC as a function of age, APOE ∈4 status, baseline MMSE, and treatment group was constructed. No significant differences were observed for any of the MRI rate measures for either donepezil or vitamin E.
Table 2.
MRI Annualized Percent Change Measures by Treatment Group
| Placebo total |
Placebo APOE ∈4+ |
Placebo APOE ∈4− |
Vit E total | Vit E APOE ∈4+ |
Vit E APOE ∈4− |
Donepezil total |
Donepezil APOE ∈4+ |
Donepezil APOE ∈4− |
|
|---|---|---|---|---|---|---|---|---|---|
| Hippocampus | −5.44 (3.20) [−16.40,−1.79] | −6.14 (3.49) [−16.40,−1.79] | −4.04 (1.94) [−9.12, −1.97] | −4.86 (2.50) [−11.83, −1.90] | −5.08 (2.59) [−11.83, −2.10] | −4.86 (2.50) [−10.94, −1.90] | −4.60 (2.18) [−10.71, −1.26] | −4.50 (2.28) [−10.71, −1.51] | −4.78 (2.07) [−7.54, −1.26] |
| ERC | −11.58 (9.27) [−44.99, 5.11] | −12.84 (9.36) [−44.99, 5.11] | −9.06 (8.80) [−44.43, −3.62] | −10.90 (5.84) [−27.17, −4.23] | −11.17 (6.16) [−26.78, −4.74] | −10.90 (5.84) [−27.17, −4.23] | −10.13 (5.63) [−29.59, −3.84] | −10.07 (5.11) [−23.80, −3.84] | −10.24 (6.68) [−29.59, −4.27] |
| Whole brain | −0.64 (0.80) [−2.85, 1.85] | −0.83 (0.94) [−2.85, 1.85] | −0.31 (0.32) [−1.06, 0.11] | −0.46 (0.54) [−2.02, 0.70] | −0.53(0.63) [−2.02, 0.70] | −0.46(0.54) [−0.72, 0.21] | −0.50 (0.52) [−1.97, 0.33] | −0.55(0.49) [−1.97, 0.21] | −0.41(0.59) [−1.73, 0.33] |
| Ventricle | 4.54 (3.00) [0.75, 14.68] | 5.48 (3.17) [1.77, 14.68] | 2.89 (1.77) [0.75, 5.97] | 3.99 (2.45) [−1.14, 9.68] | 4.08 (2.56) [−1.14, 8.95] | 3.99(2.45) [1.73, 9.68] | 3.77 (2.36) [−0.53, 8.89] | 4.18(2.01) [1.59, 8.89] | 2.88(2.91) [−0.53, 8.79] |
Mean (standard deviation) [minimum, maximum] Annualized Percent Change
APC values for the hippocampus, ERC, and brain are negative reflecting brain shrinkage, while values for the ventricle are positive reflecting ex vacuo ventricular expansion.
In the main treatment study, a stratified analysis by APOE ∈4 status was pre-specified and the protective effect of donepezil against conversion to AD was found primarily in APOE ∈4 carriers. We therefore tested that a relationship existed between MRI APC and treatment status in the APOE ∈4 carrier group alone. MRI APCs unadjusted for subject-related variables are found by treatment group split into APOE∈4 carriers and non carriers in Table 2. In APOE ∈4 carriers only (treated plus placebo), a trend toward lower rates of hippocampal atrophy in subjects treated with donepezil (n=23) than in those treated with placebo (n=36) (Wilcoxon rank sum, p=0.07 with permutation adjustment) (Figure 2). We followed this suggestive finding by constructing a linear regression model with MRI APC as a function of age, baseline MMSE, and treatment group in APOE ∈4 carriers. In this analysis a non-significant trend (p = 0.07 with permutation adjustment) toward lower hippocampal APC was again seen in APOE ∈4 carriers treated with donepezil than in APOE ∈4 carriers treated with placebo. However, when we evaluated these suggestive results by assessing interactions in a general linear model which included hippocampal volume, age, APOE status, and treatment arm, the interaction between treatment with donepezil and APOE 4 status was not significant. In addition, the difference in the rate of clinical conversion to AD between the donepezil and placebo subjects included in the hippocampal MRI analysis was not significant.
