Abstract
During infection, signals from the periphery are known to reach draining lymph nodes (DLNs), but how these molecules, such as inflammatory cytokines, traverse the significant distances involved without dilution or degradation remains unclear. We show that peripheral mast cells, upon activation, release stable submicrometer heparin-based particles containing tumor necrosis factor and other proteins. These complexes enter lymphatic vessels and rapidly traffic to the DLNs. This physiological drug delivery system facilitates communication between peripheral sites of inflammation and remote secondary lymphoid tissues.
The draining LNs (DLNs) are dynamic lymphoid structures that coordinate the development of specific immune responses after microbial or vaccine challenge. In response to these peripheral events, the DLN quickly undergoes significant structural changes, including rapid growth and vascular remodeling (McLachlan et al., 2003; Soderberg et al., 2005). This enlargement, which is largely attributable to enhanced recruitment and retention of naive lymphocytes from the circulation, increases the probability that rare lymphocytes bearing relevant specificities will be present to interact with activated tissue-derived APCs, which must migrate from inflamed tissues via afferent lymphatic vessels. This interaction between lymphocytes and APCs occurring within DLNs is the critical initiating event in the development of the adaptive immune response.
For these rapid and impressive changes in DLNs to occur after microbial or vaccine challenge, not only should lymphocytes and APCs be able to reach the DLN but, importantly, LNs must receive signals from the periphery to create the appropriate environment to facilitate this interaction. It has been suggested that the periphery signals the DLN by “remote control” (Baekkevold et al., 2001; Palframan et al., 2001; Huang et al., 2008). For example, a chemokine, monocyte chemoattractant protein-1, has been suggested to drain via afferent lymphatics into the DLN (Palframan et al., 2001). A potential peripheral source of immunomodulatory mediators is the mast cell (MC), a cell type which is strategically situated beneath epithelial barriers. In addition to their presence at potential sites of pathogen entry, MCs express numerous pattern recognition and other receptors for rapidly recognizing pathogens (Prodeus et al., 1997; Malaviya et al., 1999; Supajatura et al., 2001), as well as the capacity to release large amounts of inflammatory mediators, many of which are concentrated within prominent cytoplasmic granules and are available for immediate release. One such prestored mediator is TNF (Gordon and Galli, 1990), a multifunctional cytokine which is implicated in modulating immune cell trafficking. Recent studies have shown that as rapidly as 1 h after peripheral MC activation, increased levels of TNF are detected in prenodal lymph (Frangogiannis et al., 1998) and in the DLNs (McLachlan et al., 2003). The lack of any corresponding change in DLN TNF message suggested that it was not newly synthesized locally but was derived from peripheral sources (McLachlan et al., 2003). Because a significant period of time is required for the secretion of de novo cytokine after stimulation, this peripheral source appears to be preformed MC TNF, a conclusion supported by the finding that most MCs in the vicinity of infection were degranulated (McLachlan et al., 2003). One might presume that TNF, secreted by peripheral MCs into the extracellular fluid, enters lymphatic vessels and then is carried to DLNs as a soluble molecule in the lymph. However, considering the route of trafficking and the significant distances involved, it is unclear how peripherally derived TNF could reach the DLNs without degradation, dilution to ineffective concentrations, or interaction with extracellular matrix components. This scenario is especially difficult to rationalize early in the response when it is expected that only small amounts of TNF are elaborated, and because TNF has a very short half-life in vivo (Rampart et al., 1989). This uncertainty is supported by the observation that although peripherally injected chemokines can be detected in DLNs, it typically requires the application of large quantities of recombinant protein (Stein et al., 2000; Baekkevold et al., 2001).
One conceivable explanation for the long-distance action of MC-derived TNF is its trafficking in a form resistant to dilution and degradation. To investigate this possibility, we examined the process of MC exocytosis in detail. When MCs undergo secretory exocytosis of granules (degranulation), in addition to releasing some prestored mediators as freely soluble molecules (e.g., histamine and β-hexosaminidase), they also release distinct insoluble granular particles, comprised primarily of heparin proteoglycans and positively charged proteases (Schwartz et al., 1981). Other than isolated studies describing their uptake by phagocytic cells (Welsh and Geer, 1959; Miyata and Takaya, 1985), the fate of these particles after MC degranulation is largely unknown. MC granule particles are insoluble because of the large numbers of electrostatic interactions between the highly cationic MC-specific proteases and highly anionic heparin proteoglycans (Schwartz et al., 1981). Because heparin is also known to bind strongly to a variety of extracellular signaling proteins (Roberts et al., 1988; Webb et al., 1993), it is probable that some MC-derived mediators are borne within the particle structure even after their release. This possibility led us to hypothesize that MC particles might function as extracellular chaperones for MC mediators (e.g., TNF) in vivo, carrying these signaling molecules from the periphery to the DLN.
RESULTS
Activated MCs release stable particles containing inflammatory mediators
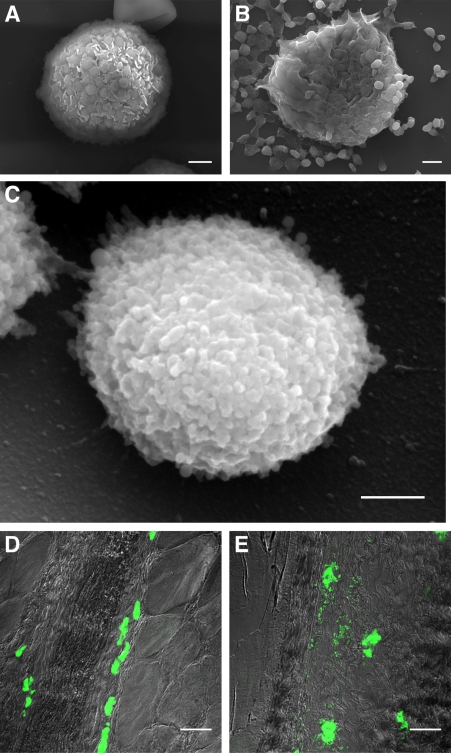
First, we investigated whether MCs released stable particles upon activation. Fig. 1 (A and B) shows scanning electron micrographs (SEMs) of isolated rat peritoneal MCs before and after 15 min of exposure to compound 48/80, a potent small molecule MC activator. After MC activation, a large number of extracellular spherical particles were dispersed around each MC (Fig. 1 B), which had a mean diameter of 917 nm. Examination at higher magnification revealed that each of these particles was comprised of many smaller, mostly spherical, subunits with a mean diameter of 59.4 nm (Fig. 1 C). Video microscopy of these events revealed the dynamics of particle release by MCs (Video 1). Interestingly, particles were released relatively gradually and continued to be shed for at least a minute after treatment.
Figure 1.
