Abstract
Neutrophils are rapidly and massively recruited to the site of Leishmania inoculation, where they phagocytose the parasites, some of which are able to survive within these first host cells. Neutrophils can thus provide a transient safe shelter for the parasites, prior to their entry into macrophages where they will replicate. In addition, neutrophils release and synthesize rapidly several factors including cytokines and chemokines. The mechanism involved in their rapid recruitment to the site of parasite inoculation, as well as the putative consequences of their massive presence on the microenvironment of the focus of infection will be discussed in the context of the development of the Leishmania-specific immune response.
1. Introduction
Obligate intracellular protozoa of the genus Leishmania are sand fly-transmitted parasites capable of infecting various mammalian hosts including rodents, dogs, and humans. In man, infection with different species of Leishmania leads to a large spectrum of clinical manifestations including cutaneous, mucocutaneous, and visceral forms. Spontaneous cure of skin ulcers and life-long immunity to reinfection are the most common outcome in cutaneous leishmaniasis (CL), but diffuse or mucocutaneous forms of the disease may also develop. Mucocutaneous leishmaniasis (MCL) is mostly related to Leishmania species of the New World and can lead to partial or total destruction of the mucosal epithelia of the mouth, nose, throat, and associated tissues. Visceral leishmaniasis (VL) may display several clinical manifestations ranging from subclinical infection to potentially lethal forms if not treated, with heavy parasite burdens in the spleen, liver, and bone marrow, associated with anemia, splenomegaly, hepatomegaly, fever, and loss of weight. Differences in clinical outcome and manifestations of leishmaniasis result from several parameters including the nature of the infecting Leishmania species and host genetic factors. There are currently no available vaccines.
In order to decipher the mechanisms of the immune response involved in susceptibility or resistance to infection, the most widely used experimental model of cutaneous leishmaniasis relies on the infection of mice with L. major. Subcutaneous infection with L. major promastigotes leads in most strains of mice (the so-called “resistant” strains, e.g., C57BL/6, C3H, CBA) to the development of a small, self-healing lesion, to the control of parasite replication, and to immunity against reinfection. In contrast, in a few (susceptible) strains of mice such as BALB/c, sustained inflammatory lesions develop and parasite replication is not controlled, with spreading of parasites to nondraining lymph nodes and spleen. Susceptibility or resistance to infection was shown to result from the development of a subset of T cells distinguished by the cytokines they secrete. Emergence of Leishmania-specific Th2 cells, characterized by the secretion of IL-4 and IL-13, was shown to correlate with susceptibility to infection. In contrast, resistance to infection was associated with the IL-12 driven secretion of IFNγ by Th1 cells. IFNγ activates the microbicidal properties of phagocytes leading to parasite clearing and healing of the lesions [1, 2].
The driving events leading to the development of either a protective response or nonhealing lesions were reported to occur within the first hours to 3 days after infection with L. major, at a time during which neutrophils are massively recruited as a result of infection. Indeed neutrophils are the first cells to be mobilized and arrive within hours to the site of tissue damage and parasite entry. Together with macrophages they phagocytose Leishmania, but only macrophages function as definitive host cells for Leishmania. The rapid recruitment of neutrophils to the site of infection was first described following needle inoculation of a large number of parasites into the skin [3, 4] and elegantly confirmed following infection with the natural vector (infected Phlebotomus dubosqui sand flies) using two photon intravital microscopy [5]. Importantly, in resistant strains of mice, neutrophils are recruited within hours of parasite inoculation but their level decreases to 1%–2% of the cellular infiltrate 3 days after infection. In contrast, in susceptible BALB/c mice neutrophils are still recruited and detected in large numbers at the site of infection more than 10 days after parasite inoculation. The importance of the newly migrating polymorphonuclear leukocytes (PMN) in the subsequent development of Leishmania-protective immune response will be the subject of this review.
