Abstract
Interleukin (IL)-17–producing T helper (Th17) cells play a critical role in the pathophysiology of several autoimmune disorders. The differentiation of Th17 cells requires the simultaneous presence of an unusual combination of cytokines: IL-6, a proinflammatory cytokine, and transforming growth factor (TGF) β, an antiinflammatory cytokine. However, the molecular mechanisms by which TGF-β exerts its effects on Th17 cell differentiation remain elusive. We report that TGF-β does not directly promote Th17 cell differentiation but instead acts indirectly by blocking expression of the transcription factors signal transducer and activator of transcription (STAT) 4 and GATA-3, thus preventing Th1 and Th2 cell differentiation. In contrast, TGF-β had no effect on the expression of retinoic acid receptor–related orphan nuclear receptor γt, a Th17-specific transcription factor. Interestingly, in Stat-6−/−T-bet−/− mice, which are unable to generate Th1 and Th2 cells, IL-6 alone was sufficient to induce robust differentiation of Th17 cells, whereas TGF-β had no effect, suggesting that TGF-β is dispensable for Th17 cell development. Consequently, BALB/c Stat-6−/−T-bet−/− mice, but not wild-type BALB/c mice, were highly susceptible to the development of experimental autoimmune encephalomyelitis, which could be blocked by anti–IL-17 antibodies but not by anti–TGF-β antibodies. Collectively, these data provide evidence that TGF-β is not directly required for the molecular orchestration of Th17 cell differentiation.
IL-17 plays a central role in several types of autoimmune inflammation and provides resistance to certain infections (Weaver et al., 2006; Reiner, 2007; Rudner et al., 2007; Zelante et al., 2007). Mice that lack IL-17 or IL-17 receptor are resistant to multiple autoimmune conditions and are susceptible to certain infections. In humans, IL-17 levels in the serum correlate well with the severity of several autoimmune and inflammatory conditions, suggesting that IL-17 is a promising target for these diseases (Iwakura and Ishigame, 2006; Burlingham et al., 2007). A major source of IL-17 is a distinct subset of Th cells termed Th17 cells. These cells produce abundant amounts of IL-17, IL-21, IL-22, and IL-6 (Weaver et al., 2006; Reiner, 2007; Zhou et al., 2007).
Differentiation of Th17 cells is guided by retinoic acid receptor–related orphan nuclear receptors (RORs) α and γt (Ivanov et al., 2007; Yang et al., 2008b), which are activated by various stimuli such as IL-1, TGF-β, and IL-6 (Bettelli et al., 2006; Mangan et al., 2006). Additionally, IL-6– and IL-21–induced activation of transcription factor STAT-3 also plays a key role in Th17 cell differentiation (Yang et al., 2007). Thus, mice deficient in RORγt or STAT-3 are unable to generate Th17 cells (Zhou et al., 2007). On the other hand, IL-2–induced activation of STAT-5 dramatically inhibits Th17 cell differentiation (Laurence et al., 2007). Th17 cells do not express the Th1- and Th2-specific transcription factors T-bet and GATA-3. Interestingly, activation of these Th subset lineage-specific transcription factors hinders the differentiation of Th17 cells (Harrington et al., 2005; Mathur et al., 2006; Tato et al., 2006; Veldhoen et al., 2006).
The roles of several cytokines in Th17 cell differentiation have been studied. Initially, it was thought that IL-23 played a critical role in Th17 cell differentiation (Langrish et al., 2005; Kikly et al., 2006). However, recent studies have indicated that IL-23 is involved in promoting the effector functions of Th17 cells (Khader et al., 2007). IL-21, a cytokine of the IL-2 family, was found to profoundly promote Th17 cell expansion and assist the differentiation of Th17 cells (Korn et al., 2007; Nurieva et al., 2007; Zhou et al., 2007), whereas IL-2 drastically inhibited Th17 cell differentiation (Laurence et al., 2007; Stockinger, 2007). Similarly, many other cytokines, including IL-4, IL-12, IL-27, and type I and II IFNs, have also been shown to inhibit Th17 cell differentiation (Harrington et al., 2005; Park et al., 2005; Weaver et al., 2007; Guo et al., 2008).
Recent studies have demonstrated that the differentiation of Th17 cells in vitro requires an unusual but key combination of two cytokines: IL-6, a proinflammatory cytokine, and TGF-β, an antiinflammatory cytokine (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006). Although TGF-β enhances Th17 cell differentiation, it also inhibits Th1 and Th2 cell differentiation (Li et al., 2007). Additionally, both Th1 and Th2 cytokines have been shown to potently inhibit the development of Th17 cells (Park et al., 2005). To determine the mechanism by which TGF-β promotes Th17 differentiation, we investigated whether TGF-β acts directly on the molecular orchestration of Th17 cell differentiation or indirectly through its inhibition of the differentiation of Th1 and Th2 cells. In this paper, we show that mice that are incapable of mounting both Th1 and Th2 cell responses (Stat-6−/−T-bet−/− mice) develop robust experimental autoimmune encephalomyelitis (EAE), even in a genetic background (BALB/c) that is normally resistant to the development of EAE. The CD4+ T cells isolated from these mice generated vigorous Th17 cell responses in IL-6–supplemented cultures regardless of the presence of TGF-β. The presence of TGF-β strongly inhibited STAT-4 and GATA-3 expression in differentiating and committed Th1 and Th2 cells derived from wild-type mice, whereas RORγt expression remained unaffected in either condition. Collectively, these data indicate that TGF-β is dispensable for the molecular orchestration of Th17 cell differentiation and instead promotes Th17 cell differentiation by inhibiting the differentiation of Th1 and Th2 cells.
