Abstract
AIM: To investigate the role of tumor infiltrating lymphocytes (TIL) in primary hepatocellular and cholangiolar carcinomas of the liver.
METHODS: Immunohistochemical analysis was performed including antibodies to CD3, CD4, CD8, CD20, CD56 and TIA-1 in formalin-fixed and paraffin-embedded tissue of 35 liver resection specimens of hepatocellular or cholangiocellular carcinomas. Semiquantitative evaluation was performed with emphasis on the area of the tumor itself and of the tumor/liver interface.
RESULTS: All hepatocellular carcinomas showed infiltration of lymphocytes predominantly around the tumor in the tumor/liver interface consisting mainly of CD3+ CD4+ T lymphocytes [164.3/10 high power fields (HPF)] and in the tumor itself of CD8+ cells (54.9/10 HPF). Cholangiocarcinomas contained a heterogeneous amount of TIL, composed mainly of CD3+ T cells with a predominance of CD8+ cells in the tumor tissue (52.6/10 HPF) and of CD4+ cells in the interface region (223.1/10 HPF). CD56+ cells of the innate immune system were scarce. There was no significant difference between hepatocellular or cholangiolar carcinoma. No correlation with the clinicopathological data was seen.
CONCLUSION: Liver TIL consists of intratumoral CD8+ T cells and peritumoral CD4+ T cells independent of histogenetic origin. Different functions of lymphocytes in these regions seem possible.
Keywords: Liver neoplasms, Hepatocellular carcinoma, Lymphocytes, Immunologic factors, Cholangiocarcinoma
INTRODUCTION
Tumor infiltrating lymphocytes (TIL) are part of the tumor surveillance system[1]. This immune response is thought to be a result of changes of surface components of tumor cells. The innate as well as the adaptive immune system is involved in tumor destruction with cell-mediated mechanisms playing the main role. They are frequently present in human solid tumors. Some CD8+ T cells are seen as final effector cells with the ability to induce apoptosis of cancer cells. Subpopulations of CD4+ T cells have a helper function, activating other immune cells via cytokine secretion or antigen processing. TIL are a target for immunotherapeutic strategies[2].
The liver can be regarded as an immunological organ, specially equipped with liver-associated lymphocytes, mainly T lymphocytes and natural killer cells[3]. They play an important role in the barrier function of the liver between the gastrointestinal tract and an organism. They do not only function as part of the defense system but also as a regulator of immune tolerance.
Hepatocellular carcinoma is the leading cause of malignant cancer deaths worldwide and the morbidity is increasing year on year. It accounts for approximately 6% of all human cancers and up to 1 million deaths per year. The second most common primary malignancy of the liver, cholangiolar carcinoma also has a bad prognosis. Its resectability rate is very low, but surgical resection is the only treatment which can change outcome significantly[4,5].
We studied the frequency and composition of TIL in primary liver cancers with special attention to the morphological distribution. The subtyping was performed to clarify their putative role in host response, immunotolerance and as a therapeutic target.
MATERIALS AND METHODS
Patients
Formalin-fixed and paraffin-embedded tissue of 35 liver resection specimens were investigated. The specimens were obtained from 8 women and 27 men with a median age of 60.5 years (38-82 years). Twenty seven of the cases were diagnosed as hepatocellular carcinoma (8 × T1, 3 × T2, 12 × T3 4 × T4; 6 × G1, 14 × G2, 7 × G3) and 8 as cholangiolar carcinoma ( 2 × T1, 1 × T2, 2 × T3, no T-stage available in 3; 6 × G2, 2 × G3) with a mean diameter of 7.9 cm and 8.3 cm, respectively.
Immunohistochemistry
A panel of immunohistochemical stains was performed including antibodies to CD3, CD4, CD8, CD20, CD56 and TIA-1. The specifications and titers are given in Table 1.
Table 1.
