Abstract
AIM: To identify the clinical features and outcomes of infrequently reported leptomeningeal carcinomatosis (LMC) of gastric cancer.
METHODS: We analyzed 54 cases of cytologically confirmed gastric LMC at four institutions from 1994 to 2007.
RESULTS: The male-to-female ratio was 32:22, and the patients ranged in age from 28 to 78 years (median, 48.5 years). The majority of patients had advanced disease at initial diagnosis of gastric cancer. The clinical or pathologic tumor, node and metastasis stage of the primary gastric cancer was IV in 38 patients (70%). The median interval from diagnosis of the primary malignancy to the diagnosis of LMC was 6.3 mo, ranging between 0 and 73.1 mo. Of the initial endoscopic findings for the 45 available patients, 23 (51%) of the patients were Bormann type III and 15 (33%) patients were Bormann type IV. Pathologically, 94% of cases proved to be poorly differentiated adenocarcinomas. Signet ring cell component was also observed in 40% of patients. Headache (85%) and nausea/vomiting (58%) were the most common presenting symptoms of LMC. A gadolinium-enhanced magnetic resonance imaging was conducted in 51 patients. Leptomeningeal enhancement was noted in 45 cases (82%). Intrathecal (IT) chemotherapy was administered to 36 patients-primarily methotrexate alone (61%), but also in combination with hydrocortisone/± Ara-C (39%). The median number of IT treatments was 7 (range, 1-18). Concomitant radiotherapy was administered to 18 patients, and concomitant chemotherapy to seven patients. Seventeen patients (46%) achieved cytological negative conversion. Median overall survival duration from the diagnosis of LMC was 6.7 wk (95% CI: 4.3-9.1 wk). In the univariate analysis of survival duration, hemoglobin, IT chemotherapy, and cytological negative conversion showed superior survival duration (P = 0.038, P = 0.010, and P = 0.002, respectively). However, in our multivariate analysis, only cytological negative conversion was predictive of relatively longer survival duration (3.6, 6.7 and 14.6 wk, P = 0.030, RR: 0.415, 95% CI: 0.188-0.918).
CONCLUSION: Although these patients had a fatal clinical course, cytologic negative conversion by IT chemotherapy may improve survival.
Keywords: Carcinomatosis, Gastric cancer, Intrathecal chemotherapy, Leptomeningeal
INTRODUCTION
Leptomeningeal carcinomatosis (LMC) is defined as malignant infiltration of the pia mater and the arachnoid membrane. LMC is one of the most serious complications that can occur in cancer patients[1]. According to the results of a large autopsy study, the incidence of LMC was 5%-8% in cancer patients[2]. As a significant proportion of these patients have asymptomatic microscopic disease, the clinical diagnosis of LMC has been established in 2%-4% of patients during the course of their malignancy[1]. LMC is frequently detected in patients with leukemia, breast cancer, lymphoma, and lung cancer[3]. Among solid tumors, LMC is observed more frequently in cases of disseminated and progressive disease. Although a subset of patients, particularly those with lymphoma or breast cancer, may survive for more than 12 mo with a reasonable quality of life, leptomeningeal metastasis from solid tumors is associated with a poor overall prognosis. The treatment of LMC is palliative and unsatisfactory. No evidence demonstrating the superiority of intrathecal (IT) treatment compared to best palliative care is currently available from clinical trials.
Furthermore, the development of LMC from a gastric cancer is a very rare occurrence. Some articles have reported that the incidence of LMC in patients with gastric cancer was responsible for 0.16% of all cases of gastric cancer[4]. Due to its rarity, the clinical features and prognostic factors of LMC as a metastasis from gastric carcinoma have yet to be clearly characterized. The benefits of IT chemotherapy are also currently a matter of some contention.
Gastric cancer is the most common malignancy in Korea[5]. Because of the high prevalence of gastric cancer in Korea, we took the opportunity to study gastric cancer patients with LMC. The principal objective of this study was to review our experience with LMC associated with gastric cancer, and to evaluate its clinical features and the efficacy of a variety of treatment modalities in terms of neurological status and overall survival (OS).
MATERIALS AND METHODS
Patients
From 1995 to 2007, 22 154 patients were diagnosed with gastric cancer at four independent institutions. Among them, 54 patients who were diagnosed with leptomeningeal seeding metastasis of gastric cancer were analyzed. Although it is not representative of the cohort of patients, the prevalence of LMC was 0.24%.
