Abstract
Background
Conventional methods describing daily glycemic variability (i.e., standard deviation and coefficient of variation) do not express risk. Low and High Blood Glucose Indices (LBGI and HBGI, respectively) and Average Daily Risk Range (ADRR) are parameters derived from self-monitored blood glucose (SMBG) data that quantify risk of glycemic excursions and temporal aspects of variability. In the present study, variability parameters were used to assess effects of exenatide and insulin glargine on risk of acute blood glucose extremes.
Methods
New (LBGI, HBGI, and ADRR) and conventional variability analyses were applied retrospectively to SMBG data from patients with type 2 diabetes suboptimally controlled with metformin and a sulfonylurea plus exenatide or insulin glargine as a next therapeutic step. Exenatide- (n = 282) and insulin glargine-treated (n = 267) patients were well matched.
Results
Exenatide treatment reduced ADRR overall (exenatide, mean ± SEM, 16.33 ± 0.45; insulin glargine, 18.54 ± 0.49; P = 0.001). Seventy-seven percent of exenatide-treated patients were at low risk for glucose variability compared with 62% of glargine-treated patients (P = 0.00023). LBGI for exenatide remained minimal for all categories and significantly lower than glargine for all comparisons, and HBGI for exenatide remained low or moderate for all categories and significantly lower than glargine after the morning and evening meals. Reduced variability in exenatide-treated patients was shown by conventional methods but provided no indications of risk.
Conclusions
Average glycemic control was similar for both treatment groups. However, exenatide treatment minimized risk for glycemic variability and extremes to a greater degree than insulin glargine treatment.
Introduction
Cross-sectional and longitudinal studies indicate that glycemic variability, including acute postprandial glucose excursions, increases the risk of diabetes complications.1–9 Unfortunately, long-term initiatives to prospectively evaluate this relationship with patient-oriented outcomes have not been undertaken.10 Although the authors of the Diabetes Control and Complications Trial concluded that a factor independent of hemoglobin A1c level (A1C) may contribute to diabetes complications,11 further analysis demonstrated that most of the risk of complications between the conventional and intensive therapeutic groups may be explained by A1C level.12 Nonetheless, most investigators who evaluate the relationship between glycemic variability and patient outcomes have used conventional variability statistics, such as standard deviation, coefficient of variation, postprandial peaks, or area under the curve.13 Conventional methods, which rely on blood glucose (BG) values to predict the risk of hyperglycemia or hypoglycemia, are substantively limited in that risk is not normally distributed over the usual BG scale. However, when the BG scale is made symmetric through a nonlinear transformation, risk for glycemic extremes may be predicted more readily13; thus, sensitivity and predictive power of the BG scale are enhanced.14
To address the need to predict risk for daily glycemic extremes, Kovatchev and co-workers13–19 have developed an approach based on aspects of BG variation to estimate the risk of the frequency and magnitude of hypoglycemic and hyperglycemic excursions: Low BG Index (LBGI), High BG Index (HBGI), and the Average Daily Risk Range (ADRR). These measures use the self-monitored BG (SMBG) data stored in most contemporary glucose meters and have been standardized in patients with type 1 and type 2 diabetes.
This retrospective analysis used seven-point SMBG data from a 26-week, open-label, intervention trial that assessed the addition of exenatide, an incretin mimetic with substantive effects on postprandial glucose excursions, or insulin glargine in patients with type 2 diabetes suboptimally controlled with oral antihyperglycemic therapies.20 In the open-label trial, both treatments resulted in similar improvements in overall glycemic control (A1C) at study end point. In the present analysis, LBGI, HBGI, ADRR, and conventional variability parameters were calculated to ascertain whether exenatide and insulin glargine had different effects on patterns relative to the temporal direction, magnitude, and risk of acute BG extremes.
Materials and Methods
The research design and methods of the prospective portion of this analysis have previously been published in full.20 In brief, the study was a multicenter, randomized, open-label, phase 3 clinical trial in patients with type 2 diabetes suboptimally controlled with combination metformin and sulfonylurea therapy. Patients added exenatide (before morning and evening meals) or insulin glargine (at bedtime) to their current therapy regimen. Exenatide-treated patients initially received a fixed dose (5 μg) of exenatide, twice daily for 4 weeks, and subsequently escalated the dose to 10 μg twice daily for the remainder of the study. Insulin glargine-treated patients initiated insulin therapy at 10 units/day with the use of a fixed-dose algorithm and self-titrated insulin in 2-unit increments every 3 days based on a fasting BG target of <5.6 mmol/L (<100 mg/dL).
