Abstract
Fatty acid β-oxidation is essential for seedling establishment of oilseed plants, but little is known about its role in leaf metabolism of adult plants. Arabidopsis thaliana plants with loss-of-function mutations in the peroxisomal ABC-transporter1 (PXA1) or the core β-oxidation enzyme keto-acyl-thiolase 2 (KAT2) have impaired peroxisomal β-oxidation. pxa1 and kat2 plants developed severe leaf necrosis, bleached rapidly when returned to light, and died after extended dark treatment, whereas the wild type was unaffected. Dark-treated pxa1 plants showed a decrease in photosystem II efficiency early on and accumulation of free fatty acids, mostly α-linolenic acid [18:3(n-3)] and pheophorbide a, a phototoxic chlorophyll catabolite causing the rapid bleaching. Isolated wild-type and pxa1 chloroplasts challenged with comparable α-linolenic acid concentrations both showed an 80% reduction in photosynthetic electron transport, whereas intact pxa1 plants were more susceptible to the toxic effects of α-linolenic acid than the wild type. Furthermore, starch-free mutants with impaired PXA1 function showed the phenotype more quickly, indicating a link between energy metabolism and β-oxidation. We conclude that the accumulation of free polyunsaturated fatty acids causes membrane damage in pxa1 and kat2 plants and propose a model in which fatty acid respiration via peroxisomal β-oxidation plays a major role in dark-treated plants after depletion of starch reserves.
INTRODUCTION
β-Oxidation is the major pathway for the metabolic breakdown of fatty acids. In plants, β-oxidation is localized exclusively in peroxisomes, where the enzymatic reactions involved lead to the sequential degradation of long-chain fatty acids to acetyl-CoA. This pathway is essential in oilseeds for providing growing seedlings with carbon skeletons and energy via the glyoxylate cycle in combination with either gluconeogenesis or the citrate cycle (Baker et al., 2006; Goepfert and Poirier, 2007). Many of the enzymes catalyzing reactions in β-oxidation have been identified and functionally characterized in Arabidopsis thaliana (Graham, 2008). Two long-chain acyl-CoA Synthetases (LACS6 and LACS7), activating fatty acids inside peroxisomes by esterification with CoA, are essential for mobilization of storage lipids in seeds, since lacs6 lacs7 double mutants depend on external sucrose for successful seedling establishment (Fulda et al., 2002, 2004). Sucrose dependency for seedling establishment was also found in mutants of other peroxisomal genes, such as the acyl-CoA oxidase double mutant acx1 acx2 (Adham et al., 2005; Pinfield-Wells et al., 2005) and the keto-acyl thiolase mutant kat2 (ped1) (Hayashi et al., 1998; Germain et al., 2001), all core enzymes of β-oxidation. Moreover, mutants of the peroxisomal ABC-transporter PXA1 display the same dependence on sucrose for seedling establishment (Zolman et al., 2001), providing evidence for an essential function of PXA1 in β-oxidation (Footitt et al., 2002; Hayashi et al., 2002).
PXA1 was initially identified as peroxisomal defective 3 (PED3) in a screen for Arabidopsis mutants resistant to 2,4-dichlorophenoxybutyric acid (Hayashi et al., 1998), and pxa1 mutants were subsequently shown to be resistant to indole butyric acid (IBA) (Zolman et al., 2001). Both 2,4-dichlorophenoxybutyric acid and IBA are converted to either the herbicide 2,4-D or the active auxin indole-3-acetic acid in peroxisomes through one round of β-oxidation. Later, it became evident that PED3 corresponds to the same locus as COMATOSE (Russell et al., 2000; Footitt et al., 2002) and functionally represents the full-size peroxisomal ABC transporter PXA1 (Schwacke et al., 2003; Theodoulou et al., 2006). Recently, PXA1 has also been implicated in import of substrates as diverse as 12-oxo phytodienoic acid (OPDA), an intermediate of jasmonic acid (JA) biosynthesis (Theodoulou et al., 2005) and acetate (Hooks et al., 2007), suggesting a relatively broad substrate spectrum for PXA1. However, in germinating oilseeds, the assumed function of PXA1 is the import of fatty acids into peroxisomes providing substrates for β-oxidation. Analyses of homologs in other organisms also indicate a function in fatty acid transport into peroxisomes. Mutations in the adrenoleukodystrophy protein, the closest PXA1-homolog in humans, cause the severe genetic disorder X-linked Adrenoleucodystrophy. Affected patients accumulate very-long-chain fatty acids in brain and adrenal gland tissue due to the inability to import and catabolize them in peroxisomes via β-oxidation (Berger and Gärtner, 2006). Moreover, yeast mutants defective in either of the two ABC-half transporters homologous to PXA1 are unable to grow on long-chain fatty acids like oleate as the sole carbon source and show a strong reduction of oleate degradation via β-oxidation (Hettema et al., 1996; Shani and Valle, 1996).
Arabidopsis PXA1 is highly expressed in mature and germinating seeds but also constitutively low in leaves and other vegetative tissue (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002). Microarray experiments investigating transcriptional alterations on a genome-wide scale showed that the transcripts of many genes involved in β-oxidation were also increased in abundance during dark-induced and natural senescence (Buchanan-Wollaston et al., 2005; van der Graaff et al., 2006), indicating a physiological function for β-oxidation in extended darkness.
Moreover, analyses of mutants deficient in two of the peroxisomal citrate synthases (csy2 csy3) suggested an important function for β-oxidation in fatty acid respiration in addition to gluconeogenesis via the glyoxylate cycle (Pracharoenwattana et al., 2005). Expression of both citrate synthases was strongly increased in leaves of plants exposed to extended darkness (van der Graaff et al., 2006), whereas the glyoxylate cycle key enzymes isocitrate lyase and malate synthase remained at a low level of expression. From these data it can be deduced that fatty acid respiration may be of particular significance when the status of carbon and energy is low at the end of the regular night period.
Since PXA1 was among the genes whose expression was upregulated in dark-induced senescence (Buchanan-Wollaston et al., 2005; van der Graaff et al., 2006), we studied the importance of PXA1 during periods of extended darkness by analyzing loss-of-function mutants.
Here, we demonstrate an important function for β-oxidation in metabolism of mature leaves in extended darkness through analyses of pxa1 and another mutant impaired in β-oxidation.
RESULTS
Extended Dark Conditions Are Lethal for pxa1 Plants
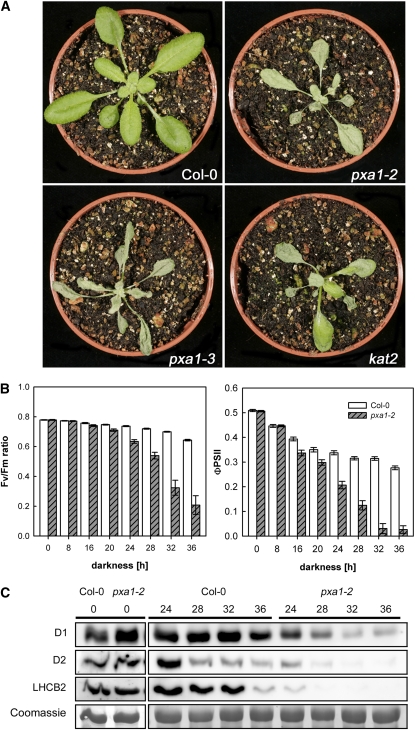
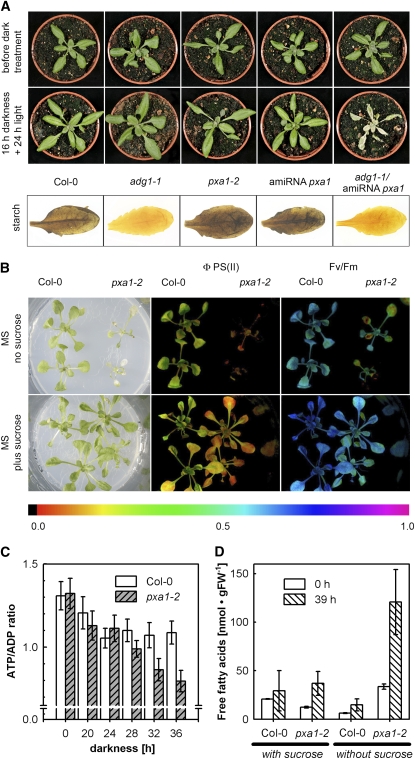
We analyzed the response of two independent mutant lines harboring T-DNA insertions in the PXA1 gene (pxa1 plants; pxa1-2 and pxa1-3) to prolonged dark conditions. As shown in Figure 1A, pxa1 plants displayed a severe phenotype when exposed to extended night conditions. After 36 h of darkness at a temperature of 24°C, the leaves of pxa1 plants appeared blue-greenish and displayed a spotty appearance compared with wild-type plants. In addition, leaves of mutants strongly bleached when transferred back into light for 24 h, and plants were unable to recover and resume growth (see Supplemental Movie online). Similarly, a mutant defective in the keto-acyl thiolase (KAT2; kat2 plants), a key enzyme of peroxisomal fatty acid β-oxidation, showed an almost identical phenotype as pxa1 plants, albeit of less severity (Figure 1A).
Figure 1.
Phenotype of pxa1 Plants and Impact on Photosynthesis after Extended Darkness.
(A) Visible phenotype of pxa1, kat2, and Col-0 wild-type plants after exposure to 36 h of darkness at 24°C.
Pictures were taken 24 h after retransferring plants into regular day/night conditions.
(B) Photosynthetic parameters Fv/Fm ratio and ΦPSII, indicating intactness of PSII measured in intact leaves of wild-type and pxa1-2 plants exposed to increasing periods of darkness. Average ± se (n = 10).
(C) Immunoblots of total leaf protein using specific antibodies against D1, D2, and LHCB2 protein. Coomassie shows protein stain of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Numbers on top refer to hours exposed to darkness.
Development of leaf necrosis in pxa1 and kat2 plants in extended night conditions was temperature and time dependent. When plants were kept at 18°C during a 36-h dark treatment, the mutant plants showed only very mild signs of leaf lesions. At 24°C, severe leaf necrosis developed and plants died (see Supplemental Figure 1A online; Figure 1A). Moreover, the phenotype appeared only after prolonged dark treatment. At a night temperature of 24°C, no visible phenotypic alterations of pxa1 and kat2 plants compared with the wild type were observed after exposure to only 16 h of darkness. However, leaf lesions increasing in size and number were detectable in response to longer exposure to darkness (see Supplemental Figure 1B online).
