Abstract
Concomitant tumor resistance refers to the ability of some large primary tumors to hold smaller tumors in check, preventing their progressive growth. Here, we demonstrate this phenomenon with a human tumor growing in a nude mouse and show that it is caused by secretion by the tumor of the inhibitor of angiogenesis, thrombospondin-1. When growing subcutaneously, the human fibrosarcoma line HT1080 induced concomitant tumor resistance, preventing the growth of experimental B16/F10 melanoma metastases in the lung. Resistance was due to the production by the tumor cells themselves of high levels of thrombospondin-1, which was present at inhibitory levels in the plasma of tumor-bearing animals who become unable to mount an angiogenic response in their corneas. Animals carrying tumors formed by antisense-derived subclones of HT1080 that secreted low or no thrombospondin had weak or no ability to control the growth of lung metastases. Although purified human platelet thrombospondin-1 had no effect on the growth of melanoma cells in vitro, when injected into mice it was able to halt the growth of their experimental metastases, providing clear evidence of the efficacy of thrombospondin-1 as an anti-tumor agent.
Concomitant tumor resistance is an unusual phenomenon wherein animals harboring large primary tumors are resistant to the growth of distant smaller tumors (reviewed in refs. 1 and 2). This resistance depends on circulating factors and disappears upon surgical resection of the primary tumor. In stunning advances over the past several years, O’Reilly and Folkman (3–7) have shown that, in syngeneic rodent systems, the circulating factors responsible for such resistance are inhibitors of angiogenesis. Two of these have been identified: angiostatin and endostatin. In these model systems, primary tumors secrete inducers of angiogenesis and also generate inhibitors of angiogenesis. In the microenvironment of the large primary tumor, the inducers are apparently sufficient to overcome the effects of the inhibitors because the new vessels essential for progressive tumor growth are present. However, when inducers and inhibitors are shed from the tumor bed into the circulation, levels of the more labile inducers fall off rapidly whereas levels of the more stable inhibitors rise, creating a systemic antiangiogenic environment that prevents small nests of metastatic cells from inducing neovascularization. As a result, these incipient tumors remain small and dormant. On removal of the primary tumor, inhibitor levels fall and the previously dormant metastases expand vigorously.
Concomitant tumor resistance likely also occurs in human cancer patients. There are a variety of anecdotal clinical reports in the literature describing the fast and disastrous development of what must have been preexisting metastases following surgical removal of a primary tumor, (for example, see ref. 8 and citations in refs. 1 and 2). One such case is that of the patient from whom the widely used human fibrosarcoma line HT1080 was derived and who died from widespread metastases within 3 mo of removal of the primary tumor (9). In addition, an occasional human tumor cell line or primary explant is able to drive nude mice in which it is growing into an antiangiogenic state in which the animals become unable to mount a corneal angiogenic response that is induced in tumor-free controls (5, 6). The molecules generated by these human tumors that are responsible for this effect have not been identified.
The HT1080 line is unusual both in that it was derived from a patient whose history is compatible with concomitant tumor resistance and in that, unlike most sarcomas that have inactivated the p53 tumor suppressor gene (10–12), it retains wild-type p53 activity (13). In fibroblasts, wild-type p53 supports the production of thrombospondin-1 (TSP-1) (14, 15), a potent inhibitor of angiogenesis (16, 17). As might be predicted for a fibroblast-derived tumor line that retains wild-type p53, HT1080 cells secrete relatively high levels of TSP-1. Here, we show that this tumor-derived TSP-1 is active and can drive tumor-bearing animals into an antiangiogenic state that blocks the growth of experimental metastases. Exogenous TSP-1 also blocked the growth of metastases. This work identifies a molecule produced directly by a human tumor that is responsible for concomitant tumor resistance and provides direct evidence for the anti-tumor efficacy of the antiangiogenic agent TSP-1.
MATERIALS AND METHODS
Cell Culture.