List of antibodies
| Name | Clone | Pre-treatment | Titer | Manufacturer |
| CD3 | PS1 | 10 min autoclave 120°C, citrate buffer pH 6 | 1:50 | DAKO, Glostrup, Denmark |
| CD4 | 1F6 | 10 min autoclave 120°C, citrate buffer pH 9 | 1:10 | Novocastra, Newcastle, UK |
| CD8 | C8/144B | 10 min autoclave 120°C, citrate buffer pH 6 | 1:100 | DAKO, Glostrup, Denmark |
| CD20 | L26 | none | 1:500 | DAKO, Glostrup, Denmark |
| CD56 | 123C3 | 2 × 7 min microwave citrate buffer pH 6 | 1:500 | Zymed, San Francisco, CA, USA |
| SCLC | ||||
| TIA-1 | TIA-1 | 2 × 7 min microwave citrate buffer pH 6 | 1:500 | Coulter Immunol., Hinleah, FL, USA |
Routinely processed formalin-fixed and paraffin-embedded tissue sections of the tumor and the tumor/liver interface with a thickness of 4 μm were used. Sections were mounted onto capillary gap slides (DAKO, Glostrup, Denmark), dried overnight at 30°C, deparaffinized with xylene and rehydrated with ethanol in a graded series to distilled water. Staining was performed using an automated immunostainer (Techmate, DAKO, Glostrup, Denmark) with AEC (3-amino-9-ethylcarbazole) for visualization. The slides were counterstained with hemalaun and a coverslip placed on top. Appropriate positive and negative tissue control samples were used throughout. Tonsils served as positive controls.
Statistical analysis
Ten high power fields (HPF) of the tumor and the tumor-liver interface were randomly selected and the frequency of TIL was counted using an ocular grid. Results are reported as mean value and standard deviation per 10 HPF. Comparison of the groups was performed using the two-tailed Students t-test (SPSS for windows). The significance level was set as P < 0.05.
RESULTS
TIL in hepatocellular carcinoma
All hepatocellular carcinomas showed an infiltration of lymphocytes which was mainly localized around the tumor in the tumor/liver interface, with less among the tumor cells (Table 2, Figure 1). The TIL consisted mainly of CD3+ T lymphocytes. CD20+ cells and CD56+ cells were rarely found. In the tumor itself, the infiltration was dominated by CD8+ cells. In contrast, in the peritumoral area the amount of CD4+ cells was higher than the amount of CD8+ cells. TIA-1 containing cells were more frequent in the peritumoral region.
Table 2.
Frequency of TIL in hepatocellular and cholangiolar carcinoma
| TIL |
Hepatocellular carcinoma |
Cholangiolar carcinoma |
||||
| Intratumoral (/10HPF) | Peritumoral (/10HPF) | Level of significance | Intratumoral (/10HPF) | Peritumoral (/10HPF) | Level of significance | |
| CD20 | 3.2 (± 9.7) | 26.4 (± 49.5) | P < 0.001 | 0.1 (± 0.3) | 11.1 (± 11.8) | P = 0.035 |
| CD3 | 85.1 (± 78.2) | 256.5 (± 90.5) | P < 0.001 | 52.6 (± 28.5) | 310.4 (± 202) | P = 0.008 |
| CD4 | 37.9 (± 42.5) | 164.3 (± 26.4) | P < 0.001 | 18 (± 22.3) | 223.1 (± 43.2) | P = 0.043 |
| CD8 | 54.9 (± 57.9) | 131.5 (± 86.8) | P ≤ 0.001 | 40.7 (± 30.5) | 118.7 (± 35.5) | P ≤ 0.001 |
| CD56 | 0.2 (± 0.6) | 0.5 (± 1.1) | P = 0.058 | 0.4 (± 1.0) | 1.9 (± 2.7) | P = 0.088 |
| TIA-1 | 50.2 (± 40.5) | 80 (± 64.5) | P = 0.036 | 41.1 (± 41.8) | 72.5 (± 37.4) | P = 0.071 |
HPF: High power fields; TIL: Tumor infiltrating lymphocytes.
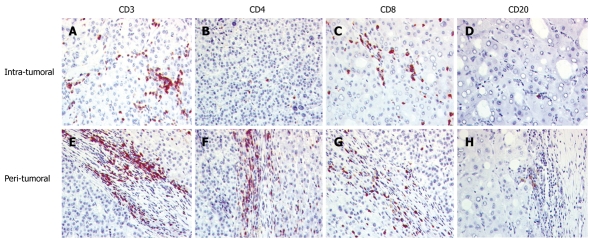
Figure 1.
Lymphocytic infiltration in the tumor tissue of hepatocellular carcinoma. A-D: Intratumoral region; E-H: Tumor/liver interface (peritumoral); A and E: CD3+ T cells are the main infiltrate with a higher amount in the interface region; B and F: CD4+ cells were mainly located in the peritumoral area; C and G: In the tumor tissue, CD8+ cells were more often seen; D and H: CD20+ cells were scarce.
TIL in cholangiolar carcinoma
Cholangiocarcinomas contained a heterogeneous amount of TIL, composed mainly of CD3+ T cells. The relationship of the subpopulations was comparable to that of hepatocellular carcinoma, with a predominance of CD8+ cells in the tumor tissue and of CD4+ cells in the interface region. CD56+ and CD20+ cells were found only in a minor proportion. Cells containing the cytotoxic granula TIA-1 occurred often in the interface region. The details are summarized in Table 2 and Figure 2.