Since PXA1 has previously been discussed to be involved in transport of the JA precursor OPDA, we examined mutants impaired exclusively in JA biosynthesis in extended dark conditions. OPR3 (OPDA reductase) is a peroxisomal enzyme unique to the biosynthetic pathway of JA, mediating the reduction of OPDA to OPC:8 (8-[3-oxo-2-cis-{(Z)-2-pentenyl}cyclopentyl]octanoic acid), which subsequently enters β-oxidation. Plants lacking a functional OPR3 enzyme did not show any leaf damage as observed in pxa1 mutants. Also, a mutant defective in allene oxide synthase (dde2), a plastidic enzyme involved in the conversion of α-linolenic acid to OPDA, was also unaffected by exposure to extended darkness (data not shown), indicating that the pxa1 and kat2 phenotype is independent of JA biosynthesis.
Although no visible phenotype was observed after short exposure to darkness (i.e., 16 h or less), leaf photosynthetic activity measured in pxa1-2 plants showed a markedly decreased electron transport efficiency of photosystem II (ΦPSII) as early as 16 h after onset of darkness compared with the wild type (Figure 1B; see Supplemental Figure 2 online). However, ΦPSII recovered to wild-type levels after a few minutes in the light at this time point. ΦPSII strongly decreased further with duration of the dark treatment (Figure 1B). Images showing ΦPSII in rosette leaves of intact plants revealed that damage of PSII started in the youngest leaves but extended to older leaves after longer periods of darkness (see Supplemental Figure 2 online). While the effect on PSII efficiency was completely reversible after 16 h, PSII efficiency did not recover at all after 36 h of darkness in pxa1-2 plants.
PSII integrity and plant health can also be measured by the ratio of variable over maximum chlorophyll a fluorescence yield (Fv/Fm), which indicates the potential maximum quantum efficiency of PSII electron transport (Maxwell and Johnson, 2000). As shown in Figure 1B, the Fv/Fm ratio in pxa1-2 plants was indistinguishable from the wild type (values of 0.78) at the end of the day, the regular night period, and after 16 h of darkness, but started to decrease after 20 h of darkness and showed a severe decrease after 36 h of darkness. Such a decline in Fv/Fm ratios is indicative of a decline in leaf health, such as seen at the onset of senescence.
To investigate the integrity of the photosynthetic apparatus, we analyzed the presence of integral membrane proteins of PSII by immunoblotting. The abundance of D1, D2, and LHCB2 protein started to strongly decrease in pxa1-2 leaves after 24 h of dark treatment compared with the wild type (Figure 1C). On the other hand, no general protein degradation could be detected in pxa1-2 leaves during 36 h of dark treatment as evidenced by the continued presence of the large subunit of ribulose-1,5-bis-phosphate carboxylase/oxygenase (Figure 1C).
Partial Darkness Prevents the pxa1 Phenotype
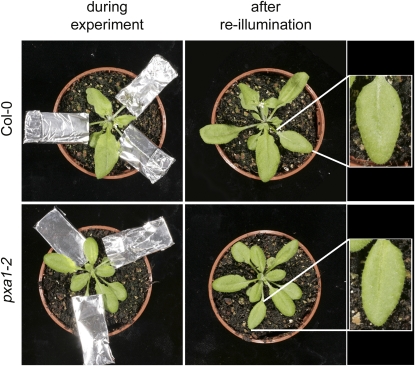
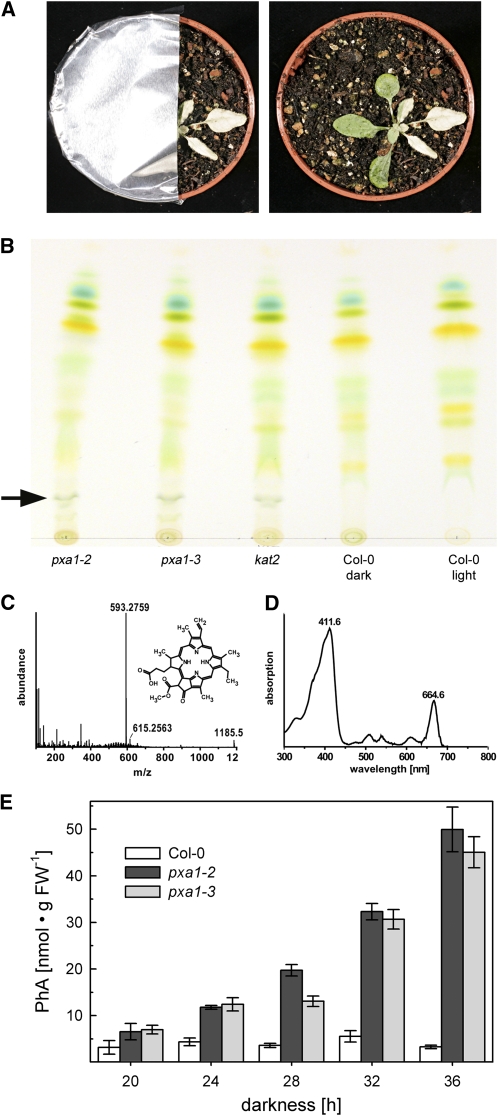
To investigate if the deleterious effect of extended darkness to pxa1 plants can be compensated by leaves maintained at the regular day/night cycle on the same plant, individual leaves were covered with aluminum foil. Figure 2 shows that leaves, even after an extended dark period of 64 h, did not show any damage, indicating that either toxic intermediates were exported from dark-treated leaves or essential metabolites were imported from leaves maintained at the regular day/night cycle.
Figure 2.
Impact of Individually Covered Leaves on Occurrence of the pxa1 Dark Treatment Phenotype.
Col-0 and pxa1-2 plants grown at a regular 16/8-h (day/night) cycle, where individual leaves were darkened for 64 h before aluminum covers were removed (left panel) and leaves reexposed to the regular day/night cycle for 24 h. No necrotic lesions could be detected in any of the dark-treated leaves.
[See online article for color version of this figure.]
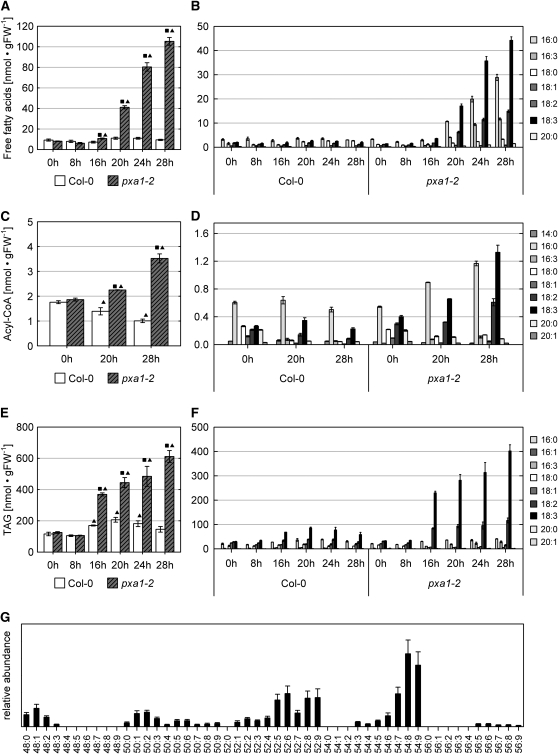
pxa1 Plants Accumulate Free Fatty Acids in Dark-Treated Leaves
Since previous studies provided evidence for the involvement of PXA1 in the import of fatty acids into peroxisomes (Hayashi et al., 1998; Zolman et al., 2001; Footitt et al., 2002), we considered the possibility that an imbalance in fatty acid metabolism could give rise to the observed phenotypes. We therefore examined the composition and concentrations of free fatty acids and acyl-CoAs in leaves of dark-treated plants after 0, 8, 16, 20, 24, and 28 h of darkness. Figure 3A shows that the fatty acid metabolism in pxa1-2 mutant plants is strongly affected upon extended darkness. The concentrations of free fatty acids started to increase significantly after 20 h of darkness in dark-treated pxa1-2 plants and reached levels up to 10 times than in the wild type after 28 h. By contrast, the levels of free fatty acids remained constantly low in wild-type leaves. In pxa1-2 plants, this increase is mainly due to elevated levels of four specific fatty acids, namely palmitic acid (16:0), 7,10,13-hexadecatrienoic acid [16:3(n-3)], linoleic acid (18:2), and α-linolenic acid [18:3(n-3)] (Figure 3B).
Figure 3.
Effects of Prolonged Dark Treatment on Fatty Acid Metabolism of pxa1 Plants.
Lipids were extracted from leaves of 21-d-old plants pregrown in a 16/8-h (day/night) cycle and exposed to darkness for the time indicated. Plants were harvested at the end of the dark treatment without reexposure to light. The values present the mean of at least three biological replicates, and error bars indicate se. Square: Significantly different values between wild-type and pxa1-2 plants at the same incubation time (P ≤ 0.05). Triangle: Significantly different within one genotype between the incubation time indicated and time 0 h (P ≤ 0.05). Data in (A) to (F) are given in nmol/gram of fresh weight [nmol • gFW−1].
(A) Total free fatty acid concentrations in leaves of pxa1 and Col-0 plants exposed to extended dark treatment.
(B) Free fatty acid profile in leaves of pxa1 and Col-0 plants.
(C) Acyl-CoA pool of pxa1 and wild-type leaves.
(D) Acyl-CoA profiles of pxa1 and wild-type leaves.
(E) Quantification of TAG in leaves of pxa1 and Col-0 plants.
(F) Fatty acid profiles of TAG. TAG was recovered from TLC plates and subjected to transmethylation prior to analysis by gas chromatography.
(G) Molecular species of triacylglycerol extracted from pxa1-2 leaves exposed to 20 h of darkness. The sum of the acyl groups are symbolized by the convention, carbon number:number of double bounds. As shown in (F), the fatty acid composition of the TAGs is based almost exclusively on C16 and C18 fatty acids. Therefore, 54:6, for example, indicates a TAG containing two C18 fatty acids and one C16 fatty acid with altogether six double bonds.