Study participants
The intent-to-treat sample was composed of 549 patients. Participants were 30–75 years of age and had been treated with stable and maximally effective doses of metformin and a sulfonylurea for at least 3 months before screening. General inclusion criteria were: A1C of >7.0% to <10.0% and a body mass index of >25 kg/m2 to <45 kg/m2. Patients meeting the inclusion criteria were randomized within 2 weeks after screening with equal probability to exenatide (n = 282) or insulin glargine (n = 267) treatment according to a central randomization table generated by the sponsor and administered by an automated interactive voice-response system. Randomization was stratified by investigative site (block size of 4).
Seven-point SMBG profiles
Two separate seven-point SMBG profiles were generated within the 2-week period before each of the following scheduled visits: baseline and Weeks 4, 8, 12, 18, and 26, as described.20 Glucose concentrations were measured before and 2 h after the start of the morning, midday, and evening meals and at 0300 h.
The number of valid BG readings recorded was 41,515. SMBG profiles for Weeks 8, 12, 18, and 26 were assessed to ensure that data represented stable doses of study medication, i.e., when insulin dose titration was completed. During the period of stable insulin dose, the average number of SMBG readings per subject was 77.
Measures
The formulas for calculating the LBGI, HBGI, and ADRR have been described.13–19 In brief, the LBGI and HBGI are non-negative numbers, the sum of which ranges from 0 to 100. The indices are based on a nonlinear transformation of the BG scale, applying symmetry to the distribution of BG readings for a subject. LBGI indicates risk for hypoglycemic excursions; HBGI indicates risk for hyperglycemic excursions. Empirically derived risk categories are as follows: LBGI, Minimal (LBGI ≤1.1), Low (1.1 <LBGI ≤2.5), Moderate (2.5 < LBGI ≤5), and High (LBGI >5.0)18; HBGI, Low (HBGI ≤4.5), Moderate (4.5 <HBGI ≤9.0), and High (HBGI >9.0).15
The ADRR reflects the risk of combined high and low glucose variability.13 For ADRR, as with LBGI and HBGI, each SMBG reading is transformed using the formula f(BG) = 1.509([ln (BG × 18)]1.084–5.381) for BG measured in mmol/L (the multiplication by 18 in the logarithm is omitted if BG is measured in mg/dL). The transformed BG readings are converted into risk values using the formula r(BG) = 10*f(BG)2. Left and right branches of the resulting parabola separately indicate the risk of hypoglycemia and hyperglycemia: rl(BG) = r(BG) if f(BG) < 0 and 0 otherwise (left branch); rh(BG) = r(BG) if f(BG) > 0 and 0 otherwise (right branch), respectively. The ADRR is a composite of hypoglycemia (or low risk [LR]) and hyperglycemia (or high risk [HR]) and is computed as the average of the risk range per day using the following formula:
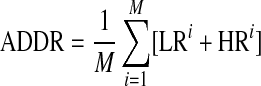 |
where 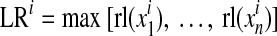 and
and 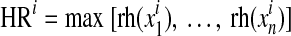 for day I, with i = 1,2, … M.
for day I, with i = 1,2, … M.
LBGI and HBGI
With the use of SMBG values recorded between Weeks 8 and 26, LBGI averages were derived for preprandial BG (before breakfast, lunch, and dinner), at 0300 h, and overall. HBGI averages were derived for 2-h postprandial BG (after breakfast, lunch, and dinner), at 0300 h, and overall.
Conventional BG parameters
With the use of SMBG values recorded between Weeks 8 and 26, averages for conventional parameters were calculated for the overall SMBG, overall SMBG SD, SMBG at 0300 h time point, daily range of SMBG (difference between minimum and maximum SMBG values), percentage of SMBG values within the American Diabetes Association-recommended target range (70–180 mg/dL), and percentage of SMBG values above and below the American Diabetes Association target range.
Statistics
An independent-samples t test (two-tailed) was performed for each of the aforementioned measures to assess if means between treatment groups differed significantly (P < 0.05). Descriptive statistics, including mean (SE), were calculated. For the ADRR, the direct comparison of the effects of exenatide versus insulin glargine was performed using a t test. The risk category of ADRR (shift to higher or lower risk for exenatide vs. insulin glargine) was assessed with a nonparametric test. To assess risk category progression with ADRR, a 2 × 6 repeated-measures analysis of variance of the ADRR (treatment × visit) was performed.