RESULTS
IL-6 is sufficient to drive Th17 cell differentiation in Stat-6−/−T-bet−/− mice
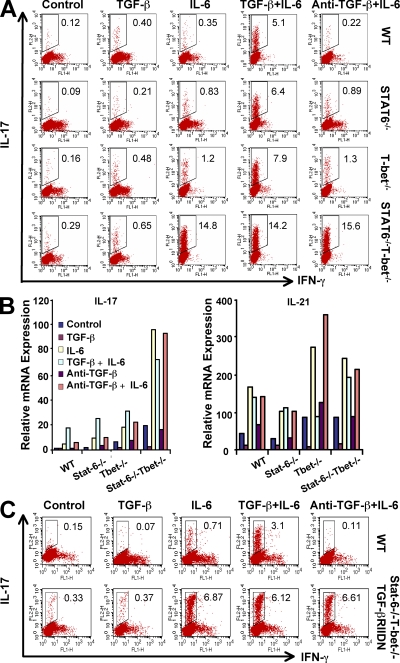
It has been previously shown that activation of the Th1 and Th2 lineage-specific transcription factors T-bet and GATA-3, respectively, or simply addition of cytokines that are produced by Th1 and Th2 cells drastically inhibits Th17 cell differentiation (Harrington et al., 2005; Mathur et al., 2006). Therefore, to study the differentiation of Th17 cells in the absence of Th1 and Th2 cells, we generated Stat-6−/−T-bet−/− double knockout mice. CD4+CD25−CD62LhiCD44lo naive T cells were sorted to a purity level of ≥99.5% from wild-type, Stat-6−/−, T-bet−/−, and Stat-6−/−T-bet−/− mice (Fig. S1 A). We found that upon activation, cells from Stat-6−/−T-bet−/− mice were unable to produce signature cytokines associated with both the Th1 and Th2 cell phenotypes. As expected, cells from T-bet−/− mice failed to produce IFN-γ, whereas cells from Stat-6−/− mice failed to produce IL-4 (Fig. S1 B). To determine the role of IL-6 and/or TGF-β in the differentiation of Th17 cells, we activated FACS-sorted naive CD4+ T cells derived from each of these mouse strains with anti-CD3 and anti-CD28 in the presence of TGF-β, IL-6, or both. We found that IL-6 was sufficient to induce a robust Th17 cell differentiation in naive CD4+ T cells isolated from Stat-6−/−T-bet−/− mice. Addition of TGF-β had no significant effect on the differentiation of Th17 cells (Fig. 1 A). In contrast, differentiation of Th17 cells from wild-type, Stat-6−/−, and T-bet−/− mice required the presence of both IL-6 and TGF-β (Fig. 1 A). By measuring the expression of IL-21 and IL-17 in the resultant cells, we found that IL-6 was sufficient to induce expression of IL-17 in Stat-6−/−T-bet−/− CD4+ T cells but not in wild-type cells (Fig. 1 B). Therefore, these results strongly suggest that TGF-β does not act directly in promoting the differentiation of Th17 cells but rather provides an environment that is conducive to the differentiation of Th17 cells, likely by inhibiting the differentiation of Th1 and Th2 cells. To test whether the production of TGF-β plays a role in the differentiation of Th17 cells in Stat-6−/−T-bet−/− mice, we performed similar experiments with anti–TGF-β antibodies. Addition of anti–TGF-β had no effect on IL-6–mediated differentiation of Th17 cells in Stat-6−/−T-bet−/− mice (Fig. 1 A). However, IL-6 alone was not sufficient to induce Th17 cell differentiation from naive CD4+ T cells derived from wild-type mice, but neutralization of TGF-β with anti–TGF-β antibodies at a concentration of 10 µg/ml completely abrogated Th17 cell differentiation (Fig. S2 A).
Figure 1.
Differentiation of Th17 cells in the absence of Th1, Th2, or Th1 and Th2 cells. (A) FACS-sorted CD25−CD62LhiCD44lo naive CD4+ T cells isolated from wild-type, Stat-6−/−, T-bet−/−, or Stat-6−/−T-bet−/− mice were activated by plate-bound anti-CD3 and anti-CD28 in the presence of 5 ng/ml TGF-β, 20 ng/ml IL-6, or both for 72 h. Intracellular cytokines were detected as described in Materials and methods. The percentage of cells positive for IL-17 is shown from one representative out of three independent experiments. (B) Expression of IL-17 and IL-21 in cells treated as in A was determined by quantitative PCR. (C) Naive CD4+ T cells from Stat-6−/−T-bet−/−/TGF-βRIIDN mice were subjected to Th17 cell differentiation as described in A. Note that these mice and their control littermates are in a mixed genetic background. Results shown are representative of three independent experiments. Four mice per group were used in each experiment.
To determine whether inhibition of Th1 cells or Th2 cells alone is sufficient to generate a robust Th17 cell response, we examined Th17 cell differentiation in CD4+ cells isolated from T-bet−/− and Stat-6−/− mice. We found that neither of these mouse strains was able to mount Th17 cell responses to levels comparable with Stat-6−/−T-bet−/− mice (Fig. 1, A and B), suggesting that simultaneous inhibition of Th1 and Th2 cell differentiation is critical for optimal Th17 cell development in the absence of TGF-β.
To verify the role of TGF-β in the differentiation of Th17 cells, we bred Stat-6−/−T-bet−/− mice with mice transgenic for a dominant-negative TGF-βRII (TGF-βRIIDN) and isolated CD4+ T cells from these Stat-6−/−T-bet−/−/TGF-βRIIDN mice. Upon activation with anti-CD3 and anti-CD28, these cells also exhibited dramatic Th17 cell differentiation in the absence of TGF-β signaling (Fig. 2 A). To further test whether this phenomenon is only limited to gene knockout and chimeric animals, we performed experiments with naive CD4+ T cells from wild-type BALB/c and C57BL/6 mice by blocking Th1 and Th2 cell differentiation with a combination of antibodies against IL-4, IFN-γ, and IL-12. We found that under these conditions, IL-6 alone was also sufficient to induce robust Th17 cell differentiation, whereas the presence of TGF-β had no substantial effect (Fig. S2 B).