The presence of 16:3 deserves special attention for two reasons. First, it cannot be detected at earlier time points in pxa1 plants (0 and 8 h of darkness) or at any time in wild-type plants, and, second, 16:3 is a specific component of lipids present in chloroplast membranes (Ohlrogge et al., 1991). These data strongly suggest that substantial amounts of the detected free fatty acids are released from chloroplast membranes during extended dark conditions and cannot be further metabolized in mutant plants due to a defect in peroxisomal function (i.e., import and subsequent degradation of fatty acids via β-oxidation).
Since changes in the pool of free fatty acids might directly impact the pool of activated fatty acids, we next analyzed the levels of acyl-CoAs after 0, 20, and 28 h of darkness. The results show again significant differences between wild-type and pxa1-2 plants. While in the wild type the levels of acyl-CoA esters continuously dropped with duration of darkness, in pxa1, the levels continuously increased (Figure 3C). Starting from the same level at the end of the light period, the acyl-CoA concentration had decreased by ∼40% in the wild type at 28 h of darkness but almost doubled in pxa1-2 during the same time. With the exception of 16:3, the overall increase is mainly due to those activated fatty acids that showed elevated levels also in the fraction of free fatty acids, illustrating the tight connection between these two pools (Figure 3D).
To extend the analysis to a more general view on lipid metabolism, we next examined changes in the concentrations of specific lipid classes upon dark treatment. Whereas no changes were detected by thin layer chromatography (TLC) for either phospholipids or glycolipids (see Supplemental Figure 3 online), a considerable accumulation of triacylglycerol (TAG) was found in leaves of pxa1-2 plants during extended dark-treatment (Figures 3E and 3F). At first glance, this increase in concentrations seems to reflect the situation of free fatty acids, but upon closer inspection, two differences can be observed. First, the accumulation of TAG starts earlier compared with the pool of free fatty acids, being significant after 16 h of darkness already. Second, at the same time the concentration of TAG increased also in the wild type, although at a much lower level (Figure 3E). Whereas in the wild type this increase seems to be transient, the accumulation in pxa1-2 continues with time. The quantification revealed a TAG concentration of 0.61 μmol/g fresh weight (se 0.04) after 28 h of darkness in pxa1-2 plants compared with 0.14 μmol/g fresh weight (se 0.02) TAG in the wild type. The fatty acid profile of the generated TAG partially reflected the situation found for the pools of free and activated fatty acids with a strong increase of 18:2 and 18:3, but the content of 16:0 remained almost unchanged (Figures 3D and 3G). Fatty acids with >18 carbon atoms were found in trace quantities only. Thus, the accumulated TAG produced in leaves during extended dark treatment differs significantly from the TAG produced as storage oil in Arabidopsis seeds that contain significant amounts of 20:1 (Graham, 2008).
Analysis of the molecular TAG species supported this observation, since the dominating species harbor either three molecules of fatty acids with 18 carbon atoms and two to three double bonds (54:7 to 54:9 in Figure 3G) or two molecules of fatty acids with 18 carbon atoms with two to three double bonds and as third fatty acid 16:3(n-3) (52:7 to 52:9 in Figure 3G). These data indicate that the accumulated TAG species are derived from plastidial lipids.
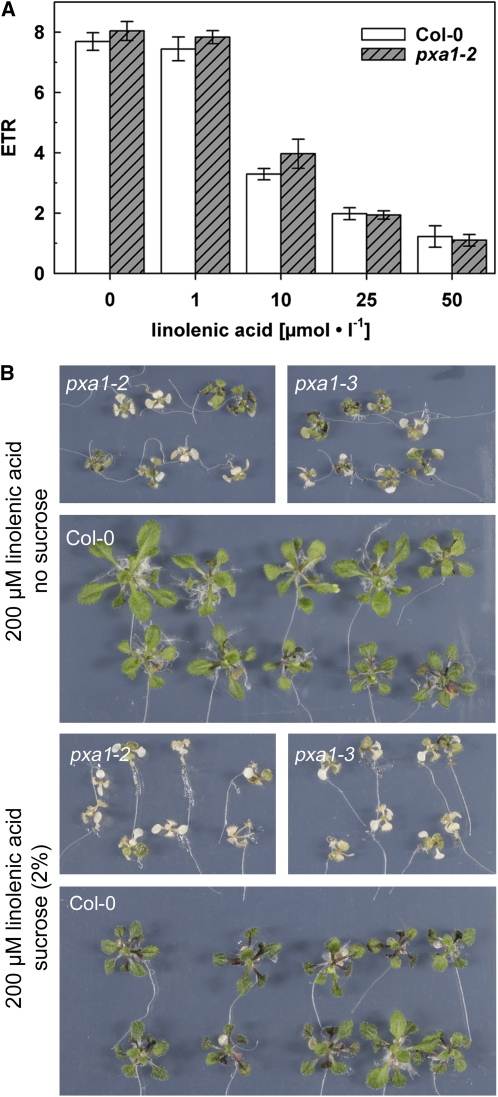
Exogenous α-Linolenic Acid Strongly Impacts Photosynthesis of Isolated Chloroplasts and Exhibits Toxicity to pxa1 and Columbia-0 Plants
Since the severe symptoms of the pxa1 mutant plants were accompanied by strongly increased concentrations of free fatty acids, we tried to estimate the impact of such elevated levels of free fatty acids on general plant vitality. As a meaningful parameter, we chose photosynthetic activity measured as electron transport rate (ETR). We exposed isolated chloroplasts to free fatty acid concentrations comparable to those found in leaves of dark-treated pxa1 plants. When challenged with 10 μM linolenic acid, the ETRs were immediately decreased to a similar extent in chloroplasts isolated from the wild type and in chloroplasts from pxa1-2 (Figure 4A). However, 1 μM linolenic acid had no effect on photosynthesis of isolated chloroplasts. ETR was further diminished at 25 μM linolenic acid and reduced by >80% at 50 μM. These data show that chloroplasts of wild-type and pxa1-2 plants are equally susceptible to increased concentrations of α-linolenic acid and that concentrations measured in pxa1-2 leaf tissue are capable of strongly reducing photosynthetic electron transport. This result suggests that elevated levels of free fatty acids interfere with chloroplast function in particular and vitality of Arabidopsis leaves in general.
Figure 4.
Impact of Exogenous α-Linolenic Acid on ETR of Isolated Chloroplasts and Plant Growth.
(A) ETR of isolated chloroplasts in response to exogenous α-linolenic acid. Bars represent averages of three independent experiments (n = 6 to 9) ± se.
(B) α-Linolenic acid toxicity in pxa1 and Col-0 plants. Seedlings were germinated and grown on half-strength MS media supplemented with 2% sucrose and transferred to plates containing α-linolenic acid at the concentration indicated after 10 d. Pictures were taken 14 d after transfer.
[See online article for color version of this figure.]
In a second approach, we compared the impact of exogenously applied free fatty acids on whole plants of the wild type and pxa1. For this purpose, plants were grown on Murashige and Skoog (MS) medium containing sucrose and after 10 d transferred to plates containing 200 μM α-linolenic acid supplemented either with or without sucrose. α-Linolenic acid toxicity was revealed as severe bleaching of pxa1 plants. While this effect was unique for both mutant lines, a reduction in plant size was observed in wild-type and pxa1 plants compared with control conditions (Figure 4B; see Supplemental Figure 4 online). Interestingly, external sucrose did not reduce mutant hypersensitivity to exogenous α-linolenic acid (Figure 4B).
The pxa1 Phenotype Is Aggravated in Starch-Free Mutants
To gain information about a putative link between starch metabolism and degradation of fatty acids, we generated a 35S-driven artificial microRNA (amiRNA) targeted against PXA1 and transformed Columbia-0 (Col-0) and starch-free adg1-1 plants. The adg1-1 mutant lacks the catalytic subunit of ADP glucose pyrophosphorylase, a key enzyme of starch biosynthesis (Lin et al., 1988). Transformed Col-0 plants expressing the PXA1 amiRNA responded exactly like the T-DNA lines pxa1-2 and pxa1-3 when exposed to 36 h of darkness.
Because they were expected to be nonviable in a long-day light regiment, adg1-1 mutants carrying the PXA1 amiRNA construct were isolated in continuous light conditions. Under these conditions, adg1-1 mutants expressing the PXA1 amiRNA already showed clearly decreased levels of ΦPSII efficiency 16 h after transfer to darkness (see Supplemental Figure 5 online) and developed severe leaf necrosis after 16 h darkness and 24 h light, while adg1-1 and PXA1 amiRNA single mutants remained symptom-free (Figure 5A).
Figure 5.
Acceleration and Alleviation of the Dark Treatment Phenotype and ATP/ADP ratios in wild-type and pxa1 plants.
(A) Leaf phenotype of 19-d-old adg1-1 plants expressing a PXA1 amiRNA construct grown in continuous light before dark treatment.
(B) Phenotype alleviation through feeding of exogenous sucrose. Three-week-old pxa1-2 and Col-0 plants grown on sterile half-strength MS medium supplemented either with (2%) or without sucrose were exposed to 39 h of darkness and subsequently returned to light for 24 h. pxa1-2 plants grown without exogenous sucrose experienced severe leaf damage and bleaching from this treatment compared with plants grown on sucrose-containing media. This finding was consistent with IMAGING-PAM (pulse amplitude modulation) fluorescence pictures showing either ΦPSII or Fv/Fm in false colors. PAM data were collected before plants were returned to light. Both values were markedly higher in mutants when sucrose was added to the media.
(C) ATP/ADP ratio in leaves of the wild type and pxa1 after different periods of darkness. Average ± se (n = 3 to 4).
(D) Total free fatty acid concentration in leaves of plants grown on half-strength MS medium supplemented with or without sucrose and harvested after 0 and 39 h of darkness. Average ± se (n = 3 to 4).