Results
Baseline characteristics
Baseline characteristics have been described in detail.20 In brief, treatment groups were well matched with respect to mean age (58–60 years old), gender (55–57% male), ethnicity (approximately 80% Caucasian), mean body weight (approximately 88 kg), mean body mass index (31 kg/m2), mean fasting plasma glucose (182–187 mg/dL), A1C (8.2–8.3%), and mean duration of diabetes (9–10 years).
ADRR
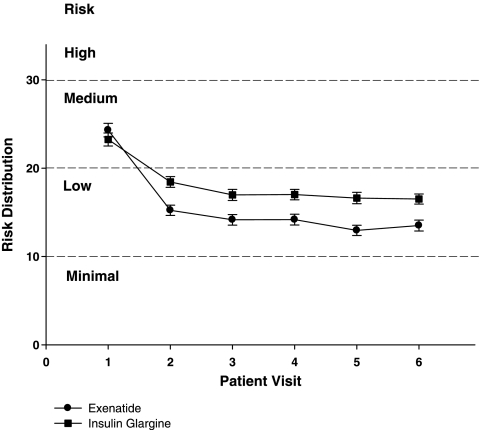
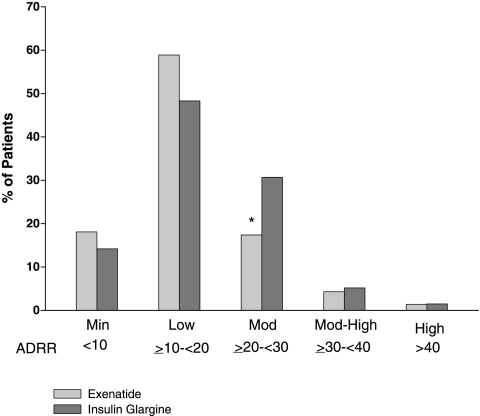
The ADRR distribution per study visit is shown in Figure 1. The overall ADRR for both exenatide-treated and glargine-treated patients was in the LR range for all visits, with exenatide-treated patients achieving a slightly lower risk (mean ± SEM, 16.33 ± 0.45, n = 282; glargine-treated, 18.54 ± 0.49, n = 267; P = 0.001). Patient distribution within each ADRR category is shown in Figure 2. As the overall ADRR is reduced for exenatide, there tended to be more exenatide-treated patients in the minimal risk and LR ranges and more glargine-treated patients in the moderate risk range; however, only the comparison within the moderate risk range was statistically different (P < 0.01). Given that there were few patients in the moderate-high risk and HR groups, ADRR categories were combined (minimal risk + LR vs. moderate risk + moderate-high risk + HR) for a more robust assessment of between-treatment risk: 77% of exenatide-treated patients were at LR of glucose variability compared with 62% of glargine-treated patients (P = 0.00023).
FIG. 1.
ADRR distribution by each study visit is shown for exenatide and insulin glargine over the course of study. Overall, ADRR was lower for exenatide-treated patients (P = 0.001). A 2 × 6 analysis of variance of ADRR per study visit after the first visit shows that variability is reduced shortly after the initiation of treatment in both groups (F = 112.5, P < 0.001), and reduction of variability is slightly more pronounced for exenatide-treated patients compared with glargine-treated patients (treatment × visit effect, F = 7.1, P < 0.0001). Data are mean ± SE values.
FIG. 2.
Percentage of exenatide- and insulin glargine-treated patients in each ADRR category (Minimal [Min], Low, Moderate [Mod], Moderate-High [Mod-High], and High). The distribution for the risk categories was compared using a nonparametric χ2 test and found to be different overall (P =0.00559). *P < 0.001.
LBGI and HBGI
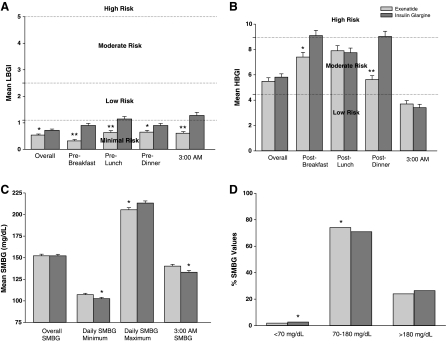
LBGI values at each time point, and overall, were significantly lower for exenatide-treated patients compared with insulin glargine-treated patients; however, none of the LBGI values exceeded the threshold associated with minimal risk-to-LR of hypoglycemia for either group (LBGI ≤2.5) (Fig. 3A). Overall HBGI values for both groups were in the moderate risk range and were lower for exenatide-treated patients compared with insulin glargine-treated patients after morning and evening meals (Fig. 3B). For both treatment groups, HBGI at 0300 h indicated LR for hyperglycemic excursions.