2. Interactions between Neutrophils and Leishmania during the First Days of Parasite Inoculation
One of the classical functions attributed to neutrophils is their capacity to phagocytose and kill microorganisms. However, some pathogens including Leishmania can survive transiently within neutrophils. To this end, the parasite has developed several protective mechanisms including the prevention of the activation of an oxidative burst, thus avoiding the generation of highly toxic reactive oxygen species [6] and the ability to be targeted to nonlytic compartments of neutrophils, as recently reported for L. donovani [7].
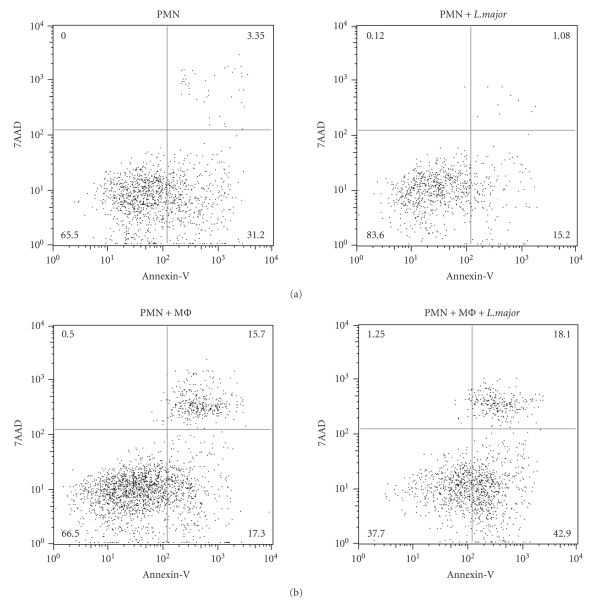
Neutrophils have a short lifespan and become rapidly apoptotic, leading to their phagocytosis by macrophages. However, following infection, their lifespan can be increased to several days. Indeed, infection of human neutrophils in vitro with L. major increased their lifespan to two days, inhibiting the processing of procaspases in the infected cells [8, 9]. In order to test if infection of mouse neutrophils with L. major also delays apoptosis, highly purified inflammatory neutrophils were isolated from the peritoneal cavity of mice four hours after injection of L. major i.p. and cultured for 24 hours alone or in the presence of L. major promastigotes. Neutrophil apoptosis was measured by FACS. Early apoptosis, characterized by the presence of phosphatidyl serine on the cell surface, was detected by Annexin-V staining, while staining with both Annexin-V and 7AAD was indicative of late apoptosis/necrosis. Exposure to L. major decreased markedly the percentage of both early and late apoptosis (Figure 1(a)). Coculture of neutrophils with an excess of macrophages (2:1 mφ:PMN ratio) was previously reported to increase significantly neutrophil apoptosis [10]. To investigate if macrophage-induced neutrophil apoptosis was modulated by L. major, neutrophils and macrophages were cocultured with or without metacyclic L. major promastigotes. No decrease in neutrophil apoptosis was measured in L. major-infected relative to noninfected cultures; indeed an increase in early apoptosis (Annexin-V+, 7AAD− neutrophils) was noted in neutrophils incubated with both macrophages and L. major (Figure 1(b)) as already shown [10]. The acceleration of neutrophil apoptosis by macrophages was reported to be mediated by the transmembrane form of Tumor necrosis factor (mTNF) on macrophages. Using mice that express a functional mTNF but do not release soluble TNF, it was shown that the sole presence of transmembrane TNF allowed the control (decrease) of neutrophil number at the site of parasite inoculation seven days postinfection, resulting in the resolution of the inflammatory lesion [10, 11].
Figure 1.
