Abstract
Group 1 CD1 (CD1a, CD1b, and CD1c)–restricted T cells recognize mycobacterial lipid antigens and are found at higher frequencies in Mycobacterium tuberculosis (Mtb)–infected individuals. However, their role and dynamics during infection remain unknown because of the lack of a suitable small animal model. We have generated human group 1 CD1 transgenic (hCD1Tg) mice that express all three human group 1 CD1 isoforms and support the development of group 1 CD1–restricted T cells with diverse T cell receptor usage. Both mycobacterial infection and immunization with Mtb lipids elicit group 1 CD1–restricted Mtb lipid–specific T cell responses in hCD1Tg mice. In contrast to CD1d-restricted NKT cells, which rapidly respond to initial stimulation but exhibit anergy upon reexposure, group 1 CD1–restricted T cells exhibit delayed primary responses and more rapid secondary responses, similar to conventional T cells. Collectively, our data demonstrate that group 1 CD1–restricted T cells participate in adaptive immune responses upon mycobacterial infection and could serve as targets for the development of novel Mtb vaccines.
Nearly one third of the human population is infected with Mycobacterium tuberculosis (Mtb). The disease caused by this bacteria, tuberculosis, remains one of the leading causes of mortality caused by infection worldwide and is a major cause of death in HIV/AIDS-affected individuals (Kaufmann and McMichael, 2005). Additionally, the emergence of multidrug-resistant Mtb strains poses a serious global health concern (Dye, 2009). However, attenuated M. bovis bacillus Calmette-Guérin (BCG), the Mtb vaccine presently available, has little to no protective efficacy in adults (Colditz et al., 1994). Thus, there is great need for the development of improved vaccines that can elicit strong cellular immune responses and confer long-term protection against Mtb in the general population. Conventional CD4+ and CD8+ T cells play critical roles in containing infection upon recognition of Mtb peptide antigens presented by MHC class II and I, respectively (Sousa et al., 2000; North and Jung, 2004; Winslow et al., 2008). Several subunit vaccine candidates have been recently developed using Mtb protein antigens that elicit potent Th1 responses (Gupta et al., 2007; Sable et al., 2007). However, little is known about the potential use of Mtb cell-wall lipids and glycolipids in vaccines against Mtb.
CD1 molecules are MHC class I–like antigen-presenting molecules that are specialized to present lipid, glycolipid, and lipopeptide antigens to T cells. Unlike MHC molecules, CD1 molecules exhibit low levels of polymorphism in their antigen-binding domains (Brigl and Brenner, 2004), making CD1 lipid antigens attractive as vaccine components that would be recognized widely in a genetically diverse human population. The human CD1 locus encodes five related proteins that are classified into two groups based on sequence similarity (Brigl and Brenner, 2004). Group 1 CD1 consists of CD1a, CD1b, and CD1c, whereas CD1d comprises group 2 CD1. The fifth human CD1 molecule, CD1e, is not expressed on the cell surface but has been shown to facilitate the loading of certain lipid antigens onto CD1b (de la Salle et al., 2005). The CD1 isoforms each have unique intracellular trafficking patterns and differ in the size and shape of their antigen-binding pocket (Brigl and Brenner, 2004; Moody et al., 2005), characteristics that enable them to access and present distinct populations of lipid antigens to T cells.
Mouse CD1d, the homologue of human CD1d, has been studied extensively. It is expressed on most hematopoietic cells and has been shown to present both self and foreign glycolipid antigens to NKT cells, a unique subset of T cells that bear NK cell receptors. Marine sponge–derived α-galactosylceramide (α-GalCer) is recognized by invariant NKT (iNKT) cells, a major subset of NKT cells with an invariant TCR α chain (Bendelac et al., 2007). These iNKT cells respond to antigenic stimulation with rapid secretion of Th1 and Th2 cytokines, and have been postulated to form a bridge between innate and adaptive immune responses. Indeed, recent studies have described roles for iNKT cells in a wide variety of immune responses, including tumor immunity, autoimmunity, and response to infection (Bendelac et al., 2007; Tupin et al., 2007). However, because mice lack homologues of CD1a, CD1b, and CD1c, many questions regarding the activation, kinetics, and in vivo function of group 1 CD1–restricted T cell responses remain unanswered.
Information on the role of group 1 CD1–restricted T cells in mycobacterial immunity has mainly come from ex vivo studies in humans. Group 1 CD1–restricted T cells have been isolated from healthy individuals, as well as from Mtb- and M. leprae–infected patients (Kawashima et al., 2003; Ulrichs et al., 2003). These T cells were found in CD4−CD8− (double-negative), CD4+, and CD8+ T cell subsets, and recognize both self and mycobacterial lipid antigens (Porcelli et al., 1992; Rosat et al., 1999; Sieling et al., 2000). CD1a has been shown to present the lipopeptide didehydroxymycobactin, which may be involved in mycobactin synthesis or degradation (Rosat et al., 1999; Moody et al., 2004). CD1b presents several distinct components of the mycobacterial cell wall, including mycolic acid, glucose monomycolate, glycerol monomycolate, lipoarabinomannan and phosphatidylinositol mannoside, diacylated sulfoglycolipids, and the self-lipid ganglioside GM1 (Beckman et al., 1994; Sieling et al., 1995; Moody et al., 1997; Shamshiev et al., 1999; Gilleron et al., 2004; Layre et al., 2009). CD1c has been shown to present isoprenoid phosphoglycolipids, such as mannosyl phosphomycoketide, to T cells (Rosat et al., 1999; Moody et al., 2000). These data suggest that group 1 CD1–restricted T cells may participate in immune responses during mycobacterial infection.
Several studies have shown that individuals who have been exposed to Mtb have increased frequencies of group 1 CD1–restricted T cells as compared with a control population (Sieling et al., 1999; Ulrichs et al., 2003; Layre et al., 2009), suggesting that Mycobacteria-specific CD1-restricted T cells are activated and expand in number after infection. The function and phenotype of activated group 1 CD1–restricted T cells in vivo are unknown. However, in vitro studies have shown that most group 1 CD1–restricted T cell lines are cytotoxic and produce IFN-γ and TNF-α upon stimulation with mycobacterial lipid antigens. These cytokines are critical for protective immunity against Mtb, suggesting that CD1-restricted T cells are capable of contributing to protection against Mtb infection in vivo. Recent studies using a guinea pig model, which expresses homologues of human CD1b and CD1c (Dascher et al., 1999), showed that immunization with mycobacterial lipids elicited antigen-specific proliferation and cytolytic ability in group 1 CD1–restricted T cells (Hiromatsu et al., 2002). In addition, Mtb lipid–vaccinated guinea pigs exhibit improved lung pathology after subsequent Mtb challenge (Dascher et al., 2003). However, because of the inherent limitations of this experimental model, it could not be confirmed that CD1-restricted T cells mediated these protective effects.
In this study, we have generated a novel transgenic mouse model that expresses the human group 1 CD1 genes under their endogenous human promoters to study the in vivo function of group 1 CD1–restricted T cells during mycobacterial infection. We found that human group 1 CD1 transgenic (hCD1Tg) mice express CD1a, CD1b, and CD1c in a pattern similar to that seen in humans and support the development of group 1 CD1–restricted T cells. Immunization with Mtb lipid antigens elicits group 1 CD1–restricted mycobacterial lipid antigen–specific Th1 T cell responses in hCD1Tg mice. The surface phenotype, kinetics of response, and tissue distribution of group 1 CD1–restricted Mtb lipid antigen–specific T cells differ from those of CD1d-restricted iNKT cells, highlighting the functional dichotomy of the two CD1 groups. Furthermore, infection of hCD1Tg mice with either Mtb or BCG activates group 1 CD1–restricted T cells that can recognize some of the same mycobacterial antigens that are recognized by humans. Collectively, our data demonstrate the viability of the hCD1Tg mouse as a functional model for the study of group 1 CD1–restricted T cells in antimycobacterial immunity.
RESULTS
Group 1 CD1 expression in hCD1Tg mice mimics human expression
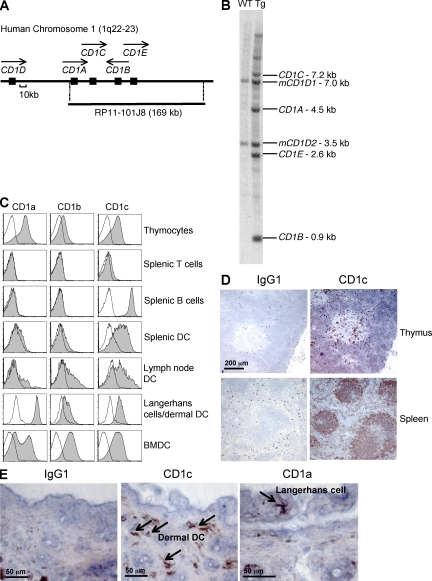
In humans, the three group 1 CD1 isoforms are differentially expressed on thymocytes and professional antigen-presenting cells, but no suitable surrogate promoters are known that replicate their complex expression patterns. We therefore introduced the entire coding region of human CD1A, B, C, and E, including candidate promoters and all open reading frames along with 5′ and 3′ flanking sequences, into the mouse on the bacterial artificial chromosome clone RP11-101J8 (Fig. 1 A) to generate hCD1Tg mice. In brief, RP11-101J8 was transfected into R1 embryonic stem cells, which were injected into C57BL/6 (B6) blastocysts to generate chimeras. Two CD1c-expressing chimeric mice were chosen as founders for the hCD1Tg 64 and 78 lines, and gene expression was verified by Southern blotting. Line 64 hCD1Tg mice express CD1A, CD1B, and CD1C (Fig. 1 B), whereas line 78 expresses CD1B and CD1C at similar levels to Tg 64 but lacks CD1A due to the loss of the 5′ promoter region that has been shown to be required for transcription (unpublished data; Colmone et al., 2006). Founders were backcrossed onto a B6 background for these studies.