Human fibrosarcoma cell line HT1080 (American Type Culture Collection) and pigmented mouse melanoma line B16 F10P (a kind gift from P. Polverini, University of Michigan) were maintained in DMEM with 10% fetal calf serum. A second highly metastatic but unpigmented strain of the B16 F10 cell line (18, 19) was kindly provided by I. J. Fidler (University of Texas M. D. Anderson Cancer Center, Houston) and maintained in MEM supplemented with glutamine, nonessential amino acids, and 10% fetal calf serum. Bovine adrenal capillary endothelial cells, BP10T8, a kind gift of J. Folkman (Children’s Hospital, Harvard Medical School, Boston) were maintained in DMEM supplemented with 10% donor calf serum, 2 mM glutamine, and 100 μg/ml endothelial cell mitogen (R & D Systems) and used at passage 14 or 15. All of the cells were incubated at 37°C at 7% CO2.
Antisense Clones.
The central fragment of human TSP-1 cDNA (nucleotides 883-2572) was amplified by PCR by using primers with HindIII linkers, 5′-ggg agctt CCGCAACTAACTAC-3′ (left) and 5′-ggg agctt GTACTGGCAGTTGTCCG-3′ (right), and cloned into the polylinker of pRC CMVneo (Invitrogen) in reverse orientation. HT1080 fibrosarcoma cells were transfected with plasmid expressing antisense TSP-1 were selected in G418, and screened individually for the loss of TSP-1 secretion by Western blotting of serum-free conditioned media. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were measured in serum-free conditioned media by using commercial kits (R & D Systems). TSP-1 was quantitated using Western blots with purified human platelet TSP-1 as a standard.
Western Analysis.
Seven to 10 μg of total protein from conditioned media, or 40 μg from mouse plasma, was resuspended in loading buffer containing 7.5% β-mercaptoethanol, boiled 3–5 min, run on 6% SDS/PAGE and blotted to Hybond-N membrane (Amersham). The membrane was probed with anti-thrombospondin mAbs A4.1 (16) or MA-1 (20), developed with an enhanced chemiluminescence kit (Amersham) and exposed to x-ray film for 1–20 s.
Thrombospondin.
TSP-1 was purified from human platelets as described (21, 22) by heparin affinity column with subsequent sizing chromatography on Sephacryl-200 (Pharmacia) and subjected to batch chromatography with gelatin Sepharose to eliminate possible fibronectin. Protein purity was assessed by 7.5% SDS/PAGE, and no contaminating factor H was detected (23). Contamination with transforming growth factor-β that can copurify with thrombospondin was measured (24) and found to be <0.1 pg/μg protein. Batch chromatography with polymyxin-B beads (Sigma) was performed to eliminate any endotoxin, and no residual activity could be detected with E-toxate kit (Sigma). TSP-1 was injected i.p. into mice and reached the circulation with good efficiency. Following an injection of 6.6 mg/Kg, 5 μg/ml intact human TSP-1 could be detected in the plasma after 3 hr and 1.4 μg/ml after 6 hr.
Angiogenesis Assays.
In vitro migration was assayed as described (25) by using BP10T8 bovine capillary endothelial cells in a modified Boyden chamber (Neuroprobe). DMEM with 0.1% BSA served as negative control, and recombinant human bFGF (10 ng/ml; R & D Systems) was used as positive control. To combine data from several different experiments, background was subtracted and data converted into percentage of maximum migration for each individual experiment. Negative values reflect migration below baseline values (BSA control).
In vivo neovascularization was assessed in rat and mouse corneas. For the rat (25), a Hydron pellet (Hydron; Interferon Sciences) of 3- to 5-μl containing test substance was formulated and implanted into avascular cornea of anesthetized rat 1.0–1.5 mm from the limbus and neovascularization assessed on day 7. Where indicated, pellets contained mouse plasma at 200 μg/ml, anti-TSP antibody A4.1 (previously shown to neutralize the antiangiogenic activity of TSP-1 in vitro and in vivo, ref. 16) at 40 μg/ml, and/or bFGF at 100 ng/ml. For mouse corneal neovascularization (26), Hydron-Sucralfate pellets of <1 μl (Aluminum sucrose sulfate; Bukh Meditec, Denmark) were formulated containing bFGF at 1 μg/ml and implanted into the cornea of a nude mouse 0.3–0.5 mm from the limbus. Angiogenesis was assessed by slit-lamp microscopy on days 3, 4, and 5 after implantation. Maximal angiogenesis was achieved on day 5.
Tumorigenicity Assays.