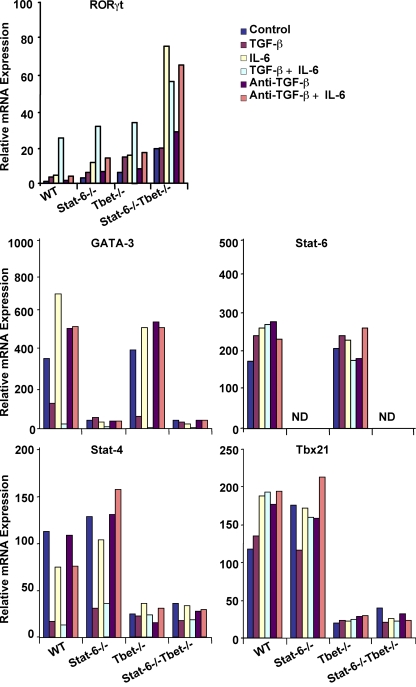
Figure 2.
Regulation of transcription factors in Th cells by TGF-β and IL-6. FACS-sorted CD25−CD62LhiCD44lo naive CD4+ T cells were activated with plate-bound anti-CD3 and anti-CD28 in the presence of 5 ng/ml TGF-β, 20 ng/ml IL-6, or both for 48 h. Anti–TGF-β was added at a concentration of 10 µg/ml. Expression levels of the transcription factors RORγt, GATA-3, Stat-6, Stat-4, and Tbx21 in the cells were determined by quantitative PCR. Results shown are representative of three independent experiments.
TGF-β plays a critical role in the regulation of the differentiation of T reg and Th17 cells (Wahl and Chen, 2003). The fine balance between RORγt and FoxP3 is critical in controlling the differentiation of T reg and Th17 cell responses (Lochner et al., 2008). Therefore, we evaluated the expression of FoxP3 in CD4+ T cells differentiated under different conditions. We did not find significant differences in FoxP3 expression in CD4+ T cells isolated from any of the mouse strains investigated and cultured under Th17 cell differentiation conditions (Fig. S3). Therefore, abnormal differentiation of T reg cells is not the cause of robust differentiation of Th17 cells in Stat-6−/−T-bet−/− mice.
TGF-β inhibits STAT-4 and GATA-3 expression in developing Th1 and Th2 cells
The experiments described in the previous section were performed with T cells that developed in the absence of Th1 and Th2 cells. To further verify that TGF-β exerts its function on Th17 cell differentiation by inhibiting Th1 and Th2 cell differentiation rather than by acting directly on Th17 cells, we treated naive CD4+ T cells from wild-type mice with TGF-β alone during their initial activation. We found that TGF-β did not significantly affect the expression of RORγt, although a dramatic increase in the expression of this orphan receptor was observed when both TGF-β and IL-6 were present (Fig. 2). Interestingly, treatment with TGF-β alone or together with IL-6 prevented the expression of STAT-4 and GATA-3 (Fig. 2). Surprisingly, expression of STAT-6 was unaltered by treatment with TGF-β, either alone or in combination with IL-6. Similarly, a very marginal effect on the expression of T-bet was noted (Fig. 2). Therefore, these findings indicate that TGF-β inhibits Th1 and Th2 cell differentiation specifically through down-regulation of STAT-4 and GATA-3. It is important to emphasize that mice deficient in STAT-4, similar to those deficient in T-bet, are defective in the generation of Th1 cell responses (Kaplan et al., 1996).
Although GATA-3 knockout mice are embryonically lethal, it has been shown that the lack of Th2 cytokine production in mice deficient in the p50 subunit of NF-κB is caused by the inability of T cells from these animals to express GATA-3 (Das et al., 2001). Therefore, to further verify that the effect of TGF-β on Th17 cell differentiation is exerted through the regulation of STAT-4 and GATA-3 expression, we examined the effect of IL-6 and TGF-β on these transcription factors in CD4+ T cells from Stat-6−/− and T-bet−/− mice. As shown in Fig. 2, CD4+ T cells from Stat-6−/− mice did not express detectable levels of GATA-3 but expressed high levels of STAT-4, and the latter became suppressed by culture in the presence of TGF-β. Conversely, T-bet−/− T cells expressed low levels of STAT-4 but high levels of GATA-3, and the latter was also inhibited by TGF-β. Therefore, we conclude that T-bet and STAT-4, as well as STAT-6 and GATA-3, coordinately promote the differentiation of Th cells.
Regulation of Th1, Th2, and Th17 signature cytokines and transcription factors by TGF-β
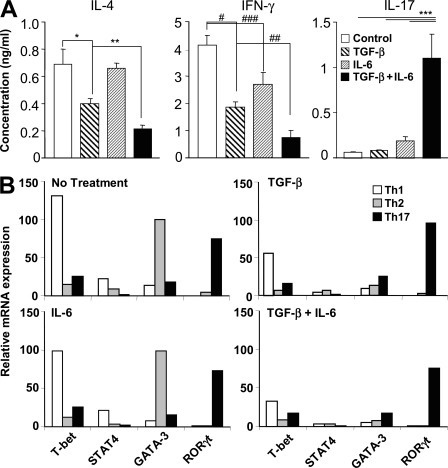
In the preceding experiments, we have shown that TGF-β inhibits the expression of STAT-4 and GATA-3 in developing T cells. Next, we examined the effect of TGF-β, alone or together with IL-6, on the cytokine expression profile of CD4+ T cells. We found that both Th1 and Th2 cytokines were suppressed by TGF-β supplementation, whereas IL-6 had a marginal effect on IFN-γ production but had no effect on IL-4 production. As shown in Fig. 1, the production of IL-17 was enhanced only in the presence of both TGF-β and IL-6 (Fig. 3 A). To further determine whether the presence of Th1 or Th2 cytokines affects the response to TGF-β and IL-6, we examined the expression of RORγt, GATA-3, STAT-4, and T-bet in differentiated Th1, Th2, and Th17 cells derived from wild-type mice treated with TGF-β, IL-6, or both. Unlike in naive CD4+ T cells (Fig. 2), IL-6 had no effect on the expression of these transcription factors in differentiated Th1, Th2, and Th17 cells. On the other hand, TGF-β alone effectively inhibited GATA-3 and STAT-4 expression, and to some extent T-bet expression, in these cells (Fig. 3 B). Similar to naive T cells, however, STAT-6 expression was unaffected in differentiating and fully differentiated Th1 and Th2 cells (unpublished data). Therefore, we conclude that TGF-β inhibits GATA-3 and STAT-4 expression in both differentiating and fully differentiated Th cells.
