Abstract
During a screen for ethylnitrosourea-induced mutations in mice affecting blood natural killer (NK) cells, we identified a strain, designated Duane, in which NK cells were reduced in blood and spleen but increased in lymph nodes (LNs) and bone marrow (BM). The accumulation of NK cells in LNs reflected a decreased ability to exit into lymph. This strain carries a point mutation within Tbx21 (T-bet), which generates a defective protein. Duane NK cells have a 30-fold deficiency in sphingosine-1-phosphate receptor 5 (S1P5) transcript levels, and S1P5-deficient mice exhibit an egress defect similar to Duane. Chromatin immunoprecipitation confirms binding of T-bet to the S1pr5 locus. S1P-deficient mice exhibit a more severe NK cell egress block, and the FTY720-sensitive S1P1 also plays a role in NK cell egress from LNs. S1P5 is not inhibited by CD69, a property that may facilitate trafficking of activated NK cells to effector sites. Finally, the accumulation of NK cells within BM of S1P-deficient mice was associated with reduced numbers in BM sinusoids, suggesting a role for S1P in BM egress. In summary, these findings identify S1P5 as a T-bet–induced gene that is required for NK cell egress from LNs and BM.
After development in the BM, NK cells are released into circulation and are found in large numbers in the spleen and liver. Recent studies have also highlighted the presence of NK cells within LNs (Martín-Fontecha et al., 2004; Chen et al., 2005; Bajénoff et al., 2006; Garrod et al., 2007; Walzer et al., 2007a), accounting for ∼0.5% of cells in mouse LNs and ∼5% in human LNs (Grégoire et al., 2007). After immunization or infection, NK cell numbers can increase rapidly within draining LNs (Martín-Fontecha et al., 2004; Lucas et al., 2007; Gustafsson et al., 2008; Watt et al., 2008). Accumulating evidence suggests that NK cells help shape the adaptive immune response emerging within a responding lymphoid tissue (Martín-Fontecha et al., 2004; Bajénoff et al., 2006; Kassim et al., 2009). LNs and spleen are also likely to be early sites of NK encounter with inflammatory stimuli presented, for example, by dendritic cells. A recent study has provided evidence that NK cells can be primed in draining LNs before returning to the circulation with enhanced effector function (Lucas et al., 2007). Moreover, several recent studies have identified requirements for NK cell entry into LNs (Martín-Fontecha et al., 2004; Chen et al., 2005; Bajénoff et al., 2006; Lucas et al., 2007); however, less is understood about how they return to circulation.
T cell egress from thymus, and T and B cell egress from peripheral lymphoid organs depends on sphingosine-1-phosphate (S1P) and S1P receptor 1 (S1P1; Allende et al., 2004; Matloubian et al., 2004; Pappu et al., 2007). In contrast, S1P1 and S1P deficiency have not been found to block B cell egress from BM (Matloubian et al., 2004; Pappu et al., 2007). FTY720, a small molecule that can down-modulate S1P1, is also effective in preventing T and B cell egress from thymus and LNs (Mandala et al., 2002; Gräler and Goetzl, 2004; Matloubian et al., 2004). However, FTY720 treatment does not deplete NK cells from circulation in mice or humans (Vaessen et al., 2006; Walzer et al., 2007b). NK cells selectively express S1P5, and in mice deficient in this receptor, NK cell distribution was altered, with reduced NK cell numbers in blood and spleen and increased numbers in LNs and BM (Walzer et al., 2007b). This work established a role for S1P5 in NK cell recirculation; however, S1P5’s role in egress was not defined.
T-bet is a T-box–containing transcription factor that is required for Th1 cell development and also functions in the generation of effector CD8+ T cells and in B cells for switching to IgG2a (Szabo et al., 2000; Peng et al., 2002; Lugo-Villarino et al., 2003; Xu and Zhang, 2005; Joshi et al., 2007; Intlekofer et al., 2008). T-bet is also required for the final maturation of NK cells and for the development of invariant NKT (iNKT cells; Townsend et al., 2004). T-bet has been shown to mediate direct effects on various promoters, to bind and modify the effects of other transcription factors, and to facilitate chromatin remodeling through the regulation of histone methyltransferase activity (Hwang et al., 2005; Mehta et al., 2005; Beima et al., 2006; Chen et al., 2006; Djuretic et al., 2007; Lewis et al., 2007). The key genes regulated by T-bet in NK cell development and maturation are not well defined.
In an effort to identify genes involved in NK cell development or trafficking, we screened mice carrying ethylnitrosourea (ENU)-induced mutations for altered blood NK cell numbers or surface phenotypes. Our characterization of a strain with low circulating NK cells led us to identify a role for T-bet in promoting S1P5 expression in several cell types and to establish a role for S1P5 in NK cell egress from LNs and BM.
RESULTS
Characterization of the ENU strain Duane
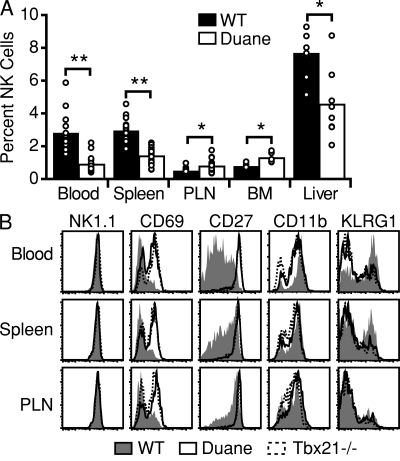
Screening peripheral blood for lymphocyte phenotypes resulting from ENU-induced mutations identified a strain, Duane, with a low percentage of NK cells in the blood, spleen, and liver, but a significantly elevated frequency of NK cells in peripheral LNs and BM (Fig. 1 A; and Fig. S1, A and B). The blood samples were also screened for CD69, with the intent of detecting mutations that altered the ability of CD69 to regulate lymphocyte egress (Shiow et al., 2006). An increased fraction of Duane NK cells was found to express CD69 (Fig. 1 B). Through further flow cytometric analysis of various developmental markers, Duane NK cells were found to have the CD27hiKLRG1lo phenotype typical of immature NK cells (Fig. 1 B). Duane had a near complete absence of mature iNKT cells (Fig. S1 A).
Figure 1.
Identification of the ENU mutant Duane. (A) NK cell frequencies within the indicated tissues of Duane and wild-type B6 mice. Bars represent mean values, and circles represent individual animals. Data are representative of at least two Duane and two wild-type mice in each of three independent experiments. (B) Representative flow cytometric analysis of NK cells from Duane, Tbx21−/−, and wild-type B6 mice for cell-surface markers. Plots are representative of at least three independent experiments, each analyzing at least two Duane and two wild-type mice. PLN, peripheral LN. *, P < 0.005; **, P < 0.0001.
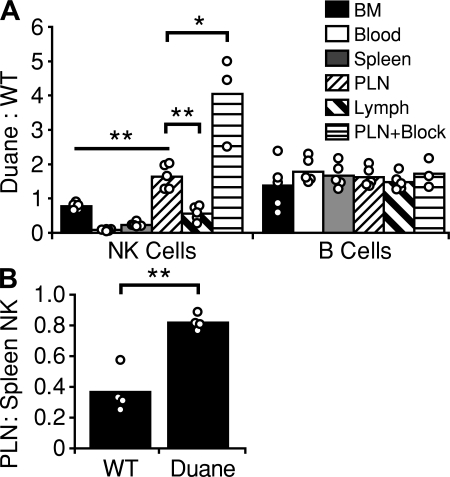
To determine if the NK cell accumulation in peripheral LNs was cell intrinsic, mixed BM chimeras were generated. In these mice, a lower percentage of Duane NK cells was observed in blood and spleen as compared with wild-type NK cells, whereas there was a relative accumulation of Duane NK cells in peripheral LNs (Fig. 2 A). The distribution of Duane and wild-type B cells was examined as a control and was equivalent in all sites (Fig. 2 A). Analysis of the lymph from these mice revealed an underrepresentation of Duane NK cells, suggesting that the LN accumulation was caused by a failure of these cells to egress (Fig. 2 A). When mixed BM chimeras were treated with α4 integrin, αL integrin, and L-selectin blocking antibodies to prevent NK cell and lymphocyte entry into LNs (von Andrian and Mempel, 2003; Bajénoff et al., 2006; Pham et al., 2008), and the extent of decay in LN cell numbers was measured after 40 h, an increase in the percentage of Duane NK cells was observed (Fig. 2 A). This finding was consistent with a reduction in the ability of Duane NK cells to exit LNs. In another test of trafficking behavior, wild-type and Duane splenocytes were each transferred into wild-type B6 recipients, and 24 h later the ratio of donor NK cells in the LN versus spleen was calculated. A greater fraction of Duane NK cells was found in the LNs as compared with wild-type NK cells, consistent with a defect in Duane NK cell egress from peripheral LNs (Fig. 2 B).