Exposure of neutrophils to L. major decreases spontaneous but not macrophage-induced apoptosis. L. major-recruited C57BL/6 neutrophils (PMNs) and macrophages (Mϕ) were isolated from the peritoneal cavity 4 hours or 24 hours after L. major injection i.p., respectively. (a) MACS-purified PMNs were cultured for 24 hours in presence or in absence of metacyclic L. major (L. major : PMN ratio 1 : 5). Cells were collected, labeled with Annexin-V, 7AAD, and the 1A8 mAb (Ly6G) and PMN apoptosis analyzed by FACS, gating on the 1A8+ PMN population. Early apoptotic cells are Annexin-V+ 7AAD−, and late apoptotic/necrotic cells are Annexin-V+7AAD+. (b) PMNs were cultured in presence of fixed Mϕ(PMN : Mϕ1 : 2) with or without parasites, and 24 hours later, neutrophils were analyzed as in A. Data are representative of three independent experiments.
Another study has revealed a further effect of the neutrophil-macrophage interaction on the fate of the intracellular parasite. Indeed in susceptible BALB/c mice, interaction between macrophages and dead neutrophils was reported to exacerbate parasite growth through the production of PGE2 and TGFβ by macrophages. In contrast, using cells from resistant C57BL/6 mice, interaction of dead neutrophils with macrophages promoted parasite killing, through secretion of TNF by macrophages [12].
Thus, it appears that at the onset of infection, neutrophils provide transiently a shelter to L. major, which in turn delays apoptosis of the cells as long as the increasing number of monocytes/macrophages at the site of parasite inoculation does not reverse this trend. When the ratio between leukocytes of the monocyte/macrophage and neutrophil increases two or three days following needle inoculation with a high dose of L. major in C57BL/6 mice, or following infection through the bite of L. major-infected sand flies, macrophages become the dominant cell population in the cellular infiltrate [3–6], favouring neutrophil apoptosis. When neutrophils become apoptotic, the parasites are transferred to macrophages where they will replicate.
The exact way live parasites present within neutrophils are transferred to macrophages is currently not clear and may include several modes of entry. Data obtained in vitro showed that macrophages can phagocytose apoptotic neutrophils containing intact Leishmania, providing the parasite with a silent “Trojan horse” mode of infection [9, 13].
Recently, Peters et al. purified Leishmania-infected neutrophils and injected them into the skin of mice that had been exposed to the bites of uninfected sand flies (to condition the site of inoculation of the infected cells to resemble as closely as possible that of a natural infection). In vivo visualization of the interaction between neutrophils and macrophages revealed that neutrophil-containing Leishmania were not directly phagocytosed by macrophages, but rather, neutrophils released the parasites that subsequently entered macrophages [5]. More studies will be needed to evaluate if there exists distinct modes of transfer from neutrophils to macrophages between different Leishmania species or between strains of differing virulence within a given species.
That neutrophils play a decisive role at the onset of infection by Leishmania parasites is also suggested by studies on experimental visceral leishmaniasis. Indeed, neutrophils were shown to have both a direct impact on parasite killing and on the protective immune response developing following infections with L. donovani or L. infantum [14–16]. A small percentage of L. donovani parasites was reported to escape direct killing in neutrophils, being directed to nonlytic compartments of the cells. Macrophages appeared to be able to phagocytose the parasitized neutrophils in vitro [7], but whether this or additional mechanism(s) of L. donovani entry exist during infection remains to be determined.
3. Neutrophils as Orchestrators of the Immune Response
In addition to their phagocytic function, neutrophils contribute to the initiation of inflammation, a process which is recognized as essential in launching immunity. The importance of neutrophils as decision shapers in the development of an immune response is only emerging as they have long been considered by immunologists as short lived, nondividing cells of poor interest [17]. This view is now changing, and neutrophils currently appear not only as key components of the inflammatory response but also as cells that display important immunoregulatory roles in different microbial infections [4, 18–21]. In this part of the review, we will focus (1) on the parameters controlling neutrophil early recruitment following L. major infection and (2) on the release by neutrophils of cytokines and chemokines that may influence the development of a protective immune response against the parasite.
