Abstract
Land plants are prone to strong thermal variations and must therefore sense early moderate temperature increments to induce appropriate cellular defenses, such as molecular chaperones, in anticipation of upcoming noxious temperatures. To investigate how plants perceive mild changes in ambient temperature, we monitored in recombinant lines of the moss Physcomitrella patens the activation of a heat-inducible promoter, the integrity of a thermolabile enzyme, and the fluctuations of cytoplasmic calcium. Mild temperature increments, or isothermal treatments with membrane fluidizers or Hsp90 inhibitors, induced a heat shock response (HSR) that critically depended on a preceding Ca2+ transient through the plasma membrane. Electrophysiological experiments revealed the presence of a Ca2+-permeable channel in the plasma membrane that is transiently activated by mild temperature increments or chemical perturbations of membrane fluidity. The amplitude of the Ca2+ influx during the first minutes of a temperature stress modulated the intensity of the HSR, and Ca2+ channel blockers prevented HSR and the onset of thermotolerance. Our data suggest that early sensing of mild temperature increments occurs at the plasma membrane of plant cells independently from cytosolic protein unfolding. The heat signal is translated into an effective HSR by way of a specific membrane-regulated Ca2+ influx, leading to thermotolerance.
INTRODUCTION
The combined effects of various environmental stresses, such as drought, elevated temperature, freezing, excess light, and salt, are a major concern for developing sustainable agriculture. Global warming is anticipated to increasingly cause droughts and peaks of temperatures that are potentially harmful (Yi and Hong-Bo, 2008), particularly to plants that are sessile organisms. Plants acquire thermotolerance by translating an initial moderate temperature increment into molecular defenses against subsequent temperature extremes, for example, by preventing and repairing damage to heat-labile proteins and membranes (Schöffl et al., 1998; Larkindale and Vierling, 2008). These molecular defenses involve the heat shock response (HSR), during which heat shock proteins (HSPs) accumulate in the cell. Among the most abundantly expressed HSPs are the highly conserved families of molecular chaperones Hsp101, Hsp90, Hsp70/40, Hsp60, and small HSPs (Vierling, 1991; Wang et al., 2004), all sharing the general ability to proofread protein structures. Some chaperones recognize misfolded proteins and prevent the formation of stable, potentially toxic, protein aggregates during heat stress. Others can act on already formed aggregates and use the energy of ATP to unfold them into natively refoldable or protease-degradable species (Hinault et al., 2006). The small heat shock proteins (sHSPs) are α-crystalline related chaperones with a central role in plant-acquired thermotolerance (Vierling, 1991). Without stress, sHSPs often form large oligomers, which in response to heat shock (HS), may transiently dissociate and expose hydrophobic surfaces that can bind misfolded protein intermediates and inhibit the formation of deleterious protein aggregates (Nakamoto and Vigh, 2007). Small HSPs thus assist the ATPase chaperones in the active unfolding of stable protein aggregates and their conversion into native proteins (Veinger et al., 1998; Mogk et al., 2003). Some heat-dissociated sHSPs can also insert into membranes and prevent membrane hyperfluidization (Török et al., 2001; Tsvetkova et al., 2002). The rapid accumulation of sHSPs in plants following a mild nondamaging rise of temperature is one of the most sensitive molecular responses of plant cells to an environmental cue (Vierling, 1991; Schöffl et al., 1998).
The general model for heat shock sensing in eukaryotes, including plants, assigns a primary role to the Hsp90 and Hsc70 (heat shock cognate 70) chaperones and some unspecified heat-labile proteins in the cytoplasm (Shi et al., 1998; Voellmy and Boellmann, 2007). This protein unfolding model is based on several observations: (1) Without HS, the heat shock transcription factors (HSFs; in Arabidopsis thaliana, mainly Hsf1a, Hsf1b, and HsfA3) are found in the cytoplasm as inactive monomers, presumably all associated with constitutively expressed cytoplasmic chaperones (Mosser et al., 1993; Kim and Schöffl, 2002; Yamada et al., 2007). (2) In response to HS, heat-damaged proteins in the cytoplasm presumably recruit the cytoplasmic chaperones, thereby allowing inactive HSFs to undergo phosphorylation, oligomerization, and translocation to the nucleus to transcribe HSP genes (Morimoto, 1998; Baniwal et al., 2004). (3) Even without HS, mutant or damaged misfolded proteins often accumulate in stressed cells alongside with higher levels of molecular chaperones (Ananthan et al., 1986). (4) Specific Hsp90 inhibitors, causing the dissociation of the Hsp90-HSF complex, can induce an isothermal HSR (Zou et al., 1998; Yamada et al., 2007). However, this model does not provide a satisfactory explanation of how very moderate temperature increments, unlikely to cause protein damage in vivo, could lead to the strong induction of HSPs and to the establishment of thermotolerance (Vierling, 1991; Saidi et al., 2005).
An alternative, possibly complementing heat-sensing mechanism places the primary temperature sensor of the cell in the plasma membrane (Vígh et al., 2007). This is substantiated by the observation that a heat-induced or chemically induced increase of membrane fluidity can generate a significant HSR in prokaryotes and eukaryotes (Vígh et al., 1998; de Marco et al., 2005; Saidi et al., 2005). Reciprocally, artificial hydrogenation, rigidifying membranes, reduces the HSR (Horváth et al., 1998). Other reports suggest that secondary messengers are involved in the plant HSR, such as Ca2+ (Gong et al., 1998), H2O2 (Volkov et al., 2006), and signal proteins like calmodulin (Liu et al., 2005), immunophilins (Aviezer-Hagai et al., 2007), and kinases (Suri and Dhindsa, 2008). These need to be integrated into a coherent cellular mechanism for plant sensing of mild temperature variations unlikely to cause protein misfolding in the cell.
The moss Physcomitrella patens is an emerging land plant model with a fully sequenced genome (Rensing et al., 2008). P. patens is widely used to study defense mechanisms against abiotic stresses (Frank et al., 2005; Cuming et al., 2007; Qudeimat et al., 2008). Its unique phylogenic position, between green algae and flowering plants, enables the study of the evolution of various stress signaling pathways in plants (Rensing et al., 2008). Recently, differences have been reported between mosses and seed plants in stress adaptation (Frank et al., 2005) and hormone signaling events (Yasumura et al., 2007). Because diurnal and seasonal variations of ambient temperatures are more extreme on land than in the ocean, the development of effective temperature-sensing mechanisms must have been central to the successful colonization of lands by plants. Here, we addressed the molecular mechanism of temperature sensing in the P. patens plant. We dissected the molecular mechanism by which moss cells perceive a mild temperature rise using pharmacological, electrophysiological, and biomonitoring approaches. Our data show that the plasma membrane contains specific Ca2+-permeable channels that act as the earliest temperature-sensing component of the plant HSR. A mild temperature rise generates a transient Ca2+ signal that triggers a specific downstream expression of HS genes, resulting in plant-acquired thermotolerance.