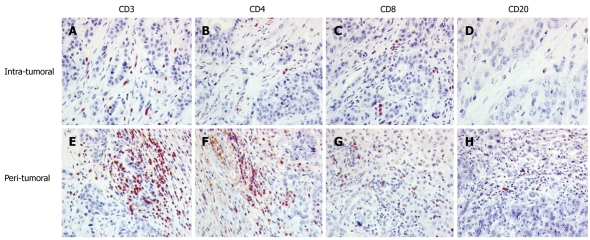
Figure 2.
The distribution of tumor infiltrating lymphocytes in cholangiocellular carcinoma. A-D: Intratumoral region; E-H: Tumor/liver interface (peritumoral); A and E: CD3+ T cells were the dominant infiltrate; B and F: The quantity of CD4+ and CD8+ cells were opposite with a higher level of CD4 cells in the peritumoral region; C and G: CD8+ cells were more often found in the tumor tissue; D and H: CD20+ cells were scarce.
No statistical differences were found in the frequency and distribution according to age, sex, size or grade of the tumor.
DISCUSSION
Liver carcinoma evolves over a long period of time from precursor lesions to invasive cancer and metastases. The exact mechanisms of this pathway are not fully understood[6]. It is known, however, that TIL have the potential to modulate this process. The extent of this modulation and the real effects of tumor growth and dissemination remain unclear.
Several methodical approaches have been used to measure TIL. Most often flow cytometry and immunohistology is used. Flow cytometry is a rapid method, in which a higher amount of cells and a panel of markers can be investigated. Fresh tissue, however, is necessary. Therefore retrospective studies, especially with human tissue from pathological files, are not possible. An exact localization of the TIL cannot be determined. Immunohistochemistry can identify different cell populations and has the advantage of direct localization of the lymphocyte subsets. Manual counting and automated counting of digital images are possible[7,8].
Using a histological approach we were able to localize TIL and could differentiate between lymphocytes in the tumor itself and in the environs of the tumor. In the tumor, CD8+ cells were more frequent, showing a closer contact with the tumor cells. As these cells can have a cytotoxic function, a direct apoptotic effect via different pathways such as the FAS/FAS-ligand pathway seems possible. Large numbers of CD8+ TILs are associated with a favorable prognosis in several solid carcinomas such as colorectal, ovarian, pancreatic and esophageal carcinoma[9-14]. Other entities such as nasopharyngeal cancer, renal cell carcinoma and non-small cell lung cancer show opposite behavior[15-17]. For hepatocellular carcinoma, further investigation is necessary.
In contrast, TIL in the interface are mostly of the CD4+ type. As CD4+ cells include helper T cells, their infiltration could activate cytotoxic killer cells by cytokine secretion or antigen presentation. Other authors, however, found an involvement of these cells, also known as regulatory cells, in immune tolerance[18]. Tumor cells adapting to these mechanisms of the CD4+ lymphocytes could use these cells to induce a tumor-friendly environment. The infiltration of CD4+ T-cells could be a sign of tumor adaptation known as enhancement[19].
Unitt et al[20,21] indicated that lymphocytic infiltration of the tumor and a high CD4+/CD8+ T cell ratio were associated with a reduced risk of tumor recurrence after liver transplantation for hepatocellular carcinoma. This ratio was beneficial in hepatocellular carcinoma. A decreased ratio was shown to imply poor tumor response[22]. CD4+ T lymphocytes can activate monocytes, macrophages and natural killer cells and can help CD8+ T lymphocytes to kill tumor cells. Thus, a positive effect of those infiltrations seems to exist. On the other hand, however, CD4+ T cells were also involved in tolerance mechanisms. As the liver is particularly involved in tolerance induction to food antigens, a supportive role of these infiltrates cannot be ruled out.
A lower quantity of CD8+ cells in the tumor and in the tumor periphery could signal a low level of T cell activation by tumor specific antigens in the liver. Primary reasons for this can be in the immune system itself such as development of self tolerance in the thymus or in the periphery[23]. The tumor can also develop various evasion strategies like converting T helper cells or lowering the expression of tumor specific antigens to below the required threshold[24-26]. These mechanisms would lead to a low quantity of TIL. Janicki et al[27], however, could show that TIL can lose their effector function even after infiltrating the tumor. One reason could be a loss of adhesion capability[28]. These results have critical implications for vaccination studies.