The ATP/ADP Ratio Is Decreased in pxa1 Leaves, and Dark-Induced Leaf Damage Can Be Alleviated by Exogenous Sucrose
The analysis of pxa1-2 plants revealed an accumulation of plastid-derived free fatty acids, acyl-CoAs, and TAGs, indicative of an important role of fatty acid β-oxidation in the metabolism of plants subjected to prolonged darkness. Because the products of fatty acid degradation via β-oxidation can be used to generate ATP in mitochondria, we determined the ATP/ADP ratio in dark-treated leaves (Figure 5C). While at the end of the day and even after 20 h of darkness there is no difference between wild-type and pxa1-2 leaves, the ATP/ADP ratio started to strongly decrease after 28 h of darkness in pxa1-2 compared with the wild type, suggesting an increasing lack of metabolic energy in pxa1-2 leaves at these later time points. Since the seedling establishment phenotype of pxa1 and kat2 mutants can be overcome by providing external sucrose (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002), we analyzed whether or not the supply of an exogenous energy source could complement the mutant phenotype in mature dark-treated leaves. Figure 5B shows that dark-treated pxa1-2 plants grown on half-strength MS plates displayed the same severe mutant phenotype as soil-grown plants. However, when the growth media was supplemented with 2% sucrose, pxa1-2 plants were almost indistinguishable from the wild type, showing only minor leaf damage after 39 h of darkness. While the total free fatty acid concentration in pxa1-2 leaves grown without sucrose increased after 39 h of darkness, pxa1-2 leaves of sucrose-grown plants showed only a minor increase and were comparable to free fatty acids concentrations found in wild-type leaves (Figure 5D). Moreover, measurement of photosynthetic activity revealed that ΦPSII in pxa1-2 plants grown in the presence of sucrose is much less decreased than when grown without sucrose (Figure 5B). However, ΦPSII in pxa1-2 is still lower than in wild-type plants, congruent with the finding that small lesions could be detected in leaves of sucrose-grown pxa1-2 plants. These data indicate that an external energy source can partially complement the severe pxa1 phenotype.
pxa1 Plants Accumulate High Concentrations of Pheophorbide a
The massive and very rapid bleaching of pxa1 leaves after transfer back into the light from darkness (Figure 1A; see Supplemental Movie online) already indicated an accumulation of phototoxic substances in mutant leaves. The observation that mutant leaves covered with aluminum foil after a 36-h dark treatment stayed green during subsequent illumination supports this assumption (Figure 6A). Therefore, the leaf pigment composition in dark-treated leaves was analyzed by TLC. As shown in Figure 6B (arrow), TLC analysis revealed an additional band in pxa1 and kat2 mutant leaf extracts that was absent from wild-type leaves. Extraction of the band and analysis of the substance by ultraperformance liquid chromatography–mass spectrometry unambiguously identified it as pheophorbide a (PhA), an intermediate of chlorophyll breakdown (Figures 6C and 6D). Quantification of PhA from leaf extracts demonstrated an increase in PhA concentrations in pxa1 leaves with longer dark incubation, reaching a maximum after 36 h of darkness. By contrast, PhA did not accumulate over time in leaves of the wild type (Figure 6E).
Figure 6.
Phototoxicity and PhA Accumulation in pxa1 and kat2 Mutants.
(A) Immediately after 36 h of dark treatment, pxa1 plants (3 weeks old, long-day grown) were partially covered with aluminum foil and illuminated for 24 h. In the uncovered right part of the plant, the phototoxic effect in the leaf becomes obvious, while the covered region maintains a blue-greenish color.
(B) Pigment extraction of 36-h dark-treated plants and control Col-0 (3 weeks old, long-day grown) were spotted on a silica gel matrix and separated with petroleum ether-isopropanol-H2O (100:15:0.25) as running solvent. While extracts of pxa1-2, pxa1-3, or kat2 showed a distinct band in the lower part of the TLC (arrow), this band was clearly absent in dark-treated and untreated Col-0.
(C) and (D) Mass spectra, chemical structure, and UV/VIS spectra of substance from unique band in pxa1 and kat2 extracts. Detected masses and UV/VIS spectrum matches a PhA (C35H36N4O5) standard.
(E) Quantification of PhA in dark-treated Col-0 and pxa1 leaves (3 weeks old, long-day grown). PhA accumulates in pxa1 plants with increasing duration of darkness, whereas levels in Col-0 plants remain close to the detection limit. Bars represent averages of three independent experiments (n = 9) ± se.
[See online article for color version of this figure.]
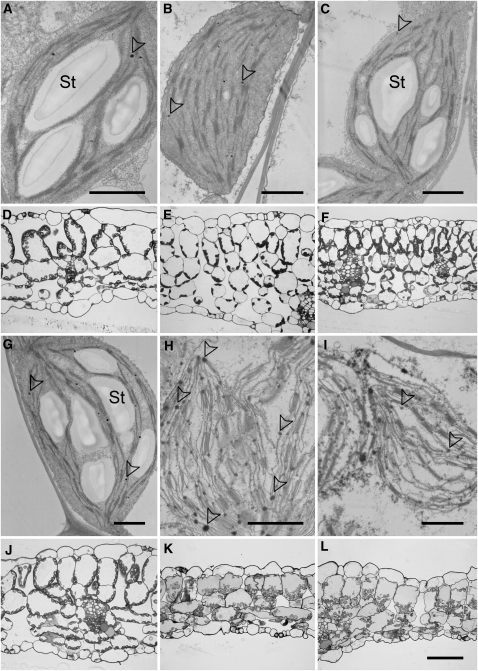
Bright-Field and Transmission Electron Micrographs Reveal Severe Damage to Leaf Tissue and Chloroplast Membrane Integrity
To gain insight into the damage of dark-treated pxa1 leaves at a microscopic scale, we analyzed leaf cross sections with bright-field and electron microscopy. At the end of the regular night period and before dark treatment, pxa1-2 and wild-type leaves are indistinguishable (Figures 7A, 7D, 7G, and 7J). Bright-field microscopy shows leaf parenchyma cells with chloroplasts localized to the cell periphery, indicating an intact subcellular compartmentation of peripheral cell plasma and a large central vacuole (Figures 7D and 7J). Chloroplasts of both wild-type and mutant leaves appeared undamaged at the ultrastructural level, containing large starch granules, grana, and stroma thylakoids and occasionally occurring plastoglobules (Figures 7A and 7G).
Figure 7.
Micrographs of Wild-Type and pxa1 Leaf Tissue at the End of the Light Period and after Extended Darkness.
Electron ([A] to [C] and [G] to [I]) and bright-field ([D] to [F] and [J] to [L]) micrographs of chloroplasts and leaf cross sections prepared from Col-0 wild-type ([A] to [F]) and pxa1-2 ([G] to [L]) leaves at the end of the regular light period (1st column), after 36 h of darkness (2nd column) and after 36 h of darkness plus 4 h of daylight (3rd column). Note the disintegrated structure and increased number of plastoglobules/lipid droplets in chloroplasts of dark-treated pxa1-2 leaves. St, starch granules. Arrowheads indicate plastoglobules. Bars = 1 μm in (A) to (C) and (G) to (I) and 50 μm in (L) (same scale for [D] to [E] and [J] to [L]), respectively.
By contrast, a striking difference between the wild type and pxa1-2 could be observed after 36 h of darkness at the tissue (Figures 7E and 7K) as well as the subcellular level (Figures 7B and 7H). Whereas leaf cells appeared turgescent in wild-type plants with intact chloroplast ultrastructure but without starch (Figures 7B and 7E), parenchyma cells of pxa1-2 leaves appeared collapsed and without cellular compartmentation (Figures 7H and 7K). Moreover, the ultrastructure of chloroplasts in pxa1-2 leaves indicated the disintegration of thylakoid and envelope membranes (Figure 7H). Interestingly, damaged chloroplasts of pxa1-2 seemed to contain an increased number of plastoglobule-like structures (Figure 7H).
The severe damage in leaves of dark-treated pxa1-2 plants could not be ameliorated by transferring plants back into the light. While 4 h of illumination subsequent to dark treatment led to an accumulation of large amounts of starch in chloroplasts of wild-type leaves (Figures 7C and 7F), chloroplasts in leaves of pxa1-2 plants still displayed a disrupted appearance without any indication of metabolic activity (Figures 7I and 7L). These microscopy observations strongly suggest that cellular disintegration is occurring in leaves of pxa1 plants during extended darkness.
DISCUSSION
It is well established that peroxisomal β-oxidation in plants is essential for germination and seedling establishment (Graham, 2008). Hence, mutants with a dysfunctional β-oxidation (e.g., pxa1, kat2, and acx1 acx2) all exhibit a reduced germination rate and are dependent on sucrose for successful seedling establishment. Here, we demonstrate a severe phenotype of mature pxa1 plants exposed to extended darkness that indicates an important role for PXA1 and peroxisomal β-oxidation during extended darkness.
The assumed function of PXA1 is the import of fatty acids into peroxisomes, providing substrates for β-oxidation and, during germination, the glyoxylate cycle. This function is supported by numerous studies in plants (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002). In Arabidopsis, PXA1 has also been associated with other substrates like acetate (Hooks et al., 2007), IBA, or OPDA, an intermediate of JA biosynthesis (Theodoulou et al., 2005). However, because opr3 and dde2 mutants, impaired specifically in JA biosynthesis, are unaffected by exposure to extended darkness (data not shown), the reported pxa1 and kat2 mutant phenotype is apparently unlinked to OPDA and/or JA biosynthesis. Exogenous feeding of IBA has been used in genetic screens for mutants impaired in β-oxidation since it has to be converted to active auxin in peroxisomes (Zolman et al., 2000). Although endogenous IBA is detectable in Arabidopsis, concentrations are ∼100 times lower than those of other auxin precursor storage forms, limiting its physiological importance (Woodward and Bartel, 2005). Moreover, indole-3-acetic acid levels are unaffected in pxa1 leaves compared with the wild type (see Supplemental Figure 6 online), excluding impaired auxin biosynthesis from being involved in the development of the darkness induced phenotype.
By contrast, loss-of-function of the KAT2 protein, which is common to both β-oxidation of fatty acids and JA biosynthesis, displayed essentially the same phenotype as pxa1 plants (Figure 1). This finding suggests that impairment of fatty acid import into peroxisomes or disabled fatty acid β-oxidation can cause the darkness-induced phenotype.