MCs release insoluble heparin-containing particles. (A) SEM of an unstimulated rat peritoneal MC (rPMC). Bar, 2 µm. (B) SEM of rPMC 15 min after treatment with compound 48/80 at 5 µg/ml. Bar, 2 µm. (C) SEM of a single extracellular granule. Bar, 500 nm. Release of particles by rat peritoneal MCs was observed repeatedly (in greater than three independent experiments). SEMs are shown from one representative experiment. For some trials (A–C), lavage was pooled from multiple rats to obtain sufficient material for analysis. (D) Confocal micrograph of MCs in mouse footpad (granule heparin labeled with Alexa Fluor 488–conjugated avidin) 30 min after PBS injection showing undegranulated MCs. Bar, 30 µm. (E) Mouse footpad MCs surrounded by extracellular particles 30 min after injection of 5 µg of compound 48/80. Bar, 30 µm. Release of particles in vivo was observed in greater than three independent experiments using groups of two to three mice each. Experiments are representative of data derived from greater than three animals.
To demonstrate the release of particles by MCs in vivo, we injected the footpads of mice with compound 48/80 and, 30 min later, thin tissue sections were prepared and examined for MC granule release. The granules were detected with Alexa Fluor 488–conjugated avidin, a probe which selectively binds heparin (Tharp et al., 1985), a major constituent of MC granules (Fig. 1, D and E). Also, when MCs were activated in vivo with another more physiological stimulus, bacterial peptidoglycan, identical particles were released into the surrounding tissue (unpublished data). This is consistent with the work of others showing that the morphology of degranulation is not highly dependent on the stimulus (Bloom and Chakravarty, 1970; Tizard and Holmes, 1974). Significant MC degranulation was observed in the footpad sections and extracellular particles moved significant distances from their parent cells, especially in areas of less dense connective tissue. Observations revealed that 30 min after MC degranulation in vivo, morphologically distinct particles containing heparin could be detectable in the immediate vicinity as well as at considerable distances (up to 150 µm in this section) from the site of release. Some of these extracellular particles were still detectable up to one hour after treatment (unpublished data).
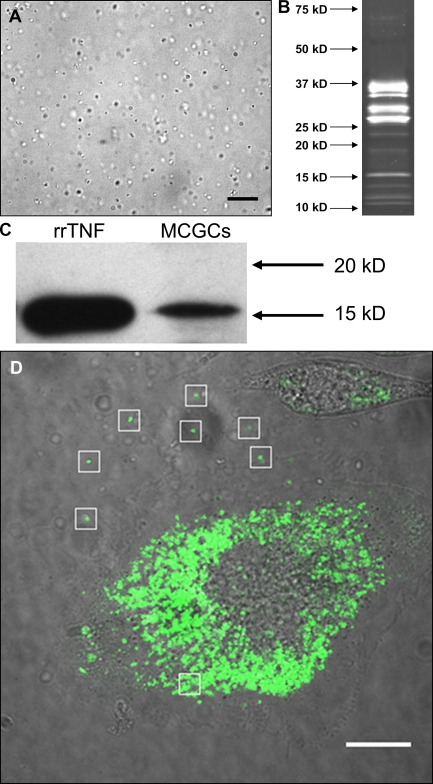
Next, we attempted to determine whether relevant signaling molecules were present in the particles released by MCs. Recent evidence shows that TNF, like many other immune molecules, binds to heparin (Kenig et al., 2008), suggesting that it may be retained in the heparin-based particles after exocytosis. To investigate this, we purified MC granule particles from rat peritoneal lavage cells using a modification of a previously described technique (Lindstedt et al., 1992). In brief, the cells were treated with compound 48/80 and the released particles were isolated by differential centrifugation. Fig. 2 A shows a micrograph of a suspension of isolated MC-derived particles. The purified particles were very stable after isolation, not changing appreciably in appearance or protein composition even after 2 mo at room temperature. To determine their bulk protein composition, we subjected them to SDS-PAGE followed by SYPRO Ruby staining (Fig. 2 B). The major bands between 25 and 37 kD correspond to the major rat MC proteases, as determined by a proteomic analysis (unpublished data), which are known to be the most abundant protein constituents of MC granules (Lagunoff and Pritzl, 1976). We also used these preparations to address whether signaling molecules, such as TNF, are present in the particles as minor components. Because TNF was shown in earlier studies to be relevant to morphological changes in the DLNs (McLachlan et al., 2003), we were especially interested in the presence of this signaling molecule. An anti-TNF immunoblot of purified granule remnant proteins revealed a band that comigrated with recombinant rat TNF (Fig. 2 C). The identity of this band was confirmed with two additional different anti-TNF antibodies (unpublished data). To further demonstrate the presence of TNF within released MC-derived particles, we expressed a TNF-GFP fusion protein in the rat MC line RBL-2H3 and in mouse BM-derived MCs (BMMCs) to follow the extracellular fate of preformed TNF after degranulation. In both cases, the fusion protein was clearly localized to vesicles, and it colocalized with known granule markers (Fig. S1). After activation, both cell types released free particles that retained their fluorescence in the extracellular environment (Fig. 2 D and Videos 2 and 3). Although the particles released by the cells have not been biochemically characterized, their origin in the granular compartment suggests that they are similar in nature to those released by peritoneal MCs. These findings strongly suggest that TNF released from activated MCs is not freely soluble but remains associated with the granular heparin matrix after exocytosis.
Figure 2.
MC-derived particles contain preformed TNF. (A) Differential interference contrast micrograph of purified MC granule particles. (B) SYPRO Ruby–stained SDS-PAGE gel of MC particle-associated proteins. (C) TNF immunoblot of SDS-PAGE–separated proteins from purified particles showing detection of rat TNF. (D) A still image from Video 2 showing that particles released from RBL-2H3 cells after activation retain overexpressed TNF-GFP fusion protein. Bars, 15 µm. White boxes highlight released particles with retained fluorescence. All panels depict data representative of experiments performed more than three times, with trials using lavage from individual rats or pooled from multiple rats, for a total n > 3.
MC-derived particles drain to local LNs via lymphatics
In order for MC-derived particles to reach the DLN, they must be able to enter lymphatic vessels. Unlike the vascular endothelium, the endothelium of lymphatic capillaries is highly permeable as a result of the presence of discontinuities between individual endothelial cells. These gaps can be larger than a micrometer in diameter and should readily admit objects the size of MC-derived particles (Leak, 1980; Trzewik et al., 2001). Indeed, during edema (which occurs almost instantaneously after MC degranulation because of the rapid action of the completely soluble mediator histamine on vascular permeability), these openings are enlarged as the relative quantity of bulk tissue flow entering the lymphatic system is greatly increased (Casley-Smith, 1980). Thus, it is likely that conditions in the tissue after MC activation favor the entry of these particles into lymphatic vessels.
