Abstract
Staphylococcus aureus infects hospitalized or healthy individuals and represents the most frequent cause of bacteremia, treatment of which is complicated by the emergence of methicillin-resistant S. aureus. We examined the ability of S. aureus to escape phagocytic clearance in blood and identified adenosine synthase A (AdsA), a cell wall–anchored enzyme that converts adenosine monophosphate to adenosine, as a critical virulence factor. Staphylococcal synthesis of adenosine in blood, escape from phagocytic clearance, and subsequent formation of organ abscesses were all dependent on adsA and could be rescued by an exogenous supply of adenosine. An AdsA homologue was identified in the anthrax pathogen, and adenosine synthesis also enabled escape of Bacillus anthracis from phagocytic clearance. Collectively, these results suggest that staphylococci and other bacterial pathogens exploit the immunomodulatory attributes of adenosine to escape host immune responses.
Staphylococcus aureus is the leading cause of bloodstream, lower respiratory tract, skin, and soft tissue infections (Klevens et al., 2006; Klevens et al., 2007) because of its unique ability to multiply in blood or other host tissues and cause persistent infections (Lowy, 1998). To survive in blood, S. aureus must escape a variety of innate immune mechanisms, such as antimicrobial peptides, complement, and phagocytic killing (Foster, 2005; Peschel and Sahl, 2006). An immediate and essential host defense against S. aureus is provided by neutrophilic PMNs (neutrophils), which comprise 60–70% of human white blood cells (Voyich et al., 2005). Staphylococci deploy a plethora of mechanisms aimed at subverting innate immune mechanisms, including secretion of factors inhibitory for complement activation and neutrophil chemotaxis (de Haas et al., 2004; Rooijakkers et al., 2005), as well as toxins that lyse neutrophils (Wang et al., 2007), neutralize antimicrobial defensins (Jin et al., 2004), or act as superantigens to inappropriately activate the host's immune system (Jardetzky et al., 1994). In this paper, we report the discovery of a hitherto unknown strategy: synthesis of the immunosuppressive signaling molecule adenosine.
In mammals, adenosine assumes an essential role in regulating innate and acquired immune responses (Thiel et al., 2003). Strong or excessive host inflammatory responses, e.g., in response to bacterial infection, exacerbate the tissue damage inflicted by invading pathogens (Thiel et al., 2003). Successful immune clearance of microbes therefore involves the balancing of pro- and antiinflammatory mediators. The cytokines IL-4, IL-10, IL-13, and TGF-β play a role in restricting excessive inflammation, but only adenosine is able to completely suppress immune responses (Németh et al., 2006). The immunoregulatory attributes of adenosine are mediated via four transmembrane adenosine receptors: A1, A2A, A2B, and A3 (Haskó and Pacher, 2008). T lymphocytes express the high affinity A2A receptor as well as the low affinity A2B receptor (Thiel et al., 2003). Depending on their activation state, macrophages and neutrophils express all four adenosine receptors, whereas B cells harbor only A2A (Thiel et al., 2003). Engagement of A2A inhibits IL-12 production, increases IL-10 in monocytes (Khoa et al., 2001) and dendritic cells (Panther et al., 2001), and decreases cytotoxic attributes and chemokine production in neutrophils (Cronstein et al., 1986; McColl et al., 2006). Generation of adenosine at sites of inflammation, hypoxia, organ injury, and traumatic shock is mediated by two sequential enzymes. Ecto-ATP diphosphohydrolase (CD39) converts circulating ATP and ADP to AMP (Eltzschig et al., 2003). CD73, expressed on the surface of endothelial cells (Deussen et al., 1993) and subsets of T cells (Thompson et al., 1987; Thompson et al., 1989; Yang et al., 2005), then converts 5′-AMP to adenosine (Zimmermann, 1992).
Although extracellular adenosine is essential for the suppression of inflammation, build-up of excess adenosine is also detrimental. This is exemplified in patients with a deficiency in adenosine deaminase, an enzyme that converts adenosine to inosine (Giblett et al., 1972). Adenosine deaminase deficiency causes severe compromised immunodeficiency syndrome, with impaired cellular immunity and severely decreased production of immunoglobulins (Buckley et al., 1997). As the regulation of extracellular adenosine is critical in maintaining immune homeostasis, perturbation of adenosine levels is likely to affect host immune responses during infection. We report in this paper that S. aureus and Bacillus anthracis, the causative agent of anthrax, use adenosine synthesis to escape host immune responses.
RESULTS
Adenosine synthase A (AdsA) is required for staphylococcal survival in blood
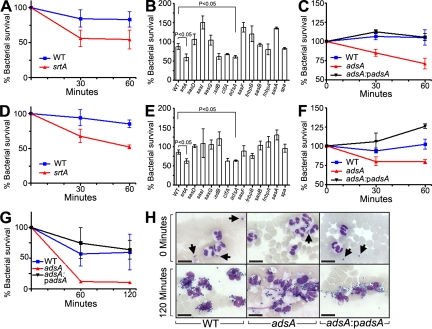
To identify the staphylococcal genes required for escape from innate immune responses, we examined the ability of S. aureus strain Newman to survive in whole blood collected from BALB/c mice or Sprague-Dawley rats by recording bacterial load at timed intervals via the formation of colonies on agar medium (Fig. 1). As expected, the blood of naive mice and rats, which lack antibodies specific for staphylococci (not depicted), were unable to kill S. aureus Newman (Fig. 1, A and D). In contrast to the wild-type strain, a variant lacking the structural gene for sortase A (srtA) displayed a defect in staphylococcal escape from phagocytic killing (P < 0.05; Fig. 1, A and D). Sortase A anchors a large spectrum of different polypeptides in the staphylococcal envelope, using a transpeptidation mechanism and LPXTG motif sorting signal at the C terminus of surface proteins (Mazmanian et al., 2002). To examine these surface proteins for their contribution to staphylococcal survival in blood, we transduced bursa aurealis insertions in surface protein genes (Bae et al., 2004) into wild-type strain S. aureus Newman and measured the survival of staphylococcal variants in blood (Fig. 1, B and E). Mutations in clfA and sasH (Staphylococcus aureus surface protein), hereafter named adsA, displayed consistent survival defects. The phenotype of clfA mutants represents an expected result, as the encoded clumping factor A product is known to precipitate fibrin and interfere with macrophage and neutrophil phagocytosis (Palmqvist et al., 2004; Higgins et al., 2006). The contribution of AdsA to pathogenesis is not yet known. AdsA harbors a 5′-nucleotidase domain with the two signature sequences ILHTnDiHGrL (residues 124–134) and YdamaVGNHEFD (residues 189–201), suggesting that the protein may catalyze the synthesis of adenosine from 5′-AMP. To further examine the importance of adsA in staphylococcal virulence, we complemented the adsA gene by cloning the entire adsA gene and upstream promoter sequences into expression vector pOS1, generating padsA. Transformation of adsA mutant staphylococci with padsA restored their ability to survive in mouse or rat blood, indicating that the observed virulence defect is indeed caused by the absence of adsA expression (Fig. 1, C and F; see Fig. 3 C for confirmation of AdsA expression). S. aureus survival was also examined in the blood of human volunteers. Similar to mouse blood, the number of adsA mutant staphylococci was reduced as compared with wild-type S. aureus Newman, and this defect was restored by transformation of adsA mutants with padsA (Fig. 1 G).
Figure 1.