RESULTS
Alternating Heat Shock Cycles Are Required to Maintain Sustained HSP Expression
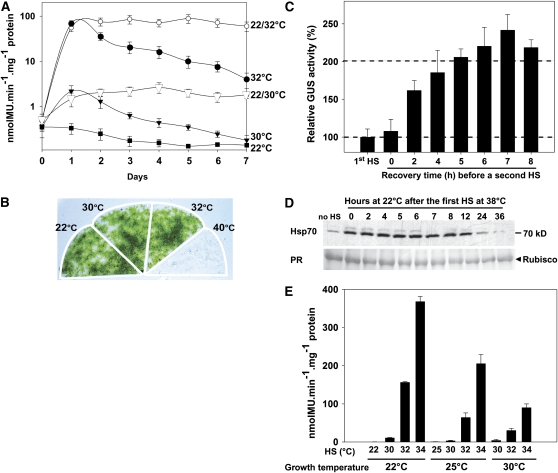
Using a highly sensitive heat-inducible reporter moss line, hsp17.3B:β-glucuronidase (HSP-GUS), we have previously shown that a continuous (120 min) nondamaging HS at 38°C induced a transient HSR, which became completely attenuated within 60 min of the heat stimulus (Saidi et al., 2005). Here, we further addressed the transient nature of the HSR during a continuous, albeit mild, temperature rise, which was maintained for up to a week (Figure 1A). A single upshift from 22 to 32°C resulted, during the first day, in a 130-fold increase of the Hsp-mediated GUS expression, which subsequently decreased about twofold each day, although the tissue was maintained at 32°C. A similar behavior, but expectedly less pronounced, was observed following a milder upshift from 22 to 30°C (Figure 1A). Seven days of continuous exposure to 30 or 32°C did not cause any observable defects to the moss tissues, unlike continuous exposure to 40°C, which was lethal (Figure 1B). Thus, following the first day of exposure to a physiological nondamaging temperature, the HS signal for de novo HSP expression became completely attenuated by a yet unclear mechanism. Remarkably, when plants were exposed to a daily regime of alternating temperatures of 1 h at 32°C (or 30°C) followed by 23 h at 22°C, the levels of GUS expression remained constantly high during the following 6 d (Figure 1A). Hence, after a week of treatment with this alternating heat regime, tissues treated for a total of 7 h at 32°C expressed 13 times more GUS than tissues continuously exposed for 168 h at 32°C.
Figure 1.
A Mild Temperature Rise Generates a Transient Signal Depending on the Temperatures of Growth and Induction.
(A) Specific GUS activity measured daily in HSP-GUS plants grown at 22°C for 3 d and then exposed continuously, during 7 d, to 22°C (closed squares), 30°C (closed triangles), or 32°C (closed circles) or exposed to seven daily cycles of 1 h at 30°C (22/30°C, open triangles) or at 32°C (22/32°C, open circles) followed by 23 h at 22°C.
(B) Appearance of moss tissues following 7 d of continuous exposure to 22, 30, 32, or 40°C.
(C) Relative GUS activities after 1 h at 38°C (1st HS) or two heat shocks (each of 1 h at 38°C) interspaced by 0 to 8 h of recovery at 22°C. GUS activities were measured after 8 h of recovery.
(D) Immunoblot analysis of Hsp70 levels in moss tissues before (no HS) or after a single HS at 38°C followed by 0 to 36 h recovery at 22°C. PR, Ponceau red staining shows ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) large subunit (arrowhead) as a control of equal loading.
(E) Specific GUS activity in HSP-GUS tissues pregrown for 1 week at 22, 25, or 30°C, followed by 1 h at 30, 32, or 34°C and 8 h recovery at 22°C. All values are means of at least three independent experiments, and standard deviations are shown.
The Full Resetting of the HSR Requires at Least 5 h at Noninducing Temperature
The above data suggest that a resetting period at noninducing temperature is needed to reactivate the plant HSR. Following an initial nondamaging 1 h HS at 38°C, HSP-GUS tissues were incubated at noninducing temperature for increasing periods before a second HS was applied. A minimum of 5 h at 22°C was found to be necessary to restore the full ability of the plant to respond to a second HS (Figure 1C). Half the resetting time was reached within <2 h, and a 7-h refractory period triggered a slightly stronger (1.3-fold) HSR (Figure 1C). Immunoblot analyses confirmed that 1 h at 38°C induced a significant accumulation of cytoplasmic Hsp70 (Figure 1D). Remarkably, the level of Hsp70s remained maximal during 8 h after HSR. Thus, the observed attenuation of the HSR following the first hour of a continuous heat treatment cannot be attributed to de novo accumulation of inhibitory Hsp70 chaperones (Figures 1A to 1D).
The Inducing Temperature Does Not Exclusively Determine the Intensity of HSR
We next explored the link between the growth temperature and the intensity of the HSR following a mild temperature upshift. HSP-GUS tissues were grown for a week, either at 22, 25, or 30°C. Interestingly, after 1 h at 32 or 34°C, the GUS levels in tissues pregrown at 22°C were 2 and 4 times higher than in tissues pregrown at 25 and 30°C, respectively (Figure 1E). Thus, the intensity of the HSR is not exclusively influenced by the inducing temperature but also by the basal growth temperature.
Heat-Induced Entry of Extracellular Calcium Regulates Heat Shock Signaling
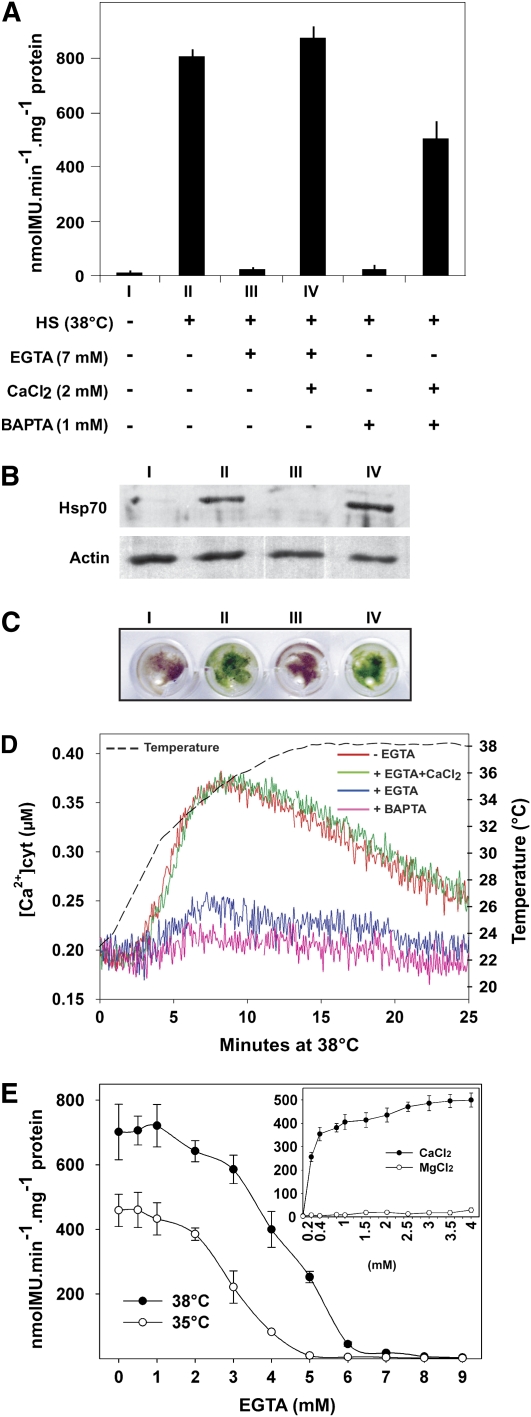
Organisms can adapt their membrane composition according to their growth temperature by modulating the lipid composition (Vígh et al., 2007). We therefore addressed the possible role of membranes as primary thermosensors of the plant cell. When HSP-GUS tissues were treated for 1 h at 38°C in the presence of 1 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) or 7 mM EGTA, the HSR was strongly inhibited (Figure 2A). The HSR was fully restored when excess Ca2+ (2 mM CaCl2) was supplemented, despite the presence of chelators. This strongly suggests that a membrane-regulated entry of extracellular Ca2+ ions is essential to mediate the plant HSR. Immunoblot analyses confirmed that when external Ca2+ was artificially chelated, the heat induction of cytosolic Hsp70 chaperones was also inhibited (Figure 2B). Ca2+ depletion also negatively impacted on the ability of the plant to develop acquired thermotolerance (Figure 2C).
Figure 2.
The Plant HSR Is Regulated by the Transient Entry of Extracellular Calcium.
(A) Specific GUS activities from HSP-GUS tissues measured after 1 h HS at 38°C in the presence (+) or absence (−) of 1 mM BAPTA, or 7 mM EGTA, with (+) or without (−) 2 mM CaCl2.
(B) Immunoblot analysis of endogenous Hsp70 and actin. Roman numerals correspond to the treatments in (A).
(C) Acquired thermotolerance test: moss tissues were treated as in (A). Following 4 h recovery at 22°C in standard liquid medium, tissues were incubated at 43°C for 2 h, and tissue survival was observed 3 d later.