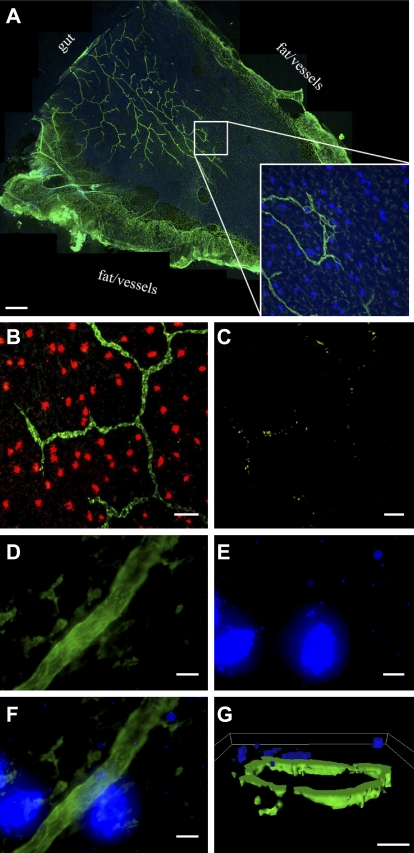
To visualize this process, we focused on the rat mesentery, because it was amenable to observation in whole mount, allowing the entire lymphatic network and connective tissue drainage to be visualized (Fig. 3 A). In mesentery isolated 30 min after the intraperitoneal instillation of compound 48/80, extensive MC degranulation occurred, and clear colocalization between extracellular MC granule particles and lymphatic capillaries was observed along the length of lymphatic vessels (Fig. 3, B and C). In contrast, there was little to no degranulation in untreated rat mesentery (Fig. S2). Close examination of lymphatic vessels in compound 48/80-treated rats, such as the area proximal to the two degranulated MCs shown in Fig. 3 (D and F), revealed that MC particles appear frequently in the center of the vessel surrounded entirely by staining for lymphatic markers. However, because the lymphatic vessels in these preparations are collapsed, it was not possible to definitively demonstrate the presence of the particles within them, although it is unlikely that there is much space outside the vessels given the extreme thinness of the tissue (15–20 µm; Barber et al., 1987). So, to corroborate these data with cross-sectioned patent lymphatic vessels, we returned to the mouse footpad. Dilated lymphatics were frequently seen in this tissue 30 min after the injection of compound 48/80. We clearly observed MC-derived particles on the luminal side of the endothelium (Fig. 3 G; Videos 4 and 5 show 3D reconstructions of this area imaged with laser-scanning confocal microscopy). DIC microscopy of the same area revealed that the particle shown in Fig. 3 G was not inside a migrating phagocyte but was apparently moving as a free particle (Fig. S3). Together, these images suggest that MC-derived particles can gain access to and traffic within the lymphatic system.
Figure 3.
Extracellular MC granule particles enter lymphatic capillaries. (A) Mosaic of 40× confocal micrographs showing association of MCs and lymphatic capillaries in whole mount rat mesentery preparation. Inset, 5× magnified view of (A). Green, LYVE-1; blue, MC heparin. Bar, 1 mm. (B) Fluorescence micrograph showing colocalization between released MC-derived particles and lymphatic capillaries 30 min after intraperitoneal injection of 500 µg of compound 48/80. Green, LYVE-1; red, MC heparin. Bar, 25 µm. (C) Same area as in B, but only areas of colocalization are shown. (D–F) Confocal micrographs of MC granule particles inside a lymphatic capillary in rat mesentery whole mount 30 min after intraperitoneal injection of 500 µg of compound 48/80. D, anti-LYVE-1; E, avidin; F, overlay. Green, LYVE-1, blue, MC heparin. Bars, 20 µm. (G) Isosurface rendering of confocal volume from mouse footpad section 30 min after injection of compound 48/80. Green, LYVE-1; blue, MC heparin. Bar, 8 µm. All images are representative of experiments repeated independently at least twice. Three rats were used for intraperitoneal compound 48/80 studies, and several groups of two to three mice were used for footpad compound 48/80 studies.
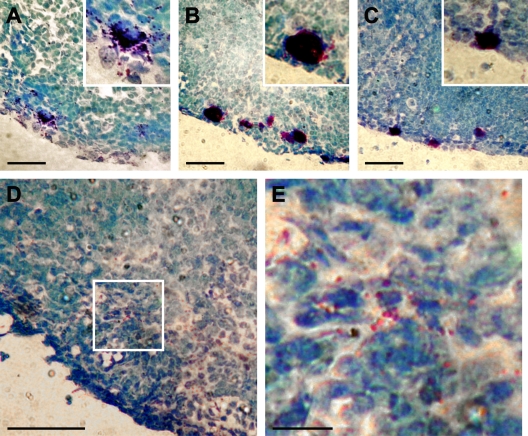
Next, we sought to demonstrate that the MC particles could reach DLNs from the periphery. Our initial attempts to detect MC particles trafficking from footpads to DLNs was confounded by the fact that the MC activator compound 48/80 that we had injected not only activated tissue resident MCs but had also drained into the DLNs, causing degranulation of LN-resident MCs (Fig. 4 A). Therefore, we selectively activated peripheral MCs in the skin by painting the footpads with a solution PMA in acetone (Wershil et al., 1988). In agreement with the literature, this treatment caused local degranulation. Footpad MCs were intact after painting with acetone alone. Next, we examined the DLNs to see if the observed MC degranulation was selective to the site of application. In contrast to DLNs harvested 2 h after administration of compound 48/80, where extensive degranulation of LN MCs occurred (Fig. 4 A), the LN-resident MCs of mice after PMA treatment (Fig. 4 C) appeared similar to vehicle alone (Fig. 4 B), without apparent degranulation. Having confirmed that the topical application of PMA selectively activated footpad MCs, we examined the DLNs more closely and observed numerous metachromatic (heparin containing) particles in the periphery of the same LN section shown in Fig. 4 C (Fig. 4 D). The subcapsular and cortical sinuses, also at the edge of the LN, are the first to receive peripheral lymph from afferent lymphatics. A higher magnification of a selected area of the DLN shows several individual particles (Fig. 4 E).
Figure 4.
Topical application of PMA activates only local MCs, allowing visualization of trafficked granules in the DLN. (A–C) Toludine blue–stained sections of DLN tissue 2 h after injection with 32 µg of compound 48/80 (A), topical application of vehicle alone (B), or topical application of 10 µg PMA (C). (D and E) Free metachromatic granules could be visualized within the DLNs of PMA-treated footpads. E is the magnified view of the white box in D. All panels show results that are representative of three independent experiments using groups of 2–3 mice each. Bars: (A–D) 50 µm; (E) 20 µm.
