Abstract
AIM: To investigate the gastroprotective effect of vardenafil against indomethacin-induced gastric damage.
METHODS: Forty-eight female Wistar albino rats were randomly divided into 6 groups. Group 1 received saline only. Group 2 (indomethacin) received indomethacin. Rats in group 3 and 4 were pretreated with different doses of famotidine. Group 5 and 6 were pretreated with different doses of vardenafil. Rats in groups 3 to 6 received 25 mg/kg indomethacin 30 min after pretreatment. The animals were sacrificed 6 h later and their stomachs were opened. Gastric lesions were counted and measured. The stomach of each animal was divided in two parts for histopathological examinations and nitric oxide (NO) and malondialdehyde (MDA) assays, respectively.
RESULTS: There were no gastric mucosal lesion in the saline group but all rats in the indomethacin group had gastric mucosal ulcerations (ulcer count; 6.25 ± 3.49, and mean ulcer area; 21.00 ± 12.35). Ulcer counts were diminished with famotidine 5 mg/kg (4.12 ± 2.47, P > 0.05), 20 mg/kg (2.37 ± 4.43, P < 0.05), vardenafil 2 mg/kg (4.37 ± 3.06), and vardenafil 10 mgkg (1.25 ± 1.38, P < 0.05) compared to the indomethacin group. Gastric mucosal lesion areas were diminished with famotidine 5 mg/kg (8.62 ± 2.97, P < 0.001) , famotidine 20 mg/kg (0.94 ± 2.06, P < 0.001), vardenafil 2 mg/kg (6.62 ± 5.87, P < 0.001), and vardenafil 10 mg/kg (0.75 ± 0.88, P < 0.001) compared to the indomethacin group. MDA levels were significantly higher in indomethacin group (28.48 ± 14.51), compared to the famotidine 5 mg/kg (6,21 ± 1.88, P < 0.05), famotidine 20 mg/kg (5.88 ± 1.60. P < 0.05), vardenafil 2 mg/kg (15.87 ± 3.93, P < 0.05), and vardenafil 10 mg/kg (10.97 ± 4.50, P < 0.05). NO concentration in gastric tissues of the famotidine groups were significantly increased (P < 0.05), but the NO increases in the vardenafil groups were not statistically significant. Histopathology revealed diminished gastric damage for pretreatment groups compared to the indomethacin group (P < 0.05).
CONCLUSION: Vardenafil affords a significant dose-dependent protection against indomethacin induced gastric mucosal lesions in rats.
Keywords: Vardenafil, Gastric ulceration, Indomethacin, Gastroprotection, Rats
INTRODUCTION
Gastric mucosal damage is a common disorder of the gastrointestinal system. The pathogenesis of gastric ulcers is based on complex interactions between aggressive and protective factors. Nonsteroidal anti-inflammatory drugs (NSAID) are known to be aggressive agents for gastric ulcer development. People of advancing age need many drugs, including NSAIDs, for the treatment of pain and inflammation due to rheumatological disturbances.
Vardenafil is a phosphodiesterase (PDE) V inhibitor that has been used for the treatment of erectile dysfunction[1] and, more recently, for pulmonary hypertension[2,3]. Recent laboratory studies demonstrated successful effects of PDE V inhibitors on cardioprotection after ischemia reperfusion injury[4,5], as well as in ischemic injury of other organs, such as the colon, liver, and brain[6,7]. Deibert et al8] revealed that vardenafil had increased portal flow and lowered portal pressure in patients with cirrhotic livers.
Diminished mucosal circulation has been blamed as one of the etiological factors in gastric ulcer formation. Like prostaglandins, the L-Arginine/nitric oxide (NO) pathway is a major protective system in gastric mucosa[9] via relaxation of the arterial smooth muscles. Inhibition of nitric oxide synthase aggravates the injury in animal models of gastric ulcers[10].
In this study, we have studied the effects of vardenafil on the acute gastric injury caused by administration of indomethacin.
MATERIALS AND METHODS
The study was approved by the Zonguldak Karaelmas University (ZKU) Animal Experiments Local Ethic Committee. The study was carried out on 48 female Wistar albino rats weighing 200-250 g, obtained from the Experimental Animal Laboratory of Medical Faculty of ZKU. The rats were kept under standard conditions (temperature; 22-24°C, and 12:12 h light/dark). The experimental procedures were carried out in accordance with international guidelines for the use and care of laboratory animals. All animals were fed with pellet food produced especially for experimental animals. Water was available ad libitum. All experiments were performed at the same time of the day to avoid diurnal variations of putative regulators of gastric functions.