To asses tumorigenicity of parental HT1080 line and antisense clones, cells were harvested, suspended in serum-free DMEM, and injected subcutaneously into hind quarters of athymic mice in quadruplicate at 105 cells/site. Latent period was scored as days elapsing before the appearance of a progressively expanding palpable tumor. Tumor-bearing animals were studied when tumors had reached a diameter of 5–10 mm. Plasma was drawn under conditions that minimize platelet aggregation, and the amount of human TSP present was estimated from Western analysis by using the human-specific antibody MA-1.
To measure the growth of metastases, B16 F10 mouse melanoma cells were grown to 80% confluence, harvested, and resuspended at 106 (for unpigmented cells) or 107 (for pigmented cells) cells/ml in PBS, and 0.1 ml was injected into the mouse tail vein using a 27-gauge needle. After 14 days for pigmented cells or 21 days in the case of unpigmented cells, animals were killed and lungs weighed and fixed overnight in formalin for pigmented cells or Bouin’s solution for the unpigmented cells, and metastases on the surface of the lung were measured using an ocular micrometer and counted. To test the effect of preexisting tumors on metastasis, tail vein injections were performed on animals harboring primary tumors that had reached a diameter of ≈5 mm.
RESULTS
HT1080 Secreted Active Antiangiogenic Thrombospondin-1.
In cells of the fibroblastic lineage, the wild-type p53 tumor suppressor gene supports the secretion of high levels of antiangiogenic thrombospondin-1 that prevents the cells from inducing angiogenesis (14, 15). As such cells progress toward tumorigenicity in vitro, wild-type p53 is inactivated and such inactivation is essential for the development of an angiogenic phenotype (27). The HT1080 human fibrosarcoma cell line is atypical in that it has retained wild-type p53 and, as expected, secretes high levels of thrombospondin. Yet, despite the TSP-1 present, the secretions of the HT1080 cells were strongly angiogenic and the cells formed tumors readily in animals. The TSP-1 secreted by the tumor cells was active because a fraction of high molecular weight secretions containing all of the TSP-1 inhibited endothelial cell migration, and this inhibitory activity was sensitive to antibodies known to neutralize TSP-1 (data not shown). To better understand how these cells became angiogenic in the face of active inhibitor, inducers were examined. Neutralizing antibodies against a variety of inducers demonstrated that VEGF was responsible for the majority of the angiogenic activity present in HT1080-conditioned medium with bFGF accounting for the remainder (data not shown). When quantitated by ELISA, these two inducers were found to be expressed at dramatically high levels, 20 pg/μg for bFGF and 510 pg/μg for VEGF, concentrations that are 10-fold over those seen in tumorigenic fibroblasts that have down-regulated TSP-1 (27).
The thrombospondin secreted by HT1080 cells was active in vivo. HT1080 cells were transfected with an antisense TSP-1 construct and a series of stable subclones isolated that expressed various reduced levels of TSP-1. All of the clones grew at similar rates in vitro; yet, when these clones were tested in vivo, their latent periods were shortened in proportion to the decrease in their TSP-1 levels (Table 1). This acceleration of tumor growth was due to TSP-1 because if antibodies were used instead of antisense to reduce TSP-1, similar control of primary tumor growth was seen. Animals in which parental HT1080 cells had been seeded and grown to a size of 5 mm in diameter were injected daily with 40 mg/kg of neutralizing TSP-1 antibody, A4.1, and tumor size was measured after 14 days of treatment. Tumor weights were 0.45 ± 0.15 g in buffer-injected animals but much larger, 2.0 ± 0.6 gm, in animals injected with anti-TSP-1 antibodies.
Table 1.
Latency of tumors growing from clones transfected with TSP-1 antisense
| Clone | TSP-1 level | μg TSP-1 per μg secreted protein | Latency, days |
|---|---|---|---|
| HT1080 (parent) | high | 0.14 | 23 ± 2 |
| HT-13 | intermediate | 0.034 | 15 ± 3* |
| HT-16 | intermediate | nd | 13 ± 4* |
| HT-18 | low | 0.0037 | 9 ± 1* |
| HT-23 | low | 0.0016 | 8 ± 2* |
nd, visually similar to HT-13 but not specifically quantitated.
*Significantly different from parental HT1080, n of 3 or 4, P < 0.02.
Tumor-Derived TSP-1 Accumulated in the Circulation Causing a Systemic Antiangiogenic Effect.