Eligibility for this study included: (1) patients with histologically confirmed adenocarcinoma of the stomach; (2) cytologically confirmed malignancy on cerebrospinal fluid (CSF) analysis, patients with suspected LMC by magnetic resonance imaging (MRI) and negative cytology were excluded; (3) no history of any other malignancies.
We retrospectively analyzed the patients’ medical records including the patients’ characteristics, clinical symptoms, laboratory and radiologic findings, treatment modality and outcomes, final follow-up, and survival duration.
Statistical analysis
Comparisons of categorical variables among groups were conducted using the chi-square test and Fisher’s exact test. OS was calculated from the cytological confirmation of LMC and plotted via the Kaplan-Meier method. Comparison of survival according to prognostic factors was evaluated via a log-rank test, and forward stepwise Cox proportional hazard models were employed to evaluate the joint effect of predictive variables. P < 0.05 was considered significant. Analysis of the data was conducted using SPSS for Windows V. 15.0 (SPSS Inc., Chicago, IL, USA) statistical software.
RESULTS
Patients’ characteristics
We analyzed 54 cases of cytologically confirmed gastric LMC at four institutions from 1994 to 2007. The clinical characteristics of these patients are summarized in Table 1. The male-to-female ratio was 32:22, and patients ranged in age from 28 to 78 years (median, 48.5 years). The majority of patients had advanced disease at initial diagnosis of gastric cancer. The clinical or pathologic tumor, node and metastasis stage of the primary gastric cancer was IV in 47 patients (87%). Stage I-III patients received curative operation. Among the stage IV patients, 13 patients had T4N1-2 or N3 (No. of nodes > 15) by pathologic features through curative operation. M1 node positive patients were counted as palliative surgery. Of the initial endoscopic findings in the available 45 patients, Bormann type III and IV were reported for 23 (51%) and 15 (33%) patients, respectively. Pathologically, 94% of cases proved to be poorly differentiated adenocarcinomas. Signet ring cell component was also observed in 40% of patients.
Table 1.
Patients’ characteristics (n = 54)
| No. of patients | n (%) |
| Gender | |
| Male/female | 32 (59.3)/22 (40.7) |
| Age (yr) | |
| Median (range) | 48 (28-78) |
| ≥ 60/< 60 | 15 (27.8)/39 (72.2) |
| Initial stage | |
| I-II | 2 (3.7) |
| III | 2 (3.7) |
| IV | 47 (87.0) |
| Not available | 3 (5.6) |
| Operation | |
| Curative | 17 (31.5) |
| Palliative | 15 (27.8) |
| Inoperable | 18 (33.3) |
| Not available | 4 (7.5) |
| Initial endoscopic finding (n = 47) | |
| Site | |
| Cardia | 1 (1.9) |
| Fundus | 1 (1.9) |
| Body | 20 (37.0) |
| Antrum, pylorus | 16 (29.7) |
| Diffuse whole stomach | 9 (19.1) |
| Borrmann type (n = 45) | |
| Early gastric cancer | 2 (4.4) |
| I (polypoid) | 1 (2.2) |
| II (ulcerative) | 4 (8.9) |
| III (ucero-infiltrative) | 23 (51.2) |
| IV (diffuse infiltrative) | 15 (33.3) |
| Differentiation (n = 47) | |
| Moderate | 3 (6.4) |
| Poor | 25 (53.2) |
| Poor with signet ring cell | 19 (40.4) |
LMC patterns
The median interval from diagnosis of the primary gastric cancer to the diagnosis of LMC was 6.3 mo, ranging from 0 to 73.1 mo. Five patients presented with initial LMC. The majority of patients (59.3%) initially presented with metastatic gastric cancer without LMC, and then progressed to LMC. One-third of the patients presented with curable disease at the initial diagnosis of gastric cancer (Table 2).
Table 2.
Patterns of leptomeningeal carcinomatosis (n = 54)
| n (%) | |
| Time to LMC (mo) | |
| Median (range) | 6.3 (0-73.1) |
| LMC presentation | |
| Curative/recurred/progression | 7 (13.0) |
| Curative/recurred LMC | 10 (18.5) |
| Metastatic/progression | 32 (59.3) |
| Initially LMC | 5 (9.3) |
LMC: Leptomeningeal carcinomatosis.