Figure 1.
hCD1Tg mice mimic human group 1 CD1 expression patterns. (A) The human CD1 locus, including the area spanned by RP11-101J8. Closed boxes indicate CD1 genes, and arrows indicate the direction of gene transcription. (B) Group 1 CD1 genes in hCD1Tg 64 and WT liver are shown in a Southern blot. Fragments containing human CD1A, B, C, and E, as well as mouse CD1D1 and CD1D2 are indicated. (C) Antibody staining of CD1a, CD1b, and CD1c on single-cell suspensions and BMDCs prepared from hCD1Tg 64 (shaded histograms) and WT (open histograms) mice as determined by flow cytometry. For thymocytes, cells were gated on the CD4+CD8+ population. B cells, B220+; CD11c+ skin cells, CD11c+MHC class II+; DCs, B220−CD11c+; T cells, TCRβ+. (D) CD1c is expressed in hCD1Tg 64 thymus and spleen, as determined by immunohistochemistry, with IgG1 staining shown as a control. (E) Expression of CD1a on Langerhans cells and CD1c on dermal DCs as described in D. Arrows indicate cells with positive staining. Results in A–E are representative of at least three independent experiments.
Similar to humans, CD1a, CD1b, and CD1c expression in hCD1Tg mice can be detected on cortical thymocytes, whereas expression is almost completely lacking on peripheral T cells (Fig. 1 C and Fig. S1 A). The expression of CD1c is readily detectable on B cells and on DCs in the thymus, spleen, and lymph node, whereas CD1a and CD1b are expressed at low levels on DCs in the lymph node and spleen (Fig. 1, C and D; and Fig. S1 A). All three group 1 CD1 isoforms are expressed in the skin; CD1a is prominently expressed on Langerhans cells, whereas CD1c is detected on dermal DCs (Fig. 1, C and E). CD1E mRNA is expressed in hCD1Tg mice in a similar pattern to CD1B (Fig. S1 B). This complex tissue distribution of group 1 CD1 in hCD1Tg mice essentially parallels the expression pattern observed in humans.
Although most monocytes and myeloid DC populations in vivo express only moderate levels of group 1 CD1, high expression levels can be induced in vitro on hCD1Tg DCs through exposure to GM-CSF and IL-4, as previously demonstrated for monocytes isolated from human PBMCs (Brigl and Brenner, 2004). The levels of CD1a, CD1b, and CD1c expression on GM-CSF– and IL-4–treated bone marrow–derived DCs (BMDCs) from hCD1Tg64 mice are comparable to those observed on human monocyte-derived DCs (Fig. 1 C and Fig. S1 C). High levels of CD1a, CD1b, and CD1c expression were also induced in vivo on splenic DCs using GM-CSF–transduced B16 melanoma cells to maintain a steady supply of cytokines (Fig. S1 D). Collectively, these data indicate that the machinery required for activation-dependent group 1 CD1 expression on DCs is functional in hCD1Tg mice.
Group 1 CD1 expressed on hCD1Tg DCs can present Mtb lipids to human T cells
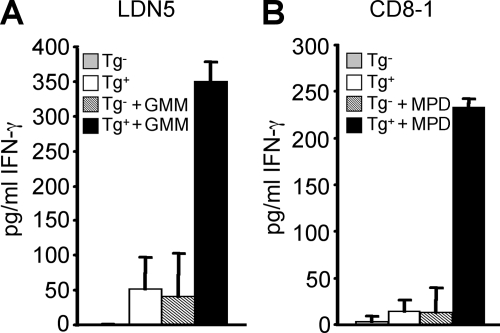
To examine whether group 1 CD1–expressing antigen-presenting cells in hCD1Tg mice are functional and can present lipid antigen to human T cells, human group 1 CD1–restricted T cell lines specific for two distinct mycobacterial lipid antigens were stimulated with BMDCs derived from hCD1Tg mice. Both LDN5, a CD1b-restricted T cell line specific for glucose monomycolate, and CD8-1, a CD1c-restricted T cell line specific for mannosyl phosphomycoketide, could be stimulated by hCD1Tg BMDCs to produce IFN-γ (Fig. 2, A and B). These interactions were antigen specific, as BMDCs that were not pulsed with lipid antigen could not stimulate these T cell lines (Fig. 2). These data demonstrate that hCD1Tg antigen-presenting cells have the capacity to process Mtb lipid antigens that are recognized by humans, and to then present these antigens to group 1 CD1–restricted T cells, resulting in their activation.
Figure 2.
hCD1Tg DCs can process and present Mtb lipids to activate human group 1 CD1–restricted Mtb lipid antigen–specific T cells. Human group 1 CD1–restricted T cell lines (A) LDN5 and (B) CD8-1 were cultured with WT (Tg−) or hCD1Tg 78 (Tg+) BMDC stimulators for 48 h. IFN-γ secretion was measured by cytokine ELISA. Some stimulators were pulsed with glucose monomycolate (GMM; A) or mannosyl phosphomycoketide (MPD; B). Error bars indicate SD. Results represent mean cytokine secretion from two pooled experiments.
hCD1Tg mice develop group 1 CD1–restricted T cells with a Th1 phenotype
To investigate the ability of human group 1 CD1 molecules to support group 1 CD1–restricted mouse T cell development, group 1 CD1–restricted T cell lines were established from hCD1Tg mice immunized either with Mtb antigens or with the ganglioside GM1. Lymphocytes from the spleen and lymph nodes of these mice were cultured and restimulated weekly with antigen-pulsed hCD1Tg BMDCs. A panel of long-term group 1 CD1–restricted T cell lines and T cell clones was generated that had similar characteristics to human-derived group 1 CD1–restricted T cells (Table I). These hCD1Tg-derived group 1 CD1–restricted T cell lines showed diverse TCR Vα and Vβ usage and expressed CD4, CD8αα, or CD8αβ coreceptors or were double negative, but did not display NK cell surface markers such as NK1.1 and Ly49A (Table I and unpublished data).
Table I.
Properties of group 1 CD1–restricted T cell lines
| Line | Coreceptor | TCR Vα usage | TCR Vβ usage | Restricted by | Cytotoxicity | Cytokine |
| 1SP9 | DN | Vα8.6 | Vβ5 | CD1a | Yes | IFN-γ |
| 2SP5 | DN | Vα8.3 | Vβ3 | CD1a | Yes | IFN-γ |
| HN2 | CD4 | * | ** | CD1a/Mtb antigen | Yes | IFN-γ |
| HN3 | CD4 | ND | ND | CD1a | Yes | IFN-γ, IL-4 |
| HN4 | CD8αβ | ND | ND | CD1a | Yes | IFN-γ |
| S2-1-16 | CD8αα/DN | ND | ND | CD1b | Yes | IFN-γ |
| HJ1 | CD8αα/DN | Vα8.5 | Vβ2 | CD1b | Yes | IFN-γ |
| S2-7-3 | CD8αα/DN | Vα8.3 | Vβ8 | CD1b | Yes | IFN-γ |
| S5-1-1 | CD8αα/DN | * | Vβ8 | CD1b | Yes | IFN-γ |
| S2-2-7 | CD8αα/DN | * | Vβ4 | CD1c | Yes | IFN-γ |
| S7-1 | CD8αβ/DN | * | Vβ5 | CD1c | Yes | IFN-γ |
| HN1 | CD8αα | Vα5 | Vβ9 | CD1c | Yes | IFN-γ |
| KF1 | CD8αα | * | Vβ4 | CD1c | Yes | IFN-γ |
| LN2 | DN | Vα3 | Vβ4 | CD1c | No | IFN-γ |
| SP2 | CD8αα | Vα5 | Vβ5 | CD1c | Yes | IFN-γ |
| S1-1-1 | CD4/DN | ND | ND | hCD1Tg | ND | IFN-γ, IL-4 |
| S10-1-1 | CD8αβ | * | Vβ11 | hCD1Tg | ND | IFN-γ |
Coreceptor and TCR usage were determined by flow cytometry. T cell line restriction was determined by 51Cr release assay or cytokine ELISA. Cytokine production was determined by ELISA. ND, not determined; *, negative for tested antibodies, including anti-Vα2, -Vα11, and -Vα8; **, negative for tested antibodies, including anti-Vβ2, -Vβ3, -Vβ5, -Vβ6, -Vβ8.1/8.2, -Vβ8.3, -Vβ11, -Vβ12, -Vβ13, and -Vβ14.