Figure 2.
Peripheral lymph node NK cell accumulation in Duane results from a cell-intrinsic egress defect. (A) Analysis of wild-type mice reconstituted with a mix of wild-type and Duane BM. Some mice (PLN+Block) received integrin and selectin blocking antibodies 40 h before analysis. Values are reported as a ratio of Duane (CD45.2) to wild-type (CD45.1) cells. At least two untreated and two treated chimeras were analyzed in each of two independent experiments. (B) Analysis of wild-type B6 mice 24 h after adoptive transfer of either Duane or wild-type splenocytes. Values are reported as a ratio of transferred NK cells recovered from the spleen (as a percentage of transferred B cells) to transferred NK cells in the peripheral LNs (as a percentage of transferred B cells). Data were obtained from two independent experiments, each composed of two recipients receiving Duane splenocytes and two recipients receiving wild-type splenocytes. Bars represent mean values, and circles represent individual animals. PLN, peripheral lymph node. *, P < 0.005; **, P < 0.001.
Identification of the Duane mutation
Mapping the causative genetic lesion in Duane was achieved by crossing an affected Duane mouse onto a wild-type C57BL/10 mouse, followed by intercrossing these F1 offspring and screening for the Duane phenotype. The seven most clearly affected and eight clearly unaffected mice were selected for the initial genome scan. Mapping was performed as previously described (Nelms and Goodnow, 2001) by using a panel of 80 Amplifluor assays that can distinguish single nucleotide polymorphisms (SNPs) between C57BL/6 and C57BL/10. The only clear linkage was for markers on the distal part of chromosome 11, and all mice were typed for these markers individually (Fig. S2 A). Because of a lack of markers that are capable of distinguishing between C57BL/6 and C57BL/10 in the region distal to 79 Mbp on chromosome 11, we set up a second round of mapping by crossing an affected Duane mouse with a wild-type CBA/J mouse, followed by backcrossing to another affected Duane mouse. The resulting N2 mice were tested for the Duane phenotype and genotyped for SNPs distinguishing CBA/J and C57BL/6 genomes on distal chromosome 11. This strategy confirmed linkage to a region between 89.3 and 116.1 Mbp on chromosome 11 (Fig. S2 B).
A search of the Mouse Genome Database (available at http://www.informatics.jax.org/; Bult et al., 2008) for genes in this interval identified Tbx21 as a likely candidate. Sequencing the genomic DNA corresponding to the gene encoding T-bet, Tbx21, identified a point mutation resulting in the replacement of a single nucleotide in the second intron (Fig. S2, C and D). This mutation disrupts an intronic splice acceptor site, thereby preventing the normal splicing of the T-bet mRNA (Fig. S2, E and F). PCR amplification and sequencing of T-bet mRNA isolated from Duane splenocytes revealed the use of a cryptic intronic splice acceptor site located 24 nucleotides into exon 3 (Fig. S2, F and G). This results in a predicted, in-frame, eight–amino acid deletion in the deduced protein sequence of Duane T-bet (Fig. S2 G). Comparison of the predicted protein structure of Duane T-bet to the known structure of the T-box domain of Xenopus laevis T protein and the predicted structures of other mammalian T-box proteins indicates that this deletion corresponds to a region adjacent to and including one of the residues involved in known protein–protein interactions (Fig. S2 H).
Expression of T-bet in Duane
PCR analysis of T-bet mRNA levels using primers spanning exons 1 and 2 indicated little difference between Duane and wild-type NK cells (Fig. S2 I). To assay T-bet protein levels, splenocytes were cultured for 48 h in conditions conducive to polyclonal T cell activation. Although both Duane and wild-type splenocytes activated to the same degree (Fig. S2 J), the amount of T-bet protein in Duane cell lysates were three- to fourfold lower than those of wild-type mice (Fig. S2 K). Interestingly, although some T-bet protein was detected in Duane, the NK phenotype of this strain was indistinguishable from that of T-bet–null mice (Fig. 1 B), and both strains exhibit a severe deficiency in iNKT cells (Fig. S1; Townsend et al., 2004).
T-bet function in Duane
To determine if the Duane mutation affected the function of T-bet, cells were transfected with an empty vector or a vector encoding wild-type or Duane T-bet, and the transcription of various gene targets was assayed. Overexpression of wild-type T-bet resulted in greater than fivefold induction in the transcription of IFN-γ, CCL3, and CXCR3 (Fig. S3 A). In contrast, overexpression of Duane T-bet did not result in the increased transcription of any of these known T-bet target genes, suggesting that the Duane mutation has rendered T-bet nonfunctional. Additionally, cotransfection of cells with a vector containing Duane T-bet and a luciferase reporter coupled to the mouse IFN-γ promoter region failed to result in an increase in luciferase activity, whereas transfection of a wild-type T-bet vector together with the reporter construct resulted in robust luciferase activity (Fig. S3 B).
To further test if any T-bet function remained in Duane, we analyzed both T and B cell differentiation in assays previously demonstrated to be T-bet dependent. Mixed cultures of wild-type and Duane T cells under Th1-inducing conditions failed to result in the expression of IFN-γ within the Duane cells despite robust expression in the co-cultured wild-type cells (Fig. S3 C). This result was similar to that observed in a wild-type and T-bet–deficient cell co-culture (Fig. S3 C). Likewise, co-culture of wild-type and Duane B cells under IgG2a-inducing conditions resulted in expression of IgG2a by the CD45.1+ wild-type cells but not the Duane B cells (Fig. S3 D). From these assays, the Duane mutation appears to result in a functionally null T-bet phenotype.
T-bet regulation of S1P5
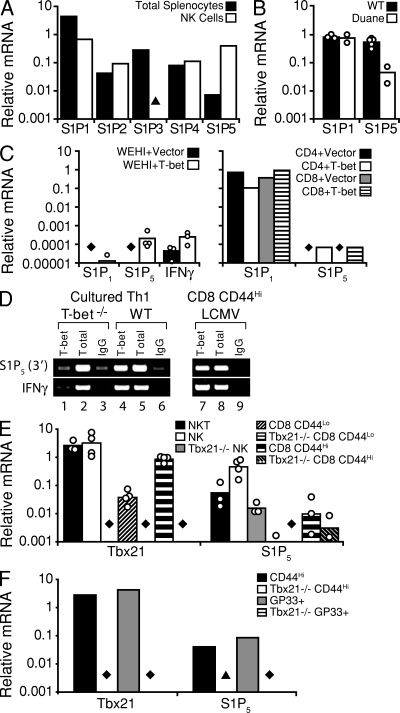
In an effort to understand the mechanism behind the NK cell accumulation in Duane LNs, we analyzed the expression of S1P receptors in NK cells. NK cells were notable in their abundant expression of S1P5 (Fig. 3 A; Barrett et al., 2007), as reported previously (Walzer et al., 2007b), but they also expressed S1P1 (Fig. 3 A). A comparison of Duane and wild-type NK cells revealed markedly reduced amounts of S1P5 in Duane NK cells but comparable amounts of S1P1 between the two groups (Fig. 3 B).
Figure 3.
Reduced S1P5 but not S1P1 expression in Duane cells and T-bet–mediated induction of S1P5 expression. (A–C) Quantitative PCR analysis of S1PR or IFN-γ transcript expression in (A) total splenocytes and sorted splenic NK cells from a wild-type mouse, (B) wild-type and Duane splenic NK cells (bars represent mean values, and circles represent sorted NK samples from individual animals), and (C) either vector- or T-bet–transduced WEHI-231 cells (left; bars represent mean values, and circles represent individual transductions) or primary T cells (right; bars represent individual transductions). (D) T-bet associates with the region 3′ of the S1pr5 gene. Primary CD4+ T cells were isolated from either Tbx21−/− (lanes 1–3) or wild-type (lanes 4–6) mice, and were polarized in Th1 conditions for 6 d (results are representative of two independent experiments, each using cells pooled from two individual animals). CD8+ T cells were isolated from mice infected with LCMV (lanes 7–9; results are representative of two independent immunoprecipitations using cells pooled from three individual animals). A standard ChIP analysis was then performed with a T-bet–specific antibody (lanes 1, 4, and 7) or an IgG control (lanes 3, 6, and 9). A standardized aliquot of the input chromatin was also examined as a control. Gene-specific primers were used to amplify the ChIP samples, as indicated to the left of the gel images. (E and F) Quantitative PCR analysis of T-bet or S1P5 transcript expression in (E) various lymphocyte subsets sorted from naive wild-type or T-bet–deficient mice (bars represent mean values, and circles represent sorted cell samples from individual animals; data were obtained from three independent experiments), and (F) in vitro–generated, sorted, polyclonal-activated (CD44hi) or LCMV antigen–specific (GP33+) CD8+ effector T cells (bars represent samples sorted from cells pooled from at least three individual animals). ▴, PCR signal < 0.001; ♦, PCR signal below detection threshold.