Famotidine and vardenafil were dissolved in distilled water. All drug solutions and suspensions were freshly prepared. Gastric ulcers were inflicted by oral administration of indomethacin 16-18 h after starvation.
Animals were randomly divided into six groups. In Group 1 (n:8) rats received only 8 mL/kg of saline by gavage. Rats in Group 2 (n:8) received 25 mg/kg indomethacin in a volume of 8 mL/kg of saline. The rats in group 3 and 4 were pretreated with famotidine, 5 mg/kg and 20 mg/kg, respectively. Rats in groups 5 and 6 were pretreated with 2 mg/kg and 10 mg/kg vardenafil, respectively. After 30 min, 25 mg/kg indomethacin in a volume of 8 mL/kg of saline were administered by gavage. Six hours after oral administration of indomethacin, all rat groups were anesthetized with an intramuscular injection of 100 mg/kg Ketamine (Ketalar®, Parke Davis-Eczacıbaşı, Istanbul, Turkey). A midline abdominal incision was then performed. All rat groups were sacrificed via cardiac puncture, and their stomachs rapidly removed, opened by an incision along the lesser curvature, and rinsed in ice-cold distilled water[11]. Gastric tissues were pinned out on a wax platform. Macroscopic damage to the gastric mucosa was assessed. Hemorrhagic and ulcerative lesions were counted and their lengths were measured on square millimeter paper. Gastric mucosal lesions were expressed as the sum of the lengths (mm) of all lesions for each stomach, which was used as the ulcer index (UI)[12,13]. Gastric lesions were judged by two independent researchers who were blinded to the protocol. The average score of the two independent observers were taken into account, and the sum of the total scores was divided by the number of animals to obtain the mean UI for each group.
The stomach of each animal was divided into two parts. One part of the stomach was excised, immersed in saline, and immediately stored at -40°C for measurement of NO and MDA levels.
Gastric tissues were homogenized in ten volumes of 150 mmol/L ice-cold KCl using a glass teflon homogenizer (Ultra Turrax IKA T18 Basic) after cutting the tissues into small pieces with scissors (for 2 min at 5000 r/min). The homogenate was then centrifuged at 5000 × g for 15 min. The supernatant was used for analysis. High-performance liquid chromatographic analysis was performed using a Shimadzu HPLC system (Kyoto, Japan) with an MDA kit (Immundiagnostik AG, Bensheim, Germany). Spectrophotometric measurements of total antioxidant status (TAS) (Randox, Crumlin, UK) was performed using a Shimadzu UV-1601 (Kyoto, Japan) spectrophotometer. Serum nitric oxide levels (nitrite + nitrate) were measured, after conversion of nitrate to nitrite by copperized cadmium granules, by a spectrophotometer at 545 nm (Shimadzu, Tokyo, Japan)[14]. Protein assays were measured on an Advia 2400 chemistry analyzer (Bayer Healthcare Instruments, Tarrytown, NY, USA). Results were expressed as μmol/g protein for NO and nmol/g protein for MDA.
The other part of the stomach was fixed in 10% neutral formalin, embedded in paraffin, and cut into 5 μm sections. The sections were stained with hematoxylin eosin (HE) and examined under the light microscope by a blinded pathologist for histological changes.
The results obtained from vardenafil groups were evaluated by comparing them with those of sham, indomethacin, and famotidine groups.
Statistical analysis
The statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 12.0 for Windows. All data are expressed as the mean ± SD. Mann-Whitney U and χ2 tests were used for statistical analysis of data among all groups. P < 0.05 was considered as statistically significant.