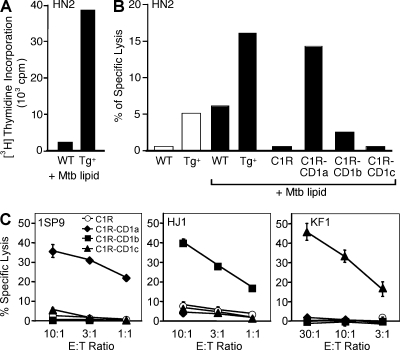
These T cell lines responded strongly to group 1 CD1–expressing (hCD1Tg) BMDCs but not to WT BMDCs, as measured by proliferation (Fig. 3 A), secretion of the Th1 cytokine IFN-γ (Table I), and lysis of group 1 CD1–expressing targets (Table I and Fig. 3 B). In contrast to CD1d-restricted iNKT cells, IL-4 secretion from group 1 CD1–restricted T cells was rarely detected, and then only in low amounts (Table I). We were able to identify T cell lines restricted to each of the group 1 isoforms by observing cytolysis of human B lymphoblasts (C1R) transfected individually with CD1a, CD1b, or CD1c (Table I and Fig. 3 C). The HN2 T cell line secreted IFN-γ only in the presence of an Mtb lipid mixture and preferentially lysed CD1a-expressing target cells that had been pulsed with Mtb lipids (Table I and Fig. 3 B), indicating Mtb lipid antigen specificity. However, the majority of the group 1 CD1–restricted T cell lines derived from hCD1Tg mice were reactive in the absence of exogenous antigens (Table I and Fig. 3 C), suggesting that they recognize self-lipids. Collectively, these experiments demonstrate that all three human group 1 CD1 molecules can indeed function as restriction elements to mouse T cells and promote the development of group 1 CD1–restricted T cells in hCD1Tg mice.
Figure 3.
hCD1Tg mice develop group 1 CD1–restricted cytotoxic T cells. T cell lines HN2, 1SP9, HJ1, and KF1 were derived from immunized hCD1Tg mice. (A and B) HN2 specifically recognizes Mtb lipid antigen–pulsed CD1a-expressing cells, as indicated by (A) [3H] incorporation and (B) lysis of 51Cr-labeled target cells. C1R, CD1-transfected C1R, and hCD1Tg (Tg+) and WT BMDCs were used as targets. Results are representative of two independent experiments. (C) 1SP9, HJ1, and KF1 lyse CD1a-, CD1b-, or CD1c-expressing target cells, respectively, in the absence of exogenous antigen. Error bars indicate SD. Results are representative of three independent experiments.
The kinetics of group 1 CD1–restricted T cell responses is distinct from group 2 CD1–restricted NKT cell responses
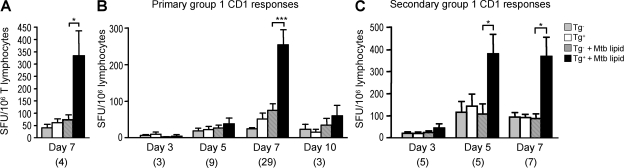
CD1d-restricted iNKT cells respond within hours upon antigenic stimulation (Bendelac et al., 2007). However, the kinetics of group 1 CD1–restricted T cell responses are largely unknown. To investigate the kinetics of group 1 CD1–restricted immune responses, we immunized hCD1Tg mice with hCD1Tg DCs pulsed with a total lipid extract from Mtb (Mtb lipid). Although we occasionally observed group 1 CD1–restricted T cell responses in hCD1Tg mice in a WT background, responses by MHC-restricted conventional T cells were generally quite strong and tended to mask group 1 CD1–restricted responses in these mice (Fig. S2). We therefore used hCD1Tg mice crossed onto an MHC class II–deficient background (MHC II−/−) for our immunization studies, as they exhibited a lower background and more consistent group 1 CD1–restricted responses. Lymphocytes from the spleen and lymph nodes of immunized mice were stimulated ex vivo with BMDCs, and IFN-γ–secreting cells were quantitated using an ELISPOT assay. Group 1 CD1–restricted responses in immunized mice were elicited by hCD1Tg+ but not Tg− stimulator cells, and demonstrated specificity to Mtb lipid (Fig. 4). In addition, preliminary observation of group 1 CD1–restricted responses in purified T cell populations confirmed T cells as the IFN-γ–secreting cell type (Fig. 4 A). These responses were minimal at 5 d after immunization, peaked on day 7, and diminished by day 10 (Fig. 4 B). WT mice immunized with Mtb lipid–pulsed DCs and hCD1Tg mice immunized with unpulsed DCs did not exhibit group 1 CD1–restricted responses, nor did they respond to Mtb lipid (unpublished data). In addition, the expression levels of group 1 CD1 on BMDC stimulators were not affected by the Mtb antigen pulsing conditions used in our studies (Fig. S3), indicating that the activation of group 1 CD1–restricted T cells by Mtb lipids is not caused by up-regulation of group 1 CD1 expression.
Figure 4.
Group 1 CD1–restricted T cell responses peak at day 7 after primary immunization and day 5 of secondary immunization. Lymphocytes from the spleen and lymph nodes of intraperitoneally immunized hCD1Tg mice were cultured with MHC II−/− (Tg−) or hCD1Tg MHC II−/− (Tg+) BMDC stimulators in an ELISPOT assay. Some stimulators were pulsed with Mtb lipid. IFN-γ–secreting cells are designated as spot-forming units (SFU). Error bars indicate SEM. The numbers of animals used for each time point are indicated in parenthesis. (A) Responses in purified T cells of hCD1Tg 64 MHC II−/− mice 7 d after immunization with Mtb lipid (mean SFU from one individual experiment). *, P = 0.0464. (B) Primary responses to Mtb lipid antigen immunization. hCD1Tg 78 MHC II−/− mice were sacrificed 3, 5, 7, and 10 d after immunization with Mtb lipid (mean SFU are pooled from 10 individual experiments). ***, P < 0.0001. (C) Secondary responses to Mtb lipid antigen immunization. Mtb lipid–immunized hCD1Tg 78 MHC II−/− mice were given booster immunizations and sacrificed 3, 5, and 7 d after secondary immunization (mean SFU are pooled from four independent experiments). *, P = 0.0265 (day 5); *, P = 0.0225 (day 7). P-values for A–C were calculated using a paired two-tailed Student's t test. Group 1 CD1–restricted responses were not detected from lymphocytes isolated from unimmunized hCD1Tg mice.
A major feature of adaptive immunity is the ability of T cells to respond more rapidly and robustly to secondary antigenic challenge. However, CD1d-restricted iNKT cells cannot be induced to expand or secrete increased amounts of IFN-γ upon stimulation with α-GalCer after either primary α-GalCer stimulation or microbial infection, but are instead unresponsive (Parekh et al., 2005; Uldrich et al., 2005; Chiba et al., 2008). To investigate the kinetics of secondary group 1 CD1–restricted immune responses, we rested Mtb lipid–immunized hCD1Tg mice for 4 wk, after which a booster immunization of Mtb lipid–pulsed DCs was administered. The number of IFN-γ–producing group 1 CD1–restricted cells was determined at days 3, 5, and 7 after boost. Although primary group 1 CD1–restricted T cell responses are weak at 5 d after immunization, responses to secondary exposure are already robust by this time (Fig. 4 C). Total numbers of IFN-γ–producing group 1 CD1–restricted T cells tend to increase during secondary responses; however, these data do not reach statistical significance because of the large variability in responses among animals. The more rapid kinetics of group 1 CD1–restricted Mtb lipid antigen–specific T cell responses upon secondary exposure suggests that Mtb lipid antigen–primed T cells are generated after initial exposure to antigen. Group 1 CD1–restricted T cells may therefore act as part of the adaptive immune system together with MHC-restricted T cells.
Group 1 CD1–restricted T cells from hCD1Tg mice have a distinct phenotype from NKT cells and resemble T cells found in humans
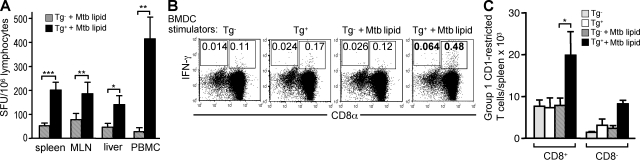
CD1d-restricted iNKT cells are most prevalent in the liver but are also found in the spleen and lymph nodes (Bendelac et al., 2007). We found that Mtb antigen–specific group 1 CD1–restricted T cell responses were present in immunized mice in nonlymphoid tissues as well as in the spleen and lymph nodes. Group 1 CD1–restricted T cells were not enriched in the liver but did appear at a slightly higher frequency in peripheral blood (Fig. 5 A). In mice, iNKT cells are either CD4+ or double negative for coreceptor expression. We performed intracellular IFN-γ staining to determine the phenotype of group 1 CD1–restricted T cells in immunized hCD1Tg mice. We found that T cells activated by group 1 CD1–expressing DCs were present in both CD8+ and CD8− T cell subsets (Fig. 5, B and C); however, group 1 CD1–restricted CD8− T cell responses were detected less often than CD8+ T cells and were present at a lower frequency (Fig. 5 C). The coreceptor phenotype of group 1 CD1–restricted T cells in hCD1Tg mice is therefore quite similar to that of human-derived group 1 CD1–restricted T cells but is distinct from that of CD1d-restricted iNKT cells.
Figure 5.