To explore the possibility that T-bet was a regulator of S1P5 expression, T-bet was overexpressed in WEHI-231 cells and in primary CD4+ and CD8+ T cells (Fig. 3 C). In each case, transduction of cells with a vector containing T-bet was sufficient to induce S1P5 expression. Although only low levels of S1P5 expression were achieved in WEHI-231 cells, the magnitude of this induction was similar to that of the known T-bet target IFN-γ (Fig. 3 C). To determine if T-bet acted directly on the S1pr5 locus, binding to conserved 5′ and 3′ regions of the gene was examined. T-bet chromatin immunoprecipitation (ChIP) using lysates from cell types known to abundantly express T-bet (Th1 cells and effector CD8+ T cells) demonstrated an interaction of T-bet with a 3′ site (Fig. 3 D).
We addressed whether other cell types that express T-bet show S1P5 expression. iNKT cells were found to have high S1P5 expression (Fig. 3 E). The failure of iNKT cells to develop in the absence of T-bet prevented us from further testing the connection between T-bet and S1P5 expression in these cells. Sorted CD44hi CD4+ and CD8+ T cells, representing memory and/or effector T cell populations, freshly isolated from adult mice showed low but measurable S1P5 expression as well as T-bet, and the S1P5 transcripts were reduced by >50-fold in cells sorted from T-bet–deficient mice (Fig. 3 E). Finally, when CD8+ T cells were stimulated with antigen under culture conditions that promote development of T-bet–expressing effector cells (Intlekofer et al., 2008), there was marked up-regulation of S1P5 and this was dependent on intrinsic T-bet expression (Fig. 3 F). Thus, T-bet promotes the expression of the egress receptor S1P5 in NK cells and CD8+ effector T cells, and likely also in iNKT cells.
S1P and S1P receptor requirement for NK cell egress from LNs
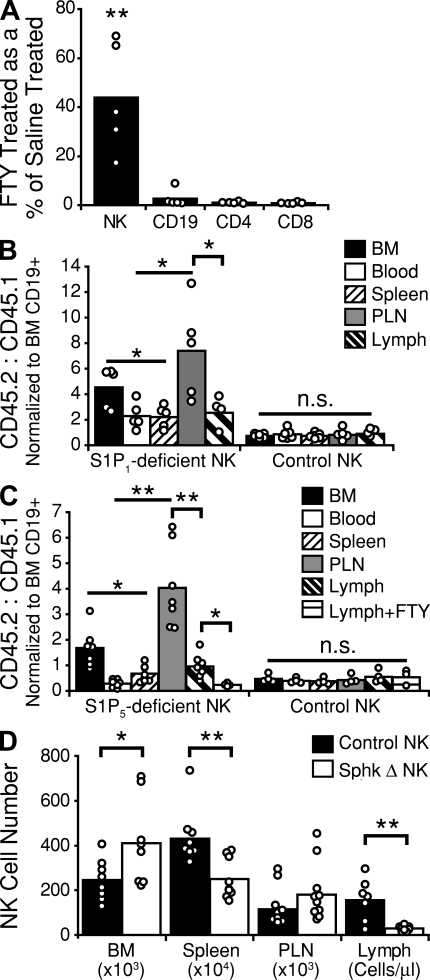
In initial experiments to elucidate the contribution of S1P receptors to NK cell egress from secondary lymphoid tissues, mice were treated for 20 h with FTY720, and analyzed for NK cell and T and B cell numbers in lymph. As previously reported, FTY720 treatment resulted in a near complete absence of T or B cells from the lymph (Fig. 4 A; Mandala et al., 2002; Matloubian et al., 2004). NK cells, however, were present in the lymph but were reduced in number as compared with saline-treated animals (Fig. 4 A). When mice were treated with integrin and selectin blocking antibodies to prevent NK cell and lymphocyte entry into lymph nodes, additional treatment with FTY720 prevented the egress-mediated decay of T cell numbers, but NK cell numbers continued to decline (Fig. S4 A), indicating the presence of an FTY720-insensitive egress mechanism. Thus, FTY720 treatment appears to reduce but not block NK cell egress from LNs into lymph. To determine the impact of FTY720 exposure on S1P receptor expression, we examined the surface abundance of FLAG-tagged S1P1 and S1P5 in transduced WEHI-231 B lymphoma cells in the presence of either S1P or FTY720-P. As previously reported (Gonzalez-Cabrera et al., 2007; Oo et al., 2007), exposure to either S1P or FTY720-P, even in small quantities, was sufficient to induce loss of cell-surface S1P1. Neither compound was able to affect the surface expression of S1P5 (Fig. S4 B). The resistance of S1P5 to down-modulation is unlikely to be caused by overexpression because the surface abundance of the receptor was lower than for S1P1 (Fig. S4 B). These observations suggest that S1P5 on lymphoid cells may be less sensitive than S1P1 to agonist-mediated down-modulation.
Figure 4.
NK cell egress requires S1P, and uses both S1P5 and an FTY720-sensitive receptor. (A) Cellular composition of lymph collected from wild-type mice treated for 20 h with 1 mg/kg FTY720. Values are reported as the number of cells in the lymph of FTY720-treated animals as a percentage of lymph cell numbers in saline-treated animals. Data are derived from at least three independent experiments. (B) Examination of wild-type B6 mice reconstituted with a mix of CD45.1+ wild-type and CD45.2+ S1pr1−/− or control S1pr1+/+ BM. Values are reported as a ratio of S1P1-deficient or control (CD45.2) to wild-type (CD45.1) cells. To account for differences between independent groups of chimeras, NK cell ratios were normalized to BM CD19+ B cell ratios for each mouse. Data are derived from at least three independent experiments. (C) Examination of wild-type B6 mice reconstituted with a mix of CD45.1+ wild-type and CD45.2+ S1pr5−/− or CD45.2+ S1pr5+/+ littermate control BM. Some mice (Lymph+FTY) received 1 mg/kg FTY720 i.p. 20 h before analysis. Values are reported as a ratio of S1P5-deficient or control (CD45.2) to wild-type (CD45.1) cells. NK cell ratios were normalized to BM CD19+ B cell ratios for each mouse. Data are derived from two independent experiments. (D) Analysis of NK cell distribution in Sphk-deficient and littermate control mice. Values for BM, spleen, and peripheral LNs (six nodes) represent absolute NK cell numbers; values for lymph represent cells per microliter of lymph. Sphk Δ represents mice that were Sphk2 null, were deficient for one allele of Sphk1, and had the second allele of Sphk1 excised. Control represents Sphk2-null mice with at least one functional allele of Sphk1. Data are derived from at least three independent experiments. Bars represent mean values, and circles represent individual animals. n.s., not significant; PLN, peripheral LN. *, P ≤ 0.05; **, P < 0.005.
The effects of FTY720 on T and B cell trafficking have been associated with intrinsic S1P1 function in these cells (Matloubian et al., 2004). To determine if S1P1 played a role in NK cell trafficking, mixed S1P1-deficient and wild-type BM chimeras were examined (Fig. 4 B). Compared with control mixed chimeras, there was a small but significant accumulation of S1P1-deficient NK cells in LNs compared with blood and spleen, and lower numbers of S1P1-deficient NK cells in lymph (Fig. 4 B). There was also a mild accumulation of S1P1-deficient cells in the BM. Thus, like FTY720 treatment, hematopoietic S1P1 deficiency reduces but does not block NK cell egress from LNs into lymph.
To understand the role of S1P5 in NK cell egress from lymphoid tissues, we generated S1P5-deficient mice (Fig. S5). The S1P5-deficient founders were backcrossed three times to B6 mice, and BM from either these mice or their S1P5+/+ littermates was used to generate mixed BM chimeras. These chimeras demonstrated a significant accumulation of S1P5-deficient NK cells in the BM and in the LNs, together with a deficit of S1P5-deficient NK cells in the blood and the spleen relative to wild-type NK cells (Fig. 4 C), in agreement with previous findings (Walzer et al., 2007b). In addition, there was a lower ratio of S1P5-deficient NK cells to wild-type NK cells in the lymph as compared with the peripheral LNs, indicating a reduced capacity of the S1P5-deficient NK cells to egress from LNs into lymph. This ratio skewed further in favor of the wild-type NK cells if the mice were treated with FTY720 for 20 h before analysis (Fig. 4 C). These results indicate that S1P5 is important for the egress of NK cells from LNs, while also indicating that some NK cell exit from LNs is S1P5 independent and can be attributed to a receptor that is modulated by FTY720, most likely S1P1.