3.1. Mediators of Early Neutrophil Recruitment
During homeostasis, neutrophils are circulating in the blood. Upon deposition of L. major in the skin, the sand fly (or the needle) causes tissue injury, favouring within hours massive neutrophil influx from the bloodstream to the site of Leishmania inoculation. The factors involved in their rapid recruitment are still not well defined and may involve chemokines, cytokines and other molecules secreted by the host and/or the parasite, as discussed below.
Neutrophils are predominantly responsive to members of the CXC chemokine family such as IL-8, a chemokine not only primarily secreted by epithelial cells, keratinocytes, fibroblasts, and endothelial cells but also by neutrophils. Indeed, in response to L. major, human neutrophils were shown to release IL-8 in vitro [22], a process that should favour their own recruitment. In the mouse IL-8 has two functional homologues, MIP-2 (CXCL2, Groβ) and KC (CXCL1, Groα). Upon L. major infection, KC mRNA has been reported to be rapidly and transiently induced in L. major infected skin, suggesting a possible association between KC transcription and granulocyte recruitment [23]. Thus IL-8 in humans and KC in mice may contribute to the early neutrophil recruitment at the site of parasite inoculation but their direct involvement during infection remains to be demonstrated in vivo.
Neutrophil recruitment can also be induced by cytokines such as IL-17 and Tumor necrosis factor (TNF). Among the family of IL-17 cytokines, IL-17A and IL-17F are able to promote the recruitment of monocytes and neutrophils via the induction of other cytokines and chemokines such as G-CSF and IL-8 by various cell types (reviewed in [24]). The role of IL-17 on neutrophil recruitment to the site of L. major inoculation has been investigated in BALB/c mice genetically deficient in this cytokine (IL-17−/− mice). During the first three weeks following L. major inoculation, neutrophil recruitment at the site of infection did not differ between IL-17−/− and control mice. However, from four weeks after infection neutrophil numbers in the infected skin were significantly lower in absence of IL-17 [25]. Thus IL-17 does not seem to be a major contributor of the early neutrophil recruitment occurring during the first days of infection but appears to be involved in promoting neutrophil influx into the infection site at later time points.
Following infection with L. major, mice deficient in TNF or in one of its receptor (TNFR1 or p55) develop nonhealing ulcers, revealing an essential role for TNF in the control of inflammatory lesions [26–31]. Furthermore, using mTNF-knock-in (mTNFΔ/Δ) mice, which express functional mTNF but do not release soluble TNF, the transmembrane form of TNF was shown to be crucial in resolution of the inflammation associated with the leishmanial ulcer. Indeed following L. major infection, (mTNFΔ/Δ) mice, unlike TNF−/− mice, were able to heal their lesion, a process associated with clearance of neutrophils from the infection site [11]. In addition, we further showed that transmembrane TNF was essential in the control of neutrophil presence one week after infection. Neutrophil numbers were still elevated at the site of infection 7 days after parasite inoculation in TNF−/− mice while L. major infected mTNFΔ/Δ mice controlled significantly better the number of neutrophils present at the infection site, which declined to levels comparable with those observed in C57BL/6 mice. Thus TNF appears to be an essential player in the control of neutrophils in the lesion however, its contribution in the rapid recruitment of neutrophils within hours of L. major inoculation still remains to be investigated.
Mast cells are thought to play a role of sentinels in the skin and are known to be activated by different factors, including live L. major [32]. When mice deficient in mast cells were inoculated with L. major, they exhibited decreased neutrophil recruitment to the infection site three weeks after infection. However no apparent difference in neutrophil numbers was detected during the first week of infection [33], suggesting that, as seen for IL-17, mast cells and the products they secrete are not major players in the rapid neutrophil influx occurring within hours of L. major inoculation but may contribute to neutrophil recruitment in a later phase of infection.
A role for the complement component C3 in neutrophil recruitment following L. major infection was investigated using BALB/c mice that express cobra venom factor (CVF) under the control of the α1-antitrypsin promoter, leading to continuous activation and consumption of C3 in the serum. Upon infection, these mice did not develop any inflammatory lesions and had lower neutrophil infiltration at the site of infection [34]. It remains difficult to assess if such decreased neutrophil recruitment is the cause or the result of the observed inhibition of lesion development.