FIG. 3.
(A) LBGI indices, (B) HBGI indices, (C) SMBG values for overall, minimum, maximum, and 0300 h time points, and (D) percentage of SMBG values falling below, within, and above the American Diabetes Association target range of 70–180 mg/dL. *P < 0.05, **P < 0.001. Data are mean ± SE values.
Conventional measures
Overall, mean daily SMBG values were similar between treatments at Week 26 (Fig. 3C).20 In spite of the similarity between SMBG values, the SD values differed between groups (exenatide-treated patients, SD = 43.0 mg/dL; insulin glargine-treated patients, SD = 47.3 mg/dL; P < 0.0001). Further, a greater percentage of exenatide-treated patients achieved SMBG values within the target glucose range of 70–180 mg/dL compared with insulin glargine-treated patients (74.1% vs. 70.9%, P < 0.05, Fig. 3D), an outcome driven primarily by a significantly higher percentage of insulin glargine-treated patients with SMBG values <70 mg/dL.
Discussion
The primary finding of this analysis is that the variability of overall glycemia between two treatments, in this case insulin glargine and exenatide, can differ markedly despite similar overall changes from baseline. The outcomes of this retrospective analysis highlight how characterizing glycemic variability with the use of newly described measures, validated in patient populations, can outline potentially important differences between treatments. The ADRR is an index of variability that captures both high and low extremes better than other conventional measures. Other conventional measures are less sensitive and robust in quantifying risk for acute BG excursions. The LBGI is a specific indicator for hypoglycemia as well as the trend toward hypoglycemic excursions and provides a continuous measure for estimating hypoglycemic risk, even when the risks are relatively low as in the current study. Thus, LBGI confirmed that although glargine-treated patients were at statistically higher risk for hypoglycemic episodes, both groups were at minimal risk to LR for severe hypoglycemia.
The HBGI identified a potentially clinically relevant difference between insulin glargine and exenatide in the treatment of patients with type 2 diabetes failing oral antihyperglycemic therapies. Although both groups were in the moderate risk range for overall HBGI values, insulin glargine-treated patients were more likely to experience significantly higher hyperglycemic excursions following breakfast and dinner.
The ADRR, a single index that combines the risk for both high and low BG measures (HBGI and LBGI), represents a sensitive indicator for glycemic extremes.13 In the present analysis, exenatide and insulin glargine treatments promptly reduced the risk of glycemic variability, which was further reduced during the course of the study. The reduction in risk was greater in exenatide-treated patients compared with insulin glargine-treated patients.
Exenatide treatment results in a better postprandial glucose profile than once-daily insulin glargine treatment,20 most likely due to the pleiotropic effect of exenatide. Sharing several glucose-lowering actions with the naturally occurring incretin hormone glucagon-like peptide-1, exenatide promotes glucose-dependent insulin secretion, inhibition of inappropriately elevated glucagon secretion, slowing of gastric emptying, and reduction of food intake.21–26 Prandial insulin mixtures also may promote a better postprandial glucose profile than once-daily preparations, given that the action of a prandial insulin mixture is likely to cover peak postprandial glucose concentrations.27 Although obesity and suboptimally controlled glycemia are considered major etiological factors contributing to the development of diabetes complications, some evidence suggests that postprandial hyperglycemia may also play a role. Several clinical trials demonstrated that markers associated with cardiovascular risk increase with acute postprandial BG rise,5,28–30 which also may serve as a risk factor for cardiovascular events.3–9,31–33 However, to date, no clinical studies targeting postprandial glucose excursions have reported clinically significant improvements in cardiovascular outcomes.