Group 1 CD1–restricted Mtb-lipid specific T cell responses are distinct from group 2 CD1–restricted responses. hCD1Tg (Tg 78 MHC II−/−) mice were immunized with Mtb lipid. 7 d later, mice were sacrificed and lymphocytes from immunized mice were cultured with MHC II−/− (Tg−) or hCD1Tg MHC II−/− (Tg+) BMDC stimulators. IFN-γ–secreting cells were quantitated in an ELISPOT assay or by intracellular cytokine staining. Error bars indicate SEM. (A) Group 1 CD1–restricted T cells detected in various tissues from immunized mice. Cells were stimulated with Mtb lipid–pulsed BMDCs (spleen, n = 17 [***, P < 0.0001]; mesenteric lymph nodes [MLN], n = 11 [**, P = 0.0024]; liver, n = 5 [*, P = 0.032]; PBMCs, n = 5 [**, P = 0.0087]). Mean SFU were pooled from nine independent experiments. (B and C) Splenocytes harvested from Mtb lipid antigen–immunized mice were stimulated with BMDCs in the presence or absence of Mtb lipid. The percentages (B) and total numbers (C) of IFN-γ–secreting CD8+ and CD8− T cells in the TCRβ+ gate were determined by intracellular staining. Of the nine animals exhibiting CD8+ group 1 CD1–restricted T cell responses, two exhibited CD8− group 1 CD1–restricted responses. *, P = 0.026. Mean numbers of IFN-γ–producing group 1 CD1–restricted T cells in C were obtained from four independent experiments. P-values for A and C were calculated using a paired two-tailed Student's t test.
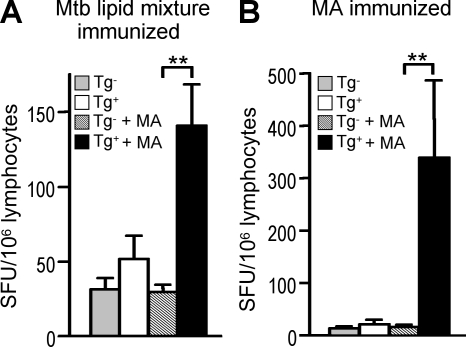
Human group 1 CD1–restricted T cells have been shown to respond to multiple mycobacterial lipid antigens, including mycolic acid and glucose monomycolate. To determine whether specific mycobacterial antigens described in humans can also be recognized by mouse T cells, we examined group 1 CD1–restricted responses to individual lipid antigens in hCD1Tg mice immunized with Mtb lipid. Lymphocytes from hCD1Tg mice immunized with Mtb lipid responded to mycolic acid-pulsed BMDCs in a group 1 CD1–restricted manner (Fig. 6 A), and group 1 CD1–restricted responses to glucose monomycolate–pulsed BMDCs were also observed (not depicted). Moreover, immunization with mycolic acid–pulsed DCs to provide exposure to a single mycobacterial lipid antigen elicited robust group 1 CD1–restricted mycolic acid–specific responses (Fig. 6 B). Thus, in addition to sharing similar phenotypic features with human-derived group 1 CD1–restricted T cells, group 1 CD1–restricted T cells from hCD1Tg mice can recognize some of the same mycobacterial lipid and glycolipid antigens.
Figure 6.
Group 1 CD1–restricted Mtb lipid–specific T cells in hCD1Tg mice respond to the same antigens recognized by human-derived T cells. hCD1Tg 78 MHC II−/− mice were immunized with either total Mtb lipid extract or mycolic acid (MA)–pulsed BMDCs. Mice were sacrificed 7 d after immunization, and lymphocytes from immunized mice were cultured with MHC II−/− (Tg−) or hCD1Tg MHC II−/− (Tg+) BMDCs in the presence or absence of MA. IFN-γ–secreting cells were quantitated in an ELISPOT assay. Error bars indicate SEM. (A) Detection of group 1 CD1–restricted MA-specific T cells in lymphocytes isolated from mice immunized with Mtb lipid mixture (n = 8; **, P = 0.0076). Mean SFU are pooled from six independent experiments. (B) Detection of group 1 CD1–restricted MA-specific T cells in lymphocytes isolated from MA-immunized mice (n = 5; **, P = 0.0087). Mean SFU were pooled from two independent experiments. P-values for A and B were calculated using a paired two-tailed Student's t test.
Mycobacterial infection of hCD1Tg mice elicits group 1 CD1–restricted immune responses
It has been proposed that mycobacterial infection stimulates and expands group 1 CD1–restricted T cell subsets, but whether infection increases or inhibits group 1 CD1 expression on antigen-presenting cells has been controversial (Stenger et al., 1998; Giuliani et al., 2001; Roura-Mir et al., 2005; Gagliardi et al., 2007; Prete et al., 2007; Hava et al., 2008). Changes in group 1 CD1 expression at sites of Mtb infection are likely to affect the magnitude of group 1 CD1–restricted T cell responses. We found that expression of all three group 1 CD1 isoforms was retained on CD11c+ DCs in the spleen and lungs of Mtb-infected hCD1Tg mice, although expression was somewhat decreased at 2 wk after infection relative to expression in naive mice (Fig. S4 A and not depicted). By 4 wk after infection, group 1 CD1 expression approached baseline levels in these tissues (Fig. S4 A and not depicted) and the number of group 1 CD1–expressing DCs was increased (Fig. S4, B and C; and not depicted), indicating that overall group 1 CD1 antigen presentation is not adversely affected by Mtb infection.
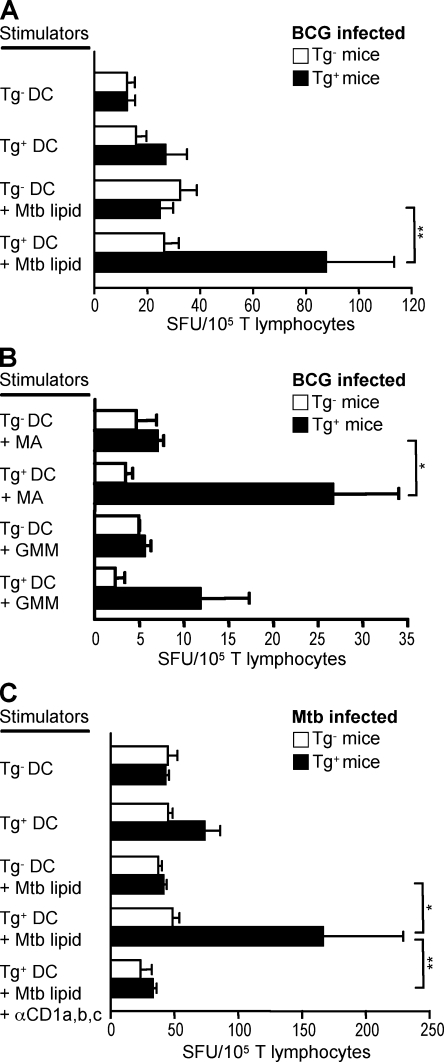
To examine group 1 CD1–restricted T cell responses to infection, we infected MHC II−/− and hCD1Tg MHC II−/− mice intravenously with BCG or via aerosol with Mtb H37Rv, and examined group 1 CD1–restricted Mtb lipid antigen–specific T cell responses at various time points after infection. We observed group 1 CD1–restricted Mtb lipid antigen–specific T cell responses in both BCG- (Fig. 7, A and B) and Mtb-infected hCD1Tg MHC II−/− mice (Fig. 7 C) at 3 and 4 wk after infection, as measured by IFN-γ secretion in an ELISPOT assay. Infected MHC II−/− mice and naive hCD1Tg MHC II−/− mice failed to respond to mycobacterial lipid antigens, thereby confirming the hCD1Tg-dependent Mtb-specific induction of group 1 CD1–restricted T cell responses (Fig. 7 and not depicted). Group 1 CD1–restricted Mtb lipid antigen–specific T cell responses were also observed in hCD1Tg mice in a WT background (Fig. S5), albeit less frequently than in the MHC II−/− background. In addition to total Mtb lipid–specific responses, group 1 CD1–restricted T cell responses specific to the individual mycobacterial antigens mycolic acid and glucose monomycolate were also detected in BCG-infected hCD1Tg MHC II−/− mice (Fig. 7 B). In a preliminary experiment, Mtb-infected mice exhibited group 1 CD1–restricted T cell responses that were abrogated in the presence of anti–group 1 CD1 monoclonal antibodies (Fig. 7 C). Although previous studies have shown a correlation between mycobacterial infection and the prevalence of group 1 CD1–restricted T cells, these data provide direct evidence that group 1 CD1–restricted T cell responses to mycobacterial lipid antigens are induced by infection in vivo and may contribute to overall immunity against Mycobacteria.
Figure 7.
Mycobacteria induce group 1 CD1–restricted responses in infected hCD1Tg mice. Splenic T cells isolated from mycobacteria-infected MHC II−/− (Tg−) and hCD1Tg MHC II−/− (Tg+) mice were cultured with Tg− or Tg+ BMDC stimulator cells in an ELISPOT assay. IFN-γ–secreting cells were quantitated as spot-forming units (SFU). Error bars indicate SEM. (A and B) Splenic T cells were isolated from mice that had been intravenously infected with BCG for 3–4 wk. Mean SFU were pooled from four independent experiments. (A) Some BMDCs were pulsed with Mtb lipid (Tg+, n = 7; Tg−, n = 6). **, P < 0.01. (B) BMDCs were pulsed with either mycolic acid (MA) or glucose monomycolate (GMM; Tg+, n = 4; Tg−, n = 3). *, P < 0.05. (C) Splenic T cells were isolated from mice 4 wk after aerosol infection with Mtb. Monoclonal antibodies against CD1a, CD1b, and CD1c were used for blocking (Tg+, n = 8; Tg−, n = 4). *, P < 0.05; **, P < 0.01. P-values for A–C were calculated using one-way analysis of variance with a Tukey's multiple comparison posttest. Mean SFU were from one experiment. Group 1 CD1–restricted responses were not detected from lymphocytes isolated from uninfected hCD1Tg mice.