The Increase of Free Fatty Acids in pxa1 Is the Primary Cause for Chloroplast Membrane Damage, Chlorophyll Degradation and Accumulation of PhA
The absence of functional β-oxidation caused, for example, by impaired fatty acid import into peroxisomes resulted in a dramatic increase in free fatty acids in pxa1 plants (Figures 3A and 3B). In addition, a significantly elevated acyl-CoA pool reflected a distorted fatty acid metabolism in dark-treated leaves of pxa1 plants (Figures 3C and 3D). Substantial amounts of the detected free fatty acids are likely derived from plastidial lipids since the observed 18:3(n-3) and 16:3(n-3) constitute major fatty acids in chloroplast membranes (Mackender and Leech, 1974). Interestingly, the changes in fatty acid metabolism in dark-treated pxa1 plants resulted in the accumulation of TAG in leaf tissue, a finding that has recently also been described by Slocombe et al. (2009). Such accumulation was reported previously for senescing Arabidopsis wild-type leaves (Kaup et al., 2002). Here, elevated levels of TAG and free fatty acids in dark-treated pxa1 plants (Figure 7) were accompanied by substantially increased size and abundance of plastoglobules. These plastoglobules are thought to be formed in the process of thylakoid membrane degradation in senescing chloroplasts (Matile, 1992) and to contain TAG as well as free fatty acids as major components (Tevini and Steinmüller, 1985). Moreover, the fatty acid composition of the detected TAG in pxa1 is distinct from TAG in seeds and reflects not only the observed mixture in the pools of free fatty acids and acyl-CoA but also the composition found in natural senescing leaves (Kaup et al., 2002). TAG accumulation might serve as a transient buffer in conditions where fatty acids are released from membrane lipids faster than β-oxidation is able to cope with them. Indeed, under conditions of prolonged darkness, TAG seems to function as transient storage for fatty acids that are finally broken down by β-oxidation (Figure 3E). In the wild type, this metabolic program is efficient and keeps the concentration of free fatty acids at a constantly low level by the combined activity of β-oxidation and TAG generation (Figure 3A). In pxa1 plants, the TAG buffer capacity is eventually exceeded; hence, the levels of free fatty acids and acyl-CoA continuously increase, reaching critical levels of >10 times that of the wild type (Figure 3A) and resulting in a series of secondary effects.
Free fatty acids can be deleterious for chloroplasts probably due to their amphipathic and detergent-like properties leading to inhibition of the photosynthetic ETR and damage to PSII (Vernotte et al., 1983). When isolated chloroplasts from wild-type and pxa1 plants were exposed to concentrations of α-linolenic acid comparable to those found in dark-treated pxa1 leaves, the ETR was drastically decreased (Figure 4A). Moreover, ETRs of wild-type and pxa1-2 chloroplasts were equally reduced, indicating that the observed impact on photosynthesis in intact, dark-treated leaves (Figure 1B; see Supplemental Figure 2 online) may not be attributed to a general difference in chloroplast viability.
Exposure of spinach (Spinacia oleracea) chloroplasts to α-linolenic acid led to severe structural damage and loss of thylakoid membrane integrity (Okamoto et al., 1977). In pxa1 plants, we observed similar damage to chloroplast membrane integrity of dark-treated plants as described for the α-linolenic acid treatment of spinach chloroplasts. In both cases, chloroplast envelope membranes seemed to be lost, and the space in between grana and stroma thylakoid membranes was increased and not filled with stroma (Figure 7; (Okamoto et al., 1977). Disintegration of chloroplasts can easily lead to release and degradation of integral membrane proteins. For example, Peters and Chin (2003) observed a loss of photosynthetic membrane proteins after treatment of isolated chloroplasts with palmitoleic acid. In agreement with this, we found decreasing abundance of integral photosynthetic membrane proteins in dark-treated pxa1 leaves (Figure 1B). Hence, it can be suggested that in dark-treated pxa1 plants, photosynthetic protein complexes are progressively degraded, probably accompanied by an increased release of chlorophyll from light-harvesting complexes. Since the structural damage and decreased photosynthetic activity were observed before reexposure to light after extended darkness, this damage cannot be due to any photooxidative processes but can most likely be explained by the damaging effect of increased free fatty acids levels. Our findings that pxa1 plants are hypersensitive to exogenous α-linolenic acid in plate experiments independent of access to exogenous sucrose (Figure 4; see Supplemental Figure 4 online) and that sucrose-grown pxa1 plants displaying reduced mutant phenotype severity in response to extended darkness accumulate less free fatty acids (Figure 5D) substantiate the notion that high levels of free fatty acids are the primary cause for the observed phenotype.
In dark-treated leaves of pxa1 and kat2 plants, we identified substantial amounts of PhA, a chlorophyll catabolite, that was almost absent in wild-type plants (Figure 6). This observation strongly indicates the onset of degradation of chlorophyll in pxa1 mutant leaves that may directly be induced by free α-linolenic acid as has been described before (Lüthy et al., 1986; Figure 4). In intact senescent leaves, PhA is converted into red chlorophyll catabolite by PhA oxygenase (PAO) (Pruzinská et al., 2003; Tanaka et al., 2003). Results obtained with plants that express a PAO antisense construct and a pao T-DNA insertion mutant indicate strong phototoxic properties of PhA, since plants with a decreased level of PAO expression accumulate high levels of PhA in dark-treated leaves and show lethal bleaching after transfer into the light (Tanaka et al., 2003; Pruzinská et al., 2005). In white goosefoot (Chenopodium album) and radish (Raphanus sativus), there is evidence that PhA degradation also occurs via pheophorbidase activity, an enzyme methylating PhA (Shioi et al., 1996; Suzuki et al., 2006). However, in Arabidopsis, this enzyme has also been linked to methylation of primary fluorescent chlorophyll catabolite, a product downstream of PAO activity in chloroplasts (Pruzinská et al., 2007). The conversion of PhA by PAO consumes reductive power in the form of reduced ferredoxin, which can be provided either by photosynthesis or the oxidative pentose phosphate pathway. Since carbohydrate resources have been depleted in plants subjected to extended darkness (Smith and Stitt, 2007), dark-treated chloroplasts of pxa1 plants probably start accumulating PhA because they cannot further catabolize chlorophyll released from fatty acid–damaged photosystems. Although the photooxidative bleaching of pxa1 plants can be readily explained by PhA accumulation, this accumulation is a secondary effect of membrane damage and release of chlorophyll caused by accumulating free fatty acids and hence is only indirectly linked to a dysfunctional β-oxidation. In wild-type plants, there is no accumulation of free fatty acids in the dark (Figure 3A) and, hence, no demand for reductive power to drive increased chlorophyll degradation.
In consequence, our data provide evidence for the release of fatty acids from chloroplasts during extended darkness, using their toxic impact on chloroplasts and photosynthesis as indicator.
Fatty Acid Degradation in the Dark Is Essential for Providing Energy but Not Carbon Skeletons
During seedling establishment, peroxisomal β-oxidation converts storage lipids into carbohydrates and, in addition, provides metabolic energy (Graham, 2008). For the efficient conversion of lipids into carbohydrates, the glyoxylate cycle needs to be active in peroxisomes. Two enzymes are specific for the glyoxylate cycle, malate synthase (MLS) and isocitrate lyase (ICL). However, neither of the two enzymes is expressed in mature Arabidopsis leaves in extended darkness or senescence conditions, as has been shown by microarray experiments (van der Graaff et al., 2006) and analysis of plants expressing the luciferase reporter gene under the control of the malate synthase promoter (Charlton et al., 2005). This indicates the absence of glyoxylate cycle activity and thus no gain of carbohydrates from the breakdown of fatty acids in mature plants (Buchanan-Wollaston et al., 2005). However, in other plant species, induction of the glyoxylate cycle upon senescence has been observed at the level of enzyme activities in leaves (Vicentini and Matile, 1993).
In contrast with ICL and MLS, PXA1 expression is strongly upregulated in dark-treated leaves along with many transcripts involved in β-oxidation (Buchanan-Wollaston et al., 2005), indicating a functional β-oxidation in leaves of dark-treated Arabidopsis plants to provide metabolic energy.
The leaf damage phenotype of pxa1 plants was partially rescued by providing an exogenous energy source (i.e., sucrose) to agar-grown plants exposed to extended darkness (Figure 5B). As with soil-grown plants, free fatty acids accumulated in leaves of pxa1 plants cultivated in the absence of sucrose compared with wild-type and sucrose-grown pxa1 plants (Figure 5D). In addition, when individual leaves of pxa1 plants were exposed to darkness, no phenotype was observed in these leaves (Figure 2A). Together, both findings suggest that pxa1 plants do not start to degrade chloroplast membranes as long as an external energy source is available, either as exogenously fed sucrose or endogenous sucrose from source leaves of the same plant. Thus, the presence of carbohydrate energy sources prevents chloroplast membrane degradation, which, in pxa1, would lead to the accumulation of toxic free fatty acids and chlorophyll breakdown products. In turn, these observations indicate that wild-type plants can use β-oxidation of lipids as an energy source in extended darkness. In line with this, we found a decreased ATP/ADP ratio in leaves of pxa1 plants (Figure 5C). However, the ATP/ADP ratio started to decrease only after >24 h of darkness, suggesting that plants can produce ATP also from other sources (e.g., amino acids) for a limited time. A recent study involving the chy1 mutant, which is impaired in peroxisomal degradation of Val, also indicated a link between peroxisome metabolism and dark-induced plant lethality (Dong et al., 2009), since mutant plants were more susceptible to extended darkness of 8 d at low temperatures. chy1 mutants also show a reduced activity of the core β-oxidation enzyme KAT2, likely through toxic intermediates of Val catabolism (Lange et al., 2004), supporting an important function of peroxisomal fatty acid β-oxidation in extended darkness.
The absence of the pxa1 phenotype in individually darkened leaves of mutant plants could also be explained by the export of toxic substances like free fatty acids. Indeed, the accumulation of free fatty acids has been observed in vascular bundles from Arabidopsis (Schad et al., 2005). However, analyses of the starch-free PXA1 amiRNA adg1-1 mutants revealed a much earlier appearance of the pxa1 phenotype in darkness in these plants (Figure 5A). These results establish a link between energy reserves and occurrence of the pxa1 phenotype and support the idea that it is delivery of sucrose from source leaves and not export of toxic fatty acids from darkened leaves that prevents the development of lesions in individually darkened leaves.