There is concern in the medical community regarding the importance of controlling postprandial glycemia. In routine clinical practice, a validated, standard approach for convenient quantification of the magnitude or frequency of acute hyper- or hypoglycemia may aid in the recognition and treatment of BG excursions. In the current analysis, similar reductions in A1C levels were observed for both treatment groups, without distinction for detecting the risk of frequent but transient BG excursions. The assessment presented here is one approach that addresses these clinical issues, demonstrating that the SMBG data stored in most glucose meters can be used to conveniently derive valid parameters for gauging risk in terms of magnitude and timing of hyperglycemic and hypoglycemic excursions. Insulin glargine-treated patients might benefit from intensifying therapy by adding mealtime insulin, before morning and evening meals, which may require more frequent SMBG monitoring, while exenatide-treated patients may require less frequent SMBG monitoring.
In summary, the methods presented herein appear promising as standardized approaches for quantifying glycemic variability, thus addressing unmet needs in this area of clinical investigation. Despite similar reductions in A1C, exenatide treatment was associated with a significant reduction in ADRR, a sensitive predictor of either hyper- or hypoglycemia. Future studies are needed to evaluate the strength of association of ADRR, LBGI, and HBGI with short-term surrogate markers of diabetes complications (e.g., measures of oxidative stress and inflammation) and to assess the relationship of these indices to clinically meaningful outcomes.
Acknowledgments
The authors wish to acknowledge Joseph Giaconia of Eli Lilly and Company for his contributions to the development of this manuscript. Funding for this study was provided by Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. The original development of the risk metrics used in this study was supported by grant RO1 DK 51562 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health and by grant and material support from Lifescan Inc., Milpitas, CA.
Author Disclosure Statement
A.L.M. and B.K. have received honoraria from Eli Lilly and Company and Amylin Pharmaceuticals, Inc. R.B. owns stock in and is an employee of Eli Lilly and Company. D.J. owns stock in and was an employee of Eli Lilly and Company during the development of the manuscript. J.C. was an employee of Amylin Pharmaceuticals, Inc. during the development of the manuscript. D.J.C. has no competing financial interests.
References
- 1.Hirsch IB. Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–181. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 2.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 3.Brunner EJ. Shipley MJ. Witte DR. Fuller JH. Marmot MG. Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care. 2006;29:26–31. doi: 10.2337/diacare.29.01.06.dc05-1405. [DOI] [PubMed] [Google Scholar]
- 4.DECODE Study Group, the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 5.Esposito K. Giugliano D. Nappo F. Marfella R Companion Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004;110:214–219. doi: 10.1161/01.CIR.0000134501.57864.66. [DOI] [PubMed] [Google Scholar]
- 6.Muggeo M. Zoppini G. Bonora E. Brun E. Bonadonna RC. Moghetti P. Verlato G. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Meigs JB. Nathan DM. D'Agostino RB., Sr Wilson PW. Framingham Offspring Study: Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002;25:1845–1850. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 8.Takei Y. Tomiyama H. Tanaka N. Yamashina A. Close relationship between sympathetic activation and coronary microvascular dysfunction during acute hyperglycemia in subjects with atherosclerotic risk factors. Circ J. 2007;71:202–206. doi: 10.1253/circj.71.202. [DOI] [PubMed] [Google Scholar]
- 9.Chiasson JL. Josse RG. Gomis R. Hanefeld M. Karasik A. Laakso M STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM Trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 10.Chiasson J-L. Josse RG. Gomis R. Hanefeld M. Karasik A. Laakso M STOP-NIDDM Trial Research Group. Acarbose for the prevention of type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia. 2004;47:969–975. doi: 10.1007/s00125-004-1409-4. [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM. Cleary PA. Backlund JY. Genuth SM. Lachin JM. Orchard TJ. Raskin P. Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lachin JM. Genuth S. Nathan DM. Zinman B. Rutledge BN. DCCT/EDIC Research Group: Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—revisited. Diabetes. 2008;57:995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 13.Kovatchev BP. Otto E. Cox D. Gonder-Frederick L. Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 14.Cox DJ. Kovatchev B. Julian D. Gonder-Frederick LA. Polonsky WH. Schlundt DG. Clarke WL. Frequency of severe hypoglycemia in IDDM can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79:1659–1662. doi: 10.1210/jcem.79.6.7989471. [DOI] [PubMed] [Google Scholar]