DISCUSSION
In this paper, we present a novel animal model for the study of group 1 CD1–restricted immune responses in vivo. The hCD1Tg mice described express group 1 CD1 in a pattern similar to humans and support the development of mouse T cells that are restricted to group 1 CD1. The group 1 CD1–restricted T cells observed in hCD1Tg mice are similar to those found in humans in that they have a diverse TCR repertoire, exhibit Th1 immune responses, are found in double-negative, CD4+, and CD8+ T cell subsets, and recognize some of the same Mtb lipid antigens. Group 1 CD1–restricted T cell responses can be induced both through the physiological route of Mtb aerosol infection or though immunization with Mtb lipids. We therefore find hCD1Tg mice to be a suitable animal model for further study of group 1 CD1–restricted immune responses both in infectious diseases and as targets for lipid antigen vaccine development.
Transcriptional regulation of all three group 1 CD1 genes is preserved in hCD1Tg mice, suggesting that the distinct tissue distribution of the group 1 CD1 proteins does not require species-specific transcription factors. Indeed, most species of mammals examined thus far, including other rodents, possess genes encoding both group 1 and group 2 CD1 homologues, suggesting diversification of the CD1 gene family in an early mammalian ancestor (Dascher, 2007). Genomic analysis has revealed that mice and rats probably deleted the group 1 CD1 locus at a recent point in their evolutionary history, most likely as the result of chromosomal rearrangement (Dascher and Brenner, 2003). The striking conservation of the human CD1 expression patterns from human promoters in hCD1Tg mice may reflect the presence of conserved trans-acting factors that were not affected when the group 1 CD1 genes were lost. Moreover, group 1 CD1 expression patterns and function are preserved in hCD1Tg mice without replacing mouse β2 microglobulin (β2m) with human β2m. We have crossed hCD1Tg mice with human β2m transgenic mouse β2m–deficient mice to generate hCD1Tg/hβ2mTg/mβ2m−/− mice. Although group 1 CD1 expression was slightly higher on hCD1Tg/hβ2mTg/mβ2m−/− BMDCs (Fig. S6 A) than on hCD1Tg BMDCs, they stimulated comparable levels of IL-2 release from a CD1b-restricted T cell hybridoma (Fig. S6 B). These data suggest that pairing with mouse β2m does not adversely affect the expression and function of human group 1 CD1 and further validate hCD1Tg mice as an adequate model of the human system.
Although several human-derived group 1 CD1–restricted T cell lines recognize Mtb antigens, most T cell lines generated from hCD1Tg mice are autoreactive. We have not identified which endogenous antigens are recognized by the autoreactive group 1 CD1–restricted T cell lines we have derived; however, these T cells are able to lyse group 1 CD1–expressing human cell lines as well as mouse hCD1Tg DCs, suggesting that the self-antigens in question are conserved between mice and humans. Several self-reactive group 1 CD1–restricted human T cell clones have been shown to exhibit an enhanced response in the presence of microbial antigens (Vincent et al., 2005). We have observed a similar phenomenon in some of our autoreactive T cell lines (unpublished data). Although these T cell lines may exhibit dual recognition of both self and microbial lipids, it is also possible that the autoreactive responses in our T cell lines are enhanced through TLR-mediated activation of DCs and increased production of endogenous glycolipids that are then presented by CD1. It has been postulated that iNKT cells can be activated in a similar manner (Mattner et al., 2005). It should also be noted that responses to two well-characterized Mtb lipid antigens in the human, mycolic acid and glucose monomycolate, are elicited after either infection or parenteral immunization of hCD1Tg mice. These data clearly demonstrate the conservation of Mtb lipid antigen–specific group 1 CD1–restricted T cell responses in our humanized mouse model.
The kinetics of Mtb antigen–specific group 1 CD1–restricted T cell responses is distinct from those of α-GalCer–stimulated iNKT cells. Group 1 CD1–restricted T cells have a delayed response to antigen stimulation when compared with iNKT cells and do not exhibit significant secretion of the Th2 cytokine IL-4. Furthermore, upon secondary antigenic challenge, group 1 CD1–restricted T cell responses are accelerated and exhibit a robust response that is equal to if not greater than the primary response. In contrast, secondary exposure to α-GalCer induces iNKT cell anergy (Parekh et al., 2005; Uldrich et al., 2005; Chiba et al., 2008). Thus, group 1 CD1–restricted Mtb lipid antigen–specific T cells more closely resemble conventional MHC-restricted T cells that are involved in adaptive immune responses against infection. However, secondary group 1 CD1–restricted responses to lipid antigens were not significantly stronger than primary responses, as is reported for immunodominant peptide antigens. Nevertheless, studies of recall responses to subdominant antigens reveal that although the kinetics of the responses these antigens elicit are similar to those of dominant antigens, they do not elicit the same magnitude of T cell expansion (Busch et al., 1998; Flynn et al., 1998). In addition, CD4 memory T cells (Homann et al., 2001; Foulds et al., 2002) and memory T cells restricted to other MHC class Ib molecules (Kerksiek et al., 1999; Bouwer et al., 2001; Kerksiek et al., 2003) respond with recall kinetics more similar to what we have found for group 1 CD1–restricted T cells. Group 1 CD1–restricted Mtb lipid antigen–specific T cells therefore appear to have a recall response, suggesting that they function as part of the adaptive immune system and that the antigens they recognize might prove useful as immunizing antigens.
It is noteworthy that our studies of in vivo group 1 CD1–restricted T cell responses were performed in MHC class II−/− hCD1Tg mice, which have low numbers of CD4+ T cells. Although group 1 CD1–restricted lipid antigen–specific immune responses can be detected in WT and MHC class Ia–deficient hCD1Tg mice, most of these mice exhibit high background responses to BMDC stimulation, possibly because of the presence of residual protein antigens in the Mtb lipid preparations. It is likely that the precursor frequency of group 1 CD1–restricted T cells is relatively low in hCD1Tg mice, and can only noticeably expand in the absence of competition with MHC class II–restricted T cells. Indeed, the removal of mouse MHC-restricted subsets has enhanced the visibility of human HLA-restricted T cells in HLA-transgenic models (Man et al., 1995). In addition, the fact that group 1 CD1–restricted T cell responses were consistently observed in both infected and immunized MHC class II−/− mice indicates that these T cells could serve as vaccine targets even in the absence of optimal CD4+ T cell numbers. This is particularly relevant when considering vaccines for AIDS patients, who have low CD4+ T cell counts and are at increased risk to Mtb infection.
As infection with Mtb induces group 1 CD1–restricted T cell responses in hCD1Tg mice, we have begun studies to investigate whether these responses are protective against disease. We examined disease pathogenesis in both the spleen and lungs of Mtb-infected hCD1Tg mice in a WT background. In this preliminary study, we found no significant differences between hCD1Tg+ and Tg− littermate controls in immunopathology or bacterial burden at 4 wk after aerosol infection with Mtb (Fig. S7, left; and not depicted). At later time points after infection, hCD1Tg mice have modestly reduced bacterial burdens (Fig. S7, right), although this reduction is not significant because of the small sample size. However, the protective capacities of group 1 CD1–restricted T cells and lipid immunization against Mtb infection remains to be formally tested in our model. In addition to investigating protection against Mtb challenge, hCD1Tg mice could be used to determine the immunogenicity of individual mycobacterial lipid antigens, just as mice transgenic for human HLA have been used to identify immunodominant peptide antigens (Man et al., 1995; Cheuk et al., 2002).
This study provides direct in vivo evidence that group 1 CD1–restricted T cell responses are induced by mycobacterial infection and demonstrates that hCD1Tg mice provide a useful tool for the continued investigation of group 1 CD1–restricted immune response in the context of infectious immunity against Mtb. The hCD1Tg mouse model may also be used to explore whether group 1 CD1–restricted autoreactive T cells play a role in infection as well as whether group 1 CD1–restricted T cells contribute to protective immunity against other pathogens. A major barrier to MHC-specific antigen immunization has been the need to design antigens that bind to a wide range of the highly diverse allelic variants of MHC proteins. As CD1 proteins show extremely low polymorphism, they may produce more consistent responses among individual humans in the population. A basic question underlying development of lipid vaccines is whether the responses are transient, as has been observed in CD1d-restricted NKT cells, or whether they persist as memory responses. Our data indicate that the group 1 CD1 molecules are indeed appealing targets for the development of new Mtb subunit vaccines. Such vaccines, which could target multiple T cell subsets with different classes of antigens, are likely to be more effective at preventing disease.
MATERIALS AND METHODS
Generation of hCD1Tg mice.