Figure 3.
TGF-β inhibits GATA-3 and STAT-4 expression in T cells during activation. (A) Naive CD4+ T cells were activated with plate-bound anti-CD3 and anti-CD28 in the presence of 5 ng/ml TGF-β, 20 ng/ml IL-6, or both for 48 h. Cytokines in the supernatants were determined by multiplexed bead array immunoassay. Data represent means ± SEM. (B) Established Th1 and Th2 cells were activated by plate-bound anti-CD3 for 6 h with or without 5 ng/ml TGF-β, 20 ng/ml IL-6, or both. The expression of transcription factors was determined by quantitative PCR. Data shown are representative of four independent experiments. *, P < 0.03; **, P < 0.002; ***, P < 0.001; #, P < 0.003; ##, P < 0.01; and ###, P < 0.005.
Stat-6−/−T-bet−/− mice develop robust EAE
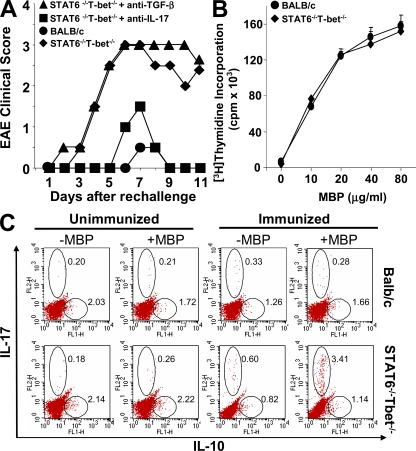
Th17 cells have been implicated in many autoimmune disorders, including EAE (Sutton et al., 2006). As an animal model for multiple sclerosis, EAE can be induced only in certain mouse strains such as SJL/J, whereas strains such as BALB/c are completely resistant. This resistance is not caused by unresponsiveness to neuroantigens, because it has been shown that BALB/c mice can generate myelin basic protein (MBP)–specific pathogenic T cells, which can induce EAE in immunodeficient mice upon adoptive transfer (Jones et al., 1993; Lafaille et al., 1997). Because we found a robust Th17 cell response in Stat-6−/−T-bet−/− mice (Fig. 1 A), we next tested whether Th1 and Th2 cells inhibit the generation of Th17 cells in vivo and if their presence prevents the development of EAE in BALB/c mice. We found that Stat-6−/−T-bet−/− mice on the BALB/c background were capable of developing MBP-induced EAE, whereas wild-type BALB/c, Stat-6−/−, and T-bet−/− mice developed very mild EAE (Fig. 4 A and Fig. S4). To test whether IL-17 is responsible for the pathology of EAE in these mice, we performed antibody neutralizations of IL-17 and TGF-β. Interestingly, administration of anti–IL-17 ameliorated EAE in Stat-6−/−T-bet−/− mice, whereas treatment with anti–TGF-β had no effect (Fig. 4 A). However, treatment with anti–IL-6 significantly inhibited the development of EAE in these animals (Fig. S4), which is in agreement with published data (Gijbels et al., 1995). Previous studies have shown that IL-6–deficient animals are resistant to EAE (Samoilova et al., 1998). Blockade of IL-6 signaling by means of antibodies is in clinical trials for treatment of multiple sclerosis and other inflammatory diseases (Paul-Pletzer, 2006). We also found that MBP-specific proliferation of T cells from Stat-6−/−T-bet−/− mice was similar to that of their wild-type littermates (Fig. 4 B), suggesting normal T cell priming events. However, the observed EAE in Stat-6−/−T-bet−/− mice could be caused by a defect in IL-10–producing cells. Therefore, to test this possibility we analyzed IL-10– and IL-17–producing cells in the lymph nodes of animals that were immunized with MBP. Intracellular cytokine staining showed a dramatic enhancement of IL-17–producing CD4+ T cells, but there was no significant difference in IL-10–producing cells between Stat-6−/−T-bet−/− mice and their wild-type littermates (Fig. 4 C). Collectively, these results provide further evidence that TGF-β does not act directly on Th17 cells in promoting their differentiation in vivo.
Figure 4.
Stat-6−/−T-bet−/− mice develop EAE in an IL-17–dependent manner. (A) Induction of EAE in wild-type and Stat-6−/−T-bet−/− mice on the BALB/c background. Stat-6−/−T-bet−/− mice were injected with neutralizing antibodies against TGF-β or IL-17 twice weekly, EAE was induced, and clinical disease was scored. Data are from five mice per group and are representative of two independent experiments. (B) Antigen-specific proliferation of T cells from wild-type and Stat-6−/−T-bet−/− mice. CD4+ T cells were isolated from MBP-immunized mice, mixed with syngeneic spleen cells at a 1:2 ratio, and challenged with MBP. After 2 d, proliferation was measured by [3H]thymidine incorporation. Data represent means ± SEM. (C) Intracellular cytokine staining for IL-10 and IL-17 was performed in lymph node cells isolated from MBP-immunized mice. Cells were stimulated ex vivo with MBP, and their intracellular cytokines were detected as described in Materials and methods. For B and C, results shown are representative of three independent experiments, and three mice were used per group.