Figure 2. Hippocampal APC in APOE ∈4 Carriers: Donepezil vs. Placebo.
Hippocampal APC of all APOE ∈4 carriers treated with donepezil (n=23) vs. placebo (n=36) with superimposed box plot (bar = median; box = inter-quartile range; whiskers = 1.5X inter-quartile range).
In order to provide clarification for future treatment studies which employ MRI as an adjunctive outcome measure we evaluated three potential confounding effects on the relationship between hippocampal APC and donepezil treatment in more depth. These were differences between treatment and placebo groups in baseline hippocampal volume, inter scan interval, and proportion of APOE ∈4 homozygotes.
The follow up MRI scan in converters was obtained at the time of conversion which could have been less than 36 months from baseline, while the follow up scan in all non converters was obtained at 36 months. This in turn could bias comparisons between the donepezil treated group, which on average had fewer conversions, and the placebo treated group which on average had a greater number of conversions. However while the mean interval between baseline and follow up MRI was 25.5 months (SD11.5) for those receiving placebo and 29.2 months (SD8.6) for those treated with donepezil among APOE ∈4 carriers, the difference was not significant (p= 0.32) (Table 1).
Natural history studies have shown that baseline MRI volumes are related to subsequent atrophy rates; rates are greater in both AD and MCI subjects with more atrophic baseline volumes (Jack et al., 2003; Mungas et al., 2005). In addition, MCI subjects with greater hippocampal atrophy at baseline are more likely to convert to AD (Jack et al., 1999; Visser et al., 1999; Killiany 2000; Dickerson et al., 2001; Killiany et al., 2002; Adak et al., 2004; DeCarli et al., 2004). In this study however, hippocampal volumes at baseline in APOE ∈4 carriers treated with donepezil (5062 mm3, SD 773 mm3) were not different from those treated with placebo (4916 mm3, SD 744) (p= 0.51) (Table 1).
The main treatment study was analyzed in terms of APOE ∈4 carrier vs. non carrier status rather than by individual APOE genotype. We noticed an imbalance in the proportion of APOE ∈4 homozygotes vs. heterozygotes in the MRI treatment sub study. Among the 54 individuals receiving placebo, the numbers of APOE ∈4 homozygotes (∈4/4), heterozygotes (∈3/4 or ∈2/4), and non carriers (∈3/3) was: 13 (24%), 23 (43%), and 18 (33%) respectively. The analogous values among the 40 subjects receiving vitamin E treatment were: 5 (12.5%), 20 (50%), and 15 (37.5%). The analogous values among subjects receiving donepezil treatment were: 2 (5%), 22 (60%), and 13 (35%). Based on prior evidence that APOE ∈4 carriers are more likely to convert from MCI to AD (Petersen et al., 1995) it seemed possible that APOE ∈4 homozygote MCI subjects might have greater intrinsic atrophy rates than APO E ∈4 heterozygotes. We assessed this possibility by comparing hippocampal APC by individual genotype in the untreated subjects in this study. Among the 54 subjects receiving placebo, the mean (SD) hippocampal APC in AOE ∈4 non carriers was −4.0% (1.9%), in ∈4 heterozygotes −6.0% (3.6%), and in ∈4 homozygotes was −6.4% (3.5%). The difference in APC between APOE ∈4 non carriers and heterozygotes was significant (p= 0.04), as was the difference in APC between non carriers and homozygotes (p= 0.01). However, the hippocampal APC was not different between APOE ∈4 homozygotes and heterozygotes (p= 0.65).
3.4 Correlation between MRI atrophy rates and change in cognitive test performance
Correlations between MRI APC vs annual change in cognitive test performance were calculated for all four MRI measures and four behavioral/cognitive measures; the MMSE, CDR sum of boxes, and ADAS – Cog 11 and 13 item scales. Each of the 16 possible correlations between change in MRI vs change in cognitive test performance was significant in the expected, biologically plausible, direction (Table 3) (Figure 3).
Table 3.
Correlation among MRI APC and Cognitive Performance APC
| Hippocampus | ERC | Ventricle | Brain | |
|---|---|---|---|---|
| MMSE | −0.38*** | −0.39*** | 0.26* | −0.26* |
| CDR | −0.49*** | −0.44*** | 0.37*** | −0.28** |
| ADAS-Cog 11 | −0.34*** | −0.44*** | 0.25* | −0.45*** |
| ADAS-Cog 13 | −0.40*** | −0.49*** | 0.35*** | −0.51*** |
Values in table are Spearman r. P-values:
p< 0.05
p < 0.01
p < 0.001.
Figure 3. Correlation between Change in MRI and Change in Cognition.
Scatter plot illustrating the correlation between annualized percent change in hippocampal volume and annualized percent change in ADAS-Cog 13.
3.5 Effect of conversion status on MRI atrophy rates
For each of the four atrophy rate measures the APCs were greater in those subjects who converted to AD vs. those who did not (Wilcoxon rank sum, all p<0.000) (Table 4). To control for age when testing for differences in MRI rates between converter and non-converter subjects, a linear regression model was constructed with MRI APC as a function of age and conversion status. The results were again highly significant for all four MRI rate measures (all p <0.000).
Table 4.
MRI Annualized Percent Change Measures by Conversion Status
| Converter | Non-converter | p-value | |
|---|---|---|---|
| Hippocampus | −6.78 (3.31) | −3.86 (1.36) | <0.000 |
| ERC | −15.08 (9.18) | −8.23 (4.07) | <0.000 |
| Whole brain | −0.88 (0.87) | −0.36 (0.38) | <0.000 |
| ventricle | 5.66 (2.95) | 3.33 (2.09) | <0.000 |
Mean (standard deviation) Annualized Percent Change
P values from Wilcoxon rank sum test
3.6 Effect of APOE ∈4 carrier status on MRI atrophy rates
Hippocampal (p <0.06), ERC (<0.04), ventricular (p<0.002), and whole brain APC (P=0.008) were greater in APOE ∈4 carriers than non-carriers (Wilcoxon rank sum) (Table 5). To control for age when testing for differences in MRI rates between APOE ∈4 carrier and non carrier subjects, a linear regression model was constructed with MRI APC as a function of age and APOE ∈4 carrier status. The results were significant for hippocampus (p = 0.04), ventricle (p= 0.003), and brain (p= 0.01).
Table 5.
MRI Annualized Percent Change Measures by APOE 4 Genotype
| ∈4 carrier | ∈4 non carrier | p-value | |
|---|---|---|---|
| Hippocampus | −5.38 (2.99) | −4.39(2.11) | 0.065 |
| ERC | −11.59 (7.50) | −9.84 (7.12) | 0.044 |
| Whole brain | −0.65 (0.74) | −0.3 (0.39) | 0.008 |
| Ventricle | 4.66 (2.72) | 3.17 (2.27) | <0.002 |
Mean (standard deviation) Annualized Percent Change
P values from Wilcoxon rank sum test
3.7 Reduction of Risk of Clinical Progression by Treatment
An analysis parallel to that conducted in the main treatment trial was conducted to determine if treatment reduced the risk of progression to AD in this 131 subject MRI cohort. The analysis was done using a Cox Proportional Hazards model that included age, baseline MMSE, and APOE ∈4 carrier status as covariates as done in the main treatment study. Neither donepezil (p = 0.24) nor vitamin E (p = 0.52) were associated with reduction in the likelihood of conversion to AD among the 131 subjects in this MRI sub study.
4.0 DISCUSSION
MRI based measurements have been employed as adjunct outcome measures in several therapeutic trials for patients with AD (Jack et al., 2003; Krishnan et al., 2003; Fox et al., 2005; Hashimoto et al., 2005). To our knowledge this is the first study evaluating the utility of MRI measurements in a therapeutic trial of MCI subjects. No effect of treatment on MRI rate was observed overall (APOE 4 carriers plus noncarriers) for any of the four MRI rate measures in this MRI sub study with either donepezil or vitamin E. A split group analysis of APOE 4 carrier and non-carrier subjects was pre-specified in the main treatment study and therefore seemed justified in this MRI sub study. A trend indicating a possible slowing of hippocampal atrophy rate by donepezil in APOE 4 carriers was evident in some of the analyses. However while we can not exclude a biological effect of donepezil on hippocampal volume, the preponderance of evidence did not support a clear cut treatment effect of donepezil on hippocampal atrophy rates. Given that among the participants in this MRI sub study, no reduction in the rate of conversion from MCI to AD was observed in those receiving donepezil, perhaps the failure to identify an effect of donepezil on MRI rates should be expected. This difference from that observed in the main study may well be due to the restricted sample size in the MRI sub study.
An aspect of this data which merits discussion is a trend (not statistically significant) toward atrophy rates that were uniformly higher among the placebo group than either the vitamin E or donepezil treatment groups in whom no effect of treatment on atrophy rates was demonstrated. We therefore sought to identify elements of the study which might have biased the results toward higher rates of atrophy in the placebo group compared to the treatment group. Obvious sources of bias could be ruled out, for example differences in age or baseline MMSE score between treatment and placebo groups. We evaluated in detail three other potential sources of bias. These were the difference in inter-scan interval between converters and non-converters, differences in baseline hippocampal volume between those in the treatment group and placebo group, and differences in the proportion of APOE 4 homozygotes between the treatment group and placebo group. None of these however seemed to explain the non-significant trend toward higher rates among subjects receiving placebo.
Another aspect of this data which merits discussion is that rates of hippocampal and ERC atrophy and ventricular expansion in the placebo subjects were greater than these rates in MCI subjects from prior observational longitudinal MRI studies from the Mayo Clinic – the imaging laboratory where the measurements for this treatment study were generated. A possible explanation for this might be that the subjects who participated in this treatment trial were different (i.e. more severely affected) than those who participate in non-therapeutic observational MRI studies at the Mayo Clinic. In order to insure a rate of conversion adequate to power the study, inclusion criteria for the main treatment study were established to select individuals whose memory performance was on average worse than that of subjects participating in observational studies at the Mayo Clinic. The proportion of APOE 4 carriers was also higher in this treatment study than those of MCI subjects in prior observational longitudinal MRI studies from the Mayo Clinic (64% here vs. averages of 48% and 32% in Mayo studies)(Jack et al., 2000; Jack et al., 2004). In addition, subjects in this treatment trial were drawn from the recruitment base for the ADCS – dementia and memory disorders referral centers. Conversely, the majority of subjects in Mayo Clinic MRI studies are drawn from a community sample (Petersen et al., 1990; Jack et al., 1997).
A second objective of this study was to assess correlations between the rates of change on MRI and concurrent change on standard instruments employed in clinical trials; MMSE, CDR, and ADAS-Cog 11 and 13 item scales (Table 3 and Figure 3). Change in MRI mapped onto concurrent change in test performance in a uniformly significant and biologically sensible manner. This tends to validate the use of MRI as a measure of disease progression in multi-site therapeutic trials for MCI. This result is also concordant with results from single site natural history studies as well as multi-site studies (Kaye et al., 1997; Fox et al., 1999; Jack et al., 2003; Jack et al., 2004; Mungas et al., 2005).
In the third objective of this study we found that concurrent rates of change of all four MRI measures were significantly greater in individuals who converted to AD than individuals who remained stable (Table 4). This is consistent with results obtained in single site observational studies of MCI subjects (Jack et al., 2000; Jack et al., 2004; Jack et al., 2005). It is also consistent with both single and multi site studies of AD patients, in which greater rates of brain atrophy are observed in individuals with more rapid concurrent clinical disease progression (Kaye et al., 1997; Jack et al., 1998; Fox et al., 1999; Jack et al., 2003; Mungas et al., 2005). Given the correlation between MRI rates and change in concurrent cognitive test performance noted in the preceding paragraph the observed correlation with conversion status is not surprising. Nonetheless, this result further supports the validity of MRI based rates of brain atrophy as an independent measure of disease progression in multi site therapeutic MCI trials.
In the final objective of this study we found that MRI atrophy rates were greater in APOE ∈4 carriers than non carriers (Table 5). This is consistent with other longitudinal MRI studies (Hashimoto et al., 2005) as well as a large body of literature on the biologic effect of APOE ∈4 indicating that carriers have an increased probability of developing AD and an increased likelihood of converting from MCI to AD than non carriers (Corder et al., 1993; Strittmatter et al., 1993; Petersen et al., 1995). Studies evaluating the relationship between APOE ∈4 status and brain morphometry have found effects consistent with greater disease severity, an independent contribution of APOE ∈4 and atrophy to clinical disease severity, and greater likelihood of clinical progression in APOE ∈4 carriers (Plassman et al., 1997; Jack et al., 1998; den Heijer et al., 2002; Adak et al., 2004; DeCarli et al., 2004; Farlow et al., 2004; Fleisher et al., 2005). The effect of APOE ∈4 on MRI rates observed in this MRI sub study is consistent with the results in the main treatment study in which APOE ∈4 carriers in each treatment group (donepezil, vitamin E, and placebo) were more likely to convert to AD than non carriers. In this MRI sub-study the risk of converting was likewise greater in APOE ∈4 carriers than non-carriers; and the mean time to conversion was less in carriers. This coupled with the observation that MRI atrophy rates were greater in carriers than non carriers suggests that enrichment of the population with APOE ∈4 carriers might be a beneficial strategy in future MCI treatment trials which employ MRI outcome metrics. Interestingly however we found in fact that among the placebo group in this study there was no difference in rates of hippocampal atrophy rates between APOE 4 heterozygotes and APOE 4 homozygotes. This is consistent with prior work suggesting that the rate of clinical change in AD patients is not necessarily greater in APOE 4 homozygotes than heterozygotes and may actually be less (Frisoni et al., 1995).
MRI was grafted onto this treatment study after the study design and data analysis plan had already been established. In addition, only ¼ of subjects baselined in the main treatment study underwent baseline MRI studies. The assessment of treatment effect on MRI atrophy rates therefore suffer from limitations imposed by small sample size and the fact that MRI was not factored into the study design prospectively. Nonetheless we believe that experience with MRI in this study has instructive lessons to offer for future clinical trials which employ imaging. Several earlier studies indicate greater power to detect change over time with structural MRI vs standard cognitive assessment instruments (Fox et al., 1999; Jack et al., 2003; Jack et al., 2004) thus improving the efficiency with which clinical trials may be conducted. This in turn would argue for performing imaging in a subset of subjects entered into clinical trials. Our experience with this study however, underscores advantages of carefully balancing demographic variables across treatment and placebo groups at baseline within the MRI sub-groups just as is done within the larger treatment group. This may avoid potential confounds in assessing possible treatment effects on MRI. For example, this study clearly indicates that atrophy rates are greater in MCI subjects who are APOE 4 carriers than non carriers and therefore the wisdom of balancing the proportion of carriers across treatment sub-groups in future therapeutic studies of MCI. Interestingly our data indicate that atrophy rates are not intrinsically greater in E4 homozygotes vs heterozygotes and therefore balancing this feature across treatment groups is unnecessary.
Acknowledgments
We thank the National Institute on Aging (AGO 10483), Pfizer, Inc., Eisai, Inc., the Institute for the Study on Aging and DSM Nutritional Products for support of this study.
Footnotes
Members of the Alzheimer's Disease Cooperative Study (ADCS) Group is listed in the Appendix.
Disclosure Statement for Authors The authors have no conflicts of interest to disclose. The industry sponsors had no role in the analysis or interpretation of these data nor in the content of the paper. Appropriate approval procedures were used concerning human subjects.
5.0 References
- Adak S, Illouz K, Gorman W, Tandon R, Zimmerman EA, Moore MM, Kaye JA. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004;63:108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Mungas D, Reed HB, Weiner M, Chui HC, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MB. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennette DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiology of Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR, He Y, S T, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- Fleisher A, Grundman M, Jack CR, Jr., Petersen RC, Taylor CJ, Kim HT, Schiller DHB, Bagwell V. Sex, apolipoprotein E e4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini Mental State": A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox N, Black RS, Gilman S, Rossor MN, Griffith S, Jenkins L, Koller M. Effects of AB immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer's disease. Journal of Magnetic Resonance Imaging. 1997;7:1069–75. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- Fox NC, Freeborough PA, Rossor MN. Visualization and quantification of rates of atrophy in Alzheimer's disease. The Lancet. 1996;348:94–97. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans on Medical Imaging. 1997;15:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- Frisoni GA, Govoni S, Geroldi C, Bianchetti A, Calabresi L, Franceschini G, Trabucchi M. Gene dose of the E4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer's disease. Ann Neurol. 1995;37:596–604. doi: 10.1002/ana.410370509. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen R, Ferris SH. Mild cognitive impairment can be distinguished from Alzheimer's disease and normal aging for clinical trials. Archives of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Gunter JL, Shiung MM, Manduca A, Jack CR., Jr. Methodological considerations for measuring rates of brain atrophy. JMRI. 2003;18:16–24. doi: 10.1002/jmri.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does Donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry. 2005;162:676–682. doi: 10.1176/appi.ajp.162.4.676. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, O'Brien PC. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu Y, O'Brien PC, Smith G. e., Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of Hippocampal Atrophy in Normal Aging, Mild Cognitive Impairment, and Alzheimer's Disease. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E. The rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu YC, O'Brien PC, Waring SC, Tangalos EG, Smith GE, Ivnik RJ, Thibodeau SN, Kokmen E. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer's disease. Annals of Neurology. 1998;43:303–310. doi: 10.1002/ana.410430307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Shiung MM, Weignad SD, O'Brien PC, Gunter JL, Boever BF, Knopman DS, Smith G, Ivnick RJ, Tangalos EG, Petersen RC. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Slomkowski M, Gracon S, Hoover TM, Felmlee JP, Stewart K, Xu YC, Shiung MM, O'Brien PC, Cha RH, Knopman DS, Petersen RC. MRI as a biomarker of disease progression in a therapeutic trial of Milameline for Alzheimer's. Neurology. 2003;60:253–260. doi: 10.1212/01.wnl.0000042480.86872.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements for MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Kaye JA, Swihart T, Howieson D, Dame A. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- Killiany R, Gomez-Isla T, Moss M. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gopmez-Isla T. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Charles HC, Doraiswamy PM, J M, Weisler R, Yu X, Perdomo C, Ieni JR, Rogers S. Randomized, placebo-controlled trial of the effects of Donepezil on neuronal markers and hippocampal volumes in Alzheimer's disease. Am J Psychiatry. 2003;160(20032011) doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65(4):565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Normal aging, mild cognitive impairment, and early Alzheimer's disease. The Neurologist. 1995;1:326–344. [Google Scholar]
- Petersen RC, Kokmen E, Tangalos EG. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC. Apolipoprotein E4 allele and hippocampal volume in twins with normal cognition. Neurology. 1997;48:985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D. Apolipoprotein E: high acidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer's disease. Proc Natl Aca Sci. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Hawkes DJ, Hill DLG. A normalized entropy measure for multimodality image alignment. Proc SPIE - The International Society for Optical Engineering. 1998;3338:132–143. [Google Scholar]
- Visser PJ, Scheltens P, Verhey FRJ, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurology. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]