Clinical symptoms
The most frequently observed presenting symptoms of LMC were nonspecific symptoms such as headache (85%) and nausea/vomiting (58%). In addition, various neurological clinical signs and symptoms were noted including altered mental status, seizure, motor weakness, sensory change, diplopia, hearing loss, and facial palsy (Table 3).
Table 3.
Symptoms of leptomeningeal carcinomatosis (n = 54)
| n (%) | |
| Cerebral symptom | |
| Headache | 46 (85.1) |
| Nausea & vomiting | 32 (59.2) |
| Dizziness | 13 (24.0) |
| Mental change | 12 (22.2) |
| Seizure | 10 (18.5) |
| Gait difficulty | 2 (3.7) |
| Dysarthria | 2 (3.7) |
| Psychosis | 1 (1.9) |
| Cranial symptom | |
| Diplopia | 3 (5.6) |
| Hearing loss | 2 (3.7) |
| Facial palsy | 1 (1.9) |
| Ptosis | 1 (1.9) |
| Spinal symptom | |
| Weakness | 5 (11.1) |
| Paresthesia | 2 (3.7) |
| Back pain | 1 (1.9) |
CSF analysis and image findings
Lumbar puncture and analysis of CSF is a crucial laboratory test in the diagnosis of LMC. All the patients presented with malignant cells on cytological analysis via the inclusion criteria. An elevated opening pressure on lumbar puncture was noted in 58% of the subjects. The mean CSF pressure in the patients was 222.1 mm CSF. 78.8% and 53.8% of patients had elevated white blood cells and protein in CSF, respectively (Table 4).
Table 4.
CSF finding of leptomeningeal carcinomatosis
| CSF | No. of > WNL1 | mean ± SD |
| Pressure (mm CSF) | 29/50 (58.0%) | 222.1 ± 158.4 |
| WBC (n/mm3) | 41/52 (78.8%) | 36.7 ± 59.0 |
| Protein (mg/dL) | 28/52 (53.8%) | 129.5 ± 250.8 |
CSF pressure > 160 mm; CSF protein > 50 mg/dL; Cell count > 5/mm3. CSF: Cerebrospinal fluid; WNL: Within normal limit; WBC: White blood cell.
Brain computed tomography was assessed in eight patients and leptomeningeal enhancement was observed only in one patient. A gadolinium-enhanced MRI was conducted in 51 patients. Leptomeningeal enhancement was noted in 45 cases (82%).
Treatment modalities and outcomes
IT chemotherapy was administered to 36 patients, principally with methotrexate (MTx) alone (61%) or in combination with hydrocortisone/± Ara-C (41%). The median number of IT treatments was 7 (range, 1-18). Seventeen patients (46%) achieved cytological negative conversion (Table 5).
Table 5.
Treatment with intrathecal chemotherapy (n = 36)
| n (%) | |
| Regimen | |
| MTx | 22 (61.1) |
| MTx + steroid | 4 (11.1) |
| MTx + Ara-C + steroid | 10 (27.8) |
| Concurrent/sequential | |
| + alone | 16 (44.4) |
| + radiotherapy | 13 (36.1) |
| + chemotherapy | 2 (5.6) |
| + chemoradiotherapy | 5 (13.9) |
| No. of cycles | |
| Median (range) | 7 (1-18) |
| Cytological response | |
| (-) conversion | 17 (47.2) |
MTx: Methotrexate; Ara-C: Cytarabine.
Thirteen patients were treated with whole brain irradiation coupled with IT chemotherapy. 6 patients received radiation treatment alone.
Additional systemic chemotherapy was given to 10 patients. Three patients were treated with the orally available 5-fluorouracil (5-FU) drugs - capecitabine, S-1, and tegafur-uracil. Irinotecan/leucovorin/5-FU, 5-FU/cisplatin, and paclitaxel/cisplatin were administered to four, two and one patients, respectively. Seven patients were treated with chemotherapy plus IT chemotherapy. Three patients received chemotherapy alone. The median number of cycles administered was 2 (range, 1-6). Among the treated patients, only one exhibited a detectable response to treatment.