To address the role of S1P in NK cell egress, we analyzed mice deficient in Sphk2 and conditionally ablated for Sphk1, and that lacked detectable circulatory S1P (Pappu et al., 2007). In these mice, we observed a significant reduction in the number of NK cells in the spleen but found these mice to have a normal or slightly elevated number of NK cells in peripheral LNs (Fig. 4 D). These mice also demonstrated a significant accumulation of NK cells in the BM. Analysis of lymph from these animals revealed a significant reduction in the number of NK cells, indicating a failure to egress from the peripheral lymphoid tissues in the absence of S1P (Fig. 4 D).
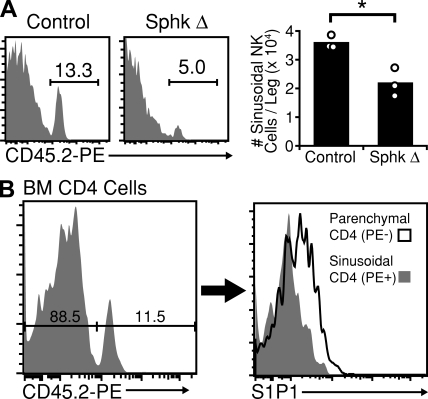
Reduced NK cell numbers in BM sinusoids of S1P-deficient mice
Cellular egress from the BM occurs via BM sinusoids. Recent work has shown that a 2-min in vivo exposure to PE-coupled anti-CD45 antibody selectively labels sinusoidal cells among BM cells (Pereira et al., 2009). Using this labeling procedure, we found that ∼14% of BM NK cells were situated inside sinusoids (Fig. 5 A). In S1P-deficient mice, this frequency fell to <6% (Fig. 5 A). Previous studies have shown a tight relationship between S1P1 surface abundance on T cells and local S1P concentrations (Lo et al., 2005; Schwab et al., 2005; Pappu et al., 2007). Analysis of T cells in the BM showed that S1P1 was present in higher amounts on parenchymal compared with sinusoidal cells (Fig. 5 B), suggesting the existence of an increasing S1P gradient between parenchyma and sinusoid. These observations, coupled with the lower total NK cell numbers in the periphery, suggest that the ability of NK cells to exit from BM parenchyma to sinusoid is diminished in the absence of S1P.
Figure 5.
Reduced numbers of NK cells in BM sinusoids of S1P-deficient mice and S1P1 surface expression on BM T cells. (A, left) NK cells present in the BM sinusoids of Sphk-deficient and littermate control mice were labeled in vivo by a 2-min i.v. treatment with PE-conjugated anti-CD45.2. Numbers indicate the frequency of PE+ (sinusoidal) cells among total NK cells. (right) Summary of data for three mice. Data are representative of two independent experiments, each involving at least two Sphk-deficient and two littermate control mice. Bars represent mean values, and circles represent individual animals. (B, left) Control mice were treated i.v. with PE-conjugated anti-CD45.2 for 2 min. Cells were gated on CD4+, TCRβ+, NK1.1− cells and resolved for sinusoidal labeling. (right) Comparison of S1P1 surface expression levels between PE− (parenchymal) and PE+ (sinusoidal) CD4+ T cells. Data are representative of two experiments, each analyzing two animals. *, P < 0.05.
S1P5 mediates the egress of activated NK cells
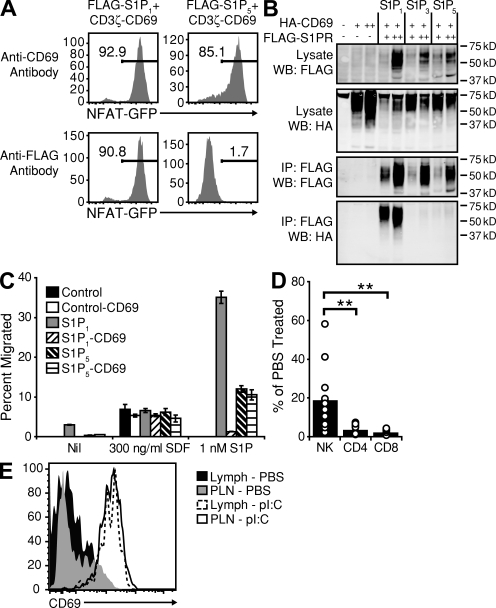
CD69 is rapidly expressed at high levels on activated NK cells and may have a role in NK cell–mediated cytotoxicity (Karlhofer and Yokoyama, 1991). CD69 has also been shown to bind and inhibit the function of S1P1 in T cell egress (Shiow et al., 2006). To test whether CD69 interacts with S1P5, we used a coexpression system whereby a FLAG-tagged S1P receptor was coexpressed with a fusion molecule consisting of the extracellular and transmembrane domains of CD69 coupled to the intracellular domain of CD3ζ (Fig. S6). CD69 is known to physically associate with S1P1, and cross-linking of FLAG-tagged S1P1 results in the induction of CD3ζ signaling, as evident by the expression of an NFAT-regulated GFP reporter (Fig. 6 A). When FLAG-S1P5 was cross-linked, no expression of GFP was detected, indicating a failure of CD69 to associate with S1P5. Further, as previously reported (Shiow et al., 2006), immunoprecipitation of FLAG-S1P1 resulted in the pull down of hemagglutinin (HA)-tagged CD69 (Fig. 6 B). Immunoprecipitation of FLAG-S1P5, however, even from cells expressing higher amounts of the S1P receptor (S1P5 ++ vs. S1P1 +), failed to pull down any detectable HA-CD69 (Fig. 6 B). In agreement with the lack of interaction between CD69 and S1P5, CD69 showed no ability to inhibit S1P5-dependent S1P chemotaxis in Transwell assays with transduced cells (Fig. 6 C).
Figure 6.
CD69 neither associates with nor inhibits S1P5 function. (A) NFAT-GFP reporter expression in 2B4 cells transduced with the indicated constructs and treated with anti-CD69 antibody (top) or anti-FLAG antibody (bottom). Numbers indicate percentages of cells expressing the reporter. Data are representative of six independent experiments. (B) Western blot analysis of total lysates or anti-FLAG immunoprecipitates from WEHI-231 cells transduced with the indicated transgenes and sorted for CD69 and FLAG expression (+, low; ++, high). WB indicates the antibody used for Western blotting, and IP indicates the antibody used for immunoprecipitation. Data are representative of two independent experiments. (C) Transwell migration assay of untransduced WEHI-231 cells (control) or cells expressing S1PR transgenes alone or together with a CD69 transgene. Values are reported as the percentage of input cells migrating to the lower chamber during the 3-h assay. Columns represent the means of duplicate wells, and the bars represent the range within a single experiment. Data are representative of three independent experiments. (D) Cellular composition of lymph from animals treated with poly I:C for 6 h before analysis. Values are reported as the number of cells in the lymph of poly I:C–treated animals as the percentage of cells in the lymph of the PBS-treated animals. Data are representative of at least three independent experiments. Bars represent mean values, and circles represent individual animals. (E) Representative flow cytometric analysis of lymph and LN NK cells from saline- or poly I:C–treated mice. Data are representative of at least three independent experiments. PLN, peripheral LN. **, P < 0.005.
These observations suggested that, compared with T cells, NK cells may be resistant to LN egress inhibition caused by CD69 up-regulation. To assess the ability of CD69 to block NK egress in vivo, we analyzed the cellular composition of lymph from animals treated with the type I IFN–inducing agent poly I:C for 6 h. Treatment of mice with poly I:C has been previously demonstrated to efficiently block T cell egress from peripheral LNs (Shiow et al., 2006). Although nearly all T cell egress into lymph was inhibited by poly I:C treatment, a significant percentage of NK cells was found to retain the ability to enter the lymph (Fig. 6 D). Flow cytometric analysis confirmed that all NK cells in the lymph appeared activated and expressed high amounts of CD69 (Fig. 6 E), indicating that S1P5 is able to mediate egress of activated NK cells even in the presence of CD69.
DISCUSSION
T-bet has been previously demonstrated to be important for normal NK cell development and survival (Townsend et al., 2004). NK cells from T-bet–deficient mice appear phenotypically immature and hyperactivated, and undergo increased rates of cell death. Although these observations may partly explain the observed deficit of NK cells in some peripheral sites of T-bet–deficient mice (blood, spleen, and liver), they do not explain the observed increase in NK cell number within the BM and peripheral LNs. Our findings indicate that S1P5 is likely the key gene acting downstream of T-bet to allow the normal LN and BM egress of NK cells, and thus, recirculation to other sites. T-bet also promotes expression of CXCR3, a receptor involved in NK cell entry to inflamed LNs (Martín-Fontecha et al., 2004), indicating that part of the T-bet–induced gene expression program acts to coordinate NK cell trafficking. ChIP analysis identified the ability of T-bet to bind a conserved element 3′ of the S1pr5 gene. This finding, together with our observation of T-bet–induced expression of S1P5 in three different transduced cell types, as well as the T-bet dependence of S1P5 expression in NK and CD8+ T cells, establishes that S1P5 is a common component of the T-bet gene expression program.