RESULTS
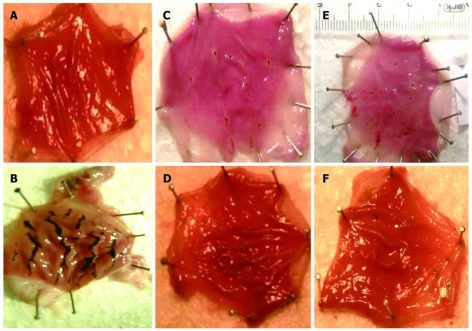
Macroscopic analysis showed that there was no gastric mucosal lesion in the sham group. There were gastric mucosal lesions in all stomachs of the indomethacin 25 mg/kg treated group. The mean ulcer area was 21.00 ± 12.35 in the indomethacin group. Gastric mucosal damage was significantly reduced by famotidine 20 mg/kg and vardenafil 10 mg/kg pretreatments. In both groups, the mean count of ulceration and the mean count of ulcer area were significantly lower than the control group. Gastric mucosal lesion areas were significantly diminished in rats pretreated with famotidine 5 mg/kg and vardenafil 2 mg/kg, when compared with the control group, but this did not reach statistical significance in respect to ulcer count. The mean ulcer area in the vardenafil 2 mg/kg group and vardenafil 10 mg/kg group were 6.62 ± 5.87 and 0.75 ± 0.88, respectively. Macroscopic evaluation of gastric mucosal lesion counts and gastric mucosal lesion areas for each group are presented in Table 1. In damaged stomachs, mucosal lesions of various sizes and forms were dispersed to all stomach surfaces. Those lesions consisted of elongated bands parallel to the long axis of the stomach. Lesions of the gastric mucosa in each group are shown in Figure 1. Tissue MDA and NO levels are presented in Table 2 for each group.
Table 1.
Macroscopic evaluation of gastric mucosa
| Groups | n | Weight (gr) | GML count | GML area mm2 |
| Sham | 8 | 223.50 ± 18.40 (200-249) | 0 | 0 |
| Indomethacin | 8 | 225.00 ± 13.77 (201-248) | 6.25 ± 3.49 (1-11)a | 21.00 ± 12.35 (1-36)b |
| Famotidine 5 (F5) | 8 | 223.25 ± 13.13 (203-236) | 4.12 ± 2.47 (2-8) | 8.62 ± 2.97 (3-12) |
| Famotidine 20 (F20) | 8 | 224.75 ± 14.78 (200-247) | 2.37 ± 4.43 (0-13) | 0.94 ± 2,06 (0-6)d |
| Vardenafil 2 (V2) | 8 | 221.12 ± 13.27 (204-242) | 4.37 ± 3.06 (0-8) | 6.62 ± 5.87 (0-16)e |
| Vardenafil 10 (V10) | 8 | 224.12 ± 15.16 (202-250) | 1.25 ± 1.38 (0-3)cg | 0.75 ± 0.88 (0-2)dg |
GML: Gastric mucosal lesion. The values are presented as mean ± SD, (min-max).
P < 0.05,
P < 0.001 vs all other groups;
P < 0.05,
P < 0.001 vs group F5;
P < 0.05 vs group F20;
P < 0.05 vs group V2.
Figure 1.
Gross appearance of the opened stomachs in the experimental groups. A: Appearance of normal mucosa of the stomach (Saline); B: Severe mucosal injury (Indomethacin); C: Diminished mucosal injury (Group F5); D: Gastric mucosa without any lesion (Group F20); E: Partially protected gastric mucosa against the harmful effect of indomethacin (Group V2); F: Lesion free gastric mucosa (Group V10).
Table 2.
MDA and NO levels in gastric tissues in each group
| Group | n | MDA (nmol/g protein) | NO (μmol/g protein) |
| Sham | 8 | 9.4 ± 4.47 (3.6-14.3) | 35.67 ± 5.69 (30.21-46.63) |
| Indomethacin | 8 | 28.48 ± 14.51 (7.1-45)a | 27.20 ± 6.25 (20.0-38.78) |
| Famotidine 5 (F5) | 8 | 6.21 ± 1.88 (3.4-9.5) | 40.82 ± 9.42 (31.08-59.92)c |
| Famotidine20 (F20) | 8 | 5.88 ± 1.60 (3.3-7.7) | 51.22 ± 15.27 (34.24-77.50)c |
| Vardenafil 2 (V2) | 8 | 15.87 ± 3.93 (11.6-23.8)e | 31.01 ± 20.27 (21-55.15)e |
| Vardenafil 10 (V10) | 8 | 10.97 ± 4.50 (5.4-19.9)gh | 33.55 ± 9.29 (22.16-48.51)g |
The values are presented as mean ± SD, (min-max).
P < 0.05 vs the other group;
P < 0.05 vs indomethacin group;
P < 0.05 vs famotidine groups (F5 and F20);
P < 0.05 vs group F20;
P < 0.01 vs group F20.