(D) In vivo effect of calcium depletion on the concentration of cytosolic Ca2+ during a temperature upshift from 22 to 38°C. UBI-AEQ tissues were treated with 1 mM BAPTA (pink line), 7 mM EGTA (blue line), or 7 mM EGTA supplemented with 2 mM CaCl2 (green line). Red line shows control conditions without calcium chelator. The dashed line represents the sample temperature.
(E) Dose-dependent effect of increasing EGTA concentrations on heat-induced GUS expression following 1 h at 35 or 38°C as in (A). Inset: Specific GUS activity in HSP-GUS tissues treated 1 h at 35°C in the presence of 5 mM EGTA and increasing concentrations of CaCl2 or MgCl2. GUS expression values are means of at least three independent experiments, and standard deviations are shown.
To follow the fluctuations of cytoplasmic Ca2+ concentrations during a mild temperature upshift, we generated a stable transgenic line, UBI-AEQ, that constitutively expresses the Aequorea victoria apoaequorin under control of the strong maize (Zea mays) Ubiquitin-1 promoter (Christensen and Quail, 1996; see Methods). Because the stability of apoaequorin (Gong et al., 1998) and the activity of the Ubiquitin-1 promoter (Saidi et al., 2005) are not affected by the range of HS conditions used here, this line is particularly appropriate for temperature variation studies. The relative concentration of cytoplasmic Ca2+ was monitored in intact tissues during a single temperature upshift from 22 to 38°C (Figure 2D). Around 3 min after the start of HS, when the effective temperature in the sample reached 28°C, a strong increase of cytoplasmic Ca2+ was observed, which lasted about 5 min. Once the temperature reached 34°C, the concentration of cytoplasmic Ca2+ leveled and thereafter slowly decreased. After 25 min of HS, calcium levels were returned to near basal concentrations, despite maintaining the inducing temperature (Figure 2D). Interestingly, applying higher temperatures induced a more intense and rapid Ca2+ transient, which proportionally translated into a subsequent stronger HSR (see Supplemental Figure 1 online). Pretreatments with EGTA or BAPTA significantly reduced the heat-induced Ca2+ influx, an inhibition that was abrogated when an excess of free Ca2+ (2 mM CaCl2) was provided (Figure 2D). A Ca2+ dose response on Ca2+-depleted moss tissues, without chelators, showed that 100 μM CaCl2 could drive half of the optimal HSR (see Supplemented Figure 2A online). Interestingly, at lower temperature (35°C), lower concentrations of EGTA were required to achieve half inhibition of the HSR (3 mM at 35°C instead of 4 mM at 38°C; Figure 2E). Under these experimental conditions (pH 6.25), the free Ca2+ concentration in the medium was estimated to be 1.77 and 1.2 μM, respectively. A dose–response analysis at 35°C showed that, in the presence of 5 mM EGTA, as little as 0.2 mM CaCl2, corresponding to a calculated 0.37 μM free Ca2+ in the medium, could restore half of the maximal HSR (Figure 2E, inset). Interestingly, when extracellular Ca2+ was immobilized using EGTA, strontium and barium could effectively substitute for the calcium ions and restore a full HSR (see Supplemental Figure 2B online), a selectivity sequence that is similar to Ca2+ channels in animal neurons (Marom et al., 2007).
Electrophysiological Evidence for a Heat-Sensitive Plasma Membrane Ca2+-Permeable Channel
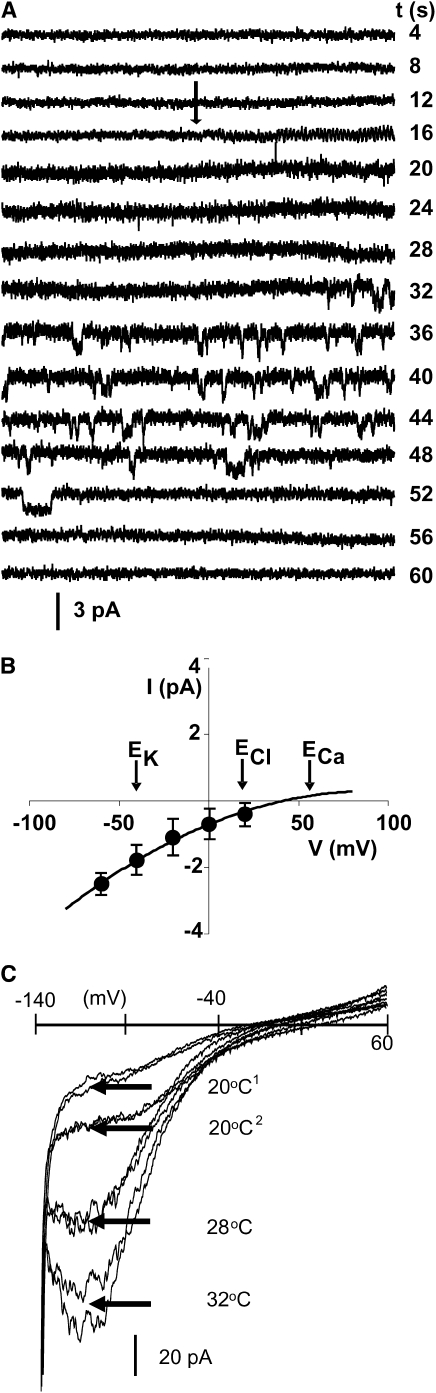
The data obtained with the UBI-AEQ reporter line strongly suggest the presence of a temperature-sensitive ion channel in the plasma membrane that can generate a Ca2+ transient to initiate the moss HSR. We therefore used electrophysiological techniques to determine whether temperature increases, similar to those described in intact tissues, evoked corresponding alterations in channel activity in the plasma membrane of isolated P. patens protoplasts and excised membrane patches.
As reported earlier (Johannes et al., 1997), we found that the currents across the P. patens plasma membrane are dominated by K+-selective outward rectifying channels, with a unitary conductance of around 45 pS. The activity of these channels was not significantly affected by changes in bath temperature. By contrast, an increase of the bath temperature from 22 to 38°C evoked the opening of a specific ion channel in excised membrane patches (n = 5), usually within 10 to 15 s of the applied temperature change (Figure 3A). Channel activation was transient and it spontaneously disappeared after periods ranging from 30 to 60 s. No or little voltage dependence was observed for the channel open probability, and current voltage plots (Figure 3B) indicate a unitary conductance of around 26 pS with a reversal potential near ECa. Using the Goldman equation (Hille, 2001), a relative permeability PK/Ca of around 0.35 was calculated, indicating that the majority of the current is carried by a Ca2+ influx in the applied conditions (20 mM K+ and 10 mM Ca2+ on the apoplastic side). Whole-cell recordings from protoplasts exposed to 20 mM CaCl2 in a K+ free bath solution (Figure 3C) show a reversible increase in inward current when bath temperatures are raised from 20 to 28°C or to 32°C. The negative slope conductance at more hyperpolarized membrane voltages is characteristic of hyperpolarization activated Ca2+ channels (Demidchik and Maathuis, 2007).
Figure 3.
Electrophysiology Analysis Showing Activation of Ca2+-Permeable Channels in Response to Mild Temperature Increments.
(A) Transient channel activity in outside-out membrane patches recorded before and ∼45 s after the bath solution temperature was raised from 22 to 38°C (arrow). The activity spontaneously decreased after around 30 s. Holding potential was −40 mV, bath solution contained 20 mM KCl and 10 mM CaCl2, pipette solution contained 100 mM KCl and 0.1 mM CaCl2, and elapsed time is indicated at the end of each trace.
(B) Current voltage relationship of the recorded channels indicates a reversal potential (i.e., the voltage where the current is zero) near the theoretical Erev of Ca2+ and a unitary conductance of 26 pS. Theoretical reversal potentials for the solutions used in (A) are EK = -41, ECa= 58, and ECl= 22 mV for K+, Ca2+, and Cl− (indicated by arrows).