AdsA, a cell wall–anchored surface protein, is required for staphylococcal survival in blood. Comparison of the survival of 105 CFU of wild-type S. aureus Newman (WT) or the isogenic srtA variants in 1 ml of blood from (A) BALB/c mice or (D) Sprague-Dawley rats. Data are the means, and error bars represent ± SEM from three independent analyses. To assess the relative contribution of sortase A–anchored cell-wall surface proteins for staphylococci survival in blood, isogenic mutants with transposon insertions in the indicated genes were incubated in blood from mice (B) or rats (E) for 60 min. Expression of padsA rescues staphylococcal survival of an adsA mutant in blood from mice (C), rats (F), or human volunteers (G). 105 CFU of staphylococci were incubated with 1 ml of human blood for 0 or 120 min, and Giemsa-stained samples were viewed by microscopy (H). At 0 min, only extracellular staphylococci were detected (arrows), whereas after 120 min of incubation staphylococci were mostly associated with neutrophils. The abundance of adsA mutants was reduced compared with wild-type staphylococci or adsA mutants harboring the complementing plasmid (padsA). Data are representative of three independent analyses with blood from three different human donors. Bars, 10 µm.
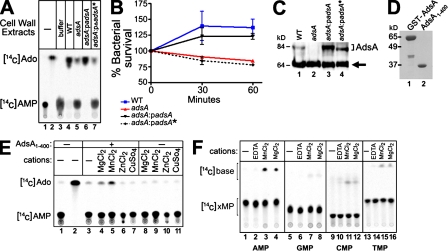
Figure 3.
5′-Nucleotidase activity of AdsA is essential for virulence. (A) Lysostaphin-generated cell-wall extracts of staphylococcal strains were incubated with radiolabeled [14C]AMP. The generation of [14C]adenosine ([14C]Ado) was measured by TLC using a PhosphorImager (Storm; GE Healthcare). Data are representative of two independent analyses. (B) S. aureus strains in A were analyzed for their ability to survive in lepirudin-anticoagulated fresh blood obtained from BALB/c mice. Data are representative of two independent analyses conducted in triplicate; error bars represent the SEM. (C) The abundance of AdsA in cell-wall lysates was measured by immunoblotting analyses with anti-AdsA rabbit polyclonal sera. The following strains were tested: S. aureus Newman wild-type (WT), adsA, adsA:padsA (harboring the padsA plasmid for the expression of wild-type adsA), and adsA:padsA* (harboring the padsA* plasmid for the expression of an adsA variant lacking the 5′-nucleotidase signature sequence. The arrow denotes protein A immunoreactive signals. Data are representative of four independent analyses (D) Recombinant GST-AdsA was purified from E. coli and cleaved with thrombin to generate AdsA1-400, and purified proteins were analyzed by Coomassie-stained SDS-PAGE. Data are representative of five independent analyses. (E) Radiolabeled [14C]AMP was incubated in the presence or absence of 2 µM of purified AdsA1-400 and in the presence or absence of 5 mM of various metal ions. Radioactive signals for [14C]AMP and [14C]Ado after TLC were captured by a PhosphorImager. Data are representative of six independent analyses. (F) Radiolabeled [14C]AMP (lanes 1–4), [14C]GMP (lanes 5–8), [14C]CMP (lanes 9–12), and [14C]TMP (lanes 13–16) were incubated in the presence or absence of 2 µM of purified AdsA1-400 in the presence or absence of 5 mM of various metal ions, as indicated. Radioactive signals for [14C]xMP (nucleotide monophosphate) and [14C]nucleotide free base after TLC were captured by a PhosphorImager. Data are representative of three independent analyses.
AdsA is required for staphylococci virulence and abscess formation
To investigate the contribution of adsA to invasive staphylococcal disease, BALB/c mice were infected by intravenous inoculation with 107 CFU of wild-type S. aureus Newman or its isogenic adsA variant. Animals were killed 5 d after infection and both kidneys were removed. The right kidney was homogenized, and staphylococcal load was enumerated by plating on agar and colony formation (Fig. 2 A). The left kidney was fixed with formalin, embedded in paraffin, thin sectioned, and analyzed for histopathology (Fig. 2 B). As expected, wild-type S. aureus Newman formed abscesses in kidneys with a mean bacterial load of 3 × 107 CFU per gram of tissue. In contrast, adsA mutant staphylococci were unable to form abscesses and displayed a >10-fold reduction in bacterial load as compared with the wild type (P < 0.03; Fig. 2, A and B).
Figure 2.
AdsA is a virulence factor that enables staphylococcal replication and abscess formation in vivo. Staphylococcal burden in kidneys after intravenous challenge of BALB/c mice (n = 10) with (A) 107 CFU of wild-type S. aureus Newman or its isogenic adsA mutant and (C) S. aureus USA300 wild-type or its isogenic adsA mutant was enumerated as log10 CFU per gram of tissue 5 d after infection. Data are representative of two independent analyses. Microscopic images of hematoxylin-eosin–stained kidney tissue were obtained after necropsy of mice infected with (B) wild-type S. aureus Newman and adsA mutants or (D) wild-type S. aureus USA300 and corresponding adsA mutants. Black arrows denote the abscess lesions. Data are representative samples of cohorts of five animals per bacterial strain and two independent analyses. Bars: (top) 1 mm; (bottom) 100 µm. (E) Staphylococcal burden—wild-type (WT), adsA, or adsA:padsA S. aureus Newman—in blood of BALB/c mice (n = 10) was measured as log10 CFU per 0.5 ml of blood retrieved by cardiac puncture at 30 and 90 min after intravenous challenge with 107 CFU. Horizontal red bars represent mean CFUs in each cohort. Data are representative of three independent analyses using cohorts of 10 mice per bacterial strain per time point. Unpaired Student's t tests were used for statistical analysis.
Staphylococcal strains that cause community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) have been characterized by pulsed-field gel electrophoresis and DNA sequencing. Currently, the major CA-MRSA clone is USA300 (McDougal et al., 2003), the predominant cause of skin and soft tissue infections as well as bacteremia (Carleton et al., 2004). To assess the contribution of adsA toward virulence of USA300, we isolated an isogenic adsA mutant using phage transduction and S. aureus strain LAC (USA300; Bae et al., 2004). BALB/c mice were infected by intravenous injection of 107 CFU. 5 d after challenge, staphylococci were enumerated in homogenized kidney tissue and histopathology was visualized in hematoxylin-eosin–stained thin sections (Fig. 2 C). Similar to S. aureus Newman, we observed a 1-log reduction in CFUs recovered from the kidneys of animals infected with the adsA mutant of S. aureus USA300. Further, we observed fewer abscesses and smaller lesions in the kidneys of mice infected with the adsA variant (Fig. 2 D and Fig. S1). Collectively, these results suggest a significant contribution of adsA toward virulence of two clinical isolates, S. aureus strains Newman and USA300.
Differences in abscess formation and recovery of CFUs from the kidneys of infected mice may stem from enhanced bacterial clearance in the blood stream, causing fewer bacteria to reach peripheral organ tissues. Alternatively, adsA could play a direct role in the formation of abscesses and infectious foci. To discern between these possibilities, BALB/c mice were infected by intravenous inoculation with 107 CFU and peripheral blood was sampled at timed intervals by cardiac puncture. In agreement with observations of enhanced clearance of adsA mutant staphylococci in vitro, significantly fewer CFUs of adsA mutant staphylococci were retrieved 90 min after infection as compared with the wild-type parent strain S. aureus Newman (P < 0.05). Transformation of the adsA mutant strain with padsA restored its ability to survive in blood after intravenous challenge (Fig. 2 E). Although we cannot rule out the possibility that adsA also contributes specifically to abscess formation, these data suggest that the reduced virulence of adsA mutant staphylococci results from their decreased survival in blood.