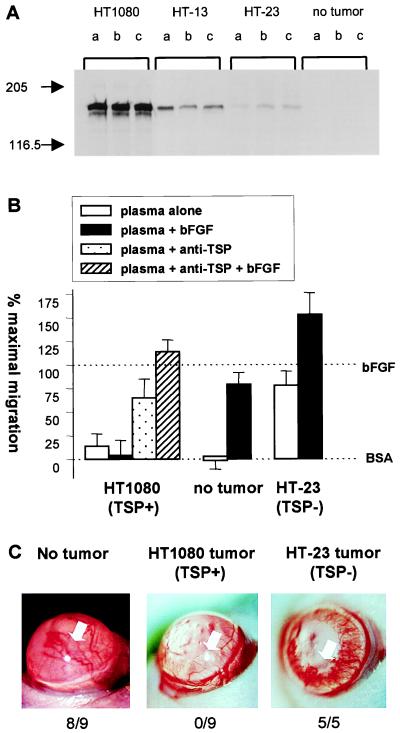
To determine whether the TSP-1 secreted at such high levels by HT1080 tumor cells accumulated in the circulation of tumor-bearing animals, plasma samples were collected and tested by Western analysis for human TSP-1 using an antibody, MA-1, which fails to recognize mouse TSPs (Fig. 1A). In all cases, the amount of TSP-1 in the circulation of tumor-bearing mice was directly proportional to the amount of soluble TSP-1 the cells secreted in vitro. Dilution curves run on Western blots using human platelet TSP-1 as a control suggested that the actual circulating levels of human TSP-1 in these animals was 6.2 ± 0.8 μg/ml of plasma for those bearing HT1080 tumors, 0.82 ± 0.18 μg/ml of plasma for those bearing HT-13 tumors, and 0.13 ± 0.036 μg/ml of plasma for those bearing HT-23 tumors.
Figure 1.
Systemic effects of tumors producing TSP-1. (A) Nude mice bearing tumors derived from cells producing various amounts of TSP-1 were examined for human TSP-1 levels present in plasma of tumor-bearing animals by Western analysis. Letters denote individual animals and cells that gave rise to tumors are indicated above each set of samples. (B) In vitro angiogenic activity of the plasma of tumor-bearing animals. Plasma of animals bearing no tumor or tumors derived from HT1080, producing high levels of TSP-1, or from HT-23, producing negligible levels of TSP-1, were tested for the ability to inhibit the migration of capillary endothelial cells toward bFGF in the presence and in the absence of neutralizing anti-TSP-1 antibodies. Data is presented as percent (%) of maximum migration, with 0% set to background levels (BSA) and 100% to the migration toward bFGF. The 100% varied in these experiments; between 43 and 87 cells migrated per ten high powered fields. When tested alone the antibodies did not influence basal or induced migration. (C) Ability of tumor-bearing animals to mount an angiogenic response. Hydron-sucralfate pellets containing bFGF were implanted in the avascular cornea of untreated mice (Left) or of mice bearing tumors formed by parental HT1080 (Middle) or by HT-23 (Right). Note vigorous vessel ingrowth on Left and Right only. Total positive responses/number of implants are tallied below pictures. White arrow indicates position of pellet.
The presence of TSP-1 in the plasma made it antiangiogenic. Plasma from normal mice, when tested at 20 or 50 μg/ml protein, was not stimulatory or inhibitory in vitro (Fig. 1B, Center) or in vivo (Table 2, line 1). However, plasma derived from animals bearing HT1080 tumors producing high levels of TSP-1 was distinctly different. It inhibited both migration (Fig. 1B, Left) and neovascularization (Table 2) induced by bFGF, and this inhibition was due to TSP-1 because neutralizing antibodies alleviated the effect. Plasma from animals bearing tumors that produced low, negligible levels of TSP-1 was weakly angiogenic in vitro and in vivo (Fig. 1B, Right; Table 2), possibly due to the massive amounts of angiogenic inducers that escape from these tumors.
Table 2.
Angiogenic activity of plasma from tumor-bearing animals was due to TSP-1
| Primary tumor | Secreted TSP-1 | Positive corneas/total implanted
|
|||
|---|---|---|---|---|---|
| Tested alone | + bFGF | + anti-TSP | + bFGF and anti-TSP | ||
| none | none | 0/3 | 3/3 | nd | nd |
| HT-23 | low | 2*/3 | nd | 3*/3 | nd |
| HT1080 | high | 0/3 | 0/3 | 3*/3 | 3/3 |
Pellets containing plasma derived from tumor-bearing mice mixed with additives where indicated were implanted into avascular rat carneas and vigorous vessel ingrowth at 7 days and scored as a positive response.