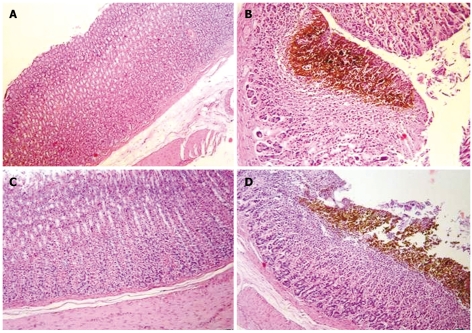
On histopathological examination, erosion, inflammation, hemorrhage, and necrosis were abundant in the indomethacin group. Those lesions were encountered with increasing frequency in the Famotidine 20 mg/kg, vardenafil 10 mg/kg, famotidine 5 mg/kg, and vardenafil 2 mg/kg groups. There were statistically significant (P < 0.05) differences between the indomethacin group and pretreatment groups. Famotidine 20 mg/kg pretreatment had the most efficient protective effect against indomethacin-induced gastric mucosal lesions. Minimal hemorrhage, minimal focal necrosis, and superficial erosions were observed in 25% of the rats given 2 mg/kg vardenafil. A high dose (10 mg/kg) of vardenafil had a potent protective effect against indomethacin-induced gastric mucosal lesions, but vardenafil in low doses (2 mg/kg) did protect the gastric mucosa against the harmful effects of indomethacin, similarly famotidine 5 mg/kg did. Microscopic views of the normal and damaged gastric mucosa are shown in Figure 2. In our study, vardenafil has gastroprotective effects against indomethacin-induced gastric mucosal damage. The potency of this effect is stronger in high doses than low doses.
Figure 2.
Normal rat gastric mucosa in the saline group and Group V10 (A and C, HE; × 100); gastric mucosal hemorrhage and necrosis in indomethacin group and Group V2 (B and D, HE; × 200, × 100) are shown.
DISCUSSION
Despite the great progress in the treatment protocols, peptic ulcers are still a major ongoing heath problem. The gastric barrier protects the mucosa against damage of its deeper structures by noxious substances. Mucosal microcirculation of the stomach has an important role in gastric mucosal protection[15]. Prostaglandins and NO are the main factors that regulate gastric blood flow. NSAIDs cause gastric mucosal damage by inhibiting endogenous prostaglandins due to inhibition of COX-1 and COX-2[16,17]. Prospective data from the Arthritis, Rheumatism, and Aging Medical Information System (ARAMIS) states that 13 of every 100 patients with rheumatoid arthritis treated with NSAID for one year suffer from serious gastrointestinal complications related to the NSAIDs[18]. Indomethacin administration increases aggressive factors but decreases protective factors[19,20].
There is no doubt on the protective effect of H2 blockers. The gastroprotective effects of H2 blockers are significantly greater when given in high doses than in low doses. The results in our experiment, either in low or in high doses of H2 blockers, were in accordance with the literature. Widespread use of H2 blockers could not prevent peptic ulcer related disorders. Therefore, the search for new alternatives with novel mechanisms of action is ongoing.
PDE type-5 inhibitors, which were developed as cardioprotective drugs, are commonly used in the treatment of erectile dysfunction. Sildenafil citrate has shown gastroprotective effects in experimental studies[21-23], and its gastroprotective effect was dose-dependent. Vardenafil is more potent than sildenafil. The gastroprotective effect of vardenafil has not yet been studied. In our study, the antiulcer activity of vardenafil was investigated against indomethacin-induced gastric mucosal damage. Vardenafil decreased indomethacin-induced gastric mucosal lesions significantly at high doses (10 mg/kg). Macroscopically, vardenafil at a dose of 2 mg/kg has a protective effect on the gastric mucosa similar to famotidine at 5 mg/kg.
Macroscopic evaluations of gastric tissues revealed that vardenafil given in 2 mg/kg protects gastric mucosa better than famotidine at a dose of 5 mg/kg. Vardenafil has clinically important gastroprotective effects at high doses (10 mg/kg). Thus, the gastroprotective effect of vardenafil was dose dependent. In the stomach tissue of rats given indomethacin, the level of the lipid peroxidation product, MDA, increased significantly compared to the sham operated group. Tissues exposed to oxidative stress include large amounts of toxic oxygen radicals, which induce lipid peroxidation leading to MDA formation[24,25]. The lowest MDA values were detected in the famotidine groups. The mean MDA values in the vardenafil groups were similar to the sham group (Table 2). Thus, vardenafil pretreatment has inhibited MDA production in indomethacin treated rats.