5′-Nucleotidase activity is the mediator of the virulence attributes of AdsA
Given that AdsA harbors a 5′nucleotidase signature sequence, we asked whether AdsA can synthesize adenosine from AMP. Cell-wall peptidoglycan of S. aureus wild type, adsA, and the adsA:padsA strain was degraded with lysostaphin (Schindler and Schuhardt, 1964), and cell-wall extracts were incubated with radiolabeled [14C]AMP. The relative abundance of AdsA in the staphylococcal envelope was assessed by immunoblotting (Fig. 3 C), and the production of adenosine was monitored by TLC (Fig. 3 A). Lysostaphin extracts of adsA mutant staphylococci displayed reduced adenosine synthase activity, which could be restored to wild-type levels when adsA mutants were transformed with padsA, a plasmid-encoded wild-type allele (Fig. 3 A). AdsA harbors 5′-nucleotidase signature sequences, domains critical for the catalytic activity of mammalian enzymes (Zimmermann, 1992). To assess the role of adenosine synthase activity toward staphylococcal virulence, the YdamaVGNHEFD signature sequence (AdsA residues 189–201) was removed to generate AdsA*. Immunoblotting revealed the expression of AdsA* in the envelope of adsA mutant staphylococci that had been transformed with padsA* (Fig. 3 C). Expression of AdsA* restored neither the defects in adenosine synthase activity (Fig. 3 A) nor the increased clearance of adsA mutant staphylococci in blood (Fig. 3 C). Incubation of staphylococci with adenosine 5′-(α,β-methylene) diphosphate (Knöfel and Sträter, 2001), a known 5′-nucleotidase inhibitor, reduced adenosine production and survival of wild-type S. aureus Newman in blood (Fig. S2). Collectively, these experiments suggest that AdsA adenosine synthase activity may be responsible for the virulence attributes of this surface protein.
To characterize the enzymatic activity of AdsA, we expressed a soluble affinity-tagged recombinant form of S. aureus AdsA (residues 1–400) in Escherichia coli. Purified AdsA, removed of its affinity tag (Fig. 3 D), cleaved [14C]AMP to generate adenosine (Fig. 3 E). Maximal activity (KM = 44 nM) was observed in the presence of 5 mM MgCl2 or 5 mM MnCl2, similar to the metal requirements of other adenosine synthases (Fig. 3 E; Zimmermann, 1992). On the other hand, incubation of AdsA with 5 mM ZnCl2 or 5 mM CuSO4 before the addition of [14C]AMP inhibited adenosine synthase activity (Fig. 3 E, lanes 6 and 7). A similar inhibitory effect was observed when EDTA, a divalent metal ion chelator, was added to the enzyme (Fig. 3 F). AdsA activity appears specific for adenosine monophospate, as other nucleotide monophosphates were either not cleaved or were cleaved at a much reduced rate (Fig. 3 F).
Staphylococcal AdsA synthesizes adenosine during infection
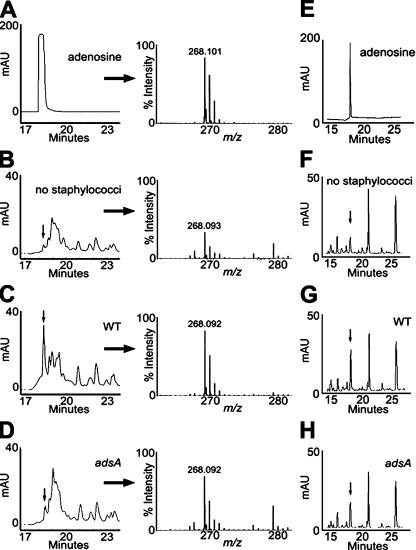
Under physiological conditions, the concentration of AMP in the extracellular milieu is estimated to be in the nanomolar range. Immunological insult or tissue injury, however, causes release of AMP with concentrations up to 100 µM. To us, it seemed plausible that these AMP stores may be converted to adenosine during staphylococcal infection. To assess the relative abundance of adenosine during staphylococcal infection, 1 ml of mouse blood was infected in vitro with 105 CFU S. aureus for 60 min. Plasma was recovered by centrifugation, protein was removed, and samples were subjected to reversed-phase HPLC (RP-HPLC). For calibration, we separated chemically pure adenosine and determined its molecular mass in the eluate (Fig. 4 A). Chromatography of uninfected blood revealed the adenosine absorption peak, whose identity was confirmed by mass spectrometry (Fig. 4 B). The adenosine peak in blood was increased 10-fold after infection with S. aureus Newman (Fig. 4 C), whereas infection with the isogenic adsA mutant produced a <2-fold increase in adenosine (Fig. 4 D). To examine adenosine production in vivo, mice were infected by intravenous inoculation of 107 CFU S. aureus and plasma adenosine abundance was quantified 60 min after infection. Animals infected with wild-type S. aureus Newman (Fig. 4 G) displayed increased amounts of adenosine in blood when compared with mice that had been mock infected (Fig. 4 F) or challenged with the adsA variant (Fig. 4 H). Thus, in agreement with the conjecture that staphylococci synthesize adenosine during infection, both the in vitro and the in vivo inoculation of mouse blood with S. aureus Newman cause an adsA-dependent increase in adenosine.
Figure 4.
S. aureus AdsA synthesizes adenosine in blood. (A) RP-HPLC to quantify 100 µM adenosine (left) and identify its monoisotopic ions by MALDI-MS (right). 1 ml of lepirudin-anticoagluated mouse blood was incubated without (B) or with 105 CFU of wild-type S. aureus Newman (WT; C) or its isogenic adsA variants (D) for 1 h. Plasma was deproteinized, filtered, and subjected to RP-HPLC to quantify adenosine (left) and identify its monoisotopic ions by MALDI-MS (right). Arrows denote corresponding adenosine peaks. Calculated abundance of adenosine in plasma extrapolated from the purified adenosine control was 1.1 µM (B, no staphylococci), 13.2 µM (C, WT S. aureus Newman), and 2.1 µM (D, adsA mutant staphylococci). Data are representative of three independent analyses. (E–H) RP-HPLC analyses of 50 µM adenosine (E) or plasma collected from mice that had been mock infected (F), or from animals that were challenged with 107 CFU wild-type (WT; G) or (H) adsA mutant bacteria. Data are representative of two independent analyses. The calculated abundance of plasma adenosine in mice extrapolated from the purified adenosine control was 2.8 ± 0.6 µM (F, no staphylococci), 16.4 ± 2.1 µM (G, WT S. aureus Newman), and 7.4 ± 3.2 µM (H, adsA mutant staphylococci). mAU, milliabsorbance units of HPLC eluate.
Adenosine reduces killing of staphylococci by neutrophils
We hypothesized that staphylococci escape phagocytic clearance in blood by synthesizing adenosine. If so, the survival defect of adsA mutant staphylococci in blood should be rescued by exogenous supplies of adenosine. This was tested, revealing a specific increase in the survival of adsA mutant staphylococci in the presence of 5 and 15 µM adenosine (Fig. S3) but not with guanosine (Fig. 5 A). We wondered whether enhanced growth rates of S. aureus were not observed when adenosine was added to staphylococci incubated in laboratory media (Fig. 5 B), suggesting that the ability of adenosine to increase the bacterial load in blood may be exerted by its effect on phagocytic cells that otherwise kill the invading pathogen. In agreement with this hypothesis, no difference in bacterial survival or growth was observed when wild-type and adsA mutant staphylococci were incubated in human serum or plasma (Fig. 5 C).
Figure 5.
Immunosuppressive effects of adenosine contribute to S. aureus survival in blood. (A) Survival of adsA S. aureus Newman in mouse blood in the presence of increasing concentrations of adenosine (left) or guanosine (right) as indicated. Extracellular adenosine concentration was quantified by RP-HPLC immediately before enumerating bacterial CFUs, as described in Fig. S3. Data are representative of two independent analyses conducted in duplicate (left) and are representative of five independent analyses (right); error bars represent the SEM. (B) Growth of wild-type and adsA S. aureus Newman in laboratory growth media with 0–500 µM adenosine (Ado) for 15 and 45 min. Data are means of two independent analyses conducted in triplicate. Error bars represent the SEM. (C) Survival of wild-type, adsA, or adsA:padsA S. aureus Newman in human plasma. Data are averaged of two independent analyses conducted in triplicate; error bars indicate SEM. (D) The adsA gene is required for staphylococcal escape from neutrophil killing. Mouse blood was inoculated with S. aureus Newman expressing GFP, comparing the wild type (WT) and its isogenic adsA variant. At the indicated time points, samples were treated with 10 µg/ml−1 lysostaphin, followed by isolation of neutrophils by FACS using anti-GR1 staining and phagocytosis and survival of staphylococci measured as mean GFP fluorescence. Data are representative of two independent analyses conducted in triplicate; error bars indicate SEM.