The bacterial artificial chromosome clone RP11-101J8 was obtained from BACPAC Resources Center. Strain 129/sv–derived R-1 embryonic stem cells were electroporated with 25 µg PI-SCeI–digested RP11-101J8 and 1 µg KpnI-digested pGK-Neo plasmid. Embryonic stem cell clones were selected for G418 resistance and screened for RP11-101J8 insertion using primers for T7 (forward, 5′-CTGTATGGAACTAACACTCCAA-3′; reverse, 5′-AGTCTCTCTTAGCACCTGCCTGGA-3′), CD1A (forward, 5′-GCCGTACTTTGCTAACTGTGC-3′; reverse, 5′-TGCAGGCAAGATCCTTCAC-3′), CD1E (forward, 5′-TGTGGGTCAGTGAAAAATG-3′; reverse, 5′-AGCATGTGACCTGTGCAGAC-3′), and SP6 (forward, 5′-TCTAGATGCAGAGGAGAACTCA-3′; reverse, 5′-CAGACCTTAATGGGATGTATCA-3′). Chimeric mice were screened for CD1c expression on peripheral blood lymphocytes using flow cytometry. EcoRI-digested genomic DNA was probed for the conserved CD1 exon 4 region in a Southern blot, as described previously (Martin et al., 1986). The densities of bands corresponding to the group 1 CD1 genes in both hCD1Tg 64 and 78 mice were comparable to those of bands corresponding to endogenous CD1D (Fig. 1 B and not depicted), suggesting that the copy number of the group 1 CD1 transgenes is two or close to two for both lines. hCD1Tg founders were backcrossed onto a B6 background for 12 generations. hCD1Tg mice were also crossed onto B6 MHC class Ia (H2-KbDb)–deficient, MHC class II (I-Aβ)–deficient (Taconic), and hβ2mTg/mβ2m−/− (provided by A.V. Chervonsky, University of Chicago, Chicago, IL) backgrounds. Animal work was performed at the University of Chicago, Northwestern University, and Harvard Medical School, and was approved by their Institutional Animal Care and Use Committees.
Antibodies, flow cytometry, immunohistochemistry, and mRNA expression.
Allophycocyanin-conjugated antibodies specific for human CD11c (B-ly6), mouse CD11c (HL3), IFN-γ (XMG1.2), and TCRβ (H57-597); FITC-conjugated antibodies specific for B220 (RA3-6B2), CD8α (53-6.7), and Vβ2 (B20.6); PE-conjugated antibodies specific for Vα2 (B20.1), Vβ3 (KJ25), Vβ4 (KT4), and Vβ11 (RR3-15); and PerCP-conjugated anti-CD4 (RM4-5) were purchased from BD. Biotinylated antibodies specific for CD1a (CB-T6), CD1b (K5-1B8), and CD1c (M241) were purchased from Ancell. FITC-conjugated anti-mCD1d (5C6; Mandal et al., 1998) and anti–MHC class II (M5114) were generated in house. Secondary staining of biotinylated antibodies was performed with PE-conjugated streptavidin. Cells were incubated with 2.4G2 FcR blocking antibody for 15 min, and stained in HBSS containing 2% FBS (HyClone) for 30 min at 4°C. For intracellular staining, cells were fixed after surface staining in 2% paraformaldehyde for 5 min at room temperature. Cells were washed, permeabilized in PBS containing 1% bovine serum albumin, and 0.1% saponin for 10 min, and stained for intracellular cytokine for 30 min in PBS/bovine serum albumin/saponin. Flow cytometric analysis was performed using a FACSCanto (BD) with FlowJo software (Tree Star, Inc.). Tissue samples were mounted in optimal cutting temperature compound (Tissue-Tek), frozen in liquid nitrogen, and stored at −80°C. Immunohistochemistry using biotinylated antibodies against CD1a (O10) and CD1c (BDCA1) was performed on 5-µm-thick frozen tissue sections that were fixed in acetone for 10 min, air dried, and stained using a substrate kit for peroxidase (NovaRED; Vector Laboratories). Sections were counterstained with hematoxylin and eosin. mRNA expression was determined by PCR on cDNA from various cell types using primers for CD1B (forward, 5′-ATATCTCTTGGGCGTCCTC-3′; reverse, 5′-TCAGGCTTCACTTGTCTTTG-3′), CD1E (forward, 5′-CTGTTCCAGTTATACTTCCATAGTT-3′; reverse, 5′-CTCAGGAAATCTGACCCTTGA-3′), and GAPDH (forward, 5′-TGCATCCTGCACCACCAACT-3′; reverse, 5′-TGCCTGCTTCACCACCTTC-3′).
Primary cell preparations and DC generation.
Single-cell suspensions were prepared from whole tissues by mechanical disruption in HBSS/2% FBS, or as previously described (Goossens et al., 1990; Larregina et al., 1996). Lymphocytes were isolated from peripheral blood supplemented with 50 U/ml heparin (Sigma-Aldrich) using Ficoll-Paque PLUS (GE Healthcare). T cells were purified from splenic lymphocytes using a Pan T Cell Isolation Kit (Miltenyi Biotec). DCs were derived from mouse bone marrow progenitors using GM-CSF and IL-4 (PeproTech) as previously described (Chun et al., 2003). Splenic DCs with robust group 1 CD1 expression were generated by injecting hCD1Tg mice subcutaneously with 5 × 105 B16–GM-CSF cells (provided by A. Ma, University of California, San Francisco, San Francisco, CA; Mach et al., 2000). Mice were sacrificed after 12 d or when tumor size reached 15 × 15 mm. Human monocyte–derived DCs were generated from human PBMCs as previously described (Porcelli et al., 1992).
Group 1 CD1–restricted T cell lines and hybridoma.
The generation of the human T cell lines CD8-1 and LND5 has been previously described (Moody et al., 1997; Rosat et al., 1999; Moody et al., 2000). To generate the mouse T cell lines HN1–HN4, hCD1Tg mice were subcutaneously immunized with 100 µg of heat-killed Mtb H37Ra sonicate (BBL; BD) in incomplete Freund's adjuvant (Sigma-Aldrich). 1 wk later, mice were given intraperitoneal booster immunizations of 2 × 106 hCD1Tg BMDCs pulsed with Mtb sonicate. The T cell lines HJ1 and SP2 were generated from hCD1Tg mice immunized with 2 × 106 ganglioside GM1 (Avanti Polor Lipids, Inc.)–pulsed hCD1Tg BMDCs, followed by three additional intraperitoneal boosts every 2 wk. The remaining T cell lines were generated by immunizing hCD1Tg mice with 2 × 106 of mycolic acid–pulsed hCD1Tg BMDCs. Lymphocytes were cultured for the first week in RPMI-10 (Chun et al., 2003), and subsequently in supplemented Mishell-Dutton medium with 20 U/ml IL-2 (partially purified from EL4.IL2 cell supernatant). T cell lines were restimulated weekly with antigen-pulsed hCD1Tg BMDCs, and T cell clones were generated through limiting dilution cloning. The hybridoma HJ1.3 was generated through fusion of the HJ1 T cell clone with BWlyt2-4 cells at a 5:1 ratio, as previously described (White et al., 2000).
T cell line functional assays.
IFN-γ production was measured by sandwich ELISA using either a Human IFN-γ ELISA Kit (eBioscience) or mouse IFN-γ antibodies from BD, alkaline phosphatase–conjugated streptavidin from Jackson ImmunoResearch Laboratories, and phosphatase substrate from Sigma-Aldrich. To measure proliferation, [3H]thymidine was added to T cell lines after 24 h of culture with 105 BMDC stimulators/well, and [3H]thymidine incorporation was measured after an additional 24 h. Cytotoxic killing was determined by measuring 51Cr release from 51Cr-labeled C1R, C1R-CD1 transfectants (provided by S. Balk, Beth Israel Deaconess Medical Center, Boston, MA), or 104 BMDCs/well that had been incubated with T cell lines for 4 h. The percentage of specific lysis was calculated as ([experimental release − spontaneous release]/[maximum release − spontaneous release]) × 100.
Antigen pulsing and immunization.
Mycobacterial lipid antigens were dried under a stream of nitrogen gas and suspended in RPMI-10 by sonication (model 2510; Bransonic). DCs were pulsed with antigen overnight at the following concentrations: 100 ng/ml αGalCer (provided by Kirin Brewery, Gunma, Japan), 10 µg/ml Mtb H37Rv glucose monomycolate, 25 µg/ml Mtb H37Ra sonicate, 20 µg/ml Mtb H37Rv total lipid mixture (chloroform/methanol extract), and 20 µg/ml Mtb mycolic acid (Sigma-Aldrich). For kinetic studies, CD11c+ DCs were purified from BMDCs or from B16/GMCSF-induced splenic lymphocytes using CD11c MicroBeads (Miltenyi Biotec) and pulsed with lipid antigen. Mice were immunized both subcutaneously in the footpad and intraperitoneally with 2 × 106 antigen-pulsed DCs.
ELISPOT.
Multiscreen-IP plates (Millipore) were coated with 10 µg/ml anti–IFN-γ, washed, and blocked with RPMI-10. 105−106 lymphocytes from immunized or infected mice and 105 BMDC stimulator cells were added to each well. Purified monoclonal antibodies specific for CD1a (OKT6), CD1b (BCD1b3), and CD1c (F10/21A3.1) were used at 10 µg/ml in blocking experiments. Plates were incubated at 37°C for 20 h and developed using an Alkaline Phosphatase Conjugate Substrate Kit according to the manufacturer's instructions (Bio-Rad Laboratories). Plates containing cells from infected tissues were given an additional incubation in 1% sodium azide. IFN-γ–producing cells were quantitated using an ImmunoSpot reader (Cellular Technology Ltd.).