Within a day after activation in peripheral LNs, “primed” NK cells can be detected at distal sites such as the spleen, lungs, and liver (Lucas et al., 2007). This apparent ability of acutely activated NK cells to traffic from LNs seemed contrary to what has been observed for activated T cells, where CD69 inhibits S1P1-mediated egress (Shiow et al., 2006). Our findings suggest that this is likely a consequence of S1P5 being resistant to CD69-mediated inhibition. The expression of S1P5 by effector CD8+ T cells suggests that these cells may also be competent to egress while in an activated state. Similar trafficking patterns of NK cells and effector CD8+ T cells have been noted (Grégoire et al., 2007). In this regard, it is notable that CD69 is a member of the NK receptor C-type lectin–like family, and some studies have suggested that it has an effector role (Karlhofer and Yokoyama, 1991), although no extrinsic ligand has yet been identified. By facilitating egress of CD69+ cells, S1P5 may allow this CD69 effector function to occur. Many iNKT cells express CD69 (Matsuda et al., 2000), and we propose that T-bet–induced S1P5 also functions to facilitate egress and recirculation of these cells.
FTY720, in the active phosphorylated form, is a receptor agonist that is also a functional antagonist of S1P1, and FTY720 treatment causes rapid down-modulation of S1P1 on T cells, an effect that has correlated tightly with FTY720-mediated inhibition of T cell egress (Matloubian et al., 2004; Pham et al., 2008). In vitro studies have shown that FTY720-P causes internalization, ubiquitination, and degradation of S1P1 (Gonzalez-Cabrera et al., 2007; Oo et al., 2007). In our studies, we found that in contrast to S1P1, S1P5 was resistant to FTY720-induced down-modulation, and our data suggest that this receptor continues to support NK cell egress in FTY720-treated hosts. The finding that NK cell egress is relatively, albeit not completely, resistant to FTY720 treatment is consistent with studies showing minimal changes in blood NK cell numbers in FTY720-treated humans (Vaessen et al., 2006) and mice (Walzer et al., 2007b). The ability of many NK cells to egress from LNs after FTY720 treatment provides further evidence against models arguing that FTY720 inhibits egress by acting on endothelial or stromal S1P receptors to close LN egress portals (Rosen and Goetzl, 2005). iNKT cell recirculation is also relatively resistant to FTY720 treatment (Allende et al., 2008). Our finding of S1P5 expression in these T-bet–dependent cells provides a possible explanation for this finding, and it will be important in future studies to track the impact of S1P5 deficiency on iNKT cell as well as NK cell and effector CD8+ T cell function.
Although S1P5 appears to be the dominant S1P receptor in NK cells, we did observe S1P1 expression in these cells, and S1P1 deficiency caused a measurable decrease in NK cell representation in lymph. Although a previous study suggested S1P1 may not be present in NK cells (Walzer et al., 2007b), S1P1 transcripts were within ∼10-fold of the amounts present in T and B cells in that study, as observed in the present work. The greater effect of S1P deficiency than S1P5 deficiency on NK cell numbers in lymph also supports the view that S1P1 plays a role in these cells, as does the finding that FTY720 treatment is able to further reduce the egress of S1P5-deficient NK cells. It remains to be determined whether these receptors are coexpressed in all NK cells or expressed in separate subsets.
Within the spleen, NK cells are largely excluded from the white pulp and are localized in the red pulp (Walzer et al., 2007a). S1P1 deficiency or FTY720 treatment depletes T and B cells from red pulp while leaving cells within the white pulp. This likely reflects a role for S1P and S1P1 in overcoming chemokine-mediated retention in the white pulp (Pham et al., 2008). The general NK cell deficiency in the spleen of T-bet–, S1P5-, and S1P-deficient mice is consistent with observations that few splenic NK cells are present within the white pulp, and with evidence that positioning in the red pulp is Gαi independent and, thus, most likely not chemokine mediated (Grégoire et al., 2007). The lack of a Gαi mediated retention mechanism may well obviate the need for S1P receptor signaling during splenic egress (Pham et al., 2008). We suspect that in the absence of S1P5 or S1P, NK cells become trapped in LNs and BM at the expense of representation in blood and the splenic red pulp. We note that the reduction in NK cell numbers in the spleens and livers of T-bet–deficient mice exceeds their numerical accumulation in LNs and BM, likely reflecting a requirement for T-bet in the maturation or maintenance of NK cells. Although T-bet has a large number of target genes, there may be a similar deficiency in the total peripheral NK cell pool in S1P5-deficient mice (Walzer et al., 2007b), raising the possibility that T-bet–driven S1P5 expression is required for normal NK cell maturation or homeostasis, a topic that will need further assessment in future studies.
The requirements for cell egress from the BM are poorly understood. CXCR4 has an established role in mediating precursor retention in the BM and there is evidence that NK cells are retained by CXCR4, and down-regulation of this receptor coincides with maturation and exit from the BM (Beider et al., 2003; Bernardini et al., 2008). However, mature NK cells retain substantial amounts of CXCR4 and are highly responsive to CXCL12 (Bernardini et al., 2008). We suggest that the requirement for S1P for efficient egress of NK cells from BM parenchyma into sinusoids reflects a role for S1P, together with S1P5 and S1P1, in overcoming CXCR4–CXCL12–mediated retention, analogously to S1P–S1P1 overcoming the CCR7–CCL21–mediated retention of T cells in lymph nodes (Pham et al., 2008). These findings point to the possibility that S1P and S1P receptors function in the BM egress of additional cell types. This has important implications for the application of therapies that interrupt S1P receptor function.
In summary, our findings establish that T-bet promotes S1P5 expression and that S1P5, and its ligand S1P, are required for NK cell egress from LNs and BM. T-bet–dependent S1P5 expression also occurs in effector CD8+ T cells. These findings extend the paradigm that S1P promotes egress through actions on migrating S1P1+ cells by showing that a second leukocyte S1P receptor is involved in egress. We propose that the differential use of S1P5 by NK and effector CD8+ T cells facilitates their egress in a CD69+ effector state. Finally, our study adds to recent findings (Walzer et al., 2007b) to suggest strongly that S1P and S1P receptors are required for efficient NK cell egress from the BM.
MATERIALS AND METHODS
Mice, ENU mutagenesis, chimeras, lymphocyte transfers, and treatments.
C57BL/6 (B6) and CD45.1-congenic B6 mice were obtained from the National Cancer Institute (NCI). C57BL/10 and CBA/J mice were obtained from the Australian Phenomics Facility, Australian National University (ANU). Tbx21−/− mice were obtained from the Jackson Laboratory. All experiments conformed to ethical principles and guidelines approved by the University of California, San Francisco Institutional Animal Care and Use Committee, the ANU Animal Experimentation Ethics Committee, and the Scripps Research Institute Animal Research Committee. The generation of S1P5-deficient mice is described in detail in the following section. S1P5-deficient mice were crossed three times to B6 mice. The generation of S1P1-deficient mice, S1P1-deficient fetal liver chimeras, and Sphk-deficient mice have been previously described (Liu et al., 2000; Matloubian et al., 2004; Pappu et al., 2007). S1P1+/− mice were provided by R. Proia (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD), and Sphk1f/− Sphk2−/− mice were provided by S. Coughlin (University of California, San Francisco, San Francisco, CA). The Duane strain was established through ENU-mediated mutagenesis of B6 mice, as previously described (Nelms and Goodnow, 2001; Shiow et al., 2008). Mapping and sequencing of the Duane mutation was performed at the Australian Cancer Research Foundation (ACRF) Biomolecular Resource Facility (John Curtin School of Medical Research [JCSMR], ANU) and is described in Genetic mapping of the Duane mutation. Once identified, the Duane strain was backcrossed onto B6 mice for three additional generations. BM chimeras were generated by i.v. transferring 3–6 × 106 BM cells into lethally irradiated (2 × 550 rads) B6-CD45.1 recipients.