DISCUSSION
Th17 cells are the newest addition to the growing family of Th cell subsets, and are distinct from Th1 and Th2 cells in their cytokine production profile, functions, and differentiation (Harrington et al., 2005). The molecular basis of Th17 cell function and differentiation is poorly understood. The molecular orchestration of Th1 and Th2 cell differentiation requires unique key cytokines, i.e., IL-12 and IL-4, respectively. However, optimal differentiation of these cell subsets requires the absence of inhibitory factors, which can be achieved experimentally with antibodies directed against these opposing cytokines (anti–IL-4 during the differentiation of Th1 cells and anti–IL-12 for Th2 cell differentiation). In contrast, current evidence indicates that Th17 cell differentiation is dependent on two cytokines: IL-6 and TGF-β. The physiological functions of these two cytokines are contrasting, and most notably, TGF-β inhibits many immunological functions, including the production and function of IL-6 (Musso et al., 1990). Thus, we examined how these two cytokines unite for a common purpose in the differentiation of Th17 cells. Our data demonstrate that TGF-β is dispensable for the molecular orchestration of Th17 cell differentiation. Rather, TGF-β potently inhibits Th1 and Th2 cell differentiation by preventing expression of STAT-4 and GATA-3. Strikingly, our in vitro studies revealed that IL-6 is responsible for the differentiation of Th17 cells only when Th1 and Th2 cell differentiation mechanisms are absent, which can be achieved by including TGF-β in the culture medium. Furthermore, similar results were obtained when Th1 and Th2 cell differentiation mechanisms were abolished by genetic ablation of essential transcription factors (Fig. 1). This conclusion is further supported by our results that in mice deficient in both Th1 and Th2 cell differentiation mechanisms, genetic interruption of TGF-β receptor signaling failed to affect Th17 cell differentiation. Therefore, Th17 cell differentiation can be achieved without the presence of TGF-β as long as Th1 and Th2 cytokines are absent.
The development of EAE is dependent on Th17 cells (Ivanov et al., 2007). Previously, it has been shown that mice that are deficient in Th1 cell differentiation exhibit a higher incidence of EAE, suggesting that Th1 cells play an inhibitory role in EAE pathogenesis (Mattner et al., 2000; Lin et al., 2006). Subsequently, it was reported that T-bet up-regulation antagonizes Th17 cell differentiation (Mathur et al., 2006). Our results indicate that CD4+ T cells from T-bet–deficient mice differentiate more effectively into Th17 cells than CD4+ T cells from wild-type animals (Fig. 1). Similarly, mice deficient in STAT-6 show increased Th17 cell differentiation compared with wild-type mice (Fig. 1). Even greater Th17 polarization was achieved in CD4+ T cells from Stat-6 and T-bet double-knockout mice. Therefore, we tested whether resistance to EAE in BALB/c mice could be attributed to the presence of Th1 and Th2 cells. Previous studies have shown that upon immunization with MBP, BALB/c mice produce MBP-specific pathogenic Th cells that can induce EAE upon adoptive transfer (Jones et al., 1993; Lafaille et al., 1997). Therefore, resistance to EAE in BALB/c mice is not MHC mediated. However, we found that Stat-6−/−T-bet−/− mice, which are deficient in both Th1 and Th2 cells, develop strong Th17 cell responses and exhibit robust EAE. These results indicate that conditions that drive the development of either Th1 or Th2 cells inhibit Th17 cell differentiation, thus mediating the resistance of BALB/c mice to EAE.
Our data demonstrate that TGF-β plays an indirect role in the differentiation of Th17 cells. In the absence of Th1 and Th2 cells, IL-6 alone was sufficient to induce Th17 cell differentiation. IL-6 is a pleiotropic cytokine that has been implicated in many autoimmune and inflammatory conditions (Schett, 2008; Schett and David, 2008). In agreement with earlier reports, our data demonstrate that IL-6 plays a central role in the differentiation of pathogenic Th17 cells. In addition, IL-6 constricts suppressor functions of T reg cells, which facilitate the generation of adaptive immune responses (Pasare and Medzhitov, 2003). Thus, IL-6–deficient animals are defective in mounting adaptive immune responses and are resistant to several autoimmune diseases (Pollard et al., 2003). Neutralization of IL-6 or blockade of its receptor by antibodies ameliorates inflammation in several autoimmune diseases. Treatment modalities that are based on IL-6 are currently in advanced clinical trials (Lin et al., 2006; Sebba, 2008). In contrast, TGF-β is well known for its immunosuppressive functions (Wahl and Chen, 2003). It inhibits both innate and adaptive immune responses, including the differentiation of Th1 and Th2 cells. Deficiency of TGF-β or impairment of TGF-β signaling leads to the spontaneous differentiation of Th1 and Th2 cells (Gorelik and Flavell, 2000). Therefore, animals deficient in TGF-β or TGF-βRIIDN develop multiple organ autoimmunity (Kulkarni and Karlsson, 1997; Gorelik and Flavell, 2000). However, our study indicates that TGF-β is incapable of inhibiting the differentiation program of Th17 cells. Thus, our study is consistent with the established immunosuppressive activities of TGF-β, which inhibits Th1 and Th2 immune responses but spares Th17 cells.