Finally, neutrophil recruitment can presumably also be actively induced by the parasite. Indeed, Leishmania parasites, including L. major, have been shown to secrete in vitro a factor that is chemotactic for granulocytes (Leishmania chemotactic factor, LCF) and capable of attracting human neutrophils [22]. However, if factors secreted by Leishmania species may play a role in the process of neutrophil recruitment, skin damage caused by uninfected sand fly bites or by a sterile needle is sufficient to induce a rapid neutrophil influx to the lesion site, suggesting that factors released by the parasites contribute only in part to the initial neutrophil ingress observed following L. major inoculation [5].
Altogether, despite the identification of many putative candidate molecules that could participate in the initial neutrophil recruitment occurring within hours of parasite inoculation, the exact contribution of individual factors and/or their combination in this process still needs to be clarified.
3.2. Influence of Neutrophils on the Local Environment during the First Days after Leishmania Inoculation
As discussed above, neutrophils are the first cells to arrive at the site of entry of infectious agents, where they are stimulated to phagocytose the foreign bodies and also to secrete factors involved in the recruitment and/or activation of other inflammatory cells. It has been shown that neutrophils can express a large number of factors including chemokines and cytokines [35] that could influence the microenvironment at the site of infection and thus the subsequent immune response. One of the strategies designed to explore the role of neutrophils during Leishmania infection has been to deplete these cells prior to infection by the injection of specific mAbs. To this end, essentially two mAbs have been used, that is, RB6-8C5 [36] and NIMP-R14 [37]. RB6-8C5 is an antibody that reacts mainly with the granulocyte differentiation Ag (Gr-1), although it also recognizes the Ly-6C- and Ly-6B.2 antigens. Thus injection of this mAb will deplete not only neutrophils but also other Gr-1-expressing cells such as monocyte-derived macrophages, eosinophils, dendritic cells, and subpopulations of lymphocytes and monocytes. The NIMP-R14 mAb [37] recognizes a 25- to 30-kDa protein present on the neutrophil membrane and depletes neutrophils more selectively, as it does not recognize Ly6C (Charmoy M. and Tacchini-Cottier F. unpublished results). Another mAb (1A8) [38] recognizes the LY6G and not the LY6C molecules, and thus it does not affect Gr-1+ blood moncytes. It has recently been used to deplete neutrophils in vivo [39]. Thus, depending on the mAb used in vivo and on the regimen of administration, one has to take into account that cells other than neutrophils may be depleted that could contribute to the phenotype observed.
The role of neutrophils in infection of mice with L. major has been investigated by several groups including ours. Depletion of neutrophils in L. major susceptible BALB/c mice prior to inoculation of the parasite dramatically changed the course of infection, leading to a significantly lower lesion size than in control mice and to modifications of the developing immune response. Indeed, more IFNγ and less IL-4 were found to be secreted by lymph nodes cells in neutrophil-depleted mice compared to their similarly infected controls [4, 12]. Neutrophil depletion in L. major-resistant C57BL/6 mice prior to parasite inoculation leads to an increase parasite burden after 35 days of infection using the RB6-8C5 or the NIMP-R14 mAb. However, this is a transient effect, as mice are finally healing their lesions with no change in the Th1-associated immune response [4, 40, 41]. In another experimental model [16], neutrophil depletion using the NIMP-R14 mAb during the first days of infection with L. donovani was shown to have an impact both on the control of parasite replication and on the development of a parasite-specific immune response. Neutrophil-depleted mice had increased parasite load in the spleen, bone marrow, and then to a lesser extent in the liver, and in these mice, development of the L. donovani-induced immune response was altered, with a significant increase in interleukin 4 (IL-4) and IL-10 levels and reduced IFNγ secretion by CD4+ and CD8+ T cells as compared to similarly infected mice injected with a control mAb.