(C) Whole-cell recordings on P. patens protoplasts at room temperature (20°C1) and after stepwise changes to higher bath temperatures of 28 and 32°C, after which the temperature was allowed to return to room temperature (20°C2). Two I/V graphs, ranging from −140 mV to 60 mV with a 500-ms duration are shown for each temperature. Bath solution contained 20 mM CaCl2, whereas the pipette solution was as in (A).
The Intensity of Calcium Influx during the First Minutes of HS Determines the Level of HSR
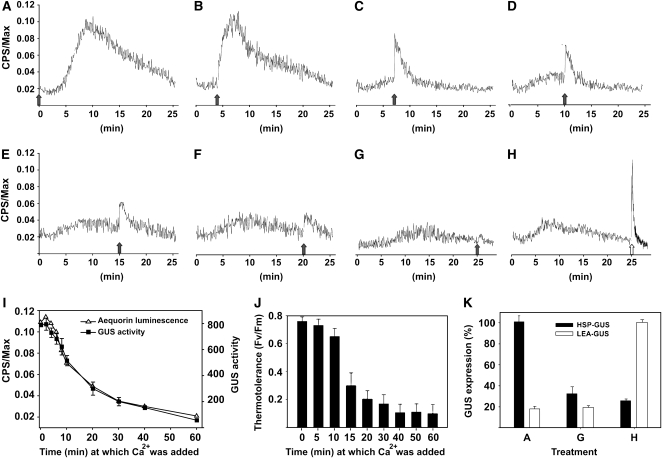
Since an elevated temperature was observed to cause transient activation of a Ca2+-permeable channel, we further investigated the correlation between an early Ca2+ influx, occurring within minutes, and the subsequent HSR developing within hours of HS onset. UBI-AEQ tissues were incubated at 38°C with 7 mM EGTA, and excess extracellular Ca2+ (2 mM) was then added with increasing time delays (Figures 4A to 4G). When Ca2+ was added simultaneously to the start of the HS (t = 0), a transient Ca2+ influx was observed that was identical to the signature without EGTA (Figure 4A). When Ca2+ was added at t = 4 min, the rate of the Ca2+ entry became greater, but with an amplitude similar to that recorded for t = 0 (Figure 4B). At t ≥ 7 min, the Ca2+ amplitude steadily declined until no longer detected after t = 25 min (Figures 4C to 4G). Together, these findings suggest that the heat-activated Ca2+-permeable channels in the plasma membrane transiently open only during the first minutes of a HS and thereafter progressively close, regardless of Ca2+ translocation and despite the ongoing heat stimulus. When the HSP-GUS line was exposed to the same treatments as in Figures 4A to 4G, activation levels of the Hsp17.3B promoter and acquired thermotolerance decreased with kinetics remarkably similar to those of the Ca2+ transient (Figures 4I and 4J).
Figure 4.
Specific Heat-Sensitive Ca2+ Channels Become Gradually Unresponsive during HS, Regardless of Ca2+ Entry.
(A) to (G) UBI-AEQ tissues treated at 38°C, in the presence of 7 mM EGTA, were supplemented with 2 mM CaCl2 at the indicated time (black arrows). t = 0, 4, 7, 10, 15, 20, and 25 min for (A) to (G), respectively. Ca2+-induced aequorin luminescence was then recorded every 3 s.
(H) Following Ca2+ addition at t = 25, 8.5% mannitol was immediately added (white arrow).
(I) Correlation between the maximal Ca2+ signal in UBI-AEQ (open triangles) and the consequent GUS expression in HSP-GUS line (closed squares), following the same treatments as in (A) to (G). Once CaCl2 was added, tissues were maintained for 1 h at 38°C, and GUS activity was measured following 8 h recovery at 22°C.
(J) Measurements of acquired thermotolerance. Moss tissues were pretreated as in (I). After 4 h recovery at 22°C, tissues were subjected to 1 h at 42°C and Fv/Fm values were measured.
(K) GUS-specific activity from HSP-GUS (closed bars) or LEA-GUS (empty bars) tissues following treatment as in (A), (G), and (H). GUS activity from HSP-GUS was measured as in (I). For LEA-GUS, once CaCl2 ([A] and [G]) or CaCl2 and mannitol (H) were added, tissues were maintained for 1 h at 38°C, and GUS activity was measured after 20 h. For each line, the highest GUS activities were set to 100%. GUS activity or Fv/Fm values are means of at least three independent experiments, and standard deviations are shown.
Whereas at t = 25 min of the HS, addition of excess calcium did not generate any detectable Ca2+ transient (Figure 4G), addition of mannitol (8.5%) concomitantly to Ca2+ generated an immediate spike of Ca2+ influx (Figure 4H). This shows that the responsiveness of the UBI-AEQ line is unaffected by 25 min of HS in the presence of EGTA. The applied osmotic shock did not activate Hsp genes despite the mannitol-induced Ca2+ influx and the ongoing HS (Figure 4K). This was confirmed using another recombinant P. patens reporter line expressing GUS under the control of an osmo-sensitive LEA promoter (Kamisugi and Cuming, 2005). This LEA-GUS line displayed a specific mannitol- but not HS-induced GUS expression, whereas the HSP-GUS line displayed a specific heat- but not mannitol-induced GUS expression (Figure 4K). This strongly suggests that during the first minutes of a temperature increase, it is the timely entry of Ca2+ via specific heat-sensitive Ca2+ channels that is central to the development of a specific HS signal. The HSR remains unaffected by Ca2+ entry by way of other stress-sensitive Ca2+ channels on the same plasma membrane.
Calcium Release from Intracellular Stores Does Not Mediate the HSR
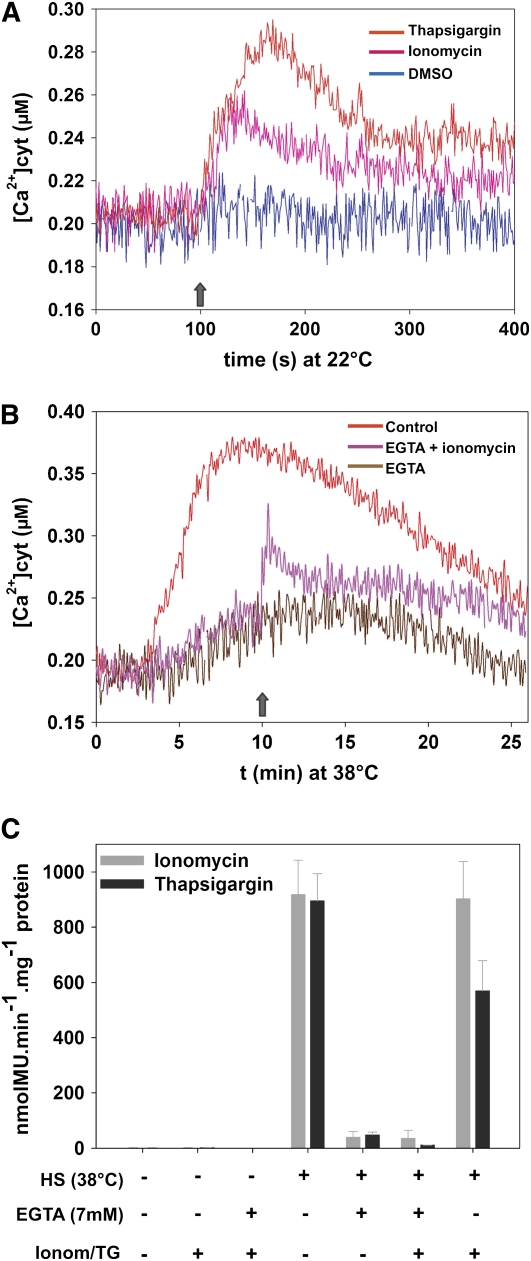
To test whether Ca2+ ions from intracellular stores contribute to the heat-induced Ca2+ transient, we used ionomycin and thapsigargin, known to cause Ca2+ release from the endocompartments of animal cells (Yoshida and Plant, 1992; Morgan and Jacob, 1994; Wang et al., 2005). Confirming that both compounds cause Ca2+ release from the endocompartments of moss cells, an addition at 22°C of 50 μM ionomycin or thapsigargin, in the presence of external EGTA, induced a significant transient elevation of cytoplasmic Ca2+ (Figure 5A). When, in the presence of EGTA, ionomycin was added at the tenth minute of a HS, a similar release of Ca2+ into the cytoplasm was observed (Figure 5B). Yet, despite ionomycin- or thapsigargin-mediated release of Ca2+ into the cytoplasm, no HSR was observed during and following HS, as long as external Ca2+ was prevented by EGTA from entering the cell (Figure 5C). Thus, confirming data from electrophysiology, the heat shock signal is initiated at the plasma membrane by a specific transient crossing of extracellular Ca2+ ions. Noticeably, without EGTA, the addition of ionomycin during HS did not alter the HSR (Figure 5C), demonstrating that this ionophore is not toxic to the moss within the time range of the experiment. Thapsigargin, however, slightly reduced the heat-induced GUS expression by 30% (Figure 5C), a possible toxic effect likely due to the high concentration used. Lower thapsigargin concentrations (1 to 8 μM), although causing an effective entry of extracellular Ca2+, failed to release Ca2+ ions from the intracellular stores of P. patens cells (see Supplemental Figure 3 online).