Survival and prognostic factors
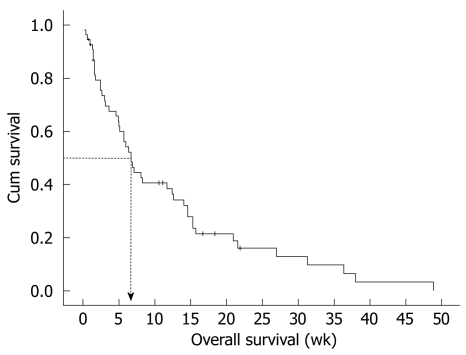
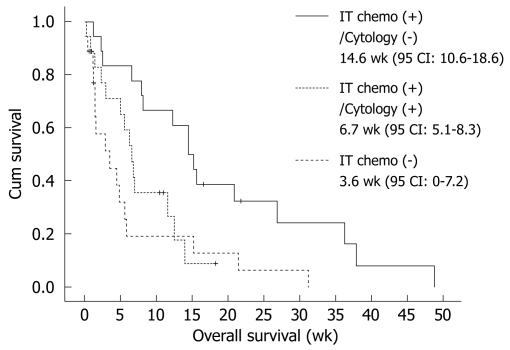
Median OS duration from diagnosis of LMC was 6.7 wk (95% CI: 4.3-9.1 wk) (Figure 1). In the univariate analysis of survival duration, hemoglobin, IT chemotherapy, and cytologic negative conversion showed superior survival duration (P = 0.038, P = 0.010, and P = 0.002, respectively). However, in the multivariate analysis, only cytologic negative conversion was predictive of relatively longer survival duration (3.6, 6.7 and 14.6 wk, P = 0.030, RR: 0.415, 95% CI: 0.188-0.918) (Table 6, Figure 2).
Figure 1.
Median overall survival (OS) duration from diagnosis of leptomeningeal carcinomatosis. Median OS was 6.7 wk (95% CI: 4.3-9.1 wk).
Table 6.
Survival duration and prognostic factors
| Factors | Median OS (mo) | Univariate | Multivariate |
| Gender | |||
| Male | 7.1 | 0.491 | - |
| Female | 6.7 | ||
| Age (yr) | |||
| > 60 | 12.4 | 0.214 | - |
| ≤ 60 | 6.4 | ||
| PS (LMC) | |||
| 0, 1 | 11.7 | 0.975 | - |
| ≥ 2 | 6.4 | ||
| Hb (LMC) | |||
| > 11 | 12.4 | 0.038 | NS |
| ≤ 11 | 5.7 | ||
| MRI enhance | |||
| Negative | 5.7 | 0.316 | - |
| Positive | 7.1 | ||
| CSF pressure | |||
| > 120 | 6.4 | 0.163 | NS |
| ≤ 120 | 8.1 | ||
| CSF protein | |||
| > 40 | 6.0 | 0.539 | - |
| ≤ 40 | 11.7 | ||
| IT chemotherapy | |||
| No | 3.6 | 0.010 | NS |
| Yes | 11.7 | ||
| Radiotherapy | |||
| No | 4.6 | 0.516 | - |
| Yes | 6.9 | ||
| Cytologic response | |||
| No | 5.1 | 0.002 | 0.030 (HR: 0.415, 95% CI: 0.188-0.918) |
| Yes | 14.6 |
OS: Overall survival; PS (LMC): Performance status scale by Eastern Cooperative Oncology Group at leptomeningeal carcinomatosis; Hb (LMC): Hemoglobin at leptomeningeal carcinomatosis; MRI: Magnetic resonance imaging; CSF: Cerebrospinal fluid; IT: Intrathecal; HR: Hazard ration; CI: Confidence interval.
Figure 2.
Cytologically negative conversion proved predictive of relatively longer survival duration (P = 0.030, RR: 0.415, 95% CI: 0.188-0.918).
DISCUSSION
Adenocarcinoma is the predominant histological type in LMC of solid tumors[6]. Among patients diagnosed with LMC, the most frequently encountered solid tumors are breast (12%-34%), lung (14%-29%), and melanoma (17%-25%)[3]. Unlike Western reports, gastric cancer is the principal etiology of LMC in solid tumors in Korea[7].
CNS metastasis is a very rare complication of gastric cancer, and occurs in 0.16%-0.69% of gastric cancer patients in general, including Korean reports[4,8,9]. Although all the included patients demonstrated CSF cytologically confirmed malignancy, the prevalence of LMC in this study was 0.24% in all gastric cancer patients.
Consistent with other studies, the majority of patients had Bormann type III or IV advanced gastric cancer of poorly differentiated or signet-ring cell histopathology, which increased the tendency for distant metastasis and poor prognosis[4,10,11]. Similar to the results of a previous study, LMC patients presented with an advanced stage and Bormann type III or IV advanced gastric cancer of poorly differentiated or signet-ring cell histopathology.