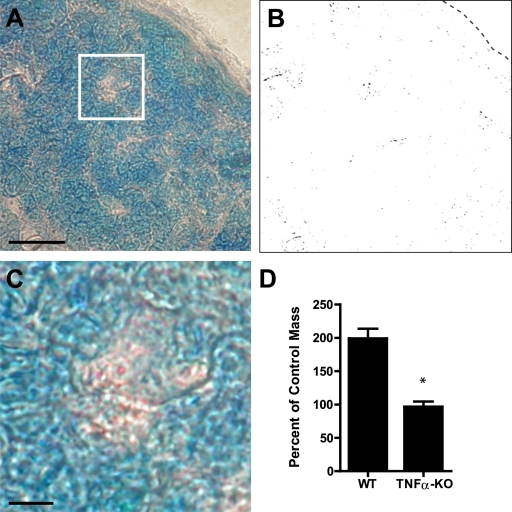
To support our conclusion that particles detected in the DLNs originated from the periphery and to determine with certainty that these particles could traffic from peripheral sites to the DLN, we isolated particles from the peritoneal MCs of mice. These particles were injected into the footpads of MC deficient KitW-sh/W-sh mice. DLNs were isolated 45 min later and cross sections were examined for the presence of granules after toluidine blue staining. At this early time point, it was extremely unlikely that the observed particles were delivered to the LN inside migrating cells, as the processes involved in migrating through tissues and across the lymphatic endothelium are complex and require changes in gene expression. Distinct particles appeared to be widely distributed in the cortical and medullary sinuses of the DLNs (Fig. 5, A and B). A close-up showing a cluster of metachromatic particles in the periphery of the DLN is shown in Fig. 5 C. To exclude the possibility that particles of this size only enter lymphatic vessels under conditions of nonphysiologic interstitial pressure (as a result of injection), we painted a footpad laceration with a suspension of fluorescent microspheres with the same size and surface charge characteristics of MC-derived particles. Microspheres were found in the DLN only 30 min later, indicating that they entered lymphatic vessels and were delivered by lymph (Fig. S4). Collectively, these experiments indicate that MC particles readily traffic to the DLNs via the lymphatic system.
Figure 5.
Injected granules traffic to the DLN and contain functional TNF. (A) Toluidine blue staining of DLN tissue sections from an MC-deficient KitW-sh/W-sh mouse injected with 9.0 × 103 isolated MC-derived particles. Bar, 50 µm. (B) Same area as in A showing only metachromatic staining. The location of the edge of the DLN is depicted by a dashed line. (C) Magnification from square in A. Bar, 10 µm. (D) LN hypertrophy 24 h after injection of 1.5 × 104 particles purified from WT or TNF−/− mice (n = 3 for each group; *, P < 0.005 for the comparison between the two groups; data analyzed by unpaired two-tailed Student’s t test; error bars indicate standard error of the mean). All panels show results that are representative of two independent experiments.
Particle-associated TNF elicits LN enlargement
Our hypothesis is that peripheral MCs are able to modulate important physiological activities at distal sites through the release of particles bearing critical mediators. Therefore, we examined the effect of footpad injection of MC particles on LN remodeling. To demonstrate the specific contribution of TNF, we injected an equal number of particles isolated from TNF−/− mice in a parallel experiment. The LN hypertrophy induced by granules from WT and TNF−/− animals was compared after 24 h to saline-injected controls. Although MC particles from wild-type mice induced a twofold increase in LN size, particles from TNF−/− mice failed to trigger any LN hypertrophy (Fig. 5 D). This finding not only demonstrates a functional role for MC-derived particles but also demonstrates the specific role played by TNF bound within them in modulating LN hypertrophy.
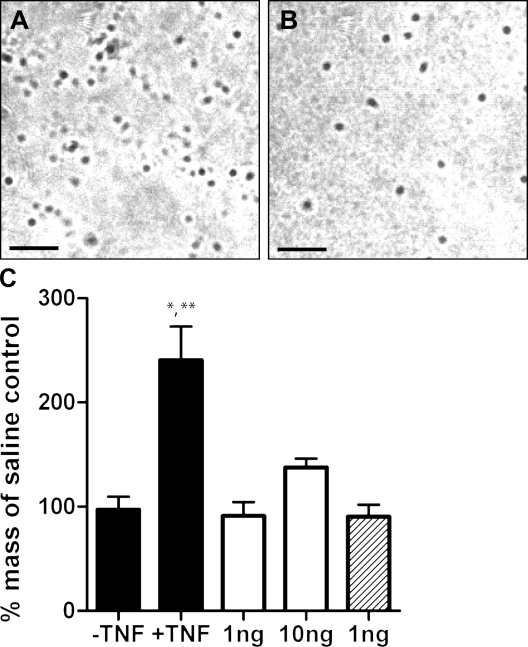
Another important component of our hypothesis is that by being packaged within stable particles, MC mediators, such as TNF, should be protected from dilution and degradation and that, therefore, minimal amounts will be required to promote biological activity at their target sites. To test this notion, we compared soluble recombinant TNF to TNF encapsulated within synthetic heparin/chitosan particles for their ability to effect remodeling of DLNs. As mentioned previously, heparin has a very high negative charge density. Chitosan, in contrast, is a polycation at pH <5.5 because of protonation of the amino groups in the side chains of the polysaccharide. When solutions containing these compounds are mixed, they rapidly phase separate to form spherical particles because of electrostatic interaction of the oppositely charged heparin and chitosan. This same process, called polyelectrolyte complexation, is thought to be the mechanism by which insoluble complexes are formed in MC granules, only with highly basic MC proteases substituting for chitosan as the polycation (Schwartz et al., 1981). The resulting particles were similar in size to those purified from rat MCs (Fig. 6, A and B). Because any molecules bound to heparin or chitosan before the mixing can be packaged within, we encapsulated recombinant TNF (rTNF) in synthetic particles by adding the mediator to the heparin solution before complexation.
Figure 6.
Encapsulation in heparin/chitosan particles greatly enhances the ability of recombinant TNF to effect popliteal LN enlargement 24 h after footpad injection. (A) DIC micrograph showing purified rat MC-derived particles. (B) Micrograph showing synthetic heparin/chitosan microparticles. These micrographs are representative of more than three independent experiments. Bars, 10 µm. (C) Less than 1 ng rTNF encapsulated in ∼1.3 × 105 synthetic particles similar to MC granule particles elicits more LN enlargement than 10 ng of soluble TNF when injected into the footpads of mice. Black bars, particles with and without encapsulated TNF; open bars, soluble TNF; hatched bar, soluble TNF mixed with soluble chitosan and heparin (n = 3 for each group; *, P < 0.05 vs. 10 ng soluble TNF; **, P < 0.01 vs. particles without TNF, 1 ng soluble TNF, and 1 ng soluble TNF mixed with soluble chitosan and heparin; data analyzed by one-way analysis of variance followed by Tukey’s Multiple Comparisons test; error bars indicate standard error of the mean). Results shown are representative of two independent experiments.
As shown in Fig. 6 C, the delivery of TNF in particle form increased its potency for causing LN enlargement by >10-fold. As the amount of TNF encapsulated was not directly measured, the 1-ng dose shown assumes 100% encapsulation. Other experiments performed during the development of these particles suggest that the true level of encapsulation using this protocol is almost never >30% (unpublished data), indicating that the 10-fold difference in potency between particle-delivered TNF and soluble TNF is a significant underestimate. In a separate experiment, we observed that even as little as 16 pg of TNF could elicit LN hypertrophy when delivered in particle form, supporting previous findings that the early increases in DLN TNF reflect the movement of a very small amount of cytokine (McLachlan et al., 2003). In addition, microparticles fabricated in exactly the same way except for the omission of TNF did not cause LN enlargement (Fig. 6 C), showing that TNF, and not the delivery vehicle, was responsible for the observed effects. Likewise, the injection of a mixture of the soluble components (combined under pH conditions under which no particles form) was not adequate to elicit LN enlargement (Fig. 6 C). Collectively, these findings reveal that, when packaged within particles of similar composition as MC particles, minimal amounts of TNF applied in the periphery can induce significant remodeling of distal DLNs.