The liver normally contains a higher quantity of cells of the innate immune system such as natural killer cells. The lack of CD56+ cells in the tumor and in the interface to the liver is surprising. This cell type is thought to be involved in tumor defense. This is in contrast to a recent study investigating human glioblastomas[29]. Half of all TIL in these tumor types were CD56+ cells, in particularly CD4+CD56+ T cells. Whether this cell type is unique for brain tumors has to be clarified by further studies.
In the liver, a primary defect or lack of cells of the innate immune systems could be regarded as an aid to tumor development. On the other hand, there is the possibility that a manifest tumor can suppress the innate immune system.
For cholangiolar carcinoma, only scarce data are available regarding TIL. Takagi and coworkers found that patients with high numbers of TIL (CD8+ or CD4+) had significantly better prognosis[30]. In our study, the amount of TILs was heterogeneous. In the tumor tissue itself, the CD8+ cells can act as cytotoxic cells. In this entity, regulatory cells are also found in the tumor environs. As in hepatocellular carcinoma, there is a lack of CD56+ cells of the innate immune system.
Earlier we investigated the lymphocytic reaction of the liver with liver metastases from different primary neoplasms[31]. In this investigation, the results were similar to those demonstrated here. Also, in liver metastases, the frequency of TIL in the interface between the liver and tumor was higher than in the tumor itself. The infiltrate in the interface was also composed mainly of CD4+ T cells. Thus, a general rule can be seen in the reaction of the liver. A manifest tumor is accompanied by a CD4+ reaction in the liver. Whether this is a defense mechanism or more like an induced protection by the tumor cells needs further clarification.
In summary, we could demonstrate for hepatocellular carcinoma and cholangiolar carcinoma that TIL consists, in the tumor itself, of CD8+ T cells and, in the peritumoral region, of CD4+ T cells such as helper cells or regulatory cells. CD20+ cells and TIA-1+ cells were scarce. There was a lack of CD56+ cells of the innate immune system. The functional interaction of TIL in liver carcinomas needs further investigation especially if considered as a target for immunotherapeutic strategies.
COMMENTS
Background
Tumor development is based on an interaction between the tumor cells and the immune system of the body. Tumor infiltrating lymphocytes (TIL) are part of the tumor surveillance system. This immune response is thought to be a result of changes of surface components of tumor cells. TIL are a target for immunotherapeutic strategies in cancer treatment.
Research frontiers
Immune therapy is one of the newer strategies in cancer therapy. The main aim is vaccination against carcinomas. A prerequisite for the development of these vaccines is an understanding of the immunological tumor reaction.
Innovations and breakthroughs
Recent reports have shown different functions of lymphocyte subsets in tumor surveillance. These cells not only have antitumor potential but growth promotion by certain lymphocytes is also documented.
Applications
This study suggests that the immune reaction to liver cancer consists of different lymphocyte subtypes. Some can enhance tumor growth, others can destroy tumor cells. A complex strategy would be necessary for successful immune therapy of liver cancer.
Peer review
The authors investigated the role of TIL in primary hepatocellular and cholangiolar carcinomas of the liver. They found that liver TIL consist of intratumoral CD8+ T cells and peritumoral CD4+ T cells independent of histogenetic origin. This article is well written and deserves publication.
Footnotes
Supported by Centre of Molecular Medicine Cologne (CMMC), Köln, Germany
Peer reviewer: Mark D Gorrell, Professor, Centenary Institute of Cancer Medicine and Cell Biology, Locked Bag No. 6, Newtown, NSW 2042, Australia
S- Editor Li LF L- Editor Cant MR E- Editor Ma WH
References
- 1.Umansky V, Schirrmacher V, Rocha M. New insights into tumor-host interactions in lymphoma metastasis. J Mol Med. 1996;74:353–263. doi: 10.1007/BF00210630. [DOI] [PubMed] [Google Scholar]
- 2.Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet. 2009;373:673–683. doi: 10.1016/S0140-6736(09)60404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selmi C, Mackay IR, Gershwin ME. The immunological milieu of the liver. Semin Liver Dis. 2007;27:129–139. doi: 10.1055/s-2007-979466. [DOI] [PubMed] [Google Scholar]
- 4.Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, Guy SR, Sheiner PA, Miller CM, Schwartz ME. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg. 1998;187:365–372. doi: 10.1016/s1072-7515(98)00203-8. [DOI] [PubMed] [Google Scholar]