For short-term adoptive transfers of splenocytes, 2.5–3.5 × 107 donor cells were transferred i.v. into each B6-CD45.1 recipient. Integrin and L-selectin blockade was mediated by the administration of 100 µg each of anti–αL integrin (clone M17/4), anti–α4 integrin (clone PS/2), and anti–L-selectin (clone MEL14) antibodies in PBS i.v. 40 h before analysis. FTY720-treated animals received 1 mg/kg of the drug injected i.p. and were analyzed 20–24 h later. For in vivo lymphocyte activation, mice received 100 µg of poly I:C (GE Healthcare) in PBS i.v. 6 h before analysis. Lymph collection and in vivo labeling of BM sinusoidal contents have been previously described (Matloubian et al., 2004; Pereira et al., 2009). For isolation of liver NK cells, livers were harvested and rinsed in PBS. The liver was disrupted by forcing through a 70-µm nylon mesh, and cells were washed in PBS, resuspended in 44% Percoll (GE Healthcare) and layered on 56% Percoll. Preparations were spun for 20 min at 2,000 rpm in a bench-top centrifuge with no brake, after which the interface containing the lymphocytes was collected and washed in PBS. Lymphocytic choriomeningitis (LCMV) infections were performed as previously described (Intlekofer et al., 2008), and CD44hi CD8+ T cells were sorted on day 8 after infection from total splenocytes.
S1pr5 targeting vector construction, embryonic stem (ES) cell manipulation, and generation of mutant mice.
A mouse genomic library was screened with an S1P5 mouse cDNA, and an S1pr5-containing genomic clone with sufficient flanking sequence on both sides of the S1P5 coding region was identified. The S1pr5 targeting construct in pBluescript (Agilent Technologies) has an ∼2.6-kb short arm and an ∼3.2-kb long arm, and a portion of the S1P5 coding region was fused with DsRed from DSRed-N1 (Takara Bio Inc.). Except for the first seven amino acids, the entire S1P5 coding region was deleted. The orientation of the neomycin cassette under the control of the phosphogylcerate kinase promoter is opposite to that of the S1pr5 genomic locus. All cloning was performed using standard molecular biology techniques.
E14Tg2a stem cells were electroporated with 25 µg of Sal I–linearized targeting vector at the Scripps Research Institute Mouse Genetics Core, and ∼150 colonies were isolated. Genomic DNA was prepared from isolated stem cells digested with BamHI and Southern blotted with a random-primed 32P-labeled external probe. Two independent ES cell clones with recombined S1pr5 loci were identified and injected into blastocysts. One line of chimeric mice derived from the identified ES cell clones transmitted the mutant allele to the germline. The resultant heterozygous mice were crossed, and the presence of wild-type, heterozygous, and null mutants was confirmed by Southern blotting of BamHI-digested tail DNA with the external probe, and PCR using S1P5- and DsRed-specific primers (Table S1). PCR was performed under standard conditions, except for the addition of 10% DMSO (final) to reaction mixes.
Genetic mapping of the Duane mutation.
Affected Duane mice were crossed onto either the C57BL/10 or the CBA/J background to generate heterozygous F1 mice. These F1 mice were either intercrossed (C57BL/10 mapping) or backcrossed to an affected Duane mouse (CBA/J mapping) to yield mice homozygous for the Duane mutation and carrying a mix of C57BL/6 and background C57BL/10 or CBA/J SNPs. Genomic DNA was isolated from both affected and unaffected mice from the C57BL10/mapping cross and was used as template material for SNP mapping using an Amplifluor assay (Millipore) with Platinum Taq (Invitrogen). SNP markers were spaced approximately every 20 Mbp throughout the genome. To confirm the linkage and to further narrow the interval, genomic DNA from both affected and unaffected mice from the CBA/J mapping cross was isolated and used as a template material for SNP typing in the suspected interval. Once a defined interval was established, the T-bet–encoding gene was sequenced from genomic DNA from both affected Duane and wild-type mice. All exons were amplified by PCR with primers (Table S1) spanning the whole exon and the flanking intronic regions. To confirm the transcript sequence, RNA was isolated with TRIzol reagent (Invitrogen) from mutant, heterozygous, and wild-type mice, and cDNA was synthesized with the SuperScript II reverse transcription kit (Invitrogen). The Duane deletion was confirmed with primers located in exons 2 and 4 (Table S1). All PCR products were amplified by using AccuPrime High Fidelity Taq (Invitrogen), gel purified, and sequenced on a sequencer (ABI 3730; Applied Biosytems) at the ACRF Biomolecular Resource Facility (JCSMR, ANU) according to the manufacturer’s protocol.
Protein modeling.
T-bet protein models were generated by using the Geno3D server (available at http://geno3d-pbil.ibcp.fr; Combet et al., 2002). Resulting models were visualized and colored using the DeepView: Swiss-PdbViewer software package (available at http://www.expasy.org/spdbv/; Guex and Peitsch, 1997). Final images were rendered with the Persistence of Vision Raytracer (POV-Ray, version 3.6.1a; Hallam Oaks Pty. Ltd.).
Cell-culture conditions.
For polyclonal T cell stimulation, splenocytes were first depleted of B220+, NK1.1+, CD11b+, CD11c+, and Gr1+ cells by using a cell separator (autoMACS; Miltenyi Biotec). The remaining T cells were resuspended in RPMI Complete (RPMI 1640 [Cellgro] supplemented with 10% FBS [Invitrogen], 10 mM Hepes [Cellgro], 100 U/ml penicillin–streptomycin [Thermo Fisher Scientific], and 50 µM 2-mercaptoethanol) and were plated at 2.5 × 106 cells/ml in 24-well plates precoated with 3 µg/ml anti-CD3 (clone 2C11) and 0.5 µg/ml anti-CD28 (clone 37.51). After 48 h of culture, cells were harvested for flow cytometric and immunoblotting analysis.
Isotype class switch assays were performed with total splenocytes. Cells were resuspended in RPMI Complete supplemented with 5 µg/ml F(ab′)2 goat anti–mouse IgM (Jackson ImmunoResearch Laboratories), 100 µg/ml IFN-γ (R&D Systems), and 20 µg/ml LPS (Sigma) and were plated at 2.5 × 105 cells/ml in 24-well plates. Fresh media was added to the cultures after 48 h, and cells were harvested for flow cytometric analysis after 96 h.
For Th1 cell differentiation assays, total splenocytes were cultured in T cell media (Iscove’s medium [Cellgro] with 10% FBS, 1 mM sodium pyruvate, 100 U/ml penicillin–streptomycin, 2.5 µM 2-mercaptoethanol, and 20 U/ml human IL-2 [NCI Preclinical Repository]) supplemented with 20 ng/ml of mouse IL-12 (R&D Systems), 10 µg/ml anti–IL-4 (clone 11B.11), 0.5 µg/ml anti-CD3 (clone 2C11), and 2 µg/ml anti-CD28 (clone 37.51). Cells were resuspended at 3 × 106 cells/ml and cultured in 24-well plates. After 72 h, cultures were transferred into 6-well plates and supplemented with fresh T cell media supplemented with IL-2, IL-12, and anti–IL-4, and then cultured for an additional 48 h. Before flow cytometric analysis, cells were stimulated with 100 ng/ml PMA (Sigma-Aldrich) and 2 µg/ml ionomycin (EMD) for 3 h in the presence of GolgiPlug (BD). CD8+ effector T cells were generated by A. Intlekofer (University of Pennsylvania, Philadelphia, PA), as previously described (Intlekofer et al., 2005).
Quantitative PCR.
For quantitative PCR, total RNA was isolated from frozen cell pellets with the RNeasy Micro Kit (QIAGEN) or from freshly dissected spinal cords with TRIzol reagent. cDNA was synthesized from cell pellets by using Moloney murine leukemia virus (Invitrogen) or from DNase-treated spinal cord RNA by using SuperScript II reverse transcription, and served as a template for subsequent amplifications using either 2× SYBR Green (Roche) or AmpliTaqGold (Applied Biosystems) PCR kits. Samples were analyzed using an ABI 7300 (Applied Biosystems).
Retroviral vector constructs and transductions.
Construction of an MSCV2.2 retroviral vector containing a FLAG-tagged mouse S1P1, IRES, and a truncated human CD4 (hCD4) has been previously described (Lo et al., 2005). MSCV2.2 vector constructs containing FLAG-tagged mouse S1P5, wild-type mouse T-bet, and Duane T-bet upstream of the IRES hCD4 elements were generated in a similar fashion. The generation of MSCV2.2 vectors containing HA-tagged mouse CD69 or a chimeric molecule of the extracellular and transmembrane domains of mouse CD69 and the cytoplasmic domain of human CD3ζ upstream of an IRES and GFP element has been described previously (Shiow et al., 2006). These vectors were used to transfect Bosc23 packaging cells, and supernatants containing infectious viral particles were collected as previously described (Lo et al., 2005). Retroviruses were subsequently used to spinfect WEHI-231 and 2B4 cells. For Western blotting and quantitative PCR analysis of T-bet overexpression in WEHI-231 cells, equal numbers of cells gated for equivalent expression of the hCD4 reporter were sorted for each transduction by using a FACSAria (BD) and snap frozen in liquid nitrogen. Transduction of primary T cells was performed as previously described (Intlekofer et al., 2005), and GFP+ cells were sorted for quantitative PCR analysis and snap frozen.