*weakly angiogenic compared to bFGF-induced responses. nd, not determined.
Moreover, animals bearing HT1080 tumors that therefore had high circulating levels of TSP-1 were systemically antiangiogenic in that they became unable to mount an angiogenic response when pellets of bFGF were implanted in their corneas (Fig. 1C) whereas in control animals bearing tumors that secreted a low level of TSP-1 or having no tumor, vigorous ingrowth of vessels was seen.
The Growth of Metastases Was Severely Curtailed by Tumors Producing TSP-1 and by Exogenous TSP-1.
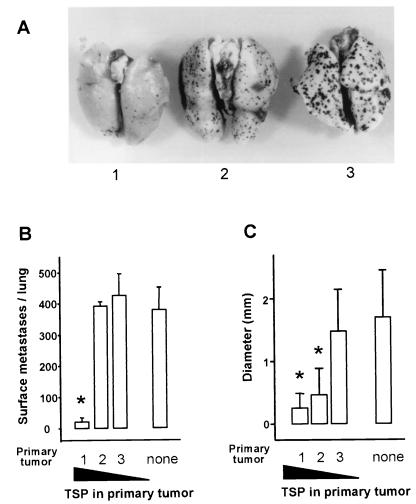
Concomitant tumor resistance could be seen in animals bearing tumors that produced high levels of TSP-1 but not in animals bearing tumors that produced low levels. Athymic mice were injected in the flank with HT1080 cells or with subclones of HT1080 carrying an antisense TSP-1 construct and therefore secreting intermediate or low levels of TSP-1. When the tumors reached ≈5 mm in diameter, tumor-bearing animals, along with a control group, were challenged with a tail vein injection of B16-F10(P) cells, a pigmented melanoma line. Fourteen days later, animals were killed and pigmented metastases in the lung were measured and counted (Fig. 2). Gross examination of the lungs showed a significant decrease in the number and size of metastases in animals with TSP-1-secreting HT1080 tumors, compared with animals bearing TSP-1-negative tumors (Fig. 2A). Quantitative analysis (Fig. 2B) showed a 15- to 20-fold reduction in the number of metastases and a sixfold reduction in their size when animals with TSP-secreting tumors were compared with animals without tumors or with tumors secreting low levels of TSP-1. Clone HT-13, which secreted intermediate levels of TSP-1, caused a notable fourfold decrease in the size of experimental lung metastases but no decrease in their number (Fig. 2C).
Figure 2.
Experimental metastases in tumor-bearing animals. Groups of four animals with no tumor (none) or bearing 5-mm diameter tumors derived from parental HT1080 that produced high levels of TSP- 1 (1), antisense clone HT-13 that produced intermediate TSP-1 levels (2), or antisense clone HT-23 that produced low levels of TSP-1 (3) and received tail vein injections of 106 melanotic B16-F10(P) cells, and 14 days later, lungs were harvested and photographed (A), metastases counted (B), and their average size measured (C). Errors are SEM. ∗Indicates significant difference from nontumor-bearing animals (none) P < 0.01.
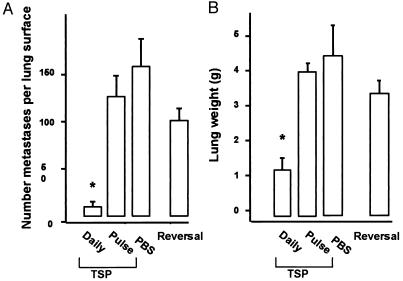
TSP-1 appeared to be solely responsible for the restricted growth of melanoma colonies in the lungs of HT1080 tumor-bearing mice because similar restriction was obtained with injections of purified human TSP-1. For these experiments, a “classic” B16-F10 melanoma line that is not pigmented but is known to complete seeding within 48 hr (28) was used. Mice were injected in the tail vein with melanoma cells on day 1, and from days 2 through 23, mice were injected i.p. twice a day with 10 mg/kg purified TSP-1. In the TSP-1-treated animals, the number of surface metastases were reduced to 6–10% of those present in the PBS-treated controls, and their lung weights were reduced to 30% that of controls (Fig. 3, A-B).