As indicated above, the outcome of NIMP-R14 mAb injection prior to inoculation of L. major differed in BALB/c and C57BL/6 mice. In order to understand the contribution of neutrophils in the distinct phenotypes observed, BALB/c and C57BL/6 neutrophils, respectively, from L. major-susceptible or resistant mice, were exposed to L. major in vitro. Different responses were observed: L. major induced selectively in C57BL/6 neutrophils TLR2, 7 and 9 mRNA, and the autocrine secretion of IL-12p70 and IL-10. In contrast exposure of BALB/c neutrophils to L. major did not increase these TLRs mRNAs, nor the secretion of IL-12p70. However, these BALB/c neutrophils secreted high levels of IL-12p40, forming the IL-12 inhibitory IL-12p80 complex [42]. TLR4 was induced similarly in neutrophils from both C57BL/6 and BALB/c mice [42]. Following infection with L. major, C57BL/6 and BALB/c neutrophils were also shown to differ in their secretion of neutrophil elastase (a molecule that contributes to parasite killing within macrophages through activation of TLR4 [43]) as indeed only C57BL/6 neutrophils secreted this molecule [12]. In most of the studies investigating neutrophil biology, the careful identification of this cell population is of utmost importance for the conclusions reached. Indeed, several cell surface neutrophil proteins are shared with other cells of the myeloid lineage. Thus, the proper identification of neutrophils requires the combination of a mAb directed against Ly-6G with several other mAbs directed against cell surface proteins also present (Ly-6C, CD11b) or absent (F4/80, MHCII, CD11c) on these cells.
The secretion of cytokines and chemokines by neutrophils is not as high as that of other cell types such as dendritic cells. However, considering the massive number of neutrophils present at the site of parasite inoculation during the first days of infection, these cells and the products they release are likely shaping the microenvironment in a way that can markedly impact on the developing immune response.
4. Conclusions
We have reviewed evidences in literature demonstrating that neutrophils play an essential role during Leishmania infection, providing a transient safe shelter for the parasite during the first day of infection, until the macrophages become the dominant population in the cellular infiltrate. In addition, factors released by these massively and rapidly recruited neutrophils likely contribute to determining the type and magnitude of the Leishmania-specific immune response. Further studies will be required to decipher the cross-talks between neutrophils and other cells, that leads to the eventual resolution of the lesion, or on the contrary to unimpaired progression of the infection (Figure 2). In this line, factors contributed by the sand fly vector also need to be considered. Indeed, recent experiments by Peters et al. revealed that sand fly inoculation maintains a localized, prolonged neutrophilic response which is different from that observed after needle injection of the parasite. This neutrophilic response impaired protection afforded in mice vaccinated using a killed Leishmania vaccine and challenged by the bite of infected sand flies, while such mice exhibited resistance against challenge infection induced through needle inoculation of live parasites [44]. Removal of the neutrophils promoted resistance against sand fly-induced infection, further pointing to the importance of neutrophils as a decisive parameter in shaping the outcome of infection by Leishmania parasites.
Figure 2.
Early neutrophil recruitment to the site of L. major infection and its potential influence on the development L. major specific-immune response. (1) L. major parasites are transmitted by the bite of the sand fly. (2) This induces rapid and massive recruitment of neutrophils to the site of parasite inoculation, a process not defined, that may involve different host and/or parasite-derived factors such as IL-8, KC(Gro-α), MIP-2 (Gro-β), IL-17, TNF, and LCF. (3) L. major parasites induce chemokine and cytokine secretion by neutrophils that attract and/or activate inflammatory cells at the site of infection. Crosstalk between neutrophils and the different cell types present or recruited to the site of infection, as well as interaction between these cells, will contribute to determine the type and magnitude of the L. major specific immune response that will develop.
Acknowledgments
The authors thank Dr. Jacques Mauël for critical reading of the manuscript. This work was supported by Grants from the Swiss National Science Foundation (320000-116197) and the Foundation Mercier, Switzerland to FTC.