DISCUSSION
In conclusion, we have demonstrated the existence of a novel form of extracellular communication over long distances. The use of the circulatory system to deliver extracellular signals is well known, as endocrine signaling involves the transport of hormones from their secreting cells to distant targets. However, this has mainly been studied in the context of signals that reach easily measurable concentrations. Such hormones, such as insulin, are secreted in great quantities by large populations of cells devoted to that purpose. This kind of signaling is relatively untargeted, insofar as high enough concentrations are reached in the circulation that the soluble mediator can be detected easily by most cells in the body or at least by a receptor-bearing subset. Endocrine signaling is also important during immunological responses, for example, the induction of the systemic acute phase response after initiation of inflammation (Suffredini et al., 1999).
This work suggests that there may exist a much less obvious, but equally important, parallel secondary system of long distance inflammatory communication earlier in responses, involving much lower levels of signals. Because most inflammatory signals are not very long lived, it is reasonable to expect that adaptations must have developed to prolong the survival of extracellular signals and to ensure their delivery to specific targets. This kind of adaptation is already well known for many blood-borne endocrine signals whose solubility and targeting are regulated by serum carrier proteins (Bischoff and Pilhorn, 1948; Gordon et al., 1952). The delivery of TNF to DLNs during inflammation by MC-derived particles appears to provide an example of such an adaptation. As opposed to the kind of untargeted signaling discussed previously, MC particles seem to be specialized for the delivery of very small quantities of cargo from one specific site (the site of inflammation) to another specific site (targets in the DLN that can mediate changes favoring the development of adaptive immunity). Heparin, the predominant component of MC particles, has the highest negative charge density of any molecule known in mammalian biology, which likely minimizes interaction with adjacent cells and other structures in the extracellular environment, with this charge favoring their movement into the lymphatic drainage (Patel et al., 1984). In contrast, their size (although likely retarding their movement through peripheral tissues) would also prevent them from moving through the DLN to the efferent lymph, resulting in their sequestration in this target organ (Hirano and Hunt, 1985). This scenario is favored by our finding that packaging TNF in similar heparin-based synthetic particles greatly enhances its potency relative to soluble TNF.
Synchronization and collaboration between the periphery and the draining secondary lymphoid tissue is crucial for a rapid and effective host response to infection. DLN enlargement alone can shorten the time between pathogen entry and antigen presentation by increasing the chances that the extremely rare circulating lymphocytes carrying receptors for antigens specific to the infection are present. The delivery of inflammatory signals by this unusual mechanism may help to explain how this MC-dependent process occurs so rapidly given the small amount of prestored cytokine available. It is also possible that particle-associated molecules other than TNF might be important in aspects of LN remodeling; MC proteases are clearly present in large quantities, and other low-abundance signaling molecules may also be present. For example, there is some evidence that MCs also prestore fibroblast growth factor 2 (Qu et al., 1998) and vascular endothelial growth factor (Grützkau et al., 1998), two well known heparin-binding growth factors which could be involved in the regulation of LN vascular remodeling during immune responses.
Although the work presented in this paper demonstrates that MC-derived particles can deliver signals from the periphery to LNs, the disposition of such signals once they reach the node remains to be elucidated. The most obvious target of peripheral TNF in the LN is the high endothelial venule (HEV) endothelium, which controls the recruitment of naive lymphocytes from the circulation into the node (a critical determinant of LN size). The up-regulation of adhesion molecules here could enhance such recruitment, and that does appear to be an aspect of MC-dependent LN enlargement (McLachlan et al., 2003). It is known that small soluble molecules can reach this site from afferent lymph via conduits that connect the sinus system to the HEV (Gretz et al., 2000). The size of the MC particles precludes their entry into this conduit system. We favor a mechanism by which cargo molecules are solubilized over time, essentially acting as slow-release capsules allowing the persistence of the signal until it has been carried most of the way to its target. Supporting this notion, experiments performed during the development of the synthetic microparticles described here showed that such particles do release encapsulated TNF over the course of 24 h (Fig. S5), suggesting that this may be the case. Alternatively, the site of action may be in the sinus system, and an amplification step may be required before the signal can be propagated to the HEVs. It is also likely that processes other than lymphocyte recruitment (such as prevention of lymphocyte egress) are also affected.
It is interesting to speculate that this process also occurs in human biology, where the distances between peripheral sites of infection or inflammation and draining secondary lymphoid tissue are even greater, presumably increasing the necessity of an adaptation such as the one described in this paper. Although there are substantial differences between human and rodent MCs, human MCs do contain preformed TNF (Walsh et al., 1991; Frangogiannis et al., 1998). There is also significant evidence to suggest that human MCs also release insoluble granule contents during degranulation (Kobayasi and Asboe-Hansen, 1969; Murphy et al., 1987; Caulfield et al., 1990; Kaminer et al., 1991; Lee and Vijayasingam, 1995; Akimoto et al., 1998). Given these factors, it is probable that a similar system operates in human immunology.
MCs reside at the crossroads between the external environment, the blood vasculature, and the lymphatic system. They are ideally situated to orchestrate events during the earliest stages of immune responses, and it seems likely that this is their physiological role in mammalian biology. In fact, their proximity to these critical structures suggests that they influence them directly in many ways in addition to those that have already been described. This work indicates one way in which these important cells may also influence events in distant lymphoid tissues.
Finally, many other immune cells including neutrophils (Niemann et al., 2007), eosinophils (Erjefält et al., 1999), basophils (Metcalfe et al., 1984), and cytotoxic T lymphocytes (Masson et al., 1990) store biologically active compounds in granules. The granular matrix in these cell types is comprised of serglycin proteoglycans, which, in addition to their role in storage, can contribute to the extracellular function of the bound mediators (Veugelers et al., 2004). To date, only the local effects of such complexes have been investigated. Our studies suggest that they may also be specialized to deliver their cargo of signaling proteins to far more distant targets.
MATERIALS AND METHODS
Animal studies.
Rat peritoneal MCs were obtained by pooling peritoneal and pleural lavage from Sprague-Dawley rats (Taconic). For compound 48/80 treatment to induce degranulation of mesenteric MCs, rats were injected intraperitoneally with 0.5 mg compound 48/80 in 10 ml PBS.
C57BL/6 mice obtained from NCI were used for most mouse experiments. Single footpad injections were done in a volume of 20 µl of vehicle in most cases. For the LN enlargement experiment, animals were injected on one side with the test substance and with vehicle on the other side to allow for within-animal controls for baseline variations in LN size.