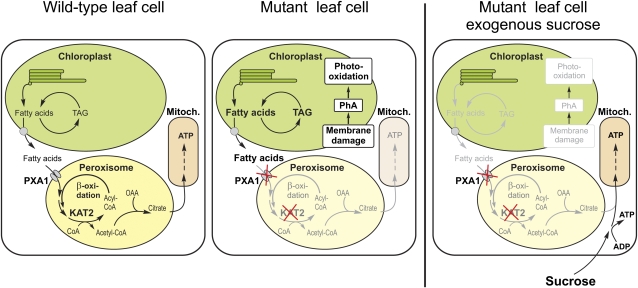
Wild-type plants apparently do not accumulate free fatty acids because they are able to oxidize them via peroxisomal β-oxidation. Pracharoenwattana et al. (2005) suggested an essential role for lipid respiration in seed germination, since a double mutant in two of the peroxisomal citrate synthase isoenzymes led to a dormant seed phenotype. An additional energy resource is necessary in dark-treated plants for maintenance of metabolism, since at the end of the regular night period transitory starch is basically exhausted (Smith and Stitt, 2007). Interestingly, in an extensive study on metabolite levels in dark-treated Arabidopsis plants, Usadel et al. (2008) observed a decrease in free fatty acids, further supporting the concept of fatty acids as an energy resource in extended darkness. Since the glyoxylate cycle is probably inactive in mature Arabidopsis leaves, we propose a model (Figure 8) in which leaves satisfy their need for ATP during extended periods of darkness from the respiration of fatty acids, probably originating from chloroplasts. In line with this model, the expression of peroxisomal citrate synthases is strongly upregulated in dark-treated leaves (Buchanan-Wollaston et al., 2005; Pracharoenwattana et al., 2005; van der Graaff et al., 2006). Peroxisomal citrate synthase combines acetyl-CoA released from fatty acid β-oxidation with imported oxaloacetate, originating from the Krebs cycle, to citrate, which in turn can be transported into mitochondria for the generation of ATP (Figure 8). Fatty acid respiration in mature leaves is probably a backup mechanism by which plants can survive transient periods of energy shortage. Additional support for this view came from the analysis of PXA1 amiRNA mutants in the starch-free adg1-1 background. Since these plants could not use transitory starch reserves during dark periods, lipid respiration had to be active and provide metabolic energy right after the onset of darkness. Consequently, the deleterious effect of increasing concentrations of free fatty acids can be observed after much shorter periods of darkness in PXA1 amiRNA adg1-1 mutants (Figure 5A).
Figure 8.
Cartoon Depicting the Suggested Model for Lipid Respiration in Mature Arabidopsis Leaves and Leaves of pxa1 and kat2 Mutants.
In extended darkness, plants respire fatty acids released from the chloroplast for ATP generation in mitochondria via peroxisomal β-oxidation and citrate synthesis. When import of fatty acids into peroxisomes or β-oxidation is impaired, plants accumulate high concentrations of free fatty acids leading to membrane damage, chlorophyll degradation, and other detrimental effects. Provision of an external energy source like sucrose largely prevents the necessity to respire fatty acids and, hence, accumulation of free fatty acids in mutant leaves since ATP can be generated from sucrose.
[See online article for color version of this figure.]
In summary, we provide evidence that peroxisomal β-oxidation is essential for mature plants to maintain the concentration of free fatty acids below toxic levels. In addition, our results suggest that respiration of lipids via β-oxidation after exhaustion of transitory starch reserves enables plants to sustain metabolism in darkness. It can be expected that other mutants defective in essential reactions of peroxisomal β-oxidation, peroxisome biogenesis, or cofactor import will show the same phenotype as pxa1 plants in prolonged darkness.
METHODS
Plant Lines
For all experiments, wild-type Arabidopsis thaliana ecotype Col-0 or Wassilewskija were used. Mutant plant lines with T-DNA insertions in the PXA1 gene (At4g39850) were isolated from the collection of sequence-indexed T-DNA lines of the SALK Institute and named according to the nomenclature used by Zolman et al. (2001), who isolated the pxa1-1 mutant (pxa1-2, SALK_019334; pxa1-3, SALK_002100) (Alonso et al., 2003). The mutant plant line with a T-DNA insertion in KAT2 was kindly provided by Steven M. Smith.
Cloning of PXA1 amiRNA
A suitable target site for the amiRNA was identified by following the instructions on http://wmd2.weigelworld.org. Cloning of the 35S:amiRNA PXA1 construct was done as previously described (Schwab et al., 2006) with minor changes (primers used: pRS300 A, 5′-CACCCTGCAAGGCGATTAAGTTGGGTAAC-3′; pRS300 B, 5′-GCGGATAACAATTTCACACAGGAAACAG-3′; PXA I miR-s, 5′-GATTATGTGTAAAGTATGGCGACTCTCTCTTTTGTATTCC-3′; PXA II miR-a, 5′-GAGTCGCCATACTTTACACATAATCAAAGAGAATCAATGA-3′; PXA III miR*s, 5′-GAGTAGCCATACTTTTCACATATTCACAGGTCGTGATATG-3′; PXA IV miR*a, 5′-GAATATGTGAAAAGTATGGCTACTCTACATATATATTCCT-3′). To use the benefits of Gateway cloning technology (Invitrogen), a CACC site was added to 5′ end of primer pRS300 A. The PCR product including the specific amiRNA (5′-TTATGTGTAAAGTATGGCGAC-3′) was subsequently cloned into pENTR/D-TOPO vector to obtain an entry clone. pGWB2 (Nakagawa et al., 2007) was used in the L/R reaction to generate a destination clone. Transgenic plants were generated by the floral dip method (Clough and Bent, 1998) and selected based on kanamycin resistance conferred by pGWB2.
Plant Cultivation
Arabidopsis seeds were surface sterilized and maintained in the dark at 4°C for 3 d. Seeds were subsequently sown on half-strength MS (Murashige and Skoog, 1962) plates containing sucrose (1%, w/v) and agar (1%, w/v, Difco agar; Becton Dickinson). Seedlings were transferred to soil after 10 d. Plants were cultivated in a 16/8-h day/night cycle (20/22°C) at ∼100 μE.
For extended darkness treatments, 21-d-old-plants were subjected to prolonged night conditions at the start of the regular night for the time indicated.
Linolenic Acid Toxicity Assay
Seeds were germinated on half-strength MS media supplemented with 2% sucrose. After 10 d of growth at a 16/8-h day/night cycle, plants were transferred to MS media with or without sucrose supplemented with linolenic acid (200 μM) (Biozol) and ethanol (1%, v/v) as solvent for linolenic acid.
Immunoblotting
Total protein was extracted from frozen, homogenized leaf tissue in a buffered solution (100 mM Tris-HCl, pH 8, 50 mM EDTA, 250 mM NaCl, 1 mM DTT, 0.75% SDS, and 1 mM PMSF). After centrifugation (14,000 rpm, Eppendorf 5430, 4°C) to pellet cell debris, the protein concentration in supernatants was determined photometrically using the Bradford assay. Ten micrograms of total protein were loaded per lane, and proteins were separated on 12.5% acrylamide gels and blotted onto PVDF membranes (Bio-Rad). Membranes were then probed with commercially available primary antibodies (Agrisera) against D1, D2, and LHCB2 proteins, and proteins were detected after secondary antibody (horseradish peroxidase conjugate) incubation using chemiluminescence (SuperSignal West substrate; Thermo Fisher Scientific) detected in a LAS-4000 Mini system (Fujifilm Europe).
ATP/ADP Measurements
ATP and ADP content were determined as described by Zhang et al. (2008). In brief, leaf samples were extracted as described by Häusler et al. (2000), and adenosine nucleotides were converted into fluorescent etheno-adenosine nucleotides (Haink and Deussen, 2003). Detection and separation were performed using a reversed phase HPLC system (Waters Alliance 2795) and a fluorescence detector.
PAM Fluorometry
In vivo chlorophyll a fluorescence assay was performed using a PAM fluorometer of the IMAGING-PAM M-Series for intact plant measurements and a WATER-PAM for measurements on isolated chloroplasts (Heinz-Walz Instruments).
For intact plants, 3-week-old dark-treated plants were taken directly out of the growth cabinet and used for fluorescence measurements. To investigate defects in the photosynthetic apparatus, a dark-light induction curve was recorded using the standard settings of the manufacturer's software, i.e., actinic light 8 (186 μmol quanta m−2 s−1), slow induction parameters: delay 40 s, clock 20 s, duration time of 315 s. At the start of every measurement, Fv/Fm was calculated. The quantum efficiency of electron flux through PSII (ΦPSII) was calculated according to Genty et al. (1989).
For isolated chloroplasts, chloroplasts were isolated from ∼10 g of rosette leaves harvested in the dark at the end of the regular night period (Kunst et al., 1988). Leaf tissue was homogenized in ice cold buffer I (450 mM sorbitol, 20 mM tricine/KOH, pH 8.4, 10 mM EDTA, and 0.1% [w/v] BSA) using a blender, filtered twice through a layer of miracloth and cheesecloth, and centrifuged for 1 min at 4000 rpm (Sorvall SS-34). The chloroplast pellet was gently resuspended and washed in buffer II (330 mM sorbitol, 20 mM tricine/KOH, pH 7.6, 2.5 mM EDTA, and 5 mM MgCl2), pelleted at 6000 rpm for 15 s, and resuspended in a small volume of buffer II. For PAM measurements, chloroplasts corresponding to 2.5 μg of chlorophyll per milliliter were diluted into 2 mL of buffer II supplemented with 5 mM K-phosphate buffer, pH 7.5, 3 mM oxaloacetate, and α-linolenic acid as indicated. Chloroplasts were added immediately before the start of measurements.