In summary, we have shown that TGF-β promotes the differentiation of Th17 cells not by acting directly on the molecular orchestration of these cells but rather by inhibiting the development of Th1 and Th2 cells. Recently, it has been shown that TGF-β is not essential for the differentiation of IL-17–producing human Th cells (Acosta-Rodriguez et al., 2007). On the other hand, some investigators have found that TGF-β plays a critical role in the differentiation of human Th17 cells from naive CD4+ T cell populations (Yang et al., 2008a). Nonetheless, the precise role of TGF-β in the molecular orchestration of human Th17 cells was not established. In our studies, we showed that mouse Th cells that lack the differentiation machinery for both Th1 and Th2 cells (Stat-6−/−T-bet−/− mice) do not require TGF-β for Th17 cell differentiation. In fact, the absence of Th1 and Th2 cells in BALB/c mice allowed the differentiation of encephalitogenic Th17 cells and susceptibility to EAE. Although TGF-β was dispensable for Th17 cell differentiation in the absence of Th1 and Th2 cell responses, IL-6 was still required. Thus, inhibition of IL-6 or its molecular signaling interferes with the differentiation of pathogenic Th17 cells, a finding with potential clinical application for the treatment of autoimmune diseases. In fact, treatment modalities based on IL-6 inhibition are currently undergoing clinical trials in patients with several types of autoimmune inflammation (Choy et al., 2002).
MATERIALS AND METHODS
Animals, cytokines, and antibodies.
BALB/c, STAT-6 knockout (Stat-6−/−), T-bet knockout (T-bet−/−), and TGF-βRIIDN transgenic mice were purchased from The Jackson Laboratory. Stat-6−/−T-bet−/− double-knockout mice were generated by crossbreeding Stat-6−/− and T-bet−/− mice. TGF-βRIIDN/Stat-6−/−T-bet−/− mice were generated by crossing TGF-βRIIDN and Stat-6−/−T-bet−/− mice. Mice of either sex were used at 8–16 wk of age. Mice were housed in a specific pathogen-free colony in the vivarium of the Robert Wood Johnson Medical School. The animal protocols for the experiments described in this paper were approved by the Institutional Animal Care and Use Committee of the Robert Wood Johnson Medical School. Fluorochrome-conjugated antibodies against IFN-γ (XMG1.2) and IL-17 (TC11-18H10), and unconjugated antibodies to CD3 (145.2C11), CD28 (37.51.1), and CD4 (GK1.5) were purchased from BD. Antibodies to cytokines (IL-4, IL-6, IL-12, IFN-γ, and TGF-β) were purchased from R&D Systems. IL-17 (MM17F3)–neutralizing antibodies were purchased from eBioscience.
Differentiation of Th cell subsets.
Naive CD25−CD62LhiCD44lo CD4+ T cells were sorted from splenocytes by using a FACSDiva (BD). These cells were differentiated under Th1- or Th2-generating conditions. In brief, 106 CD4+ lymphocytes/ml were activated with 1 µg/ml anti-CD3 bound to plastic and 2 µg/ml of soluble anti-CD28. 10 ng/ml IL-12 and 10 µg/ml anti–IL-4 were included in Th1 cultures, whereas 5 ng/ml IL-4, anti–IL-12, and anti–IFN-γ (each at 10 µg/ml) were supplied for Th2 cultures. After 24 h, IL-2 was added to all cultures. Cells were divided at a 1:4 ratio after 3 d and allowed to rest under the described cytokine conditions in the absence of anti-CD3 and anti-CD28 for another 2 d. Differentiation of Th17 cells was performed as previously described (Bettelli et al., 2006; Mangan et al., 2006). In brief, FACS-sorted naive CD4+ T cells were activated on plate-bound anti-CD3 and anti-CD28 in the presence of 5 ng/ml TGF-β, 20 ng/ml IL-6, or both. Cultures were terminated after different time points for analysis of mRNA expression and cytokines produced in the supernatants. All cell cultures were maintained in RPMI 1640 medium supplemented with 2 mMl-glutamine, 50 µM 2-mercaptoethanol, 10% heat-inactivated FBS, and 10 mM gentamycin.
Flow cytometric staining and analysis.
For cell-surface staining, cells were suspended in staining buffer (PBS, 3% FCS, 0.01% Na-azide) at a concentration of 107 cells/ml, and 50 µl of suspension was incubated with fluorescent antibodies for 30 min on ice. Cells were washed twice with staining buffer and fixed with 1% paraformaldehyde. For intracellular cytokine staining, cells were treated with 50 ng/ml PMA and 750 ng/ml ionomycin with 10 µg/ml brefeldin A (Sigma-Aldrich) added during the last 6 h of culture. For the determination of cytokines in the MBP-stimulated T cells, draining lymph nodes from EAE-induced mice were stimulated ex vivo with MBP for 24 h, and cytokine expression was determined as described. Cells were washed twice with PBS and resuspended in a permeabilization buffer (Cytofix/Cytoperm kit; BD), and stained with fluorescently conjugated antibodies. Fluorescence intensity was measured by flow cytometry (FACScan; BD).
Detection of cytokines.
Cytokine levels in culture supernatants were determined by multiplexed bead array immunoassay using Luminex technology (Bio-Plex; Bio-Rad Laboratories).
Real-time PCR.