For in vivo tracking studies, 10 µg PMA (Sigma-Aldrich) at 1 µg/µl in acetone or acetone alone were applied in two sequential treatments to the footpads of mice anesthetized with pentobarbital. Footpads were allowed to dry completely between applications. DLNs and footpads were harvested after 2 h.
For the isolation of mouse MC-derived particles, peritoneal lavage was performed on two to three mice using DME, and isolated cell suspensions were pooled and stimulated by 1 µM ionomycin (Sigma-Aldrich). Treatment with ionomycin was followed by incubation for 15 min in a cell culture incubator, after which the cellular fraction was removed by two rounds of centrifugation at 500 g for 5 min. Exocytosed particles were then pelleted by spinning at 12,000 g for 10 min at 4°C. Particles were washed and resuspended in PBS for injection and quantification using a hemocytometer. Injections were performed with a 10-µl volume. For DLN hypertrophy studies, DLN mass was determined after 24 h as compared with a paired saline control. The saline control for DLN hypertrophy studies using isolated granules contained 5% DME to control for any residual media in the preparation. For tracking studies, KitW-sh/W-sh mice (from our breeding colony) were injected with isolated particles and DLNs were recovered 45 min after particle injection for sectioning and staining with toluidine blue. Images of metachromatic areas were generated using Photoshop (Adobe). All animal experiments were done according to protocols approved by the Duke University Division of Laboratory Animal Resources and the Duke University Institutional Animal Care and Use Committee.
Cell culture.
For the generation of TNF-GFP–expressing cells, total RNA was isolated from BMMCs using an RNeasy kit (QIAGEN). Complementary DNA was made using the iScript cDNA synthesis kit (Bio-Rad Laboratories). The tnf gene was PCR amplified using the following primers: tnf forward, 5′-GATCTCGAGATGAGCACAGAAAGCATGATCCG-3′; and tnf reverse, 5′-GGTGGATCCCGCAGAGCAATGACTCCAAAGTAG-3′. The PCR product was digested with XhoI–BamHI, and then ligated with XhoI–BamHI-digested pLEGFP-N1 (BD) to generate pTNF-GFP. Sequence accuracy and whether TNF and GFP genes were in frame were confirmed by sequencing. The production of infectious viral particles and transfection of RBL-2H3 cell line and BMMCs were done as recommended by the vendor (Retroviral Gene Transfer and Expression User Manual; BD). In brief, the packaging cell line GP2-293 cells were transfected with pVSV-G (BD) and pTNF-GFP using lipofectamine 2000 (Invitrogen). Between 48 and 72 h after transfection, viral particles were collected. Healthy RBL-2H3 cells were grown to 50% confluence and then infected by collected viral particles. The infection rate of RBL-2H3 cells was 80–90%. BMMCs were cultured in the presence of 5 ng/ml rIL-3 (R&D Systems) and 5 ng/ml rSCF (R&D Systems). 2 × 105 cells/ml of healthy and actively dividing BMMCs were infected by the viral particles. The infection rate of BMMCs was around 5–10% because of the fact that these cells grow slowly and in suspension. The TNF-GFP–transfected cells were selected by adding Geneticin (Invitrogen) to a final concentration of 250 µg/ml for 5 d. TNF-GFP–expressing BMMCs were cultured in 5 ng/ml rIL-3 on a monolayer of 3T3 fibroblasts for 10 d before treatment. Both TNF-GFP–expressing cell types were observed after activation with 1 µM ionomycin.
Microscopy.
Whole mount rat mesentery preparations were made by stretching a loop of bowel over a slide so that the transparent windows were spread across them, waiting for these to dry, and then cutting away unwanted tissue. Then they were fixed with cold acetone, permeabilized, and blocked overnight in PBS with 0.3% Triton X-100 and 5% goat serum, and labeled with an anti–LYVE-1 antibody (Millipore) and fluorophore (Alexa Fluor 488 or TRITC, depending on the experiment)-labeled avidin (Sigma-Aldrich) before imaging using a laser-scanning confocal microscope. The LYVE-1 antibody was detected with an FITC-conjugated anti–rabbit IgG F(ab′)2 (Jackson ImmunoResearch Laboratories). Some images were made under epifluorescence illumination.
For sections, 10-µm frozen sections were made and fixed in cold acetone before being blocked in PBS with 1% BSA and subsequently labeled with the reagents described in the previous paragraph. For toluidine blue staining, frozen sections were rapidly fixed in 75% methanol, 20% formaldehyde, and 5% acetic acid and then stained in acidic 0.1% toluidine blue and cover slipped.
To create the image showing only metachromatic areas of toluidine blue–stained LN sections, the original image was transformed in Photoshop using the select color range function and visually identifying areas that corresponded to the metachromatic particles. Photoshop was then used to generate an image depicting only those areas, which were verified visually by comparing the original image to the generated image. This program was not used to identify particles but rather to produce an outline of metachromatic areas with more visual contrast for viewing in journal format.
For SEM, rat peritoneal lavage cells were seeded onto polylysine-coated coverslips in RPMI 1640 media with 10% FBS and incubated for 15 min at 37°C. They were then treated with compound 48/80 at 5 µg/ml for 15 min. Finally, the cells were fixed in 3% glutaraldehyde and processed for SEM. Coverslips were postfixed for one hour in 1% OsO4 (in water) before being dehydrated into ethanol and hexamethyldisilazane and finally dried. Then they were mounted and coated with osmium for imaging on a microscope (XL-30 ESEM-FEG; FEI Company).
Immunoblotting.
For the purification of MC granule particles, rat peritoneal cells were treated for 5 min with 5 µg/ml of compound 48/80 in a cell culture incubator. After the treatment, the cells were separated from granule particles by centrifugation at 450 g. The supernatant was carefully removed and thereafter spun at 12,000 g for 10 min at 4°C to pellet MC-derived particles. These were then solubilized by boiling in Laemmli buffer under reducing conditions, separating the proteins by SDS-PAGE, and transferring to a PDVF membrane. The membrane was blocked for 1 h in 5% milk in TBST and then probed with an anti-TNF antibody (Santa Cruz Biotechnology, Inc.) at 200 ng/ml at 4°C overnight. The signal was detected using enhanced chemiluminescence.
Microparticles.
Synthetic heparin/chitosan particles were generated by gradually combining 1% heparin (EMD) and 1% chitosan (Primex; both in distilled H2O) in a 1:1 ratio at approximately pH 5. To produce particles, 1 vol of 1% chitosan was added to 5 vol of 1% heparin and vortexed for 30 s. This was repeated until a 1:1 ratio of 1% chitosan to 1% heparin was achieved. After 10 min at room temperature, the pH was then adjusted to neutrality to prevent further aggregation. Particles were centrifuged at 14,000 g for 10 min at 4°C to form a pellet and washed with water before resuspension in PBS for injections or water for visualization on coverslips. To load particles with TNF, 5 ng rTNF (R&D Systems) was vortexed for 10 min in 1.25 ml 1% heparin before the addition of chitosan as described in this section.