LMC is an ultimately fatal disease[12-15]. A minority of patients, usually those with breast cancer or lymphoid malignancies, may achieve disease-free survival of a year or more, however, the median OS for patients with LMC is only 4-6 wk if untreated and 2-4 mo with therapy[6,12,15]. In our study, the median survival duration was just 6.7 wk. Although LMC patients tend to have a poor performance status, approximately two-thirds of patients who receive IT chemotherapy and 47.2% patients who responded to therapy achieved longer survival duration. The independent prognostic factor for survival was cytologic negative conversion by IT chemotherapy. Although the small sample size and inherent selection bias of the retrospective design of this study makes any conclusions regarding the outcomes of treatment somewhat difficult, the findings of our study indicate that cytologic negative conversion by IT chemotherapy may improve survival by arresting neurologic progression in selected patients.
MTx remains the most frequently utilized drug for IT administration, despite its limited success and serious complications[16,17]. Combination IT chemotherapy with MTx, arabinoside and hydrocortisone has been reported to be more effective than MTx alone in solid tumor LMC[18]. However, approximately 10% of gastric cancer patients were enrolled in this study, and the efficacy of arabinoside against gastric cancer is questionable.
Craniospinal irradiation may be a one-treatment modality. However, the additional or sequential role of radiation has been controversial[19]. In our study, additional effects of radiotherapy were not observed. As response to radiation is associated with the sensitivity or resistance of primary tumors and malignant cells circulating in the CSF space, radiation is occasionally not feasible for palliative treatment.
Systemic chemotherapy was also administered to a limited number of patients who had better performance status[6,15,20]. In our study, patients who were treated with systemic chemotherapy showed the best median OS duration (21.6 wk, 95% CI: 3.2-40 wk). However, all the treatments were administered sequentially after IT chemotherapy and the patients responded to treatment.
COMMENTS
Background
Leptomeningeal carcinomatosis (LMC) occurs in approximately 5% of cancer patients. The most common cancers involving the leptomeninges are breast and lung cancer. However, gastric adenocarcinoma has been infrequently reported in conjunction with LMC. This retrospective analysis was performed to identify the clinical features and outcomes of infrequently reported LMC of gastric cancer.
Research frontiers
This is the first large scale study on gastric LMC. LMC is a rare component in the clinical manifestation of gastric cancer. LMC usually presents at a relatively young age, at an advanced stage, and is of a poorly differentiated pathologic type.
Innovations and breakthroughs
Although gastric LMC had a fatal clinical course, the findings of our study suggest that cytologic negative conversion by intrathecal (IT) chemotherapy may improve survival by arresting neurologic progression in selected patients.
Applications
These results could provide basic clinical data on gastric LMC for physicians and demonstrate the role of IT chemotherapy.
Terminology
Leptomeninges (literally thin meninges) is a term referring to the pia mater and arachnoid mater. LMC is a condition in which a tumor diffusely spreads to the leptomeninges. Intrathecal chemotherapy involves anticancer drugs injected into the fluid-filled space between the thin layers of tissue that cover the brain and spinal cord.
Peer review
The results are interesting and suggest that cytologic negative conversion by IT chemotherapy may improve survival by arresting neurologic progression in selected patients.