Total RNA was isolated from cell pellets using an RNeasy Mini Kit. Genomic DNA was removed using the RNase-free DNase Set for DNA digestion during RNA purification. First-strand cDNA synthesis was performed using a Sensiscript RT Kit with random hexamer primers (all kits were purchased from QIAGEN). Levels of mRNA of the gene of interest were quantified by real-time PCR (ABI 7900HT Sequence Detection System; Applied Biosystems) using SYBR Green Master Mix (Applied Biosystems). Thermocycling included an initial incubation at 50°C for 2 min, then 95°C for 10 min, followed by a two-step PCR program of 95°C for 15 s and 60°C for 60 s, for a total of 40 cycles. The total amount of mRNA was normalized across samples according to endogenous β-actin mRNA. Primer sequences were as follows: β-actin, (forward) 5′-GTGGGCCGCCCTAGGCACCA-3′ and (reverse) 5′-CTCTTTGATGTCACGCACGATT-3′; GATA-3, (forward) 5′-TTATCAAGCCCAAGCGAAGG-3′ and (reverse) 5′-CATTAGCGTTCCTCCTCCAGAG-3′; STAT-4, (forward) 5′-CCTTCTCCCCATGTCTCCAAGT-3′ and (reverse) 5′-CCGTTTGCACCGTCATTCA-3′; Tbx21/T-bet, (forward) 5′-ACAACCCCTTTGCCAAAGGA-3′ and (reverse) 5′-TCCCCCAAGCAGTTGACAGTT-3′; IL-17, (forward) 5′-CTCCAGAAGGCCCTCAGACTAC-3′ and (reverse) 5′-AGCTTTCCCTCCGCATTGACACAG-3′; RORγt, (forward) 5′-CCGCTGAGAGGGCTTCAC-3′ and (reverse) 5′-TGCAGGAGTAGGCCACATTACA-3′; IL-21, (forward) 5′-CGCCTCCTGATTAGACTTCGTC-3′ and (reverse) 5′-TGCCCCTTTACATCTTGTGGA-3′; and STAT-6, (forward) 5′-GATCATGAACAACACGGTGCC-3′ and (reverse) 5′-CGCTCACAGCGCTTTATTTTCT-3′.
Induction of EAE.
Wild-type BALB/c mice, Stat-6−/− mice, T-bet−/− mice, or Stat-6−/−T-bet−/− mice on a BALB/c background were immunized s.c. at 6–8 wk of age with 200 µg of bovine MBP (4 mg/ml in saline; Sigma-Aldrich) and 200 µg of heat-killed Mycobacterium tuberculosis divided among three sites on their backs on days 0 and 7. On day 1 and again on day 8, mice were injected i.p. with 100 ng pertussis toxin (List Biologicals). For cytokine neutralization experiments, mice were injected with 100 µg anti–IL-17, anti–TGF-β, or anti–IL-6 per mouse every 72 h. Disease scores were measured as follows: 1, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 4, hind and forelimb paralysis; and 5, moribund.
Statistical analyses.
Differences in treatment groups were assessed by a paired two-tailed Student's t test. The significance levels are indicated as follows: *, P < 0.03; **, P < 0.002; ***, P < 0.001; #, P < 0.003; ##, P < 0.01; and ###, P < 0.005.
Online supplemental material.
Fig. S1 shows that Stat-6−/−T-bet−/− mice are incapable of mounting Th1 and Th2 cell responses. Fig. S2 shows that neutralization of TGF-β abrogates Th17 cell differentiation in naive CD4+ T cells from wild-type mice. Fig. S3 shows expression of FoxP3 in Th17 cells. Fig. S4 shows that anti–IL-6 abrogates EAE in Stat-6−/−T-bet−/− mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082286/DC1.
Acknowledgments
This work was supported in part by grants from the Department of Biotechnology, Government of India; the National Institutes of Health (AI057596 and RDE019932); the Chinese Academy of Sciences; and the National Space Biomedical Research Institute (IIH00405), which is supported by the National Aeronautics and Space Administration through Cooperative Agreement NCC 9-58.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- EAE
- experimental autoimmune encephalomyelitis
- MBP
- myelin basic protein
- ROR
- retinoic acid receptor–related orphan nuclear receptor
- TGF-βRIIDN
- dominant-negative TGF-βRII
References
- Acosta-Rodriguez E.V., Napolitani G., Lanzavecchia A., Sallusto F. 2007. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8:942–949 10.1038/ni1496 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Burlingham W.J., Love R.B., Jankowska-Gan E., Haynes L.D., Xu Q., Bobadilla J.L., Meyer K.C., Hayney M.S., Braun R.K., Greenspan D.S., et al. 2007. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J. Clin. Invest. 117:3498–3506 10.1172/JCI28031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E.H., Isenberg D.A., Garrood T., Farrow S., Ioannou Y., Bird H., Cheung N., Williams B., Hazleman B., Price R., et al. 2002. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 46:3143–3150 10.1002/art.10623 [DOI] [PubMed] [Google Scholar]
- Das J., Chen C.H., Yang L., Cohn L., Ray P., Ray A. 2001. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2:45–50 10.1038/83158 [DOI] [PubMed] [Google Scholar]
- Gijbels K., Brocke S., Abrams J.S., Steinman L. 1995. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol. Med. 1:795–805 [PMC free article] [PubMed] [Google Scholar]
- Gorelik L., Flavell R.A. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181 10.1016/S1074-7613(00)80170-3 [DOI] [PubMed] [Google Scholar]
- Guo B., Chang E.Y., Cheng G. 2008. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 118:1680–1690 10.1172/JCI33342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., Zhou L., Littman D.R. 2007. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19:409–417 10.1016/j.smim.2007.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ishigame H. 2006. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116:1218–1222 10.1172/JCI28508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.E., Bourdette D.N., Whitham R.H., Offner H., Vandenbark A.A. 1993. Induction of experimental autoimmune encephalomyelitis in severe combined immunodeficient mice reconstituted with allogeneic or xenogeneic hematopoietic cells. J. Immunol. 150:4620–4629 [PubMed] [Google Scholar]
- Kaplan M.H., Sun Y.L., Hoey T., Grusby M.J. 1996. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 382:174–177 10.1038/382174a0 [DOI] [PubMed] [Google Scholar]
- Khader S.A., Bell G.K., Pearl J.E., Fountain J.J., Rangel-Moreno J., Cilley G.E., Shen F., Eaton S.M., Gaffen S.L., Swain S.L., et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- Kikly K., Liu L., Na S., Sedgwick J.D. 2006. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr. Opin. Immunol. 18:670–675 10.1016/j.coi.2006.09.008 [DOI] [PubMed] [Google Scholar]