Figure 5.
The Release of Ca2+ from Intracellular Stores Does Not Mediate a HSR.
(A) Ionomycin and thapsigargin induced a transient elevation of cytoplasmic Ca2+ concentration at 22°C. The UBI-AEQ line was incubated at 22°C with 7 mM EGTA, and the cytosolic Ca2+ concentration was monitored after the addition (arrow) of 50 μM ionomycin (pink line), thapsigargin (red line), or DMSO control (0.3%; blue line).
(B) Addition of 50 μM ionomycin (pink line) at the 10th min (arrow) of a HS at 38°C, in the presence of 7 mM EGTA, induced an elevation of cytoplasmic Ca2+. Red line represents the optimal calcium signature in EGTA-free distilled water.
(C) GUS expression from HSP-GUS tissues treated with ionomycin (Ionom) or thapsigargin (TG) for 1 h at 22 or 38°C, following pretreatment with or without 7 mM EGTA as indicated. The values are means of at least three independent experiments, and standard deviations are shown.
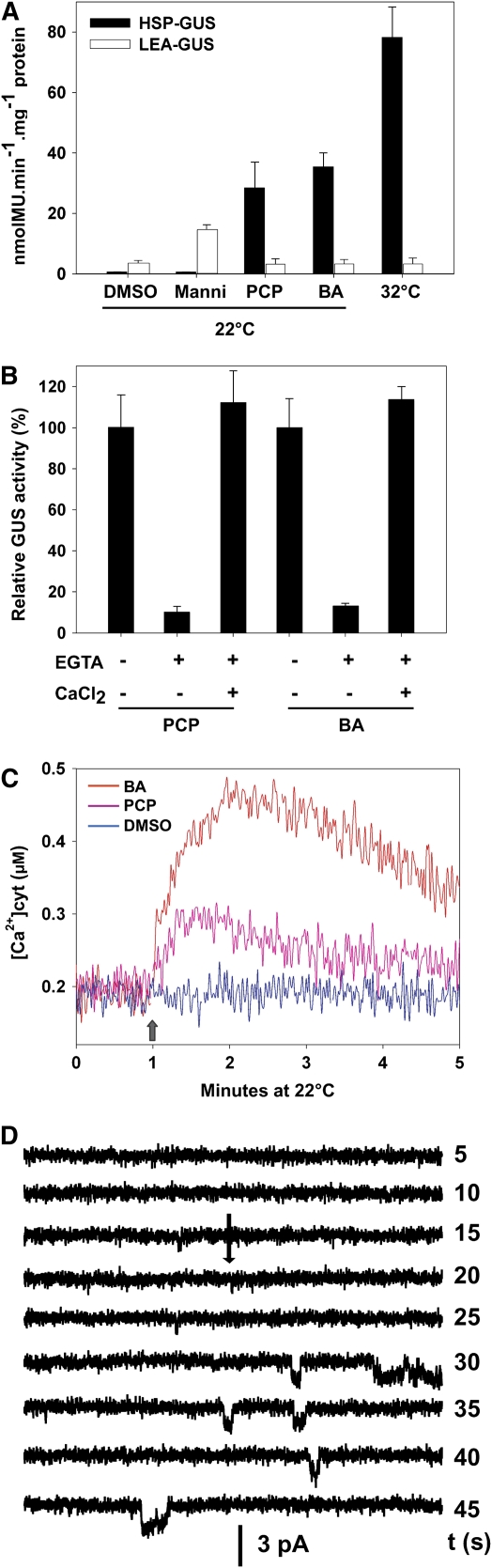
Membrane Perturbing Compounds Induce Ca2+ Influx and Specifically Activate the HSR
In bacteria and animal cells, benzyl alcohol (BA) can induce an isothermal HSR in correlation with its fluidizing effect on membranes (Horváth et al., 1998; de Marco et al., 2005). Here, we observed that millimolar amounts of BA or micromolar amounts of the hydrophobic pollutant pentachlorophenol (PCP) induced a specific HSR at noninducing temperatures (Figure 6A). Both compounds induced the plant HSR strictly in a Ca2+ entry-dependent manner (Figure 6B). Addition of BA or PCP, at 22°C, also generated a major Ca2+ transient measured in the UBI-AEQ line (Figure 6C). In addition, patch clamp recordings showed that BA isothermally activated a Ca2+-permeable ion channel (Figure 6D), resembling the activation by HS. Interestingly, BA pretreatment effectively enhanced the moss acquired thermotolerance to the same level as priming with a mild heat treatment (see Supplemental Figures 4B, 4C, and 6 online). This effect was lost when BA priming was performed in the presence of EGTA. On the other hand, isothermal mannitol pretreatment, although inducing a significant influx of external Ca2+, failed to improve thermotolerance (see Supplemental Figures 4B and 4C online).
Figure 6.
Membrane-Interacting Compounds Specifically Activate an Isothermic HSR in a Ca2+ Influx-Dependent Manner.
(A) Specific GUS activity in HSP-GUS (closed bars) or LEA-GUS (open bars) tissues, after exposure (at 22°C) to 8.5% mannitol (Manni, 20 h), PCP (10 μM PCP, 1 h), BA (25 mM BA, 1 h), DMSO control (0.1%, 1 h), or following 1 h HS at 32°C.
(B) Relative GUS activity (percentage) in HSP-GUS tissues after exposure to PCP and BA as in (A), with or without 7 mM EGTA or 2 mM CaCl2.
(C) Effect of addition (arrow) of PCP (pink trace) or BA (red trace) as in (A) on the cytosolic Ca2+ concentration using the UBI-AEQ line.
(D) Isothermal activation of inward channel activity in response to addition (arrow) of 5 mM BA to the bath solution. The induced sustained channel activity in a plasma membrane–excised patch of the moss protoplast was recorded for several minutes. The holding potential was −40 mV, and elapsed time (s) is indicated at the end of each trace. Unitary conductance is 29 ± 3 pS and channel current reversed around 30 mV. Bath solution and pipette solutions were as in Figure 3. GUS expression values are means of at least three independent experiments, and standard deviations are shown.