- 5.Lieser MJ, Barry MK, Rowland C, Ilstrup DM, Nagorney DM. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg. 1998;5:41–47. doi: 10.1007/pl00009949. [DOI] [PubMed] [Google Scholar]
- 6.Kern MA, Breuhahn K, Schuchmann M, Schirmacher P. [Molecular pathogenesis of hepatocellular carcinoma: new therapeutic approaches and predictive pathology] Pathologe. 2007;28:261–268. doi: 10.1007/s00292-007-0890-1. [DOI] [PubMed] [Google Scholar]
- 7.Hussein MR, Hassan HI. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol. 2006;59:972–977. doi: 10.1136/jcp.2005.031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loughlin PM, Cooke TG, George WD, Gray AJ, Stott DI, Going JJ. Quantifying tumour-infiltrating lymphocyte subsets: a practical immuno-histochemical method. J Immunol Methods. 2007;321:32–40. doi: 10.1016/j.jim.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501. doi: 10.1038/labinvest.3700055. [DOI] [PubMed] [Google Scholar]
- 10.Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, Barten M. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–816. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 12.Ryschich E, Cebotari O, Fabian OV, Autschbach F, Kleeff J, Friess H, Bierhaus A, Buchler MW, Schmidt J. Loss of heterozygosity in the HLA class I region in human pancreatic cancer. Tissue Antigens. 2004;64:696–702. doi: 10.1111/j.1399-0039.2004.00324.x. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher K, Haensch W, Roefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61:3932–3936. [PubMed] [Google Scholar]
- 14.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 15.Oudejans JJ, Harijadi H, Kummer JA, Tan IB, Bloemena E, Middeldorp JM, Bladergroen B, Dukers DF, Vos W, Meijer CJ. High numbers of granzyme B/CD8-positive tumour-infiltrating lymphocytes in nasopharyngeal carcinoma biopsies predict rapid fatal outcome in patients treated with curative intent. J Pathol. 2002;198:468–475. doi: 10.1002/path.1236. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi T, Takahashi H, Tobe T, Suzuki H, Mizoguchi K, Nakatsu HO, Ito H. Effect of tumor-infiltrating lymphocyte subsets on prognosis and susceptibility to interferon therapy in patients with renal cell carcinoma. Urol Int. 2002;69:51–56. doi: 10.1159/000064361. [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, Dosaka-Akita H, Nishimura M. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94:1003–1009. doi: 10.1111/j.1349-7006.2003.tb01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber H, Wu TH, Nachman J, Rowley DA. Immunological enhancement of primary tumor development and its prevention. Semin Cancer Biol. 2000;10:351–357. doi: 10.1006/scbi.2000.0331. [DOI] [PubMed] [Google Scholar]
- 20.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 21.Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol. 2006;45:246–253. doi: 10.1016/j.jhep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Sheu BC, Hsu SM, Ho HN, Lin RH, Torng PL, Huang SC. Reversed CD4/CD8 ratios of tumor-infiltrating lymphocytes are correlated with the progression of human cervical carcinoma. Cancer. 1999;86:1537–1543. doi: 10.1002/(sici)1097-0142(19991015)86:8<1537::aid-cncr21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.De Visser KE, Schumacher TN, Kruisbeek AM. CD8+ T cell tolerance and cancer immunotherapy. J Immunother. 2003;26:1–11. doi: 10.1097/00002371-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25- T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 26.Matsui M, Machida S, Itani-Yohda T, Akatsuka T. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J Gastroenterol Hepatol. 2002;17:897–907. doi: 10.1046/j.1440-1746.2002.02837.x. [DOI] [PubMed] [Google Scholar]
- 27.Janicki CN, Jenkinson SR, Williams NA, Morgan DJ. Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res. 2008;68:2993–3000. doi: 10.1158/0008-5472.CAN-07-5008. [DOI] [PubMed] [Google Scholar]
- 28.Koneru M, Monu N, Schaer D, Barletta J, Frey AB. Defective adhesion in tumor infiltrating CD8+ T cells. J Immunol. 2006;176:6103–6111. doi: 10.4049/jimmunol.176.10.6103. [DOI] [PubMed] [Google Scholar]
- 29.Waziri A, Killory B, Ogden AT 3rd, Canoll P, Anderson RC, Kent SC, Anderson DE, Bruce JN. Preferential in situ CD4+CD56+ T cell activation and expansion within human glioblastoma. J Immunol. 2008;180:7673–7680. doi: 10.4049/jimmunol.180.11.7673. [DOI] [PubMed] [Google Scholar]
- 30.Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, Iijima S, Noike T, Kobayashi A, Kawasaki S. Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Hum Pathol. 2004;35:881–886. doi: 10.1016/j.humpath.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Kasper HU, Drebber U, Zur Hausen A, Stippel D, Dienes HP, Dries V. Dominance of CD4+ alpha/beta T-cells and inferior role of innate immune reaction in liver metastases. Anticancer Res. 2003;23:3175–3181. [PubMed] [Google Scholar]