References
- 1.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature Reviews Immunology. 2002;2(11):845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 2.Louis J, Himmelrich H, Parra-Lopez C, et al. Regulation of protective immunity against Leishmania major in mice. Current Opinion in Immunology. 1998;10(4):459–464. doi: 10.1016/s0952-7915(98)80121-0. [DOI] [PubMed] [Google Scholar]
- 3.Beil WJ, Meinardus-Hager G, Neugebauer DC, et al. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. Journal of Leukocyte Biology. 1992;52(2):135–142. doi: 10.1002/jlb.52.2.135. [DOI] [PubMed] [Google Scholar]
- 4.Tacchini-Cottier F, Zweifel C, Belkaid Y, et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. Journal of Immunology. 2000;165(5):2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 5.Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321(5891):970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laufs H, Muller K, Fleischer J, et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infection and Immunity. 2002;70(2):826–835. doi: 10.1128/iai.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gueirard P, Laplante A, Rondeau C, et al. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cellular Microbiology. 2008;10(1):100–111. doi: 10.1111/j.1462-5822.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 8.Aga E, Katschinski DM, van Zandbergen G, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. Journal of Immunology. 2002;169(2):898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 9.van Zandbergen G, Klinger M, Mueller A, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. Journal of Immunology. 2004;173(11):6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 10.Allenbach C, Zufferey C, Perez C, et al. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. Journal of Immunology. 2006;176(11):6656–6664. doi: 10.4049/jimmunol.176.11.6656. [DOI] [PubMed] [Google Scholar]
- 11.Allenbach C, Launois P, Mueller C, et al. An essential role for transmembrane TNF in the resolution of the inflammatory lesion induced by Leishmania major infection. European Journal of Immunology. 2008;38(3):720–731. doi: 10.1002/eji.200737662. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro-Gomes FL, Otero AC, Gomes NA, et al. Macrophage interactions with neutrophils regulate Leishmania major Infection. Journal of Immunology. 2004;172(7):4454–4462. doi: 10.4049/jimmunol.172.7.4454. [DOI] [PubMed] [Google Scholar]
- 13.Laskay T, van Zandbergen G, Solbach W. Neutrophil granulocytes as host cells and transport vehicles for intracellular pathogens: apoptosis as infection-promoting factor. Immunobiology. 2008;213(3-4):183–191. doi: 10.1016/j.imbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau D, Demartino S, Ferrua B, et al. In vivo involvement of polymorphonuclear neutrophils in Leishmania infantum infection. BMC Microbiol. 2001;117 doi: 10.1186/1471-2180-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smelt SC, Cotterell SE, Engwerda CR, et al. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. Journal of Immunology. 2000;164(7):3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 16.McFarlane E, Perez C, Charmoy M, et al. Neutrophils contribute to development of a protective immune response during onset of infection with Leishmania donovani. Infection and Immunity. 2008;76(2):532–541. doi: 10.1128/IAI.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Reviews Immunology. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 18.Romani L, Mencacci A, Cenci E, et al. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. Journal of Immunology. 1997;158(5):2356–2362. [PubMed] [Google Scholar]
- 19.Tateda K, Moore TA, Deng JC, et al. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. Journal of Immunology. 2001;166(5):3355–3361. doi: 10.4049/jimmunol.166.5.3355. [DOI] [PubMed] [Google Scholar]
- 20.Easton A, Haque A, Chu K, et al. A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. Journal of Infectious Diseases. 2007;195(1):99–107. doi: 10.1086/509810. [DOI] [PubMed] [Google Scholar]
- 21.Tsuda Y, Takahashi H, Kobayashi M, et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21(2):215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 22.van Zandbergen G, Hermann N, Laufs H, et al. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infection and Immunity. 2002;70(8):4177–4184. doi: 10.1128/IAI.70.8.4177-4184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller K, van Zandbergen G, Hansen B, et al. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Medical Microbiology and Immunology. 2001;190(1-2):73–76. doi: 10.1007/s004300100084. [DOI] [PubMed] [Google Scholar]