Mycobacterial infection.
For intravenous infections, frozen aliquots of M. bovis BCG Pasteur or M. tuberculosis H37Rv at mid-log growth culture and supplemented with glycerol (6% vol/vol) were thawed and briefly sonicated. Bacteria were diluted in PBS–0.05% Tween 80 and passed three times through a 28-gauge needle. Mice were infected with 105 BCG or Mtb by tail vein injection. For aerosol infections, frozen aliquots of M. tuberculosis H37Rv were thawed and diluted in 0.9% saline with 0.1% Tween 80. Mtb was added to a Lovelace nebulizer attached to a nose-only aerosol exposure chamber (In-Tox Products) such that each animal received 150 CFU. Bacterial titers and tissue burden were determined by plating serial dilutions on 7H11 agar plates (BBL; BD).
Statistical analysis.
Statistical analyses were performed using Prism software (GraphPad Software, Inc.). Significant differences at 95% confidence are depicted with an asterisk on each graph.
Online supplemental material.
Fig. S1 provides additional information on the expression of CD1b and CD1c protein and CD1E mRNA in hCD1Tg mice, and compares group 1 CD1 expression levels between hCD1Tg BMDCs and human DCs. Fig. S2 shows group 1 CD1–restricted Mtb lipid antigen–specific responses in Mtb lipid antigen–immunized hCD1Tg mice in a WT background. Fig. S3 shows that group 1 CD1 expression on BMDCs is not altered by Mtb lipids. Fig. S4 shows the expression of group 1 CD1 during the course of Mtb infection. Fig. S5 shows group 1 CD1–restricted Mtb lipid antigen–specific responses in BCG-infected hCD1Tg mice in a WT background. Fig. S6 shows that CD1 expression and function in hCD1Tg mice is similar to that in hCD1Tg/hβ2mTg/mβ2m−/− mice. Fig. S7 shows the bacterial burden at 4 and 8 wk after Mtb infection in hCD1Tg 64 and Tg− littermate control mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090898/DC1.
Acknowledgments
We thank Dr. S. Balk for providing us with CD1a-, CD1b-, and CD1c-expressing C1R transfectants; Dr. A.V. Chervonsky for providing hβ2mTg/mβ2m−/− mice; Dr. A. Ma for providing B16–GM-CSF melanoma; Dr. T.-Y. Cheng for purifying glucose monomycolate; and Kirin Brewery for providing α-GalCer. The Mtb H37Rv total lipid mixture was provided by Colorado State University as part of National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) contract no. HHSN266200400091C, entitled “Tuberculosis Vaccine Testing and Research Materials.” We also acknowledge Dr. M.-Y. Zhou for his assistance with embryonic stem cell injections, and Dr. G. Raghuraman, T. King, J. Rojas, A. Rohr, H. Shim, and C. Yang for other technical assistance.
This work is supported by a Burroughs Wellcome Foundation Translational Research Award (to D.B. Moody) and by NIH R01 grants (AI049313 to D.B. Moody, AI028973 to M.B. Brenner, and AI057460 and AI040310 to C.-R. Wang).
The authors have no competing financial interests.
Footnotes
Abbreviations used:
- α-GalCer
- α-galactosylceramide
- β2m
- β2 microglobulin
- BCG
- Mycobacterium bovis bacillus Calmette-Guérin
- BMDC
- bone marrow–derived DC
- hCD1Tg
- human group 1 CD1 transgenic
- iNKT
- invariant NKT
- Mtb
- M. tuberculosis
References
- Beckman E.M., Porcelli S.A., Morita C.T., Behar S.M., Furlong S.T., Brenner M.B. 1994. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 372:691–694 10.1038/372691a0 [DOI] [PubMed] [Google Scholar]
- Bendelac A., Savage P.B., Teyton L. 2007. The biology of NKT cells. Annu. Rev. Immunol. 25:297–336 10.1146/annurev.immunol.25.022106.141711 [DOI] [PubMed] [Google Scholar]
- Bouwer H.G., Barry R.A., Hinrichs D.J. 2001. Lack of expansion of major histocompatibility complex class Ib-restricted effector cells following recovery from secondary infection with the intracellular pathogen Listeria monocytogenes. Infect. Immun. 69:2286–2292 10.1128/IAI.69.4.2286-2292.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigl M., Brenner M.B. 2004. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22:817–890 10.1146/annurev.immunol.22.012703.104608 [DOI] [PubMed] [Google Scholar]
- Busch D.H., Pilip I.M., Vijh S., Pamer E.G. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 8:353–362 10.1016/S1074-7613(00)80540-3 [DOI] [PubMed] [Google Scholar]
- Cheuk E., D'Souza C., Hu N., Liu Y., Lang H., Chamberlain J.W. 2002. Human MHC class I transgenic mice deficient for H2 class I expression facilitate identification and characterization of new HLA class I-restricted viral T cell epitopes. J. Immunol. 169:5571–5580 [DOI] [PubMed] [Google Scholar]
- Chiba A., Dascher C.C., Besra G.S., Brenner M.B. 2008. Rapid NKT cell responses are self-terminating during the course of microbial infection. J. Immunol. 181:2292–2302 [DOI] [PubMed] [Google Scholar]
- Chun T., Page M.J., Gapin L., Matsuda J.L., Xu H., Nguyen H., Kang H.S., Stanic A.K., Joyce S., Koltun W.A., et al. 2003. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J. Exp. Med. 197:907–918 10.1084/jem.20021366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz G.A., Brewer T.F., Berkey C.S., Wilson M.E., Burdick E., Fineberg H.V., Mosteller F. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 271:698–702 10.1001/jama.271.9.698 [DOI] [PubMed] [Google Scholar]
- Colmone A., Li S., Wang C.R. 2006. Activating transcription factor/cAMP response element binding protein family member regulated transcription of CD1A. J. Immunol. 177:7024–7032 [DOI] [PubMed] [Google Scholar]
- Dascher C.C. 2007. Evolutionary biology of CD1. Curr. Top. Microbiol. Immunol. 314:3–26 10.1007/978-3-540-69511-0_1 [DOI] [PubMed] [Google Scholar]
- Dascher C.C., Brenner M.B. 2003. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. 24:412–418 10.1016/S1471-4906(03)00179-0 [DOI] [PubMed] [Google Scholar]
- Dascher C.C., Hiromatsu K., Naylor J.W., Brauer P.P., Brown K.A., Storey J.R., Behar S.M., Kawasaki E.S., Porcelli S.A., Brenner M.B., LeClair K.P. 1999. Conservation of a CD1 multigene family in the guinea pig. J. Immunol. 163:5478–5488 [PubMed] [Google Scholar]
- Dascher C.C., Hiromatsu K., Xiong X., Morehouse C., Watts G., Liu G., McMurray D.N., LeClair K.P., Porcelli S.A., Brenner M.B. 2003. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int. Immunol. 15:915–925 10.1093/intimm/dxg091 [DOI] [PubMed] [Google Scholar]
- de la Salle H., Mariotti S., Angenieux C., Gilleron M., Garcia-Alles L.F., Malm D., Berg T., Paoletti S., Maître B., Mourey L., et al. 2005. Assistance of microbial glycolipid antigen processing by CD1e. Science. 310:1321–1324 10.1126/science.1115301 [DOI] [PubMed] [Google Scholar]
- Dye C. 2009. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat. Rev. Microbiol. 7:81–87 10.1038/nrmicro2048 [DOI] [PubMed] [Google Scholar]
- Flynn K.J., Belz G.T., Altman J.D., Ahmed R., Woodland D.L., Doherty P.C. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 8:683–691 10.1016/S1074-7613(00)80573-7 [DOI] [PubMed] [Google Scholar]
- Foulds K.E., Zenewicz L.A., Shedlock D.J., Jiang J., Troy A.E., Shen H. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 168:1528–1532 [DOI] [PubMed] [Google Scholar]
- Gagliardi M.C., Lemassu A., Teloni R., Mariotti S., Sargentini V., Pardini M., Daffé M., Nisini R. 2007. Cell wall-associated alpha-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the function of dendritic cell derived from infected monocyte. Cell. Microbiol. 9:2081–2092 10.1111/j.1462-5822.2007.00940.x [DOI] [PubMed] [Google Scholar]
- Gilleron M., Stenger S., Mazorra Z., Wittke F., Mariotti S., Böhmer G., Prandi J., Mori L., Puzo G., De Libero G. 2004. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 199:649–659 10.1084/jem.20031097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani A., Prete S.P., Graziani G., Aquino A., Balduzzi A., Sugita M., Brenner M.B., Iona E., Fattorini L., Orefici G., et al. 2001. Influence of Mycobacterium bovis bacillus Calmette Guérin on in vitro induction of CD1 molecules in human adherent mononuclear cells. Infect. Immun. 69:7461–7470 10.1128/IAI.69.12.7461-7470.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens P.L., Jouin H., Marchal G., Milon G. 1990. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J. Immunol. Methods. 132:137–144 10.1016/0022-1759(90)90407-M [DOI] [PubMed] [Google Scholar]
- Gupta U.D., Katoch V.M., McMurray D.N. 2007. Current status of TB vaccines. Vaccine. 25:3742–3751 10.1016/j.vaccine.2007.01.112 [DOI] [PubMed] [Google Scholar]