Calcium Depletion Prevents Hsp70 Accumulation Despite Heat-Induced Protein Unfolding in the Cytoplasm
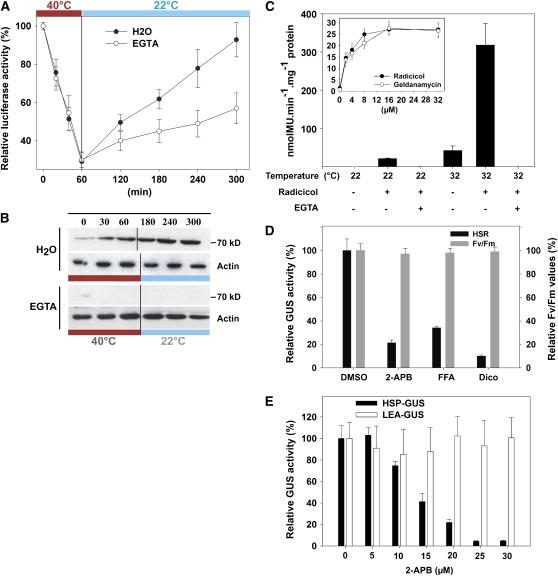
In eukaryotes, the heat-induced unfolding of cytoplasmic proteins is believed to serve as the trigger for HSR (Ananthan et al., 1986; Shi et al., 1998). To test this hypothesis, we used the 35S-LUC moss line (Saidi et al., 2007) that constitutively expresses soluble luciferase, a known thermolabile chaperone substrate (Forreiter et al., 1997; Saidi et al., 2007). When 35S-LUC tissues were treated at 40°C, with or without EGTA in the medium, ∼70% of the luciferase became inactivated within 1 h (Figure 7A). When the heat-treated tissues, without EGTA, were returned to 22°C, the initial level of luciferase activity was fully recovered within 4 h, indicative of an efficient post-HS chaperone-mediated disaggregation and refolding activity in the cytoplasm. By contrast, when the heat-treated tissues with EGTA were returned to 22°C, the rate of luciferase recovery was twice lower (Figure 7A). Immunoblot analysis showed that without EGTA, Hsp70 strongly accumulated during HS and its level remained high during the four subsequent hours of recovery (Figure 7B). However, with EGTA, no Hsp70 accumulated during and following HS, despite the pronounced denaturation of the cytosolic luciferase protein. Thus, as long as Ca2+ entry is prevented during HS, the unfolding of a chaperone substrate does not suffice to trigger a HSR.
Figure 7.
The Plant HSR Is Regulated by Membrane-Modulated Ca2+ Entry, but Not Hsp90 Inhibition or Luciferase Unfolding.
(A) Time-dependent luciferase activity in 35S-LUC tissues treated for 1 h at 40°C in the presence (open circles) or absence (closed circles) of 7 mM EGTA and during 4 h recovery at 22°C.
(B) Immunoblot analysis of Hsp70 (70 kD) and actin from the tissues treated in (A).
(C) GUS activity in HSP-GUS tissues treated 1 h at 22 or 32°C, with (+) or without (−) 8 μM radicicol, in the presence (+) or absence (−) of 7 mM EGTA, as indicated. Inset: Specific GUS activity in HSP-GUS tissues treated 1 h at 22°C with increasing concentrations of radicicol or geldanamycin.
(D) Relative GUS activity and toxicity values (in percentages) in HSP-GUS lines treated with calcium channel blockers or kinase inhibitors. HSP-GUS tissues were pretreated at 22°C for 30 min with 20 μM 2-APB, FFA, or dicoumarol (Dico) and then heat-treated for 1 h at 36°C. Toxicity analyses (Fv/Fm ratio) were performed immediately after the heat treatment, and GUS activity was measured 8 h after HS.
(E) Dose–response effect of 2-APB on the moss HSR. HSP-GUS (closed bars) or LEA-GUS (open bars) tissues were pretreated with the indicated concentrations for 30 min at 22°C and then heat treated (1 h at 36°C for HSP-GUS) or exposed to mannitol (8.5%, 20 h for LEA-GUS). The optimal GUS activities for each line (582 nmol 4-methylumbelliferone [MU]·min−1·mg−1 for HSP-GUS and 19.8 nmol MU·min−1·mg−1 for LEA-GUS) were normalized to 100%. All values are means of at least three independent experiments, and standard deviations are shown.
[See online article for color version of this figure.]
The HS-Like Response Induced by Hsp90 Inhibitors Is Ca2+ Influx Dependent
Hsp90 inhibitors can activate a specific HSR in animals, yeast, and plants, presumably by causing the dissociation of inactive cytoplasmic Hsf-1 from Hsp90 (Zou et al., 1998; Yamada et al., 2007). Here, we confirmed that 1-h exposure to increasing concentrations of radicicol or geldanamycin activated a significant HSR at 22°C, with an EC50 of 2 μM (Figure 7C, inset). Unexpectedly, we found that the HSR induced by saturating levels (8 μM) of radicicol was completely inhibited in the presence of EGTA (Figure 7C). When the UBI-AEQ line was exposed to radicicol at 22°C, a delayed transient elevation of cytosolic Ca2+ concentration was observed, ∼15 min after addition (see Supplemental Figure 5A online). When a mild heat treatment (32°C) was applied in the presence of 8 μM radicicol, a strong synergistic effect was observed, with GUS expression levels 4 times higher than the expected sum of the separate treatments (Figure 7C). This synergistic effect, also observed at the level of calcium entry (see Supplemental Figure 5B online), was completely inhibited by EGTA (Figure 7C). Altogether, this argues against Hsp90 being a direct cytosolic regulator of the plant HSR.
Calcium Channel Blockers and Kinase Inhibitors Specifically Reduce the Plant HSR
We further explored key regulators of the plant HSR by testing 12 drugs selected for their reported activity in animal cells, as Ca2+ channel blockers or protein kinase inhibitors (see Supplemental Table 1 online). Two Ca2+ channel blockers, 2-aminoethyldiphenyl borate (2-APB) and flufenamic acid (FFA), were found to significantly inhibit the moss HSR with similar IC50 values of 15 μM (Figure 7D; see Supplemental Table 1 online). The drop in Hsp-mediated GUS expression was not due to adverse effects on plant health, as reflected by the unaffected photosynthetic activity measured after the treatment (Figure 7D). Similarly, 20 μM dicoumarol, a kinase inhibitor, strongly inhibited the HSR, confirming reports about a central role of kinases in the plant HSR (Suri and Dhindsa, 2008). Expectedly, the reduced HSR caused by these compounds negatively affected acquired thermotolerance (see Supplemental Figure 6 online).
Interestingly, the calcium channel blocker 2-APB strongly inhibited the plant HSR in the HSP-GUS line but not the mannitol-induced osmotic response in the LEA-GUS line (Figure 7E). This confirms our results in Figure 4L and suggests that the plasma membrane contains at least two distinct heat- and osmo-responsive Ca2+ channels, regulating independent stress signaling pathways.
DISCUSSION
For many terrestrial organisms, the most commonly experienced change in temperature is the diurnal rhythm of the sun. In extreme continental climates, this may cause temperature variations in excess of 30°C, which are potentially harmful to fragile macromolecular complexes, such as proteins and membranes. It is essential that sessile organisms like plants register mild temperature increments and trigger the timely build up of molecular mechanisms to prevent upcoming heat damage (Mittler, 2006).
The Plant HSR Is Transient and May Occur at Mild Nondamaging Temperatures
Our data show that during a mild continuous HS, the activation of the Hsp17.3B promoter is transient and that at least 5 h at noninducing temperatures are required to fully restore the potency of a second HSR. Noticeably, the high level of Hsp70 that accumulated in P. patens cells following a first HS did not repress a subsequent HSR (Figures 1C and 1D). Thus, unlike initially shown for human cells (Abravaya et al., 1992) and earlier suggested for plants (Lee and Schöffl, 1996), the P. patens HSR is independent from cellular levels of Hsp70 chaperones. The specific inhibition of Hsp90 chaperones by radicicol produced two unexpected results: (1) It induced a minor isothermal HSR, strictly dependent on a Ca2+ influx across the plasma membrane. (2) A major heat-induced HSR is still effective in the presence of Hsp90 inhibitors. Together, this indicates that Hsp70 and Hsp90 chaperones are not largely responsible for either the repression of the HSR at noninducing temperatures or the activation of Hsfs following a moderate temperature increase.
Using thermolabile luciferase, we further tested the effect of a thermally unfolded chaperone substrate on a possible direct activation of the HSR. Calcium depletion experiments showed that the heat-induced unfolding alone of cytoplasmic luciferase did not suffice to trigger the moss HSR. This argues against unfolded cytoplasmic proteins serving as the primary heat sensors of the cell and further questions the role of molecular chaperones in the regulation of the plant response to mild HS.