Online supplemental material.
Fig. S1 shows that TNF-GFP fusion protein colocalizes with granule markers in RBL-2H3 cells. Fig. S2 shows intact MCs in untreated rat mesentery. Fig. S3 shows that intralymphatic MC-derived particle is not inside a migratory phagocyte. Fig. S4 shows that particles the size of MC-derived particles can enter lymphatics and rapidly reach the DLN under physiological conditions. Fig. S5 shows that recombinant TNF is gradually released from heparin-based microparticles. Video 1 shows DIC video microscopy of compound 48/80-activated rat peritoneal MCs releasing granule particles. Video 2 shows green fluorescent/DIC overlay video of ionomycin-activated TNF-GFP–expressing RBL-2H3 cells. Video 3 shows green fluorescent/DIC overlay video of ionomycin-activated TNF-GFP–expressing 3T3 fibroblast-cocultured BMMCs. Video 4 shows three-dimensional reconstruction of laser-scanning confocal data showing MC-derived particles inside a dermal lymphatic vessel of compound 48/80-injected mouse rear footpad. Video 5 shows isosurface reconstruction of the same region shown in Video 4. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090805/DC1.
Acknowledgments
We thank Anselmo Alonso and Leslie Eibest for assistance with electron microscopy.
This work was supported by the National Institutes of Health.
The authors declare that they have no competing financial interests.
Footnotes
Abbreviations used:
- BMMC
- BM-derived MC
- DLN
- draining LN
- HEV
- high endothelial venule
- MC
- mast cell
- SEM
- scanning electron micrograph
References
- Akimoto S., Ishikawa O., Igarashi Y., Kurosawa M., Miyachi Y. 1998. Dermal mast cells in scleroderma: their skin density, tryptase/chymase phenotypes and degranulation. Br. J. Dermatol. 138:399–406 10.1046/j.1365-2133.1998.02114.x [DOI] [PubMed] [Google Scholar]
- Baekkevold E.S., Yamanaka T., Palframan R.T., Carlsen H.S., Reinholt F.P., von Andrian U.H., Brandtzaeg P., Haraldsen G. 2001. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193:1105–1112 10.1084/jem.193.9.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber B.J., Oppenheimer J., Zawieja D.C., Zimmermann H.A. 1987. Variations in rat mesenteric tissue thickness due to microvasculature. Am. J. Physiol. 253:G549–G556 [DOI] [PubMed] [Google Scholar]
- Bischoff F., Pilhorn H.R. 1948. The state and distribution of steroid hormones in biologic systems; solubilities of testosterone, progesterone and alpha-estradiol in aqueous salt and protein solution and in serum. J. Biol. Chem. 174:663–682 [PubMed] [Google Scholar]
- Bloom G.D., Chakravarty N. 1970. Time course of anaphylactic histamine release and morphological changes in rat peritoneal mast cells. Acta Physiol. Scand. 78:410–419 10.1111/j.1748-1716.1970.tb04677.x [DOI] [PubMed] [Google Scholar]
- Casley-Smith J.R. 1980. Are the initial lymphatics normally pulled open by the anchoring filaments? Lymphology. 13:120–129 [PubMed] [Google Scholar]
- Caulfield J.P., el-Lati S., Thomas G., Church M.K. 1990. Dissociated human foreskin mast cells degranulate in response to anti-IgE and substance P. Lab. Invest. 63:502–510 [PubMed] [Google Scholar]
- Erjefält J.S., Greiff L., Andersson M., Matsson E., Petersen H., Linden M., Ansari T., Jeffery P.K., Persson C.G. 1999. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am. J. Respir. Crit. Care Med. 160:304–312 [DOI] [PubMed] [Google Scholar]
- Frangogiannis N.G., Lindsey M.L., Michael L.H., Youker K.A., Bressler R.B., Mendoza L.H., Spengler R.N., Smith C.W., Entman M.L. 1998. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 98:699–710 [DOI] [PubMed] [Google Scholar]
- Gordon J.R., Galli S.J. 1990. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 346:274–276 10.1038/346274a0 [DOI] [PubMed] [Google Scholar]
- Gordon A.H., Gross J., O’Connor D., Pitt-Rivers R. 1952. Nature of the circulating thyroid hormone-plasma protein complex. Nature. 169:19–20 10.1038/169019a0 [DOI] [PubMed] [Google Scholar]
- Gretz J.E., Norbury C.C., Anderson A.O., Proudfoot A.E., Shaw S. 2000. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192:1425–1440 10.1084/jem.192.10.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grützkau A., Krüger-Krasagakes S., Baumeister H., Schwarz C., Kögel H., Welker P., Lippert U., Henz B.M., Möller A. 1998. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol. Biol. Cell. 9:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Hunt C.A. 1985. Lymphatic transport of liposome-encapsulated agents: effects of liposome size following intraperitoneal administration. J. Pharm. Sci. 74:915–921 10.1002/jps.2600740902 [DOI] [PubMed] [Google Scholar]
- Huang V., Lonsdorf A.S., Fang L., Kakinuma T., Lee V.C., Cha E., Zhang H., Nagao K., Zaleska M., Olszewski W.L., Hwang S.T. 2008. Cutting edge: rapid accumulation of epidermal CCL27 in skin-draining lymph nodes following topical application of a contact sensitizer recruits CCR10-expressing T cells. J. Immunol. 180:6462–6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminer M.S., Lavker R.M., Walsh L.J., Whitaker D., Zweiman B., Murphy G.F. 1991. Extracellular localization of human connective tissue mast cell granule contents. J. Invest. Dermatol. 96:857–863 10.1111/1523-1747.ep12475169 [DOI] [PubMed] [Google Scholar]
- Kenig M., Gaberc-Porekar V., Fonda I., Menart V. 2008. Identification of the heparin-binding domain of TNF-alpha and its use for efficient TNF-alpha purification by heparin-Sepharose affinity chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 867:119–125 10.1016/j.jchromb.2008.03.023 [DOI] [PubMed] [Google Scholar]
- Kobayasi T., Asboe-Hansen G. 1969. Degranulation and regranulation of human mast cells. An electron microscopic study of the whealing reaction in urticaria pigmentosa. Acta Derm. Venereol. 49:369–381 [PubMed] [Google Scholar]
- Lagunoff D., Pritzl P. 1976. Characterization of rat mast cell granule proteins. Arch. Biochem. Biophys. 173:554–563 10.1016/0003-9861(76)90292-7 [DOI] [PubMed] [Google Scholar]
- Leak L.V. 1980. Lymphatic removal of fluids and particles in the mammalian lung. Environ. Health Perspect. 35:55–75 10.2307/3428974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., Vijayasingam S. 1995. Mast cells and myofibroblasts in keloid: a light microscopic, immunohistochemical and ultrastructural study. Ann. Acad. Med. Singapore. 24:902–905 [PubMed] [Google Scholar]