- Hava D.L., van der Wel N., Cohen N., Dascher C.C., Houben D., León L., Agarwal S., Sugita M., van Zon M., Kent S.C., et al. 2008. Evasion of peptide, but not lipid antigen presentation, through pathogen-induced dendritic cell maturation. Proc. Natl. Acad. Sci. USA. 105:11281–11286 10.1073/pnas.0804681105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromatsu K., Dascher C.C., LeClair K.P., Sugita M., Furlong S.T., Brenner M.B., Porcelli S.A. 2002. Induction of CD1-restricted immune responses in guinea pigs by immunization with mycobacterial lipid antigens. J. Immunol. 169:330–339 [DOI] [PubMed] [Google Scholar]
- Homann D., Teyton L., Oldstone M.B. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913–919 10.1038/90950 [DOI] [PubMed] [Google Scholar]
- Kaufmann S.H., McMichael A.J. 2005. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat. Med. 11:S33–S44 10.1038/nm1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T., Norose Y., Watanabe Y., Enomoto Y., Narazaki H., Watari E., Tanaka S., Takahashi H., Yano I., Brenner M.B., Sugita M. 2003. Cutting edge: major CD8 T cell response to live bacillus Calmette-Guérin is mediated by CD1 molecules. J. Immunol. 170:5345–5348 [DOI] [PubMed] [Google Scholar]
- Kerksiek K.M., Busch D.H., Pilip I.M., Allen S.E., Pamer E.G. 1999. H2-M3–restricted T cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 190:195–204 10.1084/jem.190.2.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerksiek K.M., Ploss A., Leiner I., Busch D.H., Pamer E.G. 2003. H2-M3-restricted memory T cells: persistence and activation without expansion. J. Immunol. 170:1862–1869 [DOI] [PubMed] [Google Scholar]
- Larregina A., Morelli A., Kolkowski E., Fainboim L. 1996. Flow cytometric analysis of cytokine receptors on human Langerhans' cells. Changes observed after short-term culture. Immunology. 87:317–325 10.1046/j.1365-2567.1996.451513.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layre E., Collmann A., Bastian M., Mariotti S., Czaplicki J., Prandi J., Mori L., Stenger S., De Libero G., Puzo G., Gilleron M. 2009. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem. Biol. 16:82–92 10.1016/j.chembiol.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Mach N., Gillessen S., Wilson S.B., Sheehan C., Mihm M., Dranoff G. 2000. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60:3239–3246 [PubMed] [Google Scholar]
- Man S., Newberg M.H., Crotzer V.L., Luckey C.J., Williams N.S., Chen Y., Huczko E.L., Ridge J.P., Engelhard V.H. 1995. Definition of a human T cell epitope from influenza A non-structural protein 1 using HLA-A2.1 transgenic mice. Int. Immunol. 7:597–605 10.1093/intimm/7.4.597 [DOI] [PubMed] [Google Scholar]
- Mandal M., Chen X.R., Alegre M.L., Chiu N.M., Chen Y.H., Castaño A.R., Wang C.R. 1998. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol. Immunol. 35:525–536 10.1016/S0161-5890(98)00055-8 [DOI] [PubMed] [Google Scholar]
- Martin L.H., Calabi F., Milstein C. 1986. Isolation of CD1 genes: a family of major histocompatibility complex-related differentiation antigens. Proc. Natl. Acad. Sci. USA. 83:9154–9158 10.1073/pnas.83.23.9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J., Debord K.L., Ismail N., Goff R.D., Cantu C., III, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 434:525–529 10.1038/nature03408 [DOI] [PubMed] [Google Scholar]
- Moody D.B., Reinhold B.B., Guy M.R., Beckman E.M., Frederique D.E., Furlong S.T., Ye S., Reinhold V.N., Sieling P.A., Modlin R.L., et al. 1997. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 278:283–286 10.1126/science.278.5336.283 [DOI] [PubMed] [Google Scholar]
- Moody D.B., Ulrichs T., Mühlecker W., Young D.C., Gurcha S.S., Grant E., Rosat J.P., Brenner M.B., Costello C.E., Besra G.S., Porcelli S.A. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 404:884–888 10.1038/35009119 [DOI] [PubMed] [Google Scholar]
- Moody D.B., Young D.C., Cheng T.Y., Rosat J.P., Roura-Mir C., O'Connor P.B., Zajonc D.M., Walz A., Miller M.J., Levery S.B., et al. 2004. T cell activation by lipopeptide antigens. Science. 303:527–531 10.1126/science.1089353 [DOI] [PubMed] [Google Scholar]
- Moody D.B., Zajonc D.M., Wilson I.A. 2005. Anatomy of CD1-lipid antigen complexes. Nat. Rev. Immunol. 5:387–399 10.1038/nri1605 [DOI] [PubMed] [Google Scholar]
- North R.J., Jung Y.J. 2004. Immunity to tuberculosis. Annu. Rev. Immunol. 22:599–623 10.1146/annurev.immunol.22.012703.104635 [DOI] [PubMed] [Google Scholar]
- Parekh V.V., Wilson M.T., Olivares-Villagómez D., Singh A.K., Wu L., Wang C.R., Joyce S., Van Kaer L. 2005. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 115:2572–2583 10.1172/JCI24762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S., Morita C.T., Brenner M.B. 1992. CD1b restricts the response of human CD4−8− T lymphocytes to a microbial antigen. Nature. 360:593–597 10.1038/360593a0 [DOI] [PubMed] [Google Scholar]
- Prete S.P., Giuliani A., D'Atri S., Graziani G., Balduzzi A., Oggioni M.R., Iona E., Girolomoni G., Bonmassar L., Romani L., Franzese O. 2007. BCG-infected adherent mononuclear cells release cytokines that regulate group 1 CD1 molecule expression. Int. Immunopharmacol. 7:321–332 10.1016/j.intimp.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Rosat J.P., Grant E.P., Beckman E.M., Dascher C.C., Sieling P.A., Frederique D., Modlin R.L., Porcelli S.A., Furlong S.T., Brenner M.B. 1999. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J. Immunol. 162:366–371 [PubMed] [Google Scholar]
- Roura-Mir C., Wang L., Cheng T.Y., Matsunaga I., Dascher C.C., Peng S.L., Fenton M.J., Kirschning C., Moody D.B. 2005. Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR-2. J. Immunol. 175:1758–1766 [DOI] [PubMed] [Google Scholar]
- Sable S.B., Kalra M., Verma I., Khuller G.K. 2007. Tuberculosis subunit vaccine design: the conflict of antigenicity and immunogenicity. Clin. Immunol. 122:239–251 10.1016/j.clim.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Shamshiev A., Donda A., Carena I., Mori L., Kappos L., De Libero G. 1999. Self glycolipids as T-cell autoantigens. Eur. J. Immunol. 29:1667–1675 [DOI] [PubMed] [Google Scholar]
- Sieling P.A., Chatterjee D., Porcelli S.A., Prigozy T.I., Mazzaccaro R.J., Soriano T., Bloom B.R., Brenner M.B., Kronenberg M., Brennan P.J., et al. 1995. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 269:227–230 10.1126/science.7542404 [DOI] [PubMed] [Google Scholar]
- Sieling P.A., Jullien D., Dahlem M., Tedder T.F., Rea T.H., Modlin R.L., Porcelli S.A. 1999. CD1 expression by dendritic cells in human leprosy lesions: correlation with effective host immunity. J. Immunol. 162:1851–1858 [PubMed] [Google Scholar]
- Sieling P.A., Ochoa M.T., Jullien D., Leslie D.S., Sabet S., Rosat J.P., Burdick A.E., Rea T.H., Brenner M.B., Porcelli S.A., Modlin R.L. 2000. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions. J. Immunol. 164:4790–4796 [DOI] [PubMed] [Google Scholar]
- Sousa A.O., Mazzaccaro R.J., Russell R.G., Lee F.K., Turner O.C., Hong S., Van Kaer L., Bloom B.R. 2000. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA. 97:4204–4208 10.1073/pnas.97.8.4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger S., Niazi K.R., Modlin R.L. 1998. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J. Immunol. 161:3582–3588 [PubMed] [Google Scholar]
- Tupin E., Kinjo Y., Kronenberg M. 2007. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5:405–417 10.1038/nrmicro1657 [DOI] [PubMed] [Google Scholar]
- Uldrich A.P., Crowe N.Y., Kyparissoudis K., Pellicci D.G., Zhan Y., Lew A.M., Bouillet P., Strasser A., Smyth M.J., Godfrey D.I. 2005. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 175:3092–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichs T., Moody D.B., Grant E., Kaufmann S.H., Porcelli S.A. 2003. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect. Immun. 71:3076–3087 10.1128/IAI.71.6.3076-3087.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.S., Xiong X., Grant E.P., Peng W., Brenner M.B. 2005. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J. Immunol. 175:6344–6351 [DOI] [PubMed] [Google Scholar]
- White J., Kappler J., Marrack P. 2000. Production and characterization of T cell hybridomas. Methods Mol. Biol. 134:185–193 [DOI] [PubMed] [Google Scholar]
- Winslow G.M., Cooper A., Reiley W., Chatterjee M., Woodland D.L. 2008. Early T-cell responses in tuberculosis immunity. Immunol. Rev. 225:284–299 10.1111/j.1600-065X.2008.00693.x [DOI] [PMC free article] [PubMed] [Google Scholar]