Isolation and Analysis of Plant Pigments
Leaf pigments were extracted from 50 mg of frozen plant material by addition of 1 mL ice-cold 100% acetone to ground tissue (Lichtenthaler, 1987). After centrifugation, 250 μL of the supernatant were spotted on a 0.2-mm Alugram SilG plate (Macherey-Nagel) and separated with petroleum ether-isopropanol-H2O (100:15:0.25) as running solvent. Bands containing PhA were scratched from the silica gel plate and dissolved in 100% acetone. Substance identity was verified by ultraperformance liquid chromatography (UPLC ACQUITY System; Waters), including a 2996 photodiode array detector (Waters) combined with a time-of-flight mass spectrometer (TOF-MS; LCT Premier; Waters). For liquid chromatography (LC), an ACQUITY UPLC BEH SHIELD RP18 column (1 × 100 mm, 1.7-μm particle size; Waters) was used at a temperature of 40°C, a flow rate of 0.2 mL/min, and with the following gradient: 0 to 0.5 min 0% B, 0.5 to 3 min from 0% B to 20% B, 3 to 8 min from 20% B to 100% B, and 8 to 10 min 95% B, 10 to 14 min from 95% B to 0% B (solvent system A: water/methanol/acetonitrile/formic acid [90:5:5:0.1, v/v/v/v]; B: acetonitrile/formic acid [100:0.1, v/v]). For photodiode array detection, a wavelength range from 190 to 700 nm with a sampling rate of 10 spectra/s was used. Parameters used for the mass detector are described in the next paragraph. The identity of PhA was confirmed by comparison of exact mass ([M+H+] 593.2753), retention time (7.86 min), and UV/VIS optima (224.57, 411.57, and 664.57 nm) with a PhA standard distributed by Frontier Scientific.
For PhA quantification, a volume corresponding to pigments extracted from 25 mg of fresh weight was spotted. PhA bands were recovered in acetone and measured in a spectrophotometer using the absorption maximum at 409 nm. Concentrations were calculated by comparison with a calibration curve obtained from an authentic PhA standard (Frontier Scientific).
Lipid Extraction and Analysis
Rosette leaves from Arabidopsis were collected at times specified. Lipids were extracted from 100 to 200 mg of ground tissue following the method of Bligh and Dyer (1959). Prior to extraction, heptadecanoic acid (17:0) was added as internal standard for free fatty acids, and triheptadecanoylglycerol was added as internal standard for esterified fatty acids. Free fatty acids from lipid extracts were methylated according to a modified protocol described previously (Stumpe et al., 2001). Briefly, the fatty acids were converted with 1-ethyl-3-(3-dimethylaminopropylcarbodiimide) (0.1 g/mL methanol) to the corresponding methylesters, which were dissolved in acetonitrile. The fatty acid methyl esters were analyzed by gas chromatography using Agilent 6890 series gas chromatograph equipped with a capillary DB-23 column (Agilent Technologies). Esterified fatty acids from lipid extracts were transmethylated according to a modified protocol described previously (Christie, 1982). In short, the lipid extract was dried under a stream of nitrogen, resuspended in 333 μL methanol/toluene (1/1, v/v), and transmethylated in the presence of 167 μL 0.5 M sodium methoxide. The reaction was stopped by adding 500 μL of 1 M NaCl and 50 μL of 32% hydrochloric acid. The methyl esters were extracted with hexane, dried down, and finally resuspended in acetonitrile and analyzed as described above.
Acyl-CoA analysis was performed as described by Larson and Graham (2001) with modifications described recently (Sayanova et al., 2006). Neutral lipids were separated by TLC in a single dimension using hexane/diethyl ether/acetic acid (80:20:1.5, v/v/v) as solvent. For the separation of phospholipids, chloroform/methanol/acetic acid (65:25:8, v/v/v) were used. Lipid spots were visualized with copper sulfate (10% in water). For quantification of triacylglycerols, the lipid spots were visualized by spraying with 0.1% aniline naphthalene sulfonic acid. Triacylglycerols were recovered from the plate, transmethylated as described above, and quantified by gas chromatography.
For a more detailed analysis, TAG species samples were analyzed by ultraperformance LC (ACQUITY UPLC system; Waters) combined with a MS (LCT Premier, TOF-MS; Waters). For LC, an ACQUITY UPLC BEH SHIELD RP18 column (1 × 100 mm, 1.7-μm particle size; Waters) was used at a temperature of 50°C, a flow rate of 0.2 mL/min, and with the following gradient: 0 to 0.5 min 80% B, 0.5 to 7 min from 80% B to 100% B, 7 to 11 min 100% B, and 11 to 13 min 80% B (solvent system A: water/methanol/acetonitrile/formic acid [90:5:5:0.1, v/v/v/v]; B: acetonitrile/formic acid [100:0.1, v/v]).
The TOF-MS was operated in positive electrospray ionization mode in W optics and with a mass resolution >10,000. Data were acquired by MassLynx software (Waters) in centroided format over a mass range of m/z 50 to 1200 with scan duration of 0.5 s and an interscan delay of 0.1 s. The capillary and the cone voltage were maintained at 2700 and 30 V and the desolvation and source temperature at 250 and 80°C, respectively. Nitrogen was used as cone (30 L/h) and desolvation gas (600 L/h). For accurate mass measurement of >5 ppm root mean squared, the TOF-MS was calibrated with phosphoric acid 0.01% (v/v) in acetonitrile/water (50:50, v/v), and the dynamic range enhancement mode was used for data recording. All analyses were monitored using Leu-enkephaline ([M+H+] 556.2771 m/z; Sigma-Aldrich) as lock spray reference compound at a concentration of 1 μg/mL in acetonitrile/water (50:50, v/v) and a flow rate of 30 μL/min. The TAG species were detectable as [M+NH4+] ion.
Plant Tissue Fixation and Microscopy
Leaf tissue was cut into pieces of 1 × 2 mm with a razor blade and incubated overnight in a 1% glutaraldehyde solution (50 mM PIPES, pH 7.2). Samples were dehydrated in an acetone series, including a 1% osmium tetroxide step at 50% acetone and gradually embedded in epoxy resin (Spurr, 1969). One-micrometer sections were stained with toluidine blue (0.8% toluidine blue, 0.8% Na2B4O7, and 0.2% Pyronin G; Fluka) for 1 min, washed with deionized water, and viewed with a Nikon Eclipse E800 microscope for bright-field microscopy. Sections (60 nm) for electron microscopy were prepared using an ultramicrotome (MT-600; RMC), stained with uranyl acetate and lead citrate, and viewed with a Philips CM 10.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession numbers At4g39850 (PXA1) and At2g33150 (KAT2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Temperature and Time Dependency of the Phenotype.
Supplemental Figure 2. Imaging PAM Measurements of Plants Exposed to Periods of Darkness.
Supplemental Figure 3. Thin Layer Chromatography of Glycolipids and Phospholipids of Dark-Treated pxa1-2 and Col-0 Plants.
Supplemental Figure 4. Impact of α-Linolenic Acid on Plant Growth.
Supplemental Figure 5. ΦPSII in Col-0, adg1-1 × amiRNA PXA1, adg1-1 Single Mutant, and amiRNA PXA1 Plants before and after 16 h Dark Treatment.
Supplemental Figure 6. Indole Acetic Acid Concentrations in Leaves of Wild-Type and pxa1 Plants Exposed to Different Periods of Darkness.
Supplemental Movie. Time-Lapse Movie Covering 24 h of Reillumination Subsequent to a 36 h Dark Incubation of Col-0, pxa1-2, and pxa1-3 Plants.
Supplementary Material
Acknowledgments
We thank Sonja Hetfeld for excellent technical assistance, Mohammad Hajirezaei for valuable help with ATP/ADP measurements, and Cornelia Göbel for valuable help with auxin measurements. We thank Henning Strissel and Anja Schneider for the kind gift of D2 and LHCB2 antibodies and Kay Marin for providing the D1 antibody and access to the Water-PAM. This research was supported by the Deutsche Forschungsgemeinschaft. K.F. was funded by the Deutsche Forschungsgemeinschaft through the DFG Research Center for Molecular Physiology of the Brain.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Markus Gierth (markus.gierth@uni-koeln.de).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Adham, A.R., Zolman, B.K., Millius, A., and Bartel, B. (2005). Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in beta-oxidation. Plant J. 41 859–874. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. [DOI] [PubMed] [Google Scholar]
- Baker, A., Graham, I.A., Holdsworth, M., Smith, S.M., and Theodoulou, F.L. (2006). Chewing the fat: Beta-oxidation in signalling and development. Trends Plant Sci. 11 124–132. [DOI] [PubMed] [Google Scholar]
- Berger, J., and Gärtner, J. (2006). X-linked adrenoleukodystrophy: Clinical, biochemical and pathogenetic aspects. Biochim. Biophys. Acta 1763 1721–1732. [DOI] [PubMed] [Google Scholar]
- Bligh, E.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37 911–917. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston, V., Page, T., Harrison, E., Breeze, E., Lim, P.O., Nam, H.G., Lin, J.F., Wu, S.H., Swidzinski, J., Ishizaki, K., and Leaver, C.J. (2005). Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 42 567–585. [DOI] [PubMed] [Google Scholar]
- Charlton, W.L., Johnson, B., Graham, I.A., and Baker, A. (2005). Non-coordinate expression of peroxisome biogenesis, beta-oxidation and glyoxylate cycle genes in mature Arabidopsis plants. Plant Cell Rep. 23 647–653. [DOI] [PubMed] [Google Scholar]
- Christie, W.W. (1982). A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 23 1072–1075. [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Dong, C.H., Zolman, B.K., Bartel, B., Lee, B.H., Stevenson, B., Agarwal, M., and Zhu, J.K. (2009). Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Mol. Plant 2 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt, S., Slocombe, S.P., Larner, V., Kurup, S., Wu, Y., Larson, T., Graham, I., Baker, A., and Holdsworth, M. (2002). Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 21 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda, M., Schnurr, J., Abbadi, A., Heinz, E., and Browse, J. (2004). Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell 16 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda, M., Shockey, J., Werber, M., Wolter, F.P., and Heinz, E. (2002). Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J. 32 93–103. [DOI] [PubMed] [Google Scholar]
- Genty, B., Briantais, J.M., and Baker, N.R. (1989). The relationship between the quantum yield of photosynthetic transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990 87–92. [Google Scholar]
- Germain, V., Rylott, E.L., Larson, T.R., Sherson, S.M., Bechtold, N., Carde, J.P., Bryce, J.H., Graham, I.A., and Smith, S.M. (2001). Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 28 1–12. [DOI] [PubMed] [Google Scholar]
- Goepfert, S., and Poirier, Y. (2007). Beta-oxidation in fatty acid degradation and beyond. Curr. Opin. Plant Biol. 10 245–251. [DOI] [PubMed] [Google Scholar]
- Graham, I.A. (2008). Seed storage oil mobilization. Annu. Rev. Plant Biol. 59 115–142. [DOI] [PubMed] [Google Scholar]
- Haink, G., and Deussen, A. (2003). Liquid chromatography method for the analysis of adenosine compounds. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 784 189–193. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., Nito, K., Takei-Hoshi, R., Yagi, M., Kondo, M., Suenaga, A., Yamaya, T., and Nishimura, M. (2002). Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid beta-oxidation. Plant Cell Physiol. 43 1–11. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., Toriyama, K., Kondo, M., and Nishimura, M. (1998). 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler, R.E., Fischer, K.L., and Flügge, U.I. (2000). Determination of low-abundant metabolites in plant extracts by NAD(P)H fluorescence with a microtiter plate reader. Anal. Biochem. 281 1–8. [DOI] [PubMed] [Google Scholar]
- Hettema, E.H., van Roermund, C.W., Distel, B., van den Berg, M., Vilela, C., Rodrigues-Pousada, C., Wanders, R.J., and Tabak, H.F. (1996). The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 15 3813–3822. [PMC free article] [PubMed] [Google Scholar]
- Hooks, M.A., Turner, J.E., Murphy, E.C., Johnston, K.A., Burr, S., and Jaroslawski, S. (2007). The Arabidopsis ALDP protein homologue COMATOSE is instrumental in peroxisomal acetate metabolism. Biochem. J. 406 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup, M.T., Froese, C.D., and Thompson, J.E. (2002). A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol. 129 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst, L., Browse, J., and Somerville, C. (1988). Altered regulation of lipid biosynthesis in a mutant of Arabidopsis deficient in chloroplast glycerol-3-phosphate acyltransferase activity. Proc. Natl. Acad. Sci. USA 85 4143–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, P.R., Eastmond, P.J., Madagan, K., and Graham, I.A. (2004). An Arabidopsis mutant disrupted in valine catabolism is also compromised in peroxisomal fatty acid [beta]-oxidation. FEBS Lett. 571 147–153. [DOI] [PubMed] [Google Scholar]
- Larson, T.R., and Graham, I.A. (2001). Technical advance: A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J. 25 115–125. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler, H.K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology, Vol. 148, S.P. Colowick and N.O. Kaplan, eds. (San Diego, CA: Academic Press), pp. 350–382.
