In the classic model of the heat shock response (HSR), the presence of denatured proteins triggers the expression of heat shock proteins (HSPs) via heat shock transcription factors (reviewed in Voellmy and Boellmann, 2007). Many HSPs are molecular chaperones that bind misfolded proteins. However, this model has increasingly been seen as inadequate to explain the entirety of HSRs (e.g., Kotak, et al., 2007). Furthermore, it has been suggested in the context of treating human diseases that, rather than being triggered by unfolded proteins alone, heat stress responses might initiate in the plasma membrane (Vigh et al., 2007). Now, work from Saidi et al. (pages 2829–2843) provides evidence that the plasma membrane in the moss Physcomitrella patens is indeed a site of heat sensing and that the subsequent responses may be induced by calcium signaling.
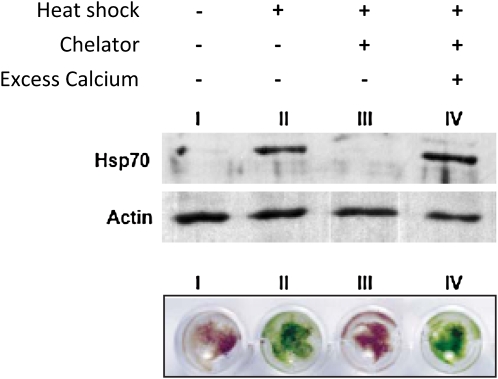
Saidi et al. used transgenic P. patens plants in which reporter gene expression was driven by a HSP promoter to observe the HSR, which was induced when plants were subjected to mild heat stress. The HSR then decreased, even when plants were kept at the higher temperature, unless a resetting period was applied. On the other hand, levels of the Hsp70 chaperone remained high, indicating that Hsp70 levels alone do not dictate the HSR. Interestingly, adding calcium chelators during heat shock almost completely inhibited the HSR. Moreover, chelator treatments inhibited Hsp70 accumulation and kept plants from acquiring thermotolerance (the ability to withstand later high temperatures after an initial exposure to heat stress). The addition of excess calcium reversed these effects (see figure), demonstrating the importance of extracellular calcium. The authors found that soon after the temperature began to rise, levels of cytoplasmic calcium also increased and subsequently decreased. Calcium channel blockers or chelators reduced this influx and excess external calcium restored it. This prompted the authors to use electrophysiological methods to identify a plasma membrane channel that opens transiently upon heat shock and then closes even if the temperature remains high. Thus, the HSR and the calcium transient have similar kinetics in high temperature, suggesting that they are intimately related. Furthermore, chemicals that fluidize the membrane induced the HSR even in the absence of a temperature change, an effect that was shown to be dependent on calcium influx.
Figure 1.
HSRs and acquired thermotolerance are calcium influx dependent. In moss tissue, heat shock–induced Hsp70 protein accumulation (immunoblot shown in top panel, with actin as a control) and acquired thermotolerance (plants shown in bottom panel after a subsequent heat treatment) required available calcium. (Adapted from Figure 2 of Saidi et al. [2009].)
The authors also showed that despite the presence of unfolded proteins after heat shock, the optimal HSR was not induced unless calcium influx occurred. Overall, Saidi et al. demonstrate that it is unlikely that chaperones such as Hsp70 act as the sensors that initiate the HSR and that unfolded proteins alone also do not suffice to trigger the HSR. Instead, the authors provide evidence that mild heating is sensed at the plasma membrane and leads to an influx of calcium that triggers a specific heat signaling pathway. This location for sensing seems reasonable given that membrane fluidity is highly sensitive to even nondenaturing physiological temperatures and can be adjusted by the cell, thereby explaining the importance of relative, rather than absolute, temperature for HSRs. It remains to be seen whether the plasma membrane is the first responder in other species/kingdoms, but the similarities of many aspects of the HSR, including reactions to drugs, suggest that it may well be and that research in plants is at the forefront of this new view of heat sensing.
References
- Kotak, S., Larkindale, J., Lee, U., von Koskull-Döring, P., Vierling, E., and Scharf, K.-D. (2007). Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 10 310–316. [DOI] [PubMed] [Google Scholar]
- Saidi, Y., Finka, A., Muriset, M., Bromberg, Z., Weiss, Y.G., Maathuis, F.J.M., and Goloubinoff, P. (2009). The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 21 2829–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh, L., Horvath, I., Maresca, B., and Harwood, J.L. (2007). Can the stress protein response be controlled by ‘membrane-lipid therapy’? Trends Biochem. Sci. 32 357–363. [DOI] [PubMed] [Google Scholar]
- Voellmy, R., and Boellmann, F. (2007). Chaperone regulation of the heat shock protein response. Adv. Exp. Med. Biol. 594 89–99. [DOI] [PubMed] [Google Scholar]