Figure 3.
Experimental metastases in animals treated with exogenous TSP-1. Animals received an injection of amelanotic B16F10 cells on day 1 and thereafter were divided into groups of four which were treated from day 2 to day 23 twice a day with i.p. injections of 10 mg/kg of purified TSP-1 (daily TSP) or an equal volume of PBS vehicle (PBS). One group was treated with TSP-1 only on the day before and the day after the B16F10 injection (Pulse of TSP). A second group was treated with TSP-1 until day 23 and then remained untreated for 14 days (Reversal). Lungs were harvested and metastases counted (A) and lung weight determined (B). Errors show SEM. ∗Indicates significant difference from PBS control, P < 0.05.
The TSP-1 used for in vivo assays had been pretreated with polymixin B to remove endotoxin, and assays indicated endotoxin levels were below detection limit (<0.05 units/μg TSP-1). To eliminate the possibility that a very low level of endotoxin was influencing metastases, an additional control was performed. Animals were injected with the usual two doses of TSP-1, once a day before the introduction of the melanoma cells, and again 1 day after the tumor cell injection. This regime did not alter the seeding or the growth of metastases (Fig. 3, pulse of TSP). TSP-1 injections also were effective against metastases derived from the melanotic B16-F10 because injections of 5 mg/kg/day TSP-1 reduced the number of visible surface metastases from this strain by 90% (data not shown).
TSP-1 was not toxic to the B16 melanoma tumor cells themselves because when TSP-1 treatments were halted, metastases grew out in numbers that were not significantly different from those seen in untreated controls (Fig. 3, reversal). In addition, the growth of the melanoma cells over 5 days in culture was unaffected by the presence of 5 μg/ml of TSP-1, which was renewed daily, and their cloning efficiency on plastic remained unchanged in concentrations of TSP-1 that ranged from 0.75 to 25 μg/ml (data not shown).
DISCUSSION
Data presented above show that the human fibrosarcoma cell line HT1080 is able to inhibit the growth of experimental metastases. These results suggest that this inhibition is due to the antiangiogenic activity of circulating TSP-1 that is released by the tumor cells in vivo, circulates at effective levels, and makes the tumor-bearing animals unable to mount an angiogenic response. The data also demonstrate the in vivo efficacy of soluble TSP-1, showing it can be used as a drug to prevent the growth of experimental metastases.
HT1080 produced concomitant tumor resistance directly by secreting active TSP-1, unlike previously studied rodent tumors that create an antiangiogenic state when the tumor cells, or associated stromal elements (29), secrete enzymes or activators of enzymes (30) that generate inhibitors from other molecules. We saw no significant evidence for in vivo breakdown of tumor-derived TSP-1 to fragments smaller than the 140 kDa monomer, a molecule that is as effective an antiangiogenic agent as the intact TSP-1 (16, 17). Although there was not enough TSP-1 available to titrate the minimal amount needed to control metastases in vivo, both 5 and 20 mg/kg/d of the 450 kDa protein were effective. These amounts are comparable on a molar basis to the 1.5 mg/kg/d of purified 45 kDa angiostatin recently shown to be needed to maintain similar control of Lewis lung metastases in vivo (31). In the tumor-bearing animals, a circulating level of 6.2 μg of TSP-1 per milliliter of plasma halted the growth of metastases whereas 0.82 μg/ml merely slowed their growth and 0.13 μg/ml was ineffective.
Although TSP-1 is a multifunctional protein, its ability to halt the growth of melanoma metastases seemed to be due to its antiangiogenic activity. TSP-1 did not directly affect the growth of the melanoma cells in two in vitro assays. Nor did it kill them in vivo because when TSP-1 treatment was withdrawn after two weeks, metastases grew out in numbers that were not significantly different from those seen in untreated control animals. The animals bearing TSP-1-secreting tumors had plasma levels of TSP-1 in the inhibitory range, as defined using in vitro experiments (16, 17), and they were clearly in an in vivo anti-angiogenic state, unable to mount a corneal angiogenic response as well as unable to support the growth of most metastases. Attempts to block this concomitant tumor resistance using neutralizing anti-TSP-1 antibodies on animals harboring TSP-1-producing tumors could not be interpreted unambiguously. The growth of metastases was stimulated by the antibodies, as was expected, but so was the growth of the primary tumor producing the TSP-1. Apparently, it eventually produced enough TSP-1 to overwhelm the antibodies. The metastases observed in these experiments were similar in size and frequency to those found in animals bearing tumors producing intermediate levels of TSP-1 (data not shown).