Immunoprecipitation and Western blotting.
Immunoprecipitation was performed as previously described (Shiow et al., 2006). For immunoblot analysis, cell pellets were lysed in 0.875% Brij97, 0.125% NP-40, 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), and 0.02% NaN3 buffer containing protease inhibitors (Sigma-Aldrich). Samples were resolved by 10% SDS-PAGE (NuPAGE; Invitrogen) and transferred to Immobilon-FL membranes (Millipore). Membranes were blocked with buffer (LI-COR Biosciences) and stained with anti–T-bet (clone 39D raised against full-length mouse T-bet; Santa Cruz Biotechnology, Inc.), rabbit anti-actin (Sigma-Aldrich), anti-FLAG (clone M1; Sigma-Aldrich), and biotin-conjugated anti-HA (clone 3F10; Roche). Products were detected with IRDye 680–conjugated goat anti–mouse Ig, IRDye 800CW–conjugated streptavidin (LI-COR Biosciences), or IRDye 700DX–conjugated donkey anti–rabbit Ig (Rockland), and imaged on an infrared imager (Odyssey; LI-COR Biosciences).
ChIP.
ChIP was performed as previously described (Miller et al., 2008). Primers specific to the 3′ region of the S1pr5 gene were as follows: forward, 5′-CACCTTGCAGTAGCTCTGAG-3′, and reverse, 5′-GGAGCCAGCCGGACTGTATC-3′.
Luciferase reporter and endogenous T-bet target transcription analyses.
Luciferase and endogenous gene quantitative RT-PCR analyses were performed as previously described (Miller et al., 2008).
NFAT-GFP reporter assay.
S1P receptor and CD69–hCD3ζ fusion proteins were cotransduced into mouse 2B4 hybridoma cells with an NFAT-GFP reporter construct and were assayed as previously described (Arase et al., 2002; Rosen et al., 2005; Shiow et al., 2006).
S1PR down-modulation assay.
WEHI-231 cells stably expressing FLAG-tagged S1PR were washed twice in RPMI 1640 medium with 10 mM Hepes and 0.5% fatty acid–free BSA (EMD; BSA-RPMI), and then incubated in BSA-RPMI for 30 min at 37°C. S1PR ligands indicated in the figures (S1P or FTY720-P) were titrated into BSA-RPMI in a 96-well U-bottom plate. Cells were added to each well, and the plate was incubated for 40 min at 37°C. The resulting surface S1PR levels were measured by flow cytometry using the anti-FLAG antibody (clone M2; Sigma-Aldrich).
Transwell migration.
Transwell migrations were performed as previously described (Matloubian et al., 2004; Lo et al., 2005). In brief, transduced WEHI-231 cells were harvested, washed, and incubated for 30 min at 37°C in migration media (RPMI 1640 supplemented with 10 mM Hepes, 100 U/ml penicillin–streptomycin, and 0.5% fatty acid–free BSA). 106 cells were loaded into 5-µm Transwells (Sigma-Aldrich) and allowed to transmigrate for 3 h in response to media alone, stromal cell–derived factor 1 (PeproTech), or S1P (Sigma-Aldrich). Contents from the bottom of each well were harvested and enumerated by flow cytometry.
Online supplemental material.
Table S1 lists nucleotide sequences for PCR primer and probe sets. Fig. S1 contains representative flow cytometric analysis and NK cell counts from various tissues from Duane and wild-type mice. Fig. S2 presents mapping and nucleotide sequencing of the causative genetic lesion within the Duane Tbx21 gene, amplification and sequencing of Duane and wild-type T-bet cDNA, and quantitative PCR and Western blot analysis of T-bet expression in Duane and wild-type mice. Fig. S3 presents assays demonstrating that the Duane T-bet mutation results in a functionally null phenotype. Fig. S4 contains analysis of NK cell numbers in peripheral LNs from mice after blocking lymphocyte entry in the presence or absence of FTY720, and representative flow cytometric analysis of untreated and S1P- or FTY720-treated 2B4 hybridoma cells transduced with either FLAG-S1P1 or FLAG-S1P5. Fig. S5 illustrates the generation of S1P5-deficient mice. Fig. S6 presents representative cell-surface staining for CD69 and FLAG on transduced 2B4 hybridoma cells used for NFAT-GFP reporter assays. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090525/DC1.
Acknowledgments
We thank S. Coughlin and R. Proia for mice; A. Intlekofer for the CD8+ effector T cells; A. Yang for generation of the S1pr5 targeting construct; J. Green, T. Pham, and B. Whittle for technical advice; and J. An for expert animal husbandry.
A. Enders was supported by a Deutsche Forschungsgemeinschaft Research Fellowship (EN 790/1-1) and the Ramaciotti Foundation. A.S. Weinmann is supported by grants from the National Institute of Allergy and Infectious Disease (AI061061) and the American Cancer Society. S.A. Miller is supported by a predoctoral training grant from the National Institute of General Medical Sciences (T3207270). C.C. Goodnow was supported by the Ramaciotti Foundation and is an Australian Research Council Federation Fellow. L.L. Lanier is an American Cancer Society Research Professor. J.G. Cyster is an investigator of the Howard Hughes Medical Institute. This work was supported by grants from the National Institutes of Health (AI52127, BAA-NIH-NIAID-DAIT-07-35, AI74847, and DA019674).
The authors have no financial conflicts of interest.
Footnotes
Abbreviations used:
- ChIP
- chromatin immunoprecipitation
- ENU
- ethylnitrosourea
- HA
- hemagglutinin
- iNKT
- invariant NKT
- LCMV
- lymphocytic choriomeningitis
- S1P
- sphingosine-1-phosphate
- S1P1
- S1P receptor 1
- SNP
- single nucleotide polymorphism
References
- Allende M.L., Dreier J.L., Mandala S., Proia R.L. 2004. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279:15396–15401 10.1074/jbc.M314291200 [DOI] [PubMed] [Google Scholar]
- Allende M.L., Zhou D., Kalkofen D.N., Benhamed S., Tuymetova G., Borowski C., Bendelac A., Proia R.L. 2008. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J. 22:307–315 10.1096/fj.07-9087com [DOI] [PubMed] [Google Scholar]
- Arase H., Mocarski E.S., Campbell A.E., Hill A.B., Lanier L.L. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 296:1323–1326 10.1126/science.1070884 [DOI] [PubMed] [Google Scholar]
- Bajénoff M., Breart B., Huang A.Y., Qi H., Cazareth J., Braud V.M., Germain R.N., Glaichenhaus N. 2006. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 203:619–631 10.1084/jem.20051474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Troup D.B., Wilhite S.E., Ledoux P., Rudnev D., Evangelista C., Kim I.F., Soboleva A., Tomashevsky M., Edgar R. 2007. NCBI GEO: mining tens of millions of expression profiles–database and tools update. Nucleic Acids Res. 35:D760–D765 10.1093/nar/gkl887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beider K., Nagler A., Wald O., Franitza S., Dagan-Berger M., Wald H., Giladi H., Brocke S., Hanna J., Mandelboim O., et al. 2003. Involvement of CXCR4 and IL-2 in the homing and retention of human NK and NK T cells to the bone marrow and spleen of NOD/SCID mice. Blood. 102:1951–1958 10.1182/blood-2002-10-3293 [DOI] [PubMed] [Google Scholar]
- Beima K.M., Miazgowicz M.M., Lewis M.D., Yan P.S., Huang T.H., Weinmann A.S. 2006. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J. Biol. Chem. 281:11992–12000 10.1074/jbc.M513613200 [DOI] [PubMed] [Google Scholar]
- Bernardini G., Sciumè G., Bosisio D., Morrone S., Sozzani S., Santoni A. 2008. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood. 111:3626–3634 10.1182/blood-2007-08-106203 [DOI] [PubMed] [Google Scholar]
- Bult C.J., Eppig J.T., Kadin J.A., Richardson J.E., Blake J.A.; Mouse Genome Database Group 2008. The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic Acids Res. 36:D724–D728 10.1093/nar/gkm961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Kawashima H., Lowe J.B., Lanier L.L., Fukuda M. 2005. Suppression of tumor formation in lymph nodes by L-selectin–mediated natural killer cell recruitment. J. Exp. Med. 202:1679–1689 10.1084/jem.20051473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.Y., Osada H., Santamaria-Babi L.F., Kannagi R. 2006. Interaction of GATA-3/T-bet transcription factors regulates expression of sialyl Lewis X homing receptors on Th1/Th2 lymphocytes. Proc. Natl. Acad. Sci. USA. 103:16894–16899 10.1073/pnas.0607926103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combet C., Jambon M., Deléage G., Geourjon C. 2002. Geno3D: automatic comparative molecular modelling of protein. Bioinformatics. 18:213–214 10.1093/bioinformatics/18.1.213 [DOI] [PubMed] [Google Scholar]
- Djuretic I.M., Levanon D., Negreanu V., Groner Y., Rao A., Ansel K.M. 2007. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 8:145–153 10.1038/ni1424 [DOI] [PubMed] [Google Scholar]