Although many different antimicrobial factors have been revealed in blood, PMNs, particularly phagocytic neutrophils, play a dominant role in innate immune defenses against staphylococci (Verdrengh and Tarkowski, 1997; Mölne et al., 2000; Voyich et al., 2005). To ascertain the fate of staphylococci in blood, we incubated S. aureus Newman expressing GFP in anticoagulated blood and measured bacterial phagocytosis and clearance by immune cells (Fig. 5 D). To distinguish extracellular from intracellular bacteria, blood and cell samples were treated with lysostaphin, a glycyl-glycine endopeptidase that quickly lyses extracellular staphylococci and abrogates GFP fluorescence (Fig. S4). Blood samples were stained with anti-GR1 and infected neutrophils were analyzed by flow cytometry (Fig. S5). 15 min after staphylococcal inoculation into blood, neutrophils had internalized similar numbers of lysostaphin-resistant wild-type and adsA mutant staphylococci, measured as GFP fluorescence of GR1-positive cells (Fig. 5 D and Fig. S5). Staphylococcal uptake involves neutrophil opsonophagocytosis, which is not affected by adenosine synthesis, as similar amounts of C3b were found deposited on the surface of wild-type and adsA mutant staphylococci (Fig. S6). Upon 30 min of incubation, adsA mutant staphylococci were killed by neutrophils, as the GFP fluorescence of GR1-positive cells declined over time (Fig. 5 D and Fig. S5). In contrast, GFP fluorescence of wild-type staphylococci within neutrophils was not diminished (Fig. 5 D). GFP fluorescence could not be identified in isolated monocytes and T or B lymphocytes of infected mouse blood, suggesting that C3b deposition primarily triggers staphylococcal phagocytosis by neutrophils (Fig. S4). Phagocytosis is thought to play a dominant role in the clearance of S. aureus from blood. Cytochalasin D, a molecule that inhibits actin polymerization, is known to block phagocytosis (Casella et al., 1981). In some cell types, e.g., macrophages, cytochalasin D can also inhibit the binding of yeast particles to complement-independent phagocytic receptors (Bos and de Souza, 2000). In our assay, where GFP-expressing staphylococci are taken up by neutrophils, 10 µM cytochalasin D inhibited phagocytosis and increased the survival of both wild-type and adsA mutant staphylococci (Fig. S7).
B. anthracis survives in blood and synthesizes adenosine
To investigate whether other pathogenic bacteria express adenosine synthases, we first examined bacterial genome sequences with BLAST searches for predicted translation products harboring the adenosine synthase domain of AdsA; homologues were identified in several different species (Table S1). For example, the genome of B. anthracis encodes an AdsA homologue (B. anthracis surface protein; available from GenBank/EMBL/DDBJ under accession no. BAS4031) with a 5′-nucleotidase signature sequence (YdvisLGNHEFN; residues 131–142), an N-terminal signal peptide, and a C-terminal LPXTG sorting signal, indicating that this surface protein is also deposited by sortase A in the cell-wall envelope (Gaspar et al., 2005). To determine whether B. anthracis AdsA functions as an adenosine synthase and contributes to escape from innate immune responses, we constructed a deletion mutant of B. anthracis adsA by allelic replacement (Fig. 6). Mutanolysin, a muralytic enzyme that cleaves N-acetylmuramyl-(β1→4)-N-acetylglucosamine within peptidoglycan (Yokogawa et al., 1974), was used to generate cell-wall lysates. Cell-wall extracts of wild-type bacilli harbored adenosine synthase activity, whereas extracts derived from adsA mutant bacilli displayed reduced activity (Fig. 6 A). Deletion of the structural gene adsA abolished the expression of adenosine synthase (Fig. 6 B), abrogated the surface display of AdsA by B. anthracis (Fig. 6 C), and reduced the ability of bacilli to synthesize adenosine (Fig. 6 A). Residual amounts of AMP hydrolysis are attributed to other phosphatases, including alkaline phosphatase. We expressed and affinity tagged B. anthracis AdsA in E. coli and purified the enzyme. Similar to S. aureus AdsA, adenosine synthase activity of B. anthracis AdsA was observed in the presence of 5 mM MnCl2 (KM = 2.01 nM), whereas 5 mM MgCl2 reduced its activity (Fig. 6 D). When inoculated into mouse blood, we observed increased phagocytic clearance of the adsA mutant, as compared with the wild-type parent B. anthracis Sterne (Fig. 6 E). Collectively, these experiments suggest that B. anthracis also utilizes AdsA to synthesize adenosine and escape host innate immune responses.
Figure 6.
5′-Nucleotidase activity of AdsA enhances B. anthracis survival in blood. (A, top) Mutanolysin extracts from B. anthracis strain Sterne (WT) or its isogenic adsA variant were incubated with radiolabeled [14C]AMP. The generation of adenosine was measured by TLC. (bottom) Quantification of relative abundance of adenosine. Data are the mean of three independent analyses; error bars indicate SEM. (B) Mutanolysin extracts were analyzed by immunoblotting with antibodies directed against B. anthracis AdsA or BasC (anti-BasC), a control protein not involved in adenosine production. Bars, 5 µm. (C) Fluorescence microscopy images of wild-type B. anthracis Sterne and its isogenic adsA mutant stained with antiserum against B. anthracis AdsA (top) or nonreactive serum (NRS) and Cy3-labeled secondary antibodies (red), as well as Hoechst staining of nucleic acids (blue). Data are representative of two independent analyses. (D) Radiolabeled [14C]AMP was incubated with 2 µM of purified B. anthracis AdsA in the presence of 5 mM of the indicated metal cations, and generation of [14C]adenosine ([14C]Ado) was measured by TLC and a PhosphorImager. Data are representative of three independent analyses. (E) Survival of wild-type and adsA B. anthracis Sterne in rat blood over time, measured as CFUs on agar plates. Data are the mean of two independent analyses; error bars indicate SEM.
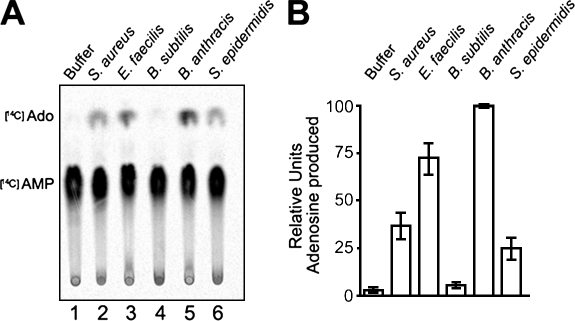
Several other Gram-positive bacteria harbor putative 5′-nucleotidase genes (Table S1), and we analyzed their ability to synthesize adenosine. Similar to S. aureus and B. anthracis, cell-wall extracts derived from Enterococcus faecilis and S. epidermidis both synthesized adenosine from AMP, but B. subtilis did not (Fig. 7). The B. subtilis chromosome encodes a gene with a 5′-nucleotidase signature motif (Kunst et al., 1997); however, it is currently not known whether or not this gene is expressed and its predicted product displays activity. Nevertheless, we surmise that the ability of bacterial pathogens to synthesize adenosine and release this immunosuppressive compound may represent an important virulence strategy in many different hosts.
Figure 7.