Possible mechanisms of gastroprotection of PDE V inhibitors are increased production of tissue NO[23,26-28] or increased tissue cGMP level without modifying NO content[22,25,29,30]. The NO levels are slightly elevated in vardenafil pretreated rats in our study; however, the level of NO in either of vardenafil groups did not surpass the level of NO determined in the sham group. Some studies revealed gastroprotective effects of some agents without significant alterations in NO or MDA levels[31]. Determination of tissue cGMP level was not included in our study design. This is a short armcoming of our study design. PDE V inhibitors might prevent indomethacin-induced gastric mucosal damage in either mechanism.
In conclusion, vardenafil reduced gastric mucosal damage significantly at a high dose. Patients treated with PDE type-5 inhibitors might benefit from the additional gastroprotective advantages of these drugs, especially in high doses.
COMMENTS
Background
Peptic ulcer is a common disorder of the gastrointestinal system. The increase in non-steroid anti inflammatory drug (NSAID) ingestion in the treatment of inflammation, fever, and pain is one of the major etiological factors for peptic ulcers. Despite the many drug treatment protocols used to date, peptic ulcer still remains a major public health problem.
Research frontiers
Vardenafil has being used in the treatment of functional impotence; however, its effects on gastric mucosa have not yet been investigated. The research’s aim was to determine its effectiveness in gastroprotection against NSAID-induced gastric lesions in comparison with famotidine.
Innovations and breakthroughs
Multiple agents have been used to prevent NSAID-induced peptic ulcer. This work is the first experimental study that show the beneficial effects of Vardenafil (a phosphodiesterase type V inhibitor) on NSAID-induced gastric ulcer. The gastroprotective effect of vardenafil against NSAID-induced peptic ulcer is dose dependent.
Applications
The study results suggest that vardenafil might be used as a potential therapeutic drug to prevent NSAID-induced gastric ulcer formation.
Peer review
This work might provide the first experimental data that directly shows the beneficial effect of a phosphodiesterase V inhibitor on gastric ulcers. In this manuscript, Karakaya et al report that administration of a phosphodiesterase V inhibitor (Vardenafil), dose-dependently suppresses indomethacin-induced gastric ulcers in rats. For comparison purposes, famotidine was used. There is only limited information suggesting the potential beneficial effect of vardenafil on ulcer healing, the data presented would be considered to provide attractive clinical information, although a clear mechanistic insight is not provided.
Footnotes
Peer reviewer: Atsushi Mizoguchi, Assistant Professor, Experimental Pathology, Massachusetts General Hospital, Simches 8234, 185 Cambridge Street, Boston, MA 02114, United States
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Shabsigh R. Therapy of ED: PDE-5 Inhibitors. Endocrine. 2004;23:135–141. doi: 10.1385/ENDO:23:2-3:135. [DOI] [PubMed] [Google Scholar]
- 2.Karatza AA, Narang I, Rosenthal M, Bush A, Magee AG. Treatment of primary pulmonary hypertension with oral sildenafil. Respiration. 2004;71:192–194. doi: 10.1159/000076684. [DOI] [PubMed] [Google Scholar]
- 3.Barnett CF, Machado RF. Sildenafil in the treatment of pulmonary hypertension. Vasc Health Risk Manag. 2006;2:411–422. doi: 10.2147/vhrm.2006.2.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salloum FN, Ockaili RA, Wittkamp M, Marwaha VR, Kukreja RC. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, Kukreja RC. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–458. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irkorucu O, Ucan BH, Cakmak GK, Emre AU, Tascilar O, Ofluoglu E, Bahadir B, Karakaya K, Demirtas C, Ankarali H, et al. Does sildenafil reverse the adverse effects of ischemia on ischemic colon anastomosis: yes, ‘no’. Int J Surg. 2009;7:39–43. doi: 10.1016/j.ijsu.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83:1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- 8.Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rossle M, Kreisel W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver -- results of a pilot study. Aliment Pharmacol Ther. 2006;23:121–128. doi: 10.1111/j.1365-2036.2006.02735.x. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 10.Konturek SJ, Brzozowski T, Majka J, Pytko-Polonczyk J, Stachura J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. Eur J Pharmacol. 1993;239:215–217. doi: 10.1016/0014-2999(93)90997-v. [DOI] [PubMed] [Google Scholar]