- 24.Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology. 2007:25821–25852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 25.Lopez Kostka S, Dinges S, Griewank K, et al. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. Journal of Immunology. 2009;182(5):3039–3046. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira LQ, Goldschmidt M, Nashleanas M, et al. mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. Journal of Immunology. 1996;157(2):827–835. [PubMed] [Google Scholar]
- 27.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. Journal of Immunology. 1998;160(11):5506–5513. [PubMed] [Google Scholar]
- 28.Kanaly ST, Nashleanas M, Hondowicz B, et al. TNF receptor p55 is required for elimination of inflammatory cells following control of intracellular pathogens. Journal of Immunology. 1999;163(7):3883–3889. [PubMed] [Google Scholar]
- 29.Chakour R, Guler R, Bugnon M, et al. Both the fas ligand and inducible nitric oxide synthase are needed for control of parasite replication within lesions in mice infected with Leishmania major whereas the contribution of tumor necrosis factor is minimal. Infection and Immunity. 2003;71(9):5287–5295. doi: 10.1128/IAI.71.9.5287-5295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritter U, Mattner J, Rocha JS, et al. The control of Leishmania (Leishmania) major by TNF in vivo is dependent on the parasite strain. Microbes and Infection. 2004;6(6):559–565. doi: 10.1016/j.micinf.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm P, Ritter U, Labbow S, et al. Rapidly fatal Leishmaniasis in resistant C57BL/6 mice lacking TNF. Journal of Immunology. 2001;166(6):4012–4019. doi: 10.4049/jimmunol.166.6.4012. [DOI] [PubMed] [Google Scholar]
- 32.Wershil BK, Galli SJ. The analysis of mast cell function in vivo using mast cell-deficient mice. Advances in Experimental Medicine and Biology. 1994:34739–34754. doi: 10.1007/978-1-4615-2427-4_5. [DOI] [PubMed] [Google Scholar]
- 33.Maurer M, Lopez Kostka S, Siebenhaar F, et al. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB Journal. 2006;20(14):2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs T, Andra J, Gaworski I, et al. Complement C3 is required for the progression of cutaneous lesions and neutrophil attraction in Leishmania major infection. Medical Microbiology and Immunology. 2005;194(3):143–149. doi: 10.1007/s00430-004-0229-y. [DOI] [PubMed] [Google Scholar]
- 35.Scapini P, Lapinet-Vera JA, Gasperini S, et al. The neutrophil as a cellular source of chemokines. Immunological Reviews. 2000:177195–177203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 36.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257(5069):548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 37.Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. British Journal of Haematology. 1984;57(3):489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 38.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow: RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. Journal of Immunology. 1993;151(5):2399–2408. [PubMed] [Google Scholar]
- 39.Daley JM, Thomay AA, Connolly MD, et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of Leukocyte Biology. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 40.Lima GM, Vallochi AL, Silva UR, et al. The role of polymorphonuclear leukocytes in the resistance to cutaneous Leishmaniasis. Immunology Letters. 1998;64(2-3):145–151. doi: 10.1016/s0165-2478(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Zhang ZH, Watanabe T, et al. The involvement of neutrophils in the resistance to Leishmania major infection in susceptible but not in resistant mice. Parasitology International. 2005;54(2):109–118. doi: 10.1016/j.parint.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Charmoy M, Megnekou R, Allenbach C, et al. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. Journal of Leukocyte Biology. 2007;82(2):288–299. doi: 10.1189/jlb.0706440. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro-Gomes FL, Moniz-de-Souza MCA, Alexandre-Moreira MS, et al. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. Journal of Immunology. 2007;179(6):3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 44.Peters NC, Kimblin N, Secundino N, et al. Vector transmission of Leishmania abrogates vaccine-induced protective immunity. PLoS Pathogens. 2009;5(6, article e1000484) doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]