Hydrolysis of AMP is observable in various Gram-positive pathogens. (A) Cell-wall proteins from the indicated bacterial strains were released with mutanolysin digestion, and mutanolysis extracts were incubated with radiolabeled [14C]AMP. Hydrolysis of AMP into adenosine was assessed by separating the compounds by TLC. (B) Quantification of the relative abundance of adenosine produced in cell-wall extracts from A. Data represent the mean of three independent analyses; error bars indicate SEM.
DISCUSSION
This study provides evidence that S. aureus and other bacterial pathogens generate adenosine to promote their survival during host infection. We identified and characterized AdsA, a cell wall–anchored protein of S. aureus with 5′-nucleotidase signature sequences, and its functional homologue in B. anthracis. We show that adsA is required for synthesis of adenosine during infection and that the structural gene contributes to the survival of S. aureus and B. anthracis in animal or human blood. Further, in a renal abscess model of S. aureus infection in mice, adsA was required for pathogen replication in organ tissues and for the formation of abscess lesions.
5′-Nucleotidases are present in many different bacterial species, but their potential as virulence factors has hitherto not been appreciated. We provide evidence that AdsA may be a clinically relevant virulence factor of CA-MRSA strain USA300, the causative agent of the majority of community-acquired skin and soft-tissue infections in the United States (Carleton et al., 2004). Roche et al. (2003) previously reported that sasH (adsA) was significantly associated with invasive disease isolates as compared with nasal carriage isolates, further distinguishing a pivotal role for adsA in staphylococcal disease. Although the extraordinary immunosuppressive attributes of adenosine are generally known, to the best of our knowledge, this study provides the first mechanistic link between bacterial pathogenesis and adenosine synthesis. S. aureus is primarily an extracellular pathogen, and the main thrust of host defenses against this pathogen relies on PMNs and phagocytic killing (Lowy, 1998). Adenosine is known to inhibit neutrophil degranulation (Bouma et al., 1997), adhesion to vascular surfaces (Firestein et al., 1995), and superoxide burst (Cronstein et al., 1990; Gunther and Herring, 1991; Kaufmann et al., 2007). Our experiments reveal the effect of adenosine on staphylococcal survival in blood at an early stage of infection (30–90 min), when tissue damage and AMP release is limited. At a later stage of infection, when S. aureus seeds abscesses and causes liquefaction necrosis, AMP levels are expected to increase as a consequence of staphylococcal toxins, e.g., α-hemolysin, leukocidins, or phenol-soluble modulins, that form pores in membranes and precipitate cellular lysis (Diep and Otto, 2008).
We observed further that adsA-deficient S. aureus were cleared more rapidly from the bloodstream of BALB/c mice than wild-type staphylococci, correlating with the reduced ability to grow during infection and/or seed abscesses; presumably, lower numbers of adsA mutant staphylococci can disseminate through the vasculature and form abscess lesions.
In addition to neutrophils, tissue macrophages are essential for the clearance of bacterial infections. Adenosine directly impairs the ability of macrophages to combat infection by decreasing the phagocytic activity of these cells (Eppell et al., 1989). Further, adenosine attenuates macrophage antibacterial activity by suppressing the production of superoxide (Edwards et al., 1994) and nitric oxide (Haskó et al., 1996; Xaus et al., 1999), both of which are integral to the killing of phagocytosed bacteria. Macrophage deactivation by adenosine also suppresses antibacterial defense mechanisms by decreasing the production of proinflammatory cytokines that both orchestrate inflammatory/immune functions of other cell types and act as regulators of macrophage function (Haskó and Szabó, 1998). For example, TNF is a regulator of neutrophil, endothelial cell, and lymphocyte function, and the production of TNF has been shown to be under negative control of adenosine. Finally, an important consideration is that adenosine, through the activation of macrophages, may decrease T cell antibacterial effector mechanisms. In support of this conjecture, adenosine decreases the expression of MHC II on macrophages (Edwards et al., 1994) and dendritic cells (Panther et al., 2001), and it decreases macrophage production of IL-12, a pivotal stimulus for Th1-type immune responses (Haskó et al., 2000).
Examination of the genomes of Gram-positive bacteria identified genes with similarities to putative 5′-nucleotidases. Many of these proteins harbor sortase A–specific sorting signals (LPXTG), indicating that their mature products are localized in the bacterial cell-wall envelope. Such localization appears relevant, as surface-exposed adenosine synthases should release their product in close proximity to phagocytic cells. Other microorganisms secrete 5′-nucleotidases into the extracellular environment, as recent studies have described a panel of nucleotide-metabolizing enzymes secreted by Trichinella spiralis parasites (Gounaris, 2002) and Vibrio cholerae, a Gram-negative pathogen (Punj et al., 2000). Collectively, our results suggest that S. aureus and other Gram-positive pathogens increase extracellular concentrations of the potent immunosuppressive molecule adenosine and, through this mechanism, are likely able to perturb immune defenses and promote their survival in host tissues.
MATERIALS AND METHODS
Bacterial strains.
S. aureus strains were grown in TSB at 37°C. S. aureus strain USA300 was obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (National Institute of Allergy and Infectious Diseases [NIAID]). All mutants used in this study were obtained from the Phoenix (ΦNΞ) library (Bae et al., 2004). Each Phoenix isolate is a derivative of the clinical isolate Newman (Duthie and Lorenz, 1952) or USA300 (Carleton et al., 2004), as indicated in the figures. All bursa aurealis insertions were transduced into wild-type S. aureus Newman or USA300 using bacteriophage ϕ85 and verified by PCR analysis. Chloramphenicol was used at 10 mg/liter−1 for plasmid and allele selection with padsA. Erythromycin was used at 10 mg/liter−1 for allele selection in S. aureus Newman and at 50 mg/liter−1 for allele selection in USA300. Mutants of B. anthracis strain Sterne were generated with pLM4, containing a thermosensitive origin of replication. Plasmids with a 1-kb DNA sequence flanking each side of the mutation were transformed into B. anthracis, and transformants were grown at 30°C (permissive temperature) in Luria broth (LB; 20 µg/ml−1 kanamycin). Cultures were diluted 1:100 and plated on LB agar (20 µg/ml−1 kanamycin) at 43°C overnight (restrictive temperature). Single colonies were inoculated into LB broth without antibiotics and grown overnight at 30°C. To ensure loss of pLM4-based plasmid, these cultures were diluted four times into fresh LB broth without antibiotic pressure and propagated at 30°C. Cultures were diluted and plated on LB agar and colonies were examined for kanamycin resistance. DNA from kanamycin-sensitive colonies was analyzed by PCR for the presence or absence of mutant alleles.
S. aureus–expressing GFP.
Plasmid pTetPro-GFP, a pCL55 derivative with a tetracycline-inducible promoter, was electroporated into S. aureus TB9 to generate TB9 (tetPro-gfp). TB9 is a variant of S. aureus Newman generated by homologous recombination to excise bacteriophage NMϕ4 from the geh lipase gene (Bae et al., 2006). pTetPro-GFP integration into the geh gene resulted in a lipase-negative phenotype, which was verified on egg-yolk plates.
Plasmids.