We show that the intensity of the HSR does not depend solely on the inducing temperature but also on the initial growth temperature. The intrinsic propensity of proteins to unfold at given temperatures is expected to remain independent from the growth temperature. By contrast, all organisms adapt their membrane fluidity in response to mild changes of ambient temperature by changing lipid saturation and composition (Vígh et al., 1998, 2007). Corroborating earlier observations in Synechocystis and Escherichia coli (Carratu et al., 1996; Horváth et al., 1998), we found that moss tissues acclimated to 30°C had a reduced HSR compared with mosses acclimated to 22°C. Moreover, isothermal treatments with the membrane fluidizer BA evoked an immediate Ca2+ transient, induced a specific HSR, and mediated the onset of acquired thermotolerance.
Early Heat Sensing Depends on a Plasma Membrane–Regulated Ca2+ Influx
Our data showed the central role of a specific plasma membrane–regulated heat-inducible Ca2+ influx for the development of the HSR. Removal of external Ca2+ prevented heat-induced Ca2+ influx, Hsp70 accumulation, and establishment of acquired thermotolerance. We found that micromolar amounts of calcium are sufficient to support an efficient HSR in P. patens (Figure 2). The apoplastic concentration of free calcium in seed plants is between 10 and 800 μM, depending on the estimation method (Sattelmacher, 2001). Thus, it is likely that a similar heat-sensing mechanism operates both in moss and higher plants. Patch clamp analyses confirmed the presence of a nonselective heat-sensitive ion channel mediating a Ca2+ influx in the plasma membrane. In the experimental conditions used, channels were activated seconds after the bath temperature was raised or following exposure to a membrane fluidizer and became spontaneously inactivated 30 to 60 s later. Severe changes in membrane fluidity have been previously found to impact on ion channel activity (Collins et al., 1993; Lee, 2003). Here, the patch clamp recordings show that heat and BA induced a rapid activation of a nonselective Ca2+ permeable channel in the plasma membrane, which were very similar in both conductance and ion selectivity. Noticeably, BA induced a much stronger and sharper Ca2+ influx than a HS at 38°C (see Supplemental Figure 4A online), whereas it induced a weaker HSR (Figure 6A). This could originate from the strong effect of BA on membrane microdomains, causing a partially futile Ca2+ influx.
Delays in the addition of external Ca2+ during HS showed that the amplitude of calcium influx within the first minutes of a temperature increase critically determines the subsequent activation level of the plant HSR. Beyond these first minutes, delayed availability of Ca2+ ions resulted in a decreased Ca2+ influx and a corresponding reduced HSR. These kinetics are slower than those observed in electrophysiology experiments, a discrepancy that can be attributed to the much faster temperature rise in the patch clamp assays and to possible differences in ionic conditions and membrane potential between protoplasts and intact tissues. Electrophysiology and biomonitoring data suggest that the putative heat-activated calcium channels behave similarly to other voltage or ion-gated channels that transiently open after a stimulus and readily become inactivated, although the stimulus persists. This explains the transient nature of the heat-induced calcium signature and justifies the need for a resetting period at noninducing temperature before a second optimal HSR can be reiterated (Figure 1C).
Ca2+-Mediated Heat Signaling Is Highly Specific
Being a ubiquitous second messenger, Ca2+ can attain signaling specificity via several mechanisms, such as amplitude and frequency modulation, and through acting on specific downstream effectors (Sathyanarayanan and Poovaiah, 2004). Although both HS and mannitol treatments induced a transient Ca2+ influx, heat specifically activated HSP but not LEA genes, and osmotic stress specifically activated LEA but not HSP genes (Figure 4K). Moreover, the fact that once all heat-sensitive Ca2+ channels were fully inactivated, a mannitol-induced Ca2+ influx was still fully operative (Figure 4H), suggests the presence of at least two distinct stress-sensitive Ca2+ channels in the same plasma membrane. This was further confirmed using the calcium channel blocker 2-APB, which strongly and specifically inhibited the HSR but not the osmotic shock response (Figure 7E).
The pharmacological release of Ca2+ from endocompartments by ionomycin or thapsigargin has no effect on the HSR, suggesting that the fluctuating concentrations of free Ca2+ in the cytoplasm do not participate per se in the transduction of the HS signaling. Rather, our data suggest that local interactions of the entering Ca2+ ions confer specificity to the heat signal. This may be achieved through structural and functional differences between Ca2+ channels and/or channels interacting with specific, local downstream components.
Protein Unfolding or Not Protein Unfolding?
Interestingly, contrary to the immediate BA effect, radicicol induced a mild Ca2+ transient 10 to 15 min following addition (see Supplemental Figure 5A online). It is tempting to speculate that during this delay, released Hsp90 clients could misfold and initiate wrong hydrophobic interactions with the cytoplasmic side of the plasma membrane, in turn triggering a classic type of Ca2+-dependent HS signal. This would explain the strong synergism that we found between mild HS and radicicol (Figure 7C). Hence, a minor component of protein unfolding could, after all, be involved in the coactivation of the plant HSR; however, this would be via membrane perturbation, rather than cytoplasmic chaperone sequestration.
Further Components of the Plant Heat Signaling Cascade
In Arabidopsis, maize, and wheat (Triticum aestivum), Ca2+ and calmodulin proteins were shown to be involved in the HSR (Li et al., 2004; Liu et al., 2005; Zhang et al., 2009). A calmodulin binding kinase was also reported to be active in heat shock signal transduction and in the onset of plant-acquired thermotolerance (Liu et al., 2008). In tobacco (Nicotiana tabacum) and Alfalfa cells, the heat-induced phosphorylation of a specific mitogen-activated protein kinase has been observed within 2 to 10 min of a temperature increase (Sangwan et al., 2002; Suri and Dhindsa, 2008). This time scale agrees with our data on heat-induced Ca2+ influx (Figure 4) and precedes the mRNA synthesis for the major HSPs (Vierling, 1991; Lohmann et al., 2004). Here, we found that a kinase inhibitor, dicoumarol, strongly inhibits the moss HSR (Figure 7D), suggesting that Ca2+-dependent activation of a mitogen-activated protein kinase is a downstream component of the heat signaling pathway, likely involved in the activation of Hsfs.
In animal cells, different environmental cues promote receptor-operated calcium entry and store-operated calcium entry, which are intertwined by the activities of transient receptor potential ion channels (TRPs) and membrane channels in the vicinity of Ca2+ stores (Liao et al., 2008). The rat TRP vanilloid-1 (TRPV1) receptor in the plasma membrane of neuron sensory cells mediates heat and pain sensations upon the transient entry of Ca2+ ions into the cytoplasm (Caterina et al., 1997). Noticeably, TRPs contain a cytoplasmic C-terminal Ca2+ calmodulin binding domain, which can provide signal specificity (Zhu, 2005). There are 19 TRP orthologs in the green alga Chlamydomonas reinhardtii (Wheeler and Brownlee, 2008), but no obvious orthologs are found in land plants, suggesting that TRP genes have been lost in land plants during evolution. However, the Arabidopsis genome contains 20 genes encoding putative ionotropic glutamate receptors and 20 others coding for putative cyclic nucleotide-gated channels, with cytoplasmic C-terminal domains for Ca2+ calmodulin and cyclic nucleotide binding (Kaplan et al., 2007; Wheeler and Brownlee, 2008). A search in the recently released P. patens genome revealed the existence of only two glutamate receptors and eight cyclic nucleotide-gated channels, suggesting a relatively simpler stress response regulation in this ancient land plant. The inhibition of the plant HSR by 2-APB and FFA, known to inhibit store-operated calcium entry (Sandoval et al., 2007), and temperature-gated TRP channels in animals (Venkatachalam and Montell, 2007), strongly suggests that the P. patens HSR is regulated by similar plasma membrane Ca2+ channels, serving as primary heat sensors. In response to temperature elevation, they trigger a specific Ca2+, calmodulin, and kinase-dependent signaling cascade, leading to an effective HSR and to the establishment of acquired thermotolerance.