- Lindstedt K.A., Kokkonen J.O., Kovanen P.T. 1992. Soluble heparin proteoglycans released from stimulated mast cells induce uptake of low density lipoproteins by macrophages via scavenger receptor-mediated phagocytosis. J. Lipid Res. 33:65–75 [PubMed] [Google Scholar]
- Malaviya R., Gao Z., Thankavel K., van der Merwe P.A., Abraham S.N. 1999. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc. Natl. Acad. Sci. USA. 96:8110–8115 10.1073/pnas.96.14.8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D., Peters P.J., Geuze H.J., Borst J., Tschopp J. 1990. Interaction of chondroitin sulfate with perforin and granzymes of cytolytic T-cells is dependent on pH. Biochemistry. 29:11229–11235 10.1021/bi00503a011 [DOI] [PubMed] [Google Scholar]
- McLachlan J.B., Hart J.P., Pizzo S.V., Shelburne C.P., Staats H.F., Gunn M.D., Abraham S.N. 2003. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 4:1199–1205 10.1038/ni1005 [DOI] [PubMed] [Google Scholar]
- Metcalfe D.D., Bland C.E., Wasserman S.I. 1984. Biochemical and functional characterization of proteoglycans isolated from basophils of patients with chronic myelogenous leukemia. J. Immunol. 132:1943–1950 [PubMed] [Google Scholar]
- Miyata K., Takaya K. 1985. Uptake of released mast cell granules by reticular cells of the rat lymph node. Cell Tissue Res. 240:49–55 10.1007/BF00217557 [DOI] [PubMed] [Google Scholar]
- Murphy G.F., Austen K.F., Fonferko E., Sheffer A.L. 1987. Morphologically distinctive forms of cutaneous mast cell degranulation induced by cold and mechanical stimuli: an ultrastructural study. J. Allergy Clin. Immunol. 80:603–611 10.1016/0091-6749(87)90015-7 [DOI] [PubMed] [Google Scholar]
- Niemann C.U., Abrink M., Pejler G., Fischer R.L., Christensen E.I., Knight S.D., Borregaard N. 2007. Neutrophil elastase depends on serglycin proteoglycan for localization in granules. Blood. 109:4478–4486 10.1182/blood-2006-02-001719 [DOI] [PubMed] [Google Scholar]
- Palframan R.T., Jung S., Cheng G., Weninger W., Luo Y., Dorf M., Littman D.R., Rollins B.J., Zweerink H., Rot A., von Andrian U.H. 2001. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194:1361–1373 10.1084/jem.194.9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H.M., Boodle K.M., Vaughan-Jones R. 1984. Assessment of the potential uses of liposomes for lymphoscintigraphy and lymphatic drug delivery. Failure of 99m-technetium marker to represent intact liposomes in lymph nodes. Biochim. Biophys. Acta. 801:76–86 [DOI] [PubMed] [Google Scholar]
- Prodeus A.P., Zhou X., Maurer M., Galli S.J., Carroll M.C. 1997. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 390:172–175 10.1038/36586 [DOI] [PubMed] [Google Scholar]
- Qu Z., Kayton R.J., Ahmadi P., Liebler J.M., Powers M.R., Planck S.R., Rosenbaum J.T. 1998. Ultrastructural immunolocalization of basic fibroblast growth factor in mast cell secretory granules. Morphological evidence for bfgf release through degranulation. J. Histochem. Cytochem. 46:1119–1128 [DOI] [PubMed] [Google Scholar]
- Rampart M., De Smet W., Fiers W., Herman A.G. 1989. Inflammatory properties of recombinant tumor necrosis factor in rabbit skin in vivo. J. Exp. Med. 169:2227–2232 10.1084/jem.169.6.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T.D., Bloomfield F., Dexter T.M. 1988. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 332:376–378 10.1038/332376a0 [DOI] [PubMed] [Google Scholar]
- Schwartz L.B., Riedel C., Caulfield J.P., Wasserman S.I., Austen K.F. 1981. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J. Immunol. 126:2071–2078 [PubMed] [Google Scholar]
- Soderberg K.A., Payne G.W., Sato A., Medzhitov R., Segal S.S., Iwasaki A. 2005. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. USA. 102:16315–16320 10.1073/pnas.0506190102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J.V., Rot A., Luo Y., Narasimhaswamy M., Nakano H., Gunn M.D., Matsuzawa A., Quackenbush E.J., Dorf M.E., von Andrian U.H. 2000. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191:61–76 10.1084/jem.191.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffredini A.F., Fantuzzi G., Badolato R., Oppenheim J.J., O’Grady N.P. 1999. New insights into the biology of the acute phase response. J. Clin. Immunol. 19:203–214 10.1023/A:1020563913045 [DOI] [PubMed] [Google Scholar]
- Supajatura V., Ushio H., Nakao A., Okumura K., Ra C., Ogawa H. 2001. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 167:2250–2256 [DOI] [PubMed] [Google Scholar]
- Tharp M.D., Seelig L.L., Jr., Tigelaar R.E., Bergstresser P.R. 1985. Conjugated avidin binds to mast cell granules. J. Histochem. Cytochem. 33:27–32 [DOI] [PubMed] [Google Scholar]
- Tizard I.R., Holmes W.L. 1974. Degranulation of sensitised rat peritoneal mast cells in response to antigen, compound 48-80 and polymyxin B. A scanning electron microscope study. Int. Arch. Allergy Appl. Immunol. 46:867–879 10.1159/000231189 [DOI] [PubMed] [Google Scholar]
- Trzewik J., Mallipattu S.K., Artmann G.M., Delano F.A., Schmid-Schönbein G.W. 2001. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 15:1711–1717 10.1096/fj.01-0067com [DOI] [PubMed] [Google Scholar]
- Veugelers K., Motyka B., Frantz C., Shostak I., Sawchuk T., Bleackley R.C. 2004. The granzyme B-serglycin complex from cytotoxic granules requires dynamin for endocytosis. Blood. 103:3845–3853 10.1182/blood-2003-06-2156 [DOI] [PubMed] [Google Scholar]
- Walsh L.J., Trinchieri G., Waldorf H.A., Whitaker D., Murphy G.F. 1991. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 88:4220–4224 10.1073/pnas.88.10.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L.M., Ehrengruber M.U., Clark-Lewis I., Baggiolini M., Rot A. 1993. Binding to heparan sulfate or heparin enhances neutrophil responses to interleukin 8. Proc. Natl. Acad. Sci. USA. 90:7158–7162 10.1073/pnas.90.15.7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R.A., Geer J.C. 1959. Phagocytosis of mast cell granule by the eosinophilic leukocyte in the rat. Am. J. Pathol. 35:103–111 [PMC free article] [PubMed] [Google Scholar]
- Wershil B.K., Murakami T., Galli S.J. 1988. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J. Immunol. 140:2356–2360 [PubMed] [Google Scholar]