The following primers were used for PCR amplification reactions: P55 (5′-TTTCCCGGGACGATCCAGCTCTAATCGCTG-3′), P56 (5′-TTTGAGCTCAAAGCAAATAGATAATCGAGAAATATAAAAAG-3′), P57 (5′-TTTGAGCTCAGTTGCTCCAGCCAGCATT-3′), mP58 (5′-TTTGAATTCAAACGGATTCATTCCAGCC-3′), FP10 (5′-TACGAATTCGACTTGGCAGGCAATTGAAAA-3′), RP10 (5′-TGTGAATTCTTAGCTAGCTTTTCTACGTCG-3′), FP3C (5′-TCGGGATCCGCTGAGCAGCATACACCAATG-3′), RPB (5′-TGTGGATCCTTATTGATTAATTTGTTCAGCTAATGC-3′), VTFD (5′-TACAGATCTTTTGGATACGATCAGTTG-3′), VTRC (5′-TACAGATCTATAACCTACTGCATTCAT-3′), #184 (5-CGGGATCCCCTCGAGTTCATGAAAAAC-3′), and #192 (5′-GGGGTACCTTATTTGTATAGTTCATCCATGCCATGTG-3′). Ligation of FP10/RP10 (adsA + 700 bp upstream from start site) PCR products into pOS1 (EcoRI) generated padsA. Triple ligation of FP10/VTRD and VTFD/RP10 (deletion of amino acids 189–201) PCR products into pOS1 (EcoRI) generated pΔadsA. Insertion of P55/P56 (basA 1-kb 5′ flanking sequence) and P57/P58 (basA 1-kb 3′ flanking sequence) PCR products into pLM4 (EcoRI, SacI, and XmaI sites) generated pJK34. This plasmid was used to delete the basA coding sequence. Ligation products were transformed into E. coli DH5α, and plasmid DNA was transformed into E. coli K1077 (dam−, dcm−), and purified (nonmethylated) plasmid DNA was transformed into B. anthracis according to a previously developed protocol (Gaspar et al., 2005). Ligation of FP3C/RPB (1.2-kb truncation of adsA starting 5′ after the signal peptide) PCR products into pGEX-2T (GE Healthcare) generated the adsA expression vector pVT1, and this plasmid was transformed into E. coli BL21. Ligation of PCR amplification products generated with primers #184/#192 into pCL55 (Lee et al., 1991) using BamHI/KpnI sites resulted in plasmid pTetPro-GFP (Gründling and Schneewind, 2007).
Animal experiments.
All experimental protocols were reviewed, approved, and performed under regulatory supervision of the University of Chicago's Institutional Biosafety Committee and Institutional Animal Care and Use Committee. BALB/c mice were purchased from Charles River, and Sprague-Dawley rats were purchased from Harlan. Overnight cultures of S. aureus strains were diluted 1:100 into fresh TSB and grown for 3 h at 37°C. Staphylococci were centrifuged, washed twice, and diluted in PBS to yield an OD600 of 0.5 (108 CFU ml−1). Viable staphylococci were enumerated by colony formation on tryptic soy agar plates to quantify the infectious dose. Mice were anaesthetized by intraperitoneal injection of 80–120 mg ketamine and 3–6 mg xylazine per kilogram of body weight. 100 µl of bacterial suspension (107 CFU) was administered intravenously via retroorbital injection into 6-wk-old female BALB/c mice. On day 5, mice were killed by compressed CO2 inhalation. Kidneys were removed and homogenized in PBS containing 1% Triton X-100. Aliquots of homogenates were diluted and plated on agar medium for triplicate determination of CFUs. The Student's t test was performed for statistical analysis using Prism software (GraphPad Software, Inc.). For histopathology, kidney tissue was incubated at room temperature in 10% formalin for 24 h. Tissues were embedded in paraffin, thin sectioned, stained with hematoxylin-eosin, and examined by microscopy.
To measure staphylococcal survival in blood, 6-wk-old female BALB/c mice were infected with 107 CFU of staphylococci by retroorbital injection. At 30 or 90 min, mice were killed by compressed CO2 inhalation and blood was collected by cardiac puncture using a 25-gauge needle. Aliquots were incubated on ice for 30 min in a final concentration of 0.5% saponin/PBS to lyse host eukaryotic cells. Dilutions were plated on TSA for enumeration of surviving CFUs at the two different time points.
Chemicals.
Mutanolysin (Sigma-Aldrich) was suspended at a concentration of 5,000 U/ml−1 in 100 mM sodium phosphate, pH 6, containing 1 mM PMSF and stored at −20°C. [14C]AMP, [14C]GMP, [14C]CMP, [14C]TMP, and [14C]adenosine were purchased from Moravek Biochemicals. Lysostaphin was purchased from AMBI and purified adenosine was purchased from Sigma-Aldrich.
Bacterial survival in blood.
Overnight cultures of S. aureus strains were diluted 1:100 into fresh TSB and grown for 3 h at 37°C. Staphylococci were centrifuged, washed twice, and diluted in PBS to yield an OD600 of 0.5 (108 CFU/ml−1). Whole blood was collected by cardiac puncture of Sprague-Dawley rats or BALB/c mice, and 5 µg/ml−1of lepirudin anticoagulant was immediately added. 100 µl of 106 CFU/ml−1 of bacteria were mixed with 900 µl of rat or mouse blood. For human blood studies, 100 µl of 108 CFU/ml−1 of bacteria was mixed with 900 µl of freshly drawn human blood. The tubes were incubated at 37°C with slow rotation for the time points indicated in the figures, at which time aliquots were incubated on ice for 30 min in a final concentration of 0.5% saponin/PBS to lyse eukaryotic cells. Dilutions of staphylococci were plated on TSA for enumeration of surviving CFUs. Experiments with blood from human volunteers involved protocols that were reviewed, approved, and performed under regulatory supervision of the University of Chicago's Institutional Review Board.
Adenosine synthase activity.
Overnight cultures of S. aureus strains were diluted 1:100 into fresh TSB and grown for 3 h at 37°C. Staphylococci were centrifuged and washed twice with PBS. 3 ml of cells was spun down and suspended in 100 µl TSM buffer (50 mM Tris-HCL [pH 7.5], 10 mM MgCl2, and 0.5 M sucrose); lysostaphin (final concentration = 70 µg/ml−1) was added and allowed to incubate for 30 min at 37°C. The solution was spun down for 5 min at 9,000 g, and supernatants containing released cell-surface proteins were collected. 15 µl of lysostaphin extracts were incubated with 3 µCi [14C]AMP for 30 min at 37°C. Samples were spotted on a silica plate, followed by separation by TLC using a (75:25 isopropanol/double-distilled H2O) 0.2-M ammonia bicarbonate solvent. For cell-wall extracts of S. aureus, E. faecilis, B. anthracis, and S. epidermidis digested with mutanolysin, mutanolysin was substituted for lysostaphin and used according to the manufacturer's recommended conditions. When assayed with purified proteins, 2 µM of purified AdsA or BasA was incubated in a final volume of 15 µl with 3 µCi [14C]AMP in the presence of the metal cations in TSM buffer indicated in the figures.
Adenosine concentration in blood.
Whole-blood killing assay with staphylococci was performed as described in Bacterial survival in blood. Extraction of plasma was performed as previously described (Mo and Ballard, 2001). In brief, after conclusion of the whole-blood killing assay, blood samples were centrifuged at 16,000 g for 5 min and noncellular plasma was collected. 600 µl of plasma was extracted with 75 µl perchloric acid (1.5 M) and 1 mM EDTA. The 500-µl supernatant was withdrawn after centrifugation for 5 min at 16,000 g and neutralized with 29 µl of 4 M KOH. After ice cooling for 10 min, the sample was again centrifuged at 16,000 g for 5 min. The pH of the supernatant was finally adjusted to pH 6–7, diluted 1:4 with PBS, and filtered with a 0.22-µm syringe filter before RP-HPLC.
HPLC and mass spectrometry.
The presence of adenosine production was determined by RP-HPLC. Samples were chromatographed on a 250 mm × 3 mm column (5-µm particle size; BDS Hypersil C18; Thermo Fisher Scientific). The mobile phase consisted of solution A (deionized H2O/0.1% trifluoroacetic acid) and solution B (acetonitrile/0.1% trifluoroacetic acid). Adenosine was eluted with a solvent B gradient from 1 to 100%, run from 5 to 50 min. The solvent flow rate was 0.5 ml/min. Peaks were detected by their UV absorbance at 260 nm. The peak of adenosine in the HPLC chromatogram was identified by comparison of its retention time to the retention time of purified adenosine used as a standard sample. Fractions containing adenosine were cospotted with matrix (α-cyano-4-hydroxycinnamic acid) and subjected to matrix-assisted laser desorption/ionization–mass spectrometry (MALDI-MS) under reflector-positive conditions.