Our data are consistent with other examples of TSP-1 influencing metastasis. A human breast cancer cell line engineered to overproduce TSP-1 metastasizes from the mammary fat pad to the lung with one-half the frequency of the parental line that produces only very low levels of TSP-1 (32). Although these effects were attributed to the local production of TSP-1 in the tumor bed, circulating levels of TSP-1 also could be playing a role in this system. Thrombospondin also has been shown to act in the opposite way. It enhances metastases in mice when injected at high levels, if the injection occurs within 5 min of the injection of the tumor cells (33). This effect requires platelet aggregation (34) and can be explained by the ability of TSP-1 to increase the size of emboli and thereby enhance the frequency with which the injected cells arrest in the lung. We saw no such enhancement in our experiments with tumor-bearing animals, possibly because the TSP-1 concentration was comparatively low in the tumor-bearing mice. In experiments in which TSP-1 was injected, the seeding of melanoma cells in the lung was complete before injections were begun.
It is not possible at this point to determine whether the patient from whose fibrosarcoma the HT1080 cell line was derived in 1974 had high levels of circulating TSP-1. His clinical record (9) is compatible with concomitant tumor immunity because he died of widespread disease within 3 mo of resection of his primary tumor, which, as shown here, can produce high circulating levels of antiangiogenic TSP-1 in vivo. TSP-1 also has the long half-life in the human circulation, estimated in one study to be ≈9 hr (35), that is thought to be essential for any mediator of concomitant tumor resistance. High plasma levels of TSP-1 have been occasionally seen in other cancer patients, some rising more than 10-fold over normal (36, 37). These levels should be sufficient to induce a systemic antiangiogenic effect, but the relationship of this phenotype to outcome after primary tumor resection in these individuals is not known. It is hoped that the identification here of TSP-1 as a human sarcoma-derived inhibitor capable of holding metastases in check, may spur the search for living patients in whom TSP-dependent concomitant resistance can be demonstrated and for whom TSP-1 replacement therapy may someday be helpful.
Acknowledgments
We are grateful to Dr. I. J. Fidler, University of Texas, Houston, TX, for melanoma cells and advice on their use, to Sara S. Tolsma, Northwestern College, Orange City, IA, for help with experimental design, and to the National Cancer Institute and National Heart, Lung and Blood Institute for their support of this work through Grants CA52750 and CA64239 (to N.P.B.) and HL28749 (to J.L.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TSP-1, thrombospondin-1; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor.
References
- 1.Prehn R T. Cancer Res. 1991;51:2–4. [PubMed] [Google Scholar]
- 2.Prehn R T. Cancer Res. 1993;53:3266–3269. [PubMed] [Google Scholar]
- 3.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 4.Holmgren L, O’Reilly M S, Folkman J. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Parangi S, Tolentino M J, Folkman J. Cancer Res. 1995;55:4230–4233. [PubMed] [Google Scholar]
- 6.O’Reilly M S, Holmgren L, Chen C, Folkman J. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E, Birkhead J R, Olsen B R, Folkman J. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker E V, Thornthwaite J, Ketcham A S. In: Cancer Invasion and Metastasis: Biological Mechanisms and Therapy. Day S B, Myers W P L, Stansly P, Garattini S, Lewis M G, editors. New York: Raven; 1977. pp. 227–240. [Google Scholar]