Footnotes
Supported by The Dong-A University Research Fund and the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST; R13-2002-044-05001-0)
Peer reviewers: Gerardo Rosati, MD, Medical Oncology Unit, “S. Carlo” Hospita, Via Potito Petrone, 1, Potenza 85100, Italy; Cuneyt Kayaalp, MD, Professor, Department of General Surgery, Turgut Ozal Medical Center, Inonu University, Malatya 44315, Turkey
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
References
- 1.Pentheroudakis G, Pavlidis N. Management of leptomeningeal malignancy. Expert Opin Pharmacother. 2005;6:1115–1125. doi: 10.1517/14656566.6.7.1115. [DOI] [PubMed] [Google Scholar]
- 2.Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–119. doi: 10.1053/ctrv.1999.0119. [DOI] [PubMed] [Google Scholar]
- 3.Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759–772. doi: 10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Kim M. Intracranial involvement by metastatic advanced gastric carcinoma. J Neurooncol. 1999;43:59–62. doi: 10.1023/a:1006156204385. [DOI] [PubMed] [Google Scholar]
- 5.Shin HR, Jung KW, Won YJ, Kong HJ, Yim SH, Sung J, Seo SW, Kim KY, Lee SY, Kong IS, et al. National cancer incidence for the year 2002 in Korea. Cancer Res Treat. 2007;39:139–149. doi: 10.4143/crt.2007.39.4.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno MK, Raizer J. Leptomeningeal metastases from solid tumors (meningeal carcinomatosis) Cancer Treat Res. 2005;125:31–52. doi: 10.1007/0-387-24199-x_3. [DOI] [PubMed] [Google Scholar]
- 7.Lee JL, Kang YK, Kim TW, Chang HM, Lee GW, Ryu MH, Kim E, Oh SJ, Lee JH, Kim SB, et al. Leptomeningeal carcinomatosis in gastric cancer. J Neurooncol. 2004;66:167–174. doi: 10.1023/b:neon.0000013462.43156.f4. [DOI] [PubMed] [Google Scholar]
- 8.Kasakura Y, Fujii M, Mochizuki F, Suzuki T, Takahashi T. Clinicopathological study of brain metastasis in gastric cancer patients. Surg Today. 2000;30:485–490. doi: 10.1007/s005950070112. [DOI] [PubMed] [Google Scholar]
- 9.York JE, Stringer J, Ajani JA, Wildrick DM, Gokaslan ZL. Gastric cancer and metastasis to the brain. Ann Surg Oncol. 1999;6:771–776. doi: 10.1007/s10434-999-0771-3. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi Y. Blood vessel invasion in gastric carcinoma. Surgery. 1990;107:140–148. [PubMed] [Google Scholar]
- 11.Adachi Y, Mori M, Maehara Y, Sugimachi K. Poorly differentiated medullary carcinoma of the stomach. Cancer. 1992;70:1462–1466. doi: 10.1002/1097-0142(19920915)70:6<1462::aid-cncr2820700603>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.DeAngelis LM, Boutros D. Leptomeningeal metastasis. Cancer Invest. 2005;23:145–154. [PubMed] [Google Scholar]
- 13.DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neurooncol. 1998;38:245–252. doi: 10.1023/a:1005956925637. [DOI] [PubMed] [Google Scholar]
- 14.Grant R, Naylor B, Greenberg HS, Junck L. Clinical outcome in aggressively treated meningeal carcinomatosis. Arch Neurol. 1994;51:457–461. doi: 10.1001/archneur.1994.00540170033013. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhary S, Chamberlain M. Leptomeningeal metastases: current concepts and management guidelines. J Natl Compr Canc Netw. 2005;3:693–703. doi: 10.6004/jnccn.2005.0039. [DOI] [PubMed] [Google Scholar]
- 16.Aiello-Laws L, Rutledge DN. Management of adult patients receiving intraventricular chemotherapy for the treatment of leptomeningeal metastasis. Clin J Oncol Nurs. 2008;12:429–435. doi: 10.1188/08.CJON.429-435. [DOI] [PubMed] [Google Scholar]
- 17.Bleyer WA, Drake JC, Chabner BA. Neurotoxicity and elevated cerebrospinal-fluid methotrexate concentration in meningeal leukemia. N Engl J Med. 1973;289:770–773. doi: 10.1056/NEJM197310112891503. [DOI] [PubMed] [Google Scholar]
- 18.Kim DY, Lee KW, Yun T, Park SR, Jung JY, Kim DW, Kim TY, Heo DS, Bang YJ, Kim NK. Comparison of intrathecal chemotherapy for leptomeningeal carcinomatosis of a solid tumor: methotrexate alone versus methotrexate in combination with cytosine arabinoside and hydrocortisone. Jpn J Clin Oncol. 2003;33:608–612. doi: 10.1093/jjco/hyg118. [DOI] [PubMed] [Google Scholar]
- 19.Mehta M, Bradley K. Radiation therapy for leptomeningeal cancer. Cancer Treat Res. 2005;125:147–158. doi: 10.1007/0-387-24199-x_9. [DOI] [PubMed] [Google Scholar]
- 20.Taillibert S, Hildebrand J. Treatment of central nervous system metastases: parenchymal, epidural, and leptomeningeal. Curr Opin Oncol. 2006;18:637–643. doi: 10.1097/01.cco.0000245323.19411.d7. [DOI] [PubMed] [Google Scholar]