- Lin, T.P., Caspar, T., Somerville, C., and Preiss, J. (1988). Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 86 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy, B., Matile, P., and Thomas, H. (1986). Properties of linolenic acid-dependent chlorophyll oxidation activity in thylakoid membranes. J. Plant Physiol. 123 169–180. [Google Scholar]
- Mackender, R.O., and Leech, R.M. (1974). Galactolipid, phospholipid, and fatty-acid composition of chloroplast envelope membranes of Vicia faba L. Plant Physiol. 53 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile, P. (1992). Chloroplast senescence. In Crop Photosynthesis: Spatial and Temporal Determinants, N.R. Baker and H. Thomas, eds (Amsterdam: Elsevier), pp. 413–440.
- Maxwell, K., and Johnson, G.N. (2000). Chlorophyll fluorescence – A practical guide. J. Exp. Bot. 51 659–668. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Nakagawa, T., Kurose, T., Hino, T., Tanaka, K., Kawamukai, M., Niwa, Y., Toyooka, K., Matsuoka, K., Jinbo, T., and Kimura, T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104 34–41. [DOI] [PubMed] [Google Scholar]
- Ohlrogge, J.B., Browse, J., and Somerville, C.R. (1991). The genetics of plant lipids. Biochim. Biophy. Acta 1082 1–26. [DOI] [PubMed]
- Okamoto, T., Katoh, S., and Murakami, S. (1977). Effects of linolenic acid on spinach choroplast structure. Plant Cell Physiol. 18 551–560. [Google Scholar]
- Peters, J.S., and Chin, C.K. (2003). Inhibition of photosynthetic electron transport by palmitoleic acid is partially correlated to loss of thylakoid membrane proteins. Plant Physiol. Biochem. 41 117–124. [Google Scholar]
- Pinfield-Wells, H., Rylott, E.L., Gilday, A.D., Graham, S., Job, K., Larson, T.R., and Graham, I.A. (2005). Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid catabolism. Plant J. 43 861–872. [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana, I., Cornah, J.E., and Smith, S.M. (2005). Arabidopsis peroxisomal citrate synthase is required for fatty acid respiration and seed germination. Plant Cell 17 2037–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinská, A., Anders, I., Aubry, S., Schenk, N., Tapernoux-Luthi, E., Muller, T., Krautler, B., and Hortensteiner, S. (2007). In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell 19 369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinská, A., Tanner, G., Anders, I., Roca, M., and Hörtensteiner, S. (2003). Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encode by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 100 15259–15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinska, A., Tanner, G., Aubry, S., Anders, I., Moser, S., Muller, T., Ongania, K.H., Krautler, B., Youn, J.Y., Liljegren, S.J., and Hortensteiner, S. (2005). Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol. 139 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, L., Larner, V., Kurup, S., Bougourd, S., and Holdsworth, M. (2000). The Arabidopsis COMATOSE locus regulates germination potential. Development 127 3759–3767. [DOI] [PubMed] [Google Scholar]
- Sayanova, O., Haslam, R., Qi, B., Lazarus, C.M., and Napier, J.A. (2006). The alternative pathway C20 Δ8-desaturase from the non-photosynthetic organism Acanthamoeba castellanii is an atypical cytochrome b5-fusion desaturase. FEBS Lett. 580 1946–1952. [DOI] [PubMed] [Google Scholar]
- Schad, M., Mungur, R., Fiehn, O., and Kehr, J. (2005). Metabolic profiling of laser microdissected vascular bundles of Arabidopsis thaliana. Plant Methods 1 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, R., Ossowski, S., Riester, M., Warthmann, N., and Weigel, D. (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwacke, R., Schneider, A., van der Graaff, E., Fischer, K., Catoni, E., Desimone, M., Frommer, W.B., Flügge, U.I., and Kunze, R. (2003). ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 131 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani, N., and Valle, D. (1996). A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl. Acad. Sci. USA 93 11901–11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi, Y., Watanabe, K., and Takamiya, K.I. (1996). Enzymatic conversion of pheophorbide a to the precursor of pyropheophorbide a in leaves of Chenopodium album. Plant Cell Physiol. 37 1143–1149. [Google Scholar]
- Slocombe, S.P., Cornah, J., Pinfield-Wells, H., Soady, K., Zhang, Q., Gilday, A., Dyer, J.M., and Graham, I.A. (2009). Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol. J. 7 694–703. [DOI] [PubMed] [Google Scholar]
- Smith, A.M., and Stitt, M. (2007). Coordination of carbon supply and plant growth. Plant Cell Environ. 30 1126–1149. [DOI] [PubMed] [Google Scholar]
- Spurr, A.R. (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26 31–43. [DOI] [PubMed] [Google Scholar]
- Stumpe, M., Kandzia, R., Gobel, C., Rosahl, S., and Feussner, I. (2001). A pathogen-inducible divinyl ether synthase (CYP74D) from elicitor-treated potato suspension cells. FEBS Lett. 507 371–376. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y., Amano, T., and Shioi, Y. (2006). Characterization and cloning of the chlorophyll-degrading enzyme pheophorbidase from cotyledons of radish. Plant Physiol. 140 716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, R., Hirashima, M., Satoh, S., and Tanaka, A. (2003). The Arabidopsis-accelerated cell death gene ACD1 is involved in oxygenation of pheophorbide a: Inhibition of the pheophorbide a oxygenase activity does not lead to the “Stay-Green” phenotype in Arabidopsis. Plant Cell Physiol. 44 1266–1274. [DOI] [PubMed] [Google Scholar]
- Tevini, M., and Steinmüller, D. (1985). Composition and function of plastoglobuli. II Lipid composition of leaves and plastoglobuli during beech leaf senescence. Planta 163 91–96. [DOI] [PubMed] [Google Scholar]
- Theodoulou, F.L., Holdsworth, M., and Baker, A. (2006). Peroxisomal ABC transporters. FEBS Lett 580 1139–1155. [DOI] [PubMed] [Google Scholar]
- Theodoulou, F.L., Job, K., Slocombe, S.P., Footitt, S., Holdsworth, M., Baker, A., Larson, T.R., and Graham, I.A. (2005). Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 137 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel, B., Blasing, O.E., Gibon, Y., Retzlaff, K., Hoehne, M., Gunther, M., and Stitt, M. (2008). Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 146 1834–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff, E., Schwacke, R., Schneider, A., Desimone, M., Flügge, U.I., and Kunze, R. (2006). Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol. 141 776–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernotte, C., Solis, C., Moya, I., Maison, B., Briantais, J.M., Arrio, B., and Johannin, G. (1983). Multiple effects of linolenic acid addition to pea thylacoids. Biochim. Biophys. Acta 725 376–383. [Google Scholar]
- Vicentini, F., and Matile, P. (1993). Gerontosomes, a multifunctional type of peroxisome in senescent leaves. J. Plant Physiol. 142 50–56. [Google Scholar]
- Woodward, A.W., and Bartel, B. (2005). Auxin: Regulation, action, and interaction. Ann. Bot. (Lond.) 95 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., Häusler, R.E., Greiten, C., Hajirezaei, M.R., Haferkamp, I., Neuhaus, H.E., Flügge, U.I., and Ludewig, F. (2008). Overriding the co-limiting import of carbon and energy into tuber amyloplasts increases the starch content and yield of transgenic potato plants. Plant Biotechnol. J. 6 453–464. [DOI] [PubMed] [Google Scholar]
- Zolman, B.K., Silva, I.D., and Bartel, B. (2001). The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid beta-oxidation. Plant Physiol. 127 1266–1278. [PMC free article] [PubMed] [Google Scholar]
- Zolman, B.K., Yoder, A., and Bartel, B. (2000). Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.