- 9.Rasheed S, Nelson-Rees W A, Toth E M, Arnstein P, Gardner M B. Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Oliner J D, Kinzler K W, Meltzer P S, George D L, Vogelstein B. Nature (London) 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 11.Leach F S, Tokino T, Meltzer P, Burrell M, Oliner J D, Smith S, Hill D E, Sidransky D, Kinzler K W, Vogelstein B. Cancer Res. 1993;53:2231–2234. [PubMed] [Google Scholar]
- 12.Cordon-Cardo C, Latres E, Drobnjak M, Oliva M R, Pollack D, Woodruff J M, Marechal V, Chen J, Brennan M F, Levine A J. Cancer Res. 1994;54:794–799. [PubMed] [Google Scholar]
- 13.Sharma S, Schwarte-Waldhoff I, Oberhuber H, Schafer R. Cell Growth Differ. 1993;4:861–869. [PubMed] [Google Scholar]
- 14.Dameron K M, Volpert O V, Tainsky M A, Bouck N. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 15.Dameron K M, Volpert O V, Tainsky M A, Bouck N. Cold Spring Harbor Symp Quant Biol. 1994;59:483–489. doi: 10.1101/sqb.1994.059.01.053. [DOI] [PubMed] [Google Scholar]
- 16.Good D J, Polverini P J, Rastinejad F, Le Beau M M, Lemons R S, Frazier W A, Bouck N P. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolsma S S, Volpert O V, Good D J, Frazier W A, Polverini P J, Bouck N. J Cell Biol. 1993;122:507–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidler I J. Nature (New Biology) 1973;242:148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- 19.Fidler I J. Cancer Res. 1975;35:218–224. [PubMed] [Google Scholar]
- 20.Lawler J, Derick L H, Connolly J E, Chen J H, Chao F C. J Biol Chem. 1985;260:3762–3772. [PubMed] [Google Scholar]
- 21.Adams J C, Lawler J. Mol Biol Cell. 1994;5:423–437. doi: 10.1091/mbc.5.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Skorstengaard K, Mosher D F. J Cell Biol. 1992;118:693–701. doi: 10.1083/jcb.118.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carron J A, Bates R C, Smith A I, Tetoz T, Arellano A, Gordon D L, Burns G F. Biochim Biophys Acta. 1996;1289:305–311. doi: 10.1016/0304-4165(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 24.Abe M, Harpel J G, Metz C N, Nunes I, Loskutoff D J, Rifkin D B. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 25.Polverini P J, Bouck N P, Rastinejad F. Methods Enzymol. 1991;198:440–450. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- 26.Kenyon B M, Voest E E, Chen C C, Flynn E, Folkman J, D’Amato R J. Invest Ophthalmol Visual Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 27.Volpert O V, Dameron K M, Bouck N. Oncogene. 1997;14:1495–1502. doi: 10.1038/sj.onc.1200977. [DOI] [PubMed] [Google Scholar]
- 28.Esumi N, Fan D, Fidler I J. Cancer Res. 1991;51:4549–4556. [PubMed] [Google Scholar]
- 29.Dong Z, Kumar R, Yang X, Fidler I J. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 30.Gately S, Twardowski P, Stack M S, Patrick M, Boggio L, Cundiff D L, Schnaper H W, Madison L, Volpert O, Bouck N, et al. Cancer Res. 1996;56:4887–4890. [PubMed] [Google Scholar]
- 31.Sim B K, O’Reilly M S, Liang H, Fortier A H, He W, Madsen J W, Lapcevich R, Nacy C A. Cancer Res. 1997;57:1329–1334. [PubMed] [Google Scholar]
- 32.Weinstat-Saslow D L, Zabrenetzky V S, VanHoutte K, Frazier W A, Roberts D D, Steeg P S. Cancer Res. 1994;54:6504–6511. [PubMed] [Google Scholar]
- 33.Tuszynski G P, Gasic T B, Rothman V L, Knudsen K A, Gasic G J. Cancer Res. 1987;47:4130–4133. [PubMed] [Google Scholar]
- 34.Tuszynski G P, Rothman V L, Deutch A H, Hamilton B K, Eyal J. J Cell Biol. 1992;116:209–217. doi: 10.1083/jcb.116.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawes J, Clemetson K J, Gogstad G O, McGregor J, Clezardin P, Prowse C V, Pepper D S. Thromb Res. 1983;29:569–581. doi: 10.1016/0049-3848(83)90212-8. [DOI] [PubMed] [Google Scholar]
- 36.Tuszynsky G P, Smith M, Rothman V L, Capuzzi D M, Joseph R R, Katz J, Besa E C, Treat J, Switalska H I. Thromb Haemostasis. 1992;67:607–611. [PubMed] [Google Scholar]
- 37.Nathan F E, Hernandez E, Dunton C J, Treat J, Switalska H I, Joseph R R, Tuszynski G P. Cancer. 1994;73:2853–2858. doi: 10.1002/1097-0142(19940601)73:11<2853::aid-cncr2820731131>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]