- Korn T., Bettelli E., Gao W., Awasthi A., Jäger A., Strom T.B., Oukka M., Kuchroo V.K. 2007. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 448:484–487 10.1038/nature05970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A.B., Karlsson S. 1997. Inflammation and TGF beta 1: lessons from the TGF beta 1 null mouse. Res. Immunol. 148:453–456 10.1016/S0923-2494(97)82669-7 [DOI] [PubMed] [Google Scholar]
- Lafaille J.J., Keere F.V., Hsu A.L., Baron J.L., Haas W., Raine C.S., Tonegawa S. 1997. Myelin basic protein–specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 186:307–312 10.1084/jem.186.2.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A., Tato C.M., Davidson T.S., Kanno Y., Chen Z., Yao Z., Blank R.B., Meylan F., Siegel R., Hennighausen L., et al. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26:371–381 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Li M.O., Wan Y.Y., Flavell R.A. 2007. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 26:579–591 10.1016/j.immuni.2007.03.014 [DOI] [PubMed] [Google Scholar]
- Lin W., Kemper A., Dupree J.L., Harding H.P., Ron D., Popko B. 2006. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 129:1306–1318 10.1093/brain/awl044 [DOI] [PubMed] [Google Scholar]
- Lochner M., Peduto L., Cherrier M., Sawa S., Langa F., Varona R., Riethmacher D., Si-Tahar M., Di Santo J.P., Eberl G. 2008. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORγt+ T cells. J. Exp. Med. 205:1381–1393 10.1084/jem.20080034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O'Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]
- Mathur A.N., Chang H.C., Zisoulis D.G., Kapur R., Belladonna M.L., Kansas G.S., Kaplan M.H. 2006. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 108:1595–1601 10.1182/blood-2006-04-015016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F., Smiroldo S., Galbiati F., Muller M., Di Lucia P., Poliani P.L., Martino G., Panina-Bordignon P., Adorini L. 2000. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur. J. Immunol. 30:498–508 [DOI] [PubMed] [Google Scholar]
- Musso T., Espinoza-Delgado I., Pulkki K., Gusella G.L., Longo D.L., Varesio L. 1990. Transforming growth factor beta downregulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood. 76:2466–2469 [PubMed] [Google Scholar]
- Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A.D., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A.M., Dong C. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483 10.1038/nature05969 [DOI] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C., Medzhitov R. 2003. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 299:1033–1036 10.1126/science.1078231 [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K. 2006. Tocilizumab: blockade of interleukin-6 signaling pathway as a therapeutic strategy for inflammatory disorders. Drugs Today (Barc). 42:559–576 10.1358/dot.2006.42.9.1025692 [DOI] [PubMed] [Google Scholar]
- Pollard K.M., Hultman P., Kono D.H. 2003. Using single-gene deletions to identify checkpoints in the progression of systemic autoimmunity. Ann. NY Acad. Sci. 987:236–239 10.1111/j.1749-6632.2003.tb06053.x [DOI] [PubMed] [Google Scholar]
- Reiner S.L. 2007. Development in motion: helper T cells at work. Cell. 129:33–36 10.1016/j.cell.2007.03.019 [DOI] [PubMed] [Google Scholar]
- Rudner X.L., Happel K.I., Young E.A., Shellito J.E. 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 75:3055–3061 10.1128/IAI.01329-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoilova E.B., Horton J.L., Hilliard B., Liu T.S., Chen Y. 1998. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161:6480–6486 [PubMed] [Google Scholar]
- Schett G. 2008. Review: Immune cells and mediators of inflammatory arthritis. Autoimmunity. 41:224–229 10.1080/08916930701694717 [DOI] [PubMed] [Google Scholar]
- Schett G., David J.P. 2008. Denosumab–a novel strategy to prevent structural joint damage in patients with RA? Nat. Clin. Pract. Rheumatol. 4:634–635 10.1038/ncprheum0927 [DOI] [PubMed] [Google Scholar]
- Sebba A. 2008. Tocilizumab: the first interleukin-6-receptor inhibitor. Am. J. Health Syst. Pharm. 65:1413–1418 10.2146/ajhp070449 [DOI] [PubMed] [Google Scholar]
- Stockinger B. 2007. Good for Goose, but not for Gander: IL-2 interferes with Th17 differentiation. Immunity. 26:278–279 10.1016/j.immuni.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato C.M., Laurence A., O'Shea J.J. 2006. Helper T cell differentiation enters a new era: le roi est mort; vive le roi! J. Exp. Med. 203:809–812 10.1084/jem.20060522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Wahl S.M., Chen W. 2003. TGF-beta: how tolerant can it be? Immunol. Res. 28:167–179 10.1385/IR:28:3:167 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Harrington L.E., Mangan P.R., Gavrieli M., Murphy K.M. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688 10.1016/j.immuni.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821–852 10.1146/annurev.immunol.25.022106.141557 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282:9358–9363 10.1074/jbc.C600321200 [DOI] [PubMed] [Google Scholar]
- Yang L., Anderson D.E., Baecher-Allan C., Hastings W.D., Bettelli E., Oukka M., Kuchroo V.K., Hafler D.A. 2008a. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 454:350–352 10.1038/nature07021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., et al. 2008b. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 28:29–39 10.1016/j.immuni.2007.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T., De Luca A., Bonifazi P., Montagnoli C., Bozza S., Moretti S., Belladonna M.L., Vacca C., Conte C., Mosci P., et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur. J. Immunol. 37:2695–2706 10.1002/eji.200737409 [DOI] [PubMed] [Google Scholar]
- Zhou L., Ivanov I.I., Spolski R., Min R., Shenderov K., Egawa T., Levy D.E., Leonard W.J., Littman D.R. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]