- 11.Xiang Z, Si JM, Huang HD. Chronic gastritis rat model and role of inducing factors. World J Gastroenterol. 2004;10:3212–3214. doi: 10.3748/wjg.v10.i21.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodie DA, Hanson HM. A study of the factors involved in the production of gastric ulcers by the restraint technique. Gastroenterology. 1960;38:353–360. [PubMed] [Google Scholar]
- 13.Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A. Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut. 1994;35:909–915. doi: 10.1136/gut.35.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–1443. [PubMed] [Google Scholar]
- 15.Bou-Abboud CF, Wayland H, Paulsen G, Guth PH. Microcirculatory stasis precedes tissue necrosis in ethanol-induced gastric mucosal injury in the rat. Dig Dis Sci. 1988;33:872–877. doi: 10.1007/BF01550978. [DOI] [PubMed] [Google Scholar]
- 16.Whittle BJ. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981;80:94–98. [PubMed] [Google Scholar]
- 17.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 19.Garcia Rodriguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007;132:498–506. doi: 10.1053/j.gastro.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Morsy MA, Fouad AA. Mechanisms of gastroprotective effect of eugenol in indomethacin-induced ulcer in rats. Phytother Res. 2008;22:1361–1366. doi: 10.1002/ptr.2502. [DOI] [PubMed] [Google Scholar]
- 21.Sawatzky DA, Megson IL, Rossi AG. Sildenafil offers protection against NSAID-induced gastric injury. Br J Pharmacol. 2005;146:477–478. doi: 10.1038/sj.bjp.0706362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos CL, Souza MH, Gomes AS, Lemos HP, Santos AA, Cunha FQ, Wallace JL. Sildenafil prevents indomethacin-induced gastropathy in rats: role of leukocyte adherence and gastric blood flow. Br J Pharmacol. 2005;146:481–486. doi: 10.1038/sj.bjp.0706361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aydinli B, Yildirgan MI, Ozturk G, Atamanalap SS, Polat KY, Basoglu M, Gundogdu C, Suleyman H, Kiziltunc A, Gursan N, et al. The role of sildenafil citrate in the protection of gastric mucosa from nonsteroidal anti-inflammatory drug-induced damage. Ulus Travma Acil Cerrahi Derg. 2007;13:268–273. [PubMed] [Google Scholar]
- 24.Talas DU, Nayci A, Polat G, Atis S, Comelekoglu U, Bagdatoglu OT, Bagdatoglu C. The effects of dexamethasone on lipid peroxidation and nitric oxide levels on the healing of tracheal anastomoses: an experimental study in rats. Pharmacol Res. 2002;46:265–271. doi: 10.1016/s1043-6618(02)00130-5. [DOI] [PubMed] [Google Scholar]
- 25.Bilici M, Ozturk C, Dursun H, Albayrak F, Saglam MB, Uyanik A, Gulaboglu M, Tekin SB. Protective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parameters. Dig Dis Sci. 2009;54:1868–1875. doi: 10.1007/s10620-008-0560-z. [DOI] [PubMed] [Google Scholar]
- 26.Jansson EA, Petersson J, Reinders C, Sobko T, Bjorne H, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic Biol Med. 2007;42:510–518. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 27.S Kwiecien S, Pawlik MW, Brzozowski T, Konturek PC, Sliwowski Z, Pawlik WW, Konturek SJ. Nitric oxide (NO)-releasing aspirin and (NO) donors in protection of gastric mucosa against stress. J Physiol Pharmacol. 2008;59 Suppl 2:103–115. [PubMed] [Google Scholar]
- 28.Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat Rev Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- 29.Buvinic S, Huidobro-Toro JP. Basal tonic release of nitric oxide coupled to cGMP production regulates the vascular reactivity of the mesenteric bed. Eur J Pharmacol. 2001;424:221–227. doi: 10.1016/s0014-2999(01)01165-7. [DOI] [PubMed] [Google Scholar]
- 30.Muscara MN, Wallace JL. Nitric Oxide. V. therapeutic potential of nitric oxide donors and inhibitors. Am J Physiol. 1999;276:G1313–G1316. doi: 10.1152/ajpgi.1999.276.6.G1313. [DOI] [PubMed] [Google Scholar]
- 31.Villa AL, Reginaldo C, Viaro F, Ramalho F, Campos AD, Evora PR. The cytoprotective effect of a nitric oxide donor drug on gastric mucous membrane of rats treated with ketoprofen, a non-steroidal anti-inflammatory drug. Arq Gastroenterol. 2006;43:233–237. doi: 10.1590/s0004-28032006000300015. [DOI] [PubMed] [Google Scholar]