- Garrod K.R., Wei S.H., Parker I., Cahalan M.D. 2007. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc. Natl. Acad. Sci. USA. 104:12081–12086 10.1073/pnas.0702867104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabrera P.J., Hla T., Rosen H. 2007. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J. Biol. Chem. 282:7254–7264 10.1074/jbc.M610581200 [DOI] [PubMed] [Google Scholar]
- Gräler M.H., Goetzl E.J. 2004. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 18:551–553 [DOI] [PubMed] [Google Scholar]
- Grégoire C., Chasson L., Luci C., Tomasello E., Geissmann F., Vivier E., Walzer T. 2007. The trafficking of natural killer cells. Immunol. Rev. 220:169–182 10.1111/j.1600-065X.2007.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N., Peitsch M.C. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 18:2714–2723 10.1002/elps.1150181505 [DOI] [PubMed] [Google Scholar]
- Gustafsson K., Ingelsten M., Bergqvist L., Nyström J., Andersson B., Karlsson-Parra A. 2008. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res. 68:5965–5971 10.1158/0008-5472.CAN-07-6494 [DOI] [PubMed] [Google Scholar]
- Hwang E.S., Szabo S.J., Schwartzberg P.L., Glimcher L.H. 2005. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 307:430–433 10.1126/science.1103336 [DOI] [PubMed] [Google Scholar]
- Intlekofer A.M., Takemoto N., Wherry E.J., Longworth S.A., Northrup J.T., Palanivel V.R., Mullen A.C., Gasink C.R., Kaech S.M., Miller J.D., et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6:1236–1244 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- Intlekofer A.M., Banerjee A., Takemoto N., Gordon S.M., Dejong C.S., Shin H., Hunter C.A., Wherry E.J., Lindsten T., Reiner S.L. 2008. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 321:408–411 10.1126/science.1159806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., Kaech S.M. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 27:281–295 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlhofer F.M., Yokoyama W.M. 1991. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK1.1 antigen. IL-2-activated NK cells possess additional specific stimulation pathways. J. Immunol. 146:3662–3673 [PubMed] [Google Scholar]
- Kassim S.H., Rajasagi N.K., Ritz B.W., Pruett S.B., Gardner E.M., Chervenak R., Jennings S.R. 2009. Dendritic cells are required for optimal activation of natural killer functions following primary infection with herpes simplex virus type 1. J. Virol. 83:3175–3186 10.1128/JVI.01907-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.D., Miller S.A., Miazgowicz M.M., Beima K.M., Weinmann A.S. 2007. T-bet’s ability to regulate individual target genes requires the conserved T-box domain to recruit histone methyltransferase activity and a separate family member-specific transactivation domain. Mol. Cell. Biol. 27:8510–8521 10.1128/MCB.01615-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wada R., Yamashita T., Mi Y., Deng C.X., Hobson J.P., Rosenfeldt H.M., Nava V.E., Chae S.S., Lee M.J., et al. 2000. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106:951–961 10.1172/JCI10905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.G., Xu Y., Proia R.L., Cyster J.G. 2005. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201:291–301 10.1084/jem.20041509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 26:503–517 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Villarino G., Maldonado-Lopez R., Possemato R., Penaranda C., Glimcher L.H. 2003. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc. Natl. Acad. Sci. USA. 100:7749–7754 10.1073/pnas.1332767100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G.J., Card D., Keohane C., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296:346–349 10.1126/science.1070238 [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A., Thomsen L.L., Brett S., Gerard C., Lipp M., Lanzavecchia A., Sallusto F. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260–1265 10.1038/ni1138 [DOI] [PubMed] [Google Scholar]
- Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- Matsuda J.L., Naidenko O.V., Gapin L., Nakayama T., Taniguchi M., Wang C.R., Koezuka Y., Kronenberg M. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192:741–754 10.1084/jem.192.5.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D.S., Wurster A.L., Weinmann A.S., Grusby M.J. 2005. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc. Natl. Acad. Sci. USA. 102:2016–2021 10.1073/pnas.0409512102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.A., Huang A.C., Miazgowicz M.M., Brassil M.M., Weinmann A.S. 2008. Coordinated but physically separable interaction with H3K27-demethylase and H3K4-methyltransferase activities are required for T-box protein-mediated activation of developmental gene expression. Genes Dev. 22:2980–2993 10.1101/gad.1689708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelms K.A., Goodnow C.C. 2001. Genome-wide ENU mutagenesis to reveal immune regulators. Immunity. 15:409–418 10.1016/S1074-7613(01)00199-6 [DOI] [PubMed] [Google Scholar]
- Oo M.L., Thangada S., Wu M.T., Liu C.H., Macdonald T.L., Lynch K.R., Lin C.Y., Hla T. 2007. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282:9082–9089 10.1074/jbc.M610318200 [DOI] [PubMed] [Google Scholar]
- Pappu R., Schwab S.R., Cornelissen I., Pereira J.P., Regard J.B., Xu Y., Camerer E., Zheng Y.W., Huang Y., Cyster J.G., Coughlin S.R. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 316:295–298 10.1126/science.1139221 [DOI] [PubMed] [Google Scholar]
- Peng S.L., Szabo S.J., Glimcher L.H. 2002. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA. 99:5545–5550 10.1073/pnas.082114899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.P., An J., Xu Y., Huang Y., Cyster J.G. 2009. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat. Immunol. 10:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.H., Okada T., Matloubian M., Lo C.G., Cyster J.G. 2008. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 28:122–133 10.1016/j.immuni.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Goetzl E.J. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5:560–570 10.1038/nri1650 [DOI] [PubMed] [Google Scholar]
- Rosen D.B., Bettadapura J., Alsharifi M., Mathew P.A., Warren H.S., Lanier L.L. 2005. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J. Immunol. 175:7796–7799 [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 309:1735–1739 10.1126/science.1113640 [DOI] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdicková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 440:540–544 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Shiow L.R., Roadcap D.W., Paris K., Watson S.R., Grigorova I.L., Lebet T., An J., Xu Y., Jenne C.N., Föger N., et al. 2008. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat. Immunol. 9:1307–1315 10.1038/ni.1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.J., Kim S.T., Costa G.L., Zhang X., Fathman C.G., Glimcher L.H. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 100:655–669 10.1016/S0092-8674(00)80702-3 [DOI] [PubMed] [Google Scholar]
- Townsend M.J., Weinmann A.S., Matsuda J.L., Salomon R., Farnham P.J., Biron C.A., Gapin L., Glimcher L.H. 2004. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 20:477–494 10.1016/S1074-7613(04)00076-7 [DOI] [PubMed] [Google Scholar]
- Vaessen L.M., van Besouw N.M., Mol W.M., Ijzermans J.N., Weimar W. 2006. FTY720 treatment of kidney transplant patients: a differential effect on B cells, naïve T cells, memory T cells and NK cells. Transpl. Immunol. 15:281–288 10.1016/j.trim.2006.02.002 [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mempel T.R. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3:867–878 10.1038/nri1222 [DOI] [PubMed] [Google Scholar]
- Walzer T., Bléry M., Chaix J., Fuseri N., Chasson L., Robbins S.H., Jaeger S., André P., Gauthier L., Daniel L., et al. 2007a. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA. 104:3384–3389 10.1073/pnas.0609692104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer T., Chiossone L., Chaix J., Calver A., Carozzo C., Garrigue-Antar L., Jacques Y., Baratin M., Tomasello E., Vivier E. 2007b. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 8:1337–1344 10.1038/ni1523 [DOI] [PubMed] [Google Scholar]
- Watt S.V., Andrews D.M., Takeda K., Smyth M.J., Hayakawa Y. 2008. IFN-gamma-dependent recruitment of mature CD27(high) NK cells to lymph nodes primed by dendritic cells. J. Immunol. 181:5323–5330 [DOI] [PubMed] [Google Scholar]
- Xu W., Zhang J.J. 2005. Stat1-dependent synergistic activation of T-bet for IgG2a production during early stage of B cell activation. J. Immunol. 175:7419–7424 [DOI] [PubMed] [Google Scholar]