- 15.Kovatchev BP. Straume M. Cox DJ. Farhy LS. Risk analysis of blood glucose data: a quantitative approach to optimizing the control of insulin dependent diabetes. J Theor Med. 2000;3:1–10. [Google Scholar]
- 16.Kovatchev BP. Cox DJ. Summers KH. Gonder-Frederick L. Clarke WL. Postprandial glucose dynamics and associated symptoms in type 2 diabetes mellitus. J Appl Res. 2003;3:449–458. [Google Scholar]
- 17.Kovatchev BP. Cox DJ. Gonder-Frederick L. Clark WL. Methods for quantifying self-monitoring blood glucose profiles exemplified by an examination of blood glucose patterns in patients with type 1 and type 2 diabetes. Diabetes Technol Ther. 2002;4:295–303. doi: 10.1089/152091502760098438. [DOI] [PubMed] [Google Scholar]
- 18.Kovatchev BP. Cox DJ. Kumar A. Algorithmic evaluation of metabolic control and risk of severe hypoglycemia in type 1 and type 2 diabetes using self-monitoring blood glucose data. Diabetes Technol Ther. 2003;5:817–825. doi: 10.1089/152091503322527021. [DOI] [PubMed] [Google Scholar]
- 19.Kovatchev BP. Clarke WL. Breton M. Brayman K. McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7:849–862. doi: 10.1089/dia.2005.7.849. [DOI] [PubMed] [Google Scholar]
- 20.Heine RJ. Van Gaal LF. Johns D. Mihm MJ. Widel MH. Brodows RG GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 21.Degn KB. Brock B. Juhl CB. Djurhuus CB. Grubert J. Kim D. Han J. Taylor K. Fineman M. Schmitz O. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes. 2004;53:2397–2403. doi: 10.2337/diabetes.53.9.2397. [DOI] [PubMed] [Google Scholar]
- 22.Edwards CM. Stanley SA. Davis R. Brynes AE. Frost GS. Seal LJ. Ghatei MA. Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281:E155–E161. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 23.Egan JM. Clocquet AR. Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab. 2002;87:1282–1290. doi: 10.1210/jcem.87.3.8337. [DOI] [PubMed] [Google Scholar]
- 24.Kolterman OG. Buse JB. Fineman MS. Gaines E. Heintz S. Bicsak TA. Taylor K. Kim D. Aisporna M. Wang Y. Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- 25.Kolterman OG. Kim DD. Shen L. Ruggles JA. Nielsen LL. Fineman MS. Baron AD. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005;62:173–181. doi: 10.1093/ajhp/62.2.173. [DOI] [PubMed] [Google Scholar]
- 26.Poon T. Nelson P. Shen L. Mihm M. Taylor K. Fineman M. Kim D. Exenatide improves glycemic control and reduces body weight in subjects with type 2 diabetes: a dose-ranging study. Diabetes Technol Ther. 2005;7:467–477. doi: 10.1089/dia.2005.7.467. [DOI] [PubMed] [Google Scholar]
- 27.Roach P. Woodworth JR. Clinical pharmacokinetics and pharmacodynamics of insulin lispro mixtures. Clin Pharmacokinet. 2002;41:1043–1057. doi: 10.2165/00003088-200241130-00003. [DOI] [PubMed] [Google Scholar]
- 28.Monnier L. Mas E. Ginet C. Michel F. Villon L. Cristol JP. Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 29.Temelkova-Kurktschiev TS. Koehler C. Henkel E. Leonhardt W. Fuecker K. Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 30.Beisswenger PJ. Howell SK. O'Dell RM. Wood ME. Touchette AD. Szwergold BS. Alpha-dicarbonyls increase in the postprandial period and reflect the degree of hyperglycemia. Diabetes Care. 2001;24:726–732. doi: 10.2337/diacare.24.4.726. [DOI] [PubMed] [Google Scholar]
- 31.Hanefeld M. Fischer S. Julius U. Schulze J. Schwanebeck U. Schmechel H. Ziegelasch HJ. Lindner J. Risk factor for myocardial infarction and death in newly detected NIDDM: the diabetes intervention study, 11-year follow-up. Diabetologia. 1996;39:1577–1583. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 32.Pyörälä M. Miettinen H. Laakso M. Pyörälä K. Hyperinsulinemia predicts coronary heart disease risk in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Circulation. 1998;98:398–404. doi: 10.1161/01.cir.98.5.398. [DOI] [PubMed] [Google Scholar]
- 33.Reichard P. Pihl M. Rosenqvist U. Sule J. Complications in IDDM are caused by elevated blood glucose level: the Stockholm Diabetes Intervention Study (SDIS) at 10-year follow up. Diabetologia. 1996;39:1483–1488. doi: 10.1007/s001250050602. [DOI] [PubMed] [Google Scholar]