METHODS
Plasmid Constructs
The EcoRI fragment bearing the 35S:nptII:CaMVter cassette, conferring resistance to G418, was excised from pBSMDII and placed in the EcoRI cut pBS-108 (Finka et al., 2008) to produce the pBS108-35SNPT-b vector. The primers (Aeq1F, 5′-CGTAACTAGTATGGTCAAGCTTACATCAGACTTCG-3′) and (Aeq2R, 5′-CGATACTAGTGAATTCATCAGTGTTTTATT-3′) were used to amplify apoaequorin cDNA from mtAEQ-pMT2 plasmid (Rizzuto et al., 1992) (kindly provided by P. Maechler, University of Geneva). The generated fragment was digested by SpeI and cloned in the corresponding site of pUBI-NOS plasmid (Finka et al., 2008). The Asp718/SmaI-digested UBI-AEQ-NOS cassette was then blunt cloned in NotI-digested pBS108-35SNPT-b to result in the pBS108-II-UBI-AEQ integrative overexpressing vector.
Plant Material and Growth Conditions
Physcomitrella patens (Gransden wild type) was grown on moss solid minimal medium overlaid with a cellophane disk as by Saidi et al. (2005) and transferred when stated to liquid minimal medium. HSP-GUS, 35S-LUC, and LEA-GUS lines were previously described by Saidi et al. (2005, 2007) and Kamisugi and Cuming (2005), respectively. To generate stable transgenic UBI-AEQ lines, a polyethylene glycol–mediated transformation of moss protoplasts was performed using pBS108-II-UBI-AEQ followed by antibiotic selection as by Saidi et al. (2005). Five independent lines were isolated and tested for the constitutive expression of the aequorin reporter. Lines A1 and A2, showing no morphological or developmental alterations (see Supplemental Figure 7A online), were used to measure the cytosolic calcium concentrations after different treatments. These two lines displayed no differences in the response to heat shock (see Supplemental Figure 7B online). The UBI-AEQ A1 line was chosen for all experiments. DNA gel blot analyses (see Supplemental Figure 8 online) confirmed that the UBI-AEQ cassette was successfully targeted to the 108 genomic locus in A1.
Heat and Chemical Treatments
Unless otherwise stated, 1-week-old HSP-GUS or 35S-LUC and 2-week-old LEA-GUS protonemal tissues were transferred to liquid minimal medium or sterile distilled water in Costar multiwell plates (Corning) and heat or chemically treated in a temperature-controlled chamber at indicated temperatures and durations. In the experiments requiring extracellular calcium depletion, protonemal tissues of all lines were washed three times with distilled water and incubated with indicated concentrations of EGTA or BAPTA for 30 min before heat or chemical treatment. The resulting free calcium concentrations in the medium were calculated using the program CALCIUM V2.1 (K.J. Foehr and W. Warchol, unpublished data).
BA, BAPTA, coelenterazine hcp, EGTA, 4-methylumbelliferone glucoronide, and PCP were purchased from Sigma-Aldrich, 2-APB, FFA, and dicomarol were obtained from the MicroSource Discovery Systems (spectrum collection). Thapsigargin, radicicol, and ionomycin were purchased from Enzo Life Sciences.
In Vivo Reconstitution of Aequorin and Ca2+-Dependent Luminescence Measurements
To monitor calcium transients, reconstitution of aequorin was performed in vivo by incubating 1-week-old UBI-AEQ protonemal tissues, in darkness, for 2 h in standard liquid medium (or distilled water) supplemented with 3 μM coelenterazine followed by washing briefly two times. Heat and chemical treatments of 1-week-old UBI-AEQ tissues were performed in liquid minimal medium or distilled water using SCREENMATES 96 well microtiter plates (Thermo Fisher Scientific), directly in the 1420 VICTOR light luminometer (Perkin-Elmer). The temperature in the medium was carefully and continuously recorded using YF-160 type-K thermocouple (Eppendorf). Luminescence counts (CPS) were integrated every 3s (in case of heat treatment) or 1s (in case of chemical treatment), and at the end of each monitoring, a discharging solution (0.1 M CaCl2, 10% ethanol, and 2% Nonidet P40) was added to measure remaining active aequorin in the sample. The concentration of cytosolic calcium was determined by calculating the pCa using the equation described by Plieth (2006). In the case of Figure 4, data (CPS) were normalized with the total activity of aequorin, maximum luminescence (Max), generated after addition of the discharging solution. All displayed traces are representative of at least three replicates.
GUS and Luciferase Assays
GUS-specific activities were determined using 2 mM of the 4-methylumbelliferone glucoronide substrate, as detailed previously (Saidi et al., 2005). GUS activity was expressed in nmol of hydrolyzed MU produced per minute per milligram of total soluble proteins. Unless otherwise stated, GUS activities were measured 8 h after the heat or chemical treatment. For luciferase activity measurements, 35S-LUC tissues were pretreated with or without EGTA for 1 h at 22°C prior to heat inactivation. Thermal inactivation of luciferase was performed at 40°C for 1 h using a temperature-controlled thermoblock. Then, tissues were transferred to 22°C for an additional 4 h. Aliquots were taken every 20 min during heat inactivation and every 60 min during recovery. These aliquots were immediately frozen in liquid nitrogen. Total soluble proteins were then extracted at 4°C using CCLR extraction buffer (Promega), and 5 μL of each extract were loaded in a SCREENMATES 96-well microtiter plate. Luciferase assays were performed in a Perkin-Elmer 1420 VICTOR light microtiter plate luminescence counter, after injection of luciferin substrate according to the manufacturer's instructions (Promega). Luciferase activities were expressed as counts per second per mg protein and were converted as percentage of the activity before inactivation at 40°C. GUS or luciferase activity values are means of at least three independent experiments and standard deviations are shown. Soluble protein concentrations in extracts were determined by the Bradford method (Bio-Rad) according to the manufacturer's instructions.
Protein Gel Blots
Protein gel blot analyses were performed as by Finka et al. (2007) with small modifications: the nitrocellulose membranes (Bio-Rad) containing the transferred proteins were incubated with a rabbit-derived polyclonal antibody against Hsp70 (Stressgen) (1/35,000, v/v) or a mouse-derived monoclonal anti-actin antibody C4 (1/1000; ICN Biomedicals). Blots were then incubated with horseradish peroxidase–conjugated anti-rabbit or anti-mouse IgG (Promega) diluted 1/15,000 (v/v). Immune complexes were visualized using the chemiluminescent Immunstar kit (Bio-Rad) according to the manufacturer's instructions. To confirm equal loading of proteins, the nitrocellulose membranes were stained with Ponceau S solution (Sigma-Aldrich) for 5 min before the incubation with antibodies.
Electrophysiological Experimentation
P. patens protoplasts were isolated as described (Johannes et al., 1997). In all patch clamp experiments, the external solution contained 20 mM KCl, 10 mM CaCl2, and 5 mM MES/Tris, pH 6, and the cytoplasmic solution contained 100 mM KCl, 0.1 mM CaCl2, and 5 mM MES/Tris, pH 7.5. Both solutions were adjusted with sorbitol to an osmotic pressure of 430 mosM. Data acquisition occurred through a CED A/D converter at 3 kHz, and data were filtered at 0.5 kHz using a 6-pole Bessel filter. Bath temperature was controlled by perfusion of bath medium with different temperatures and continuously recorded using an LN stage temperature controller.
Acquired Thermotolerance Measurement
To estimate the moss thermotolerance, chlorophyll a fluorescence measurements were made following the indicated treatments using the Plant Efficiency Analyzer (Hansatech Instruments) as described by Saidi et al. (2005). The values of Fv/Fm ratio represent the maximal photochemical efficiency of photosystem II. All Fv/Fm values are means of three independent experiments, and standard deviations are shown.
Accession Numbers
Sequence data from this article can be found in GenBank/EMBL data libraries under accession numbers GQ250943 (Pp108 sequence), L29571 (Aequorin), and GQ463722 (pBS108-35SNPT-b). Moss plants described in this article can be retrieved from the International Moss Stock Center (http://www.moss-stock-center.org/) using accession numbers 40466 (UBI-AEQ) and 40467 (HSP-GUS).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. The Intensity of the Temperature-Induced Ca2+ Influx Correlates with Subsequent Levels of HSR.
Supplemental