FACS analyses.
Freshly prepared mouse blood was inoculated with S. aureus Newman expressing GFP and incubated at 37°C as described. Aliquots were removed and incubated for an additional 5 min with 10 µg/ml−1 lysostaphin. After lysostaphin treatment, samples were diluted with ice cold PBS plus 10 µM cytochalasin D, and erythrocytes were lysed with erythrocyte lysis buffer (QIAGEN) for 30 min on ice. Blood leukocytes were washed three times by centrifuging at 400 g, and cells were suspended in PBS/1% FBS. Cells were stained with allophycocyanin-conjugated anti-GR1 or anti-F4/80 antibodies, or PE-conjugated anti-CD4, anti-CD8, or anti-B220 antibodies (eBioscience), and analyzed using a FACSCanto (BD).
Online supplemental material.
Fig. S1 examines the contribution of adsA toward S. aureus USA300 abscess formation in mice. Fig. S2 documents that adsA-mediated adenosine production is responsible for enhanced survival of staphylococci in blood and that this phenotype can be inhibited with adenosine 5′-(α,β-methylene) diphosphate. The rapid clearance of exogenously added adenosine in blood is studied in Fig. S3. Fig. S4 examines the correlation between GFP fluorescence of staphylococci and bacterial viability in the presence of lysostaphin. Fig. S5 displays representative FACS dot plots and histograms demonstrating that wild-type and adsA mutant staphylococci are phagocytosed by neutrophils, but that only adsA mutants, not the wild type, are killed by immune cells. Fig. S6 analyzes the deposition of complement (C3b) on the staphylococcal surface in the presence or absence of AdsA. Fig. S7 uses cytochalasin D to study the contribution of phagocytosis toward staphylococcal killing by neutrophils. Table S1 lists Gram-positive bacteria encoding AdsA homologues, which are anchored to the cell-wall envelope. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090097/DC1.
Acknowledgments
We thank members of our laboratory, F. Bagnoli, and G. Grandi for critical comments and discussion.
This work was supported in part by a grant from the NIAID Infectious Diseases Branch (AI42797) and by Novartis Vaccines and Diagnostics. J.W. Kern acknowledges support from the Molecular Cell Biology Training Grant (GM007183) from the University of Chicago. D.M. Missiakas and O. Schneewind are members of and supported by the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIAID, National Institutes of Health Award 1-U54-AI-057153). V. Thammavongsa received fellowship support from the Immunology Training Grant at the University of Chicago (AI07090).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- AdsA
- adenosine synthase A
- CA-MRSA
- community-acquired methicillin-resistant Staphylococcus aureus
- ClfA
- clumping factor A
- MALDI-MS
- matrix-assisted laser desorption/ionization–mass spectrometry
- RP-HPLC
- reversed-phase HPLC
References
- Bae T., Banger A.K., Wallace A., Glass E.M., Aslund F., Schneewind O., Missiakas D.M. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc. Natl. Acad. Sci. USA. 101:12312–12317 10.1073/pnas.0404728101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae T., Baba T., Hiramatsu K., Schneewind O. 2006. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 62:1035–1047 10.1111/j.1365-2958.2006.05441.x [DOI] [PubMed] [Google Scholar]
- Bos H., de Souza W. 2000. Phagocytosis of yeast: a method for concurrent quantification of binding and internalization using differential interference contrast microscopy. J. Immunol. Methods. 238:29–43 10.1016/S0022-1759(00)00132-0 [DOI] [PubMed] [Google Scholar]
- Bouma M.G., Jeunhomme T.M., Boyle D.L., Dentener M.A., Voitenok N.N., van den Wildenberg F.A., Buurman W.A. 1997. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J. Immunol. 158:5400–5408 [PubMed] [Google Scholar]
- Buckley R.H., Schiff R.I., Schiff S.E., Markert M.L., Williams L.W., Harville T.O., Roberts J.L., Puck J.M. 1997. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J. Pediatr. 130:378–387 10.1016/S0022-3476(97)70199-9 [DOI] [PubMed] [Google Scholar]
- Carleton H.A., Diep B.A., Charlebois E.D., Sensabaugh G.F., Perdreau-Remington F. 2004. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J. Infect. Dis. 190:1730–1738 10.1086/425019 [DOI] [PubMed] [Google Scholar]
- Casella J.F., Flanagan M.D., Lin S. 1981. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 293:302–305 10.1038/293302a0 [DOI] [PubMed] [Google Scholar]
- Cronstein B.N., Levin R.I., Belanoff J., Weissmann G., Hirschhorn R. 1986. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J. Clin. Invest. 78:760–770 10.1172/JCI112638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B.N., Daguma L., Nichols D., Hutchison A.J., Williams M. 1990. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J. Clin. Invest. 85:1150–1157 10.1172/JCI114547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas C.J., Veldkamp K.E., Peschel A., Weerkamp F., Van Wamel W.J., Heezius E.C., Poppelier M.J., Van Kessel K.P., van Strijp J.A. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199:687–695 10.1084/jem.20031636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussen A., Bading B., Kelm M., Schrader J. 1993. Formation and salvage of adenosine by macrovascular endothelial cells. Am. J. Physiol. 264:H692–H700 [DOI] [PubMed] [Google Scholar]
- Diep B.A., Otto M. 2008. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 16:361–369 10.1016/j.tim.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie E.S., Lorenz L.L. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95–107 [DOI] [PubMed] [Google Scholar]
- Edwards C.K., III, Watts L.M., Parmely M.J., Linnik M.D., Long R.E., Borcherding D.R. 1994. Effect of the carbocyclic nucleoside analogue MDL 201,112 on inhibition of interferon-gamma-induced priming of Lewis (LEW/N) rat macrophages for enhanced respiratory burst and MHC class II Ia+ antigen expression. J. Leukoc. Biol. 56:133–144 [DOI] [PubMed] [Google Scholar]
- Eltzschig H.K., Ibla J.C., Furuta G.T., Leonard M.O., Jacobson K.A., Enjyoji K., Robson S.C., Colgan S.P. 2003. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198:783–796 10.1084/jem.20030891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppell B.A., Newell A.M., Brown E.J. 1989. Adenosine receptors are expressed during differentiation of monocytes to macrophages in vitro. Implications for regulation of phagocytosis. J. Immunol. 143:4141–4145 [PubMed] [Google Scholar]
- Firestein G.S., Bullough D.A., Erion M.D., Jimenez R., Ramirez-Weinhouse M., Barankiewicz J., Smith C.W., Gruber H.E., Mullane K.M. 1995. Inhibition of neutrophil adhesion by adenosine and an adenosine kinase inhibitor. The role of selectins. J. Immunol. 154:326–334 [PubMed] [Google Scholar]
- Foster T.J. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958 10.1038/nrmicro1289 [DOI] [PubMed] [Google Scholar]
- Gaspar A.H., Marraffini L.A., Glass E.M., Debord K.L., Ton-That H., Schneewind O. 2005. Bacillus anthracis sortase A (SrtA) anchors LPXTG motif-containing surface proteins to the cell wall envelope. J. Bacteriol. 187:4646–4655 10.1128/JB.187.13.4646-4655.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblett E.R., Anderson J.E., Cohen F., Pollara B., Meuwissen H.J. 1972. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 300:1067–1069 10.1016/S0140-6736(72)92345-8 [DOI] [PubMed] [Google Scholar]
- Gounaris K. 2002. Nucleotidase cascades are catalyzed by secreted proteins of the parasitic nematode Trichinella spiralis. Infect. Immun. 70:4917–4924 10.1128/IAI.70.9.4917-4924.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründling A., Schneewind O. 2007. Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J. Bacteriol. 189:2521–2530 10.1128/JB.01683-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G.R., Herring M.B. 1991. Inhibition of neutrophil superoxide production by adenosine released from vascular endothelial cells. Ann. Vasc. Surg. 5:325–330 10.1007/BF02015292 [DOI] [PubMed] [Google Scholar]
- Haskó G., Pacher P. 2008. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J. Leukoc. Biol. 83:447–455 10.1189/jlb.0607359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G., Szabó C. 1998. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem. Pharmacol. 56:1079–1087 10.1016/S0006-2952(98)00153-1 [DOI] [PubMed] [Google Scholar]
- Haskó G., Szabó C., Németh Z.H., Kvetan V., Pastores S.M., Vizi E.S. 1996. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 157:4634–4640 [PubMed] [Google Scholar]
- Haskó G., Kuhel D.G., Chen J.F., Schwarzschild M.A., Deitch E.A., Mabley J.G., Marton A., Szabó C. 2000. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 14:2065–2074 10.1096/fj.99-0508com [DOI] [PubMed] [Google Scholar]
- Higgins J., Loughman A., van Kessel K.P., van Strijp J.A., Foster T.J. 2006. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol. Lett. 258:290–296 10.1111/j.1574-6968.2006.00229.x [DOI] [PubMed] [Google Scholar]
- Jardetzky T.S., Brown J.H., Gorga J.C., Stern L.J., Urban R.G., Chi Y.I., Stauffacher C., Strominger J.L., Wiley D.C. 1994. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 368:711–718 10.1038/368711a0 [DOI] [PubMed] [Google Scholar]
- Jin T., Bokarewa M., Foster T., Mitchell J., Higgins J., Tarkowski A. 2004. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J. Immunol. 172:1169–1176 [DOI] [PubMed] [Google Scholar]
- Kaufmann I., Hoelzl A., Schliephake F., Hummel T., Chouker A., Łysenko L., Peter K., Thiel M. 2007. Effects of adenosine on functions of polymorphonuclear leukocytes from patients with septic shock. Shock. 27:25–31 10.1097/01.shk.0000238066.00074.90 [DOI] [PubMed] [Google Scholar]
- Khoa N.D., Montesinos M.C., Reiss A.B., Delano D., Awadallah N., Cronstein B.N. 2001. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J. Immunol. 167:4026–4032 [DOI] [PubMed] [Google Scholar]
- Klevens R.M., Edwards J.R., Tenover F.C., McDonald L.C., Horan T., Gaynes R.; National Nosocomial Infections Surveillance System 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin. Infect. Dis. 42:389–391 10.1086/499367 [DOI] [PubMed] [Google Scholar]
- Klevens R.M., Morrison M.A., Nadle J., Petit S., Gershman K., Ray S., Harrison L.H., Lynfield R., Dumyati G., Townes J.M., et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 298:1763–1771 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- Knöfel T., Sträter N. 2001. Mechanism of hydrolysis of phosphate esters by the dimetal center of 5′-nucleotidase based on crystal structures. J. Mol. Biol. 309:239–254 10.1006/jmbi.2001.4656 [DOI] [PubMed] [Google Scholar]
- Kunst F., Ogasawara N., Moszer I., Albertini A.M., Alloni G., Azevedo V., Bertero M.G., Bessières P., Bolotin A., Borchert S., et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 390:249–256 10.1038/36786 [DOI] [PubMed] [Google Scholar]
- Lee C.Y., Buranen S.L., Ye Z.H. 1991. Construction of single-copy integration vectors for Staphylococcus aureus. Gene. 103:101–105 10.1016/0378-1119(91)90399-V [DOI] [PubMed] [Google Scholar]
- Lowy F.D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- Mazmanian S.K., Ton-That H., Su K., Schneewind O. 2002. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl. Acad. Sci. USA. 99:2293–2298 10.1073/pnas.032523999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl S.R., St-Onge M., Dussault A.A., Laflamme C., Bouchard L., Boulanger J., Pouliot M. 2006. Immunomodulatory impact of the A2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J. 20:187–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L.K., Steward C.D., Killgore G.E., Chaitram J.M., McAllister S.K., Tenover F.C. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120 10.1128/JCM.41.11.5113-5120.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo F.M., Ballard H.J. 2001. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J. Physiol. 536:593–603 10.1111/j.1469-7793.2001.0593c.xd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölne L., Verdrengh M., Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 68:6162–6167 10.1128/IAI.68.11.6162-6167.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh Z.H., Csóka B., Wilmanski J., Xu D., Lu Q., Ledent C., Deitch E.A., Pacher P., Spolarics Z., Haskó G. 2006. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 176:5616–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist N., Patti J.M., Tarkowski A., Josefsson E. 2004. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microbes Infect. 6:188–195 10.1016/j.micinf.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Panther E., Idzko M., Herouy Y., Rheinen H., Gebicke-Haerter P.J., Mrowietz U., Dichmann S., Norgauer J. 2001. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 15:1963–1970 10.1096/fj.01-0169com [DOI] [PubMed] [Google Scholar]
- Peschel A., Sahl H.G. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529–536 10.1038/nrmicro1441 [DOI] [PubMed] [Google Scholar]
- Punj V., Zaborina O., Dhiman N., Falzari K., Bagdasarian M., Chakrabarty A.M. 2000. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect. Immun. 68:4930–4937 10.1128/IAI.68.9.4930-4937.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche F.M., Massey R., Peacock S.J., Day N.P., Visai L., Speziale P., Lam A., Pallen M., Foster T.J. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology. 149:643–654 10.1099/mic.0.25996-0 [DOI] [PubMed] [Google Scholar]
- Rooijakkers S.H., van Kessel K.P., van Strijp J.A. 2005. Staphylococcal innate immune evasion. Trends Microbiol. 13:596–601 10.1016/j.tim.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Schindler C.A., Schuhardt V.T. 1964. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc. Natl. Acad. Sci. USA. 51:414–421 10.1073/pnas.51.3.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel M., Caldwell C.C., Sitkovsky M.V. 2003. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microbes Infect. 5:515–526 10.1016/S1286-4579(03)00068-6 [DOI] [PubMed] [Google Scholar]
- Thompson L.F., Ruedi J.M., Low M.G. 1987. Purification of 5′-nucleotidase from human placenta after release from plasma membranes by phosphatidylinositol-specific phospholipase C. Biochem. Biophys. Res. Commun. 145:118–125 10.1016/0006-291X(87)91295-2 [DOI] [PubMed] [Google Scholar]
- Thompson L.F., Ruedi J.M., Glass A., Low M.G., Lucas A.H. 1989. Antibodies to 5′-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J. Immunol. 143:1815–1821 [PubMed] [Google Scholar]
- Verdrengh M., Tarkowski A. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 65:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyich J.M., Braughton K.R., Sturdevant D.E., Whitney A.R., Saïd-Salim B., Porcella S.F., Long R.D., Dorward D.W., Gardner D.J., Kreiswirth B.N., et al. 2005. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175:3907–3919 [DOI] [PubMed] [Google Scholar]
- Wang R., Braughton K.R., Kretschmer D., Bach T.H., Queck S.Y., Li M., Kennedy A.D., Dorward D.W., Klebanoff S.J., Peschel A., et al. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 10.1038/nm1656 [DOI] [PubMed] [Google Scholar]
- Xaus J., Mirabet M., Lloberas J., Soler C., Lluis C., Franco R., Celada A. 1999. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J. Immunol. 162:3607–3614 [PubMed] [Google Scholar]
- Yang L., Kobie J.J., Mosmann T.R. 2005. CD73 and Ly-6A/E distinguish in vivo primed but uncommitted mouse CD4 T cells from type 1 or type 2 effector cells. J. Immunol. 175:6458–6464 [DOI] [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. 1974. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob. Agents Chemother. 6:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. 1992. 5′-Nucleotidase: molecular structure and functional aspects. Biochem. J. 285:345–365 [DOI] [PMC free article] [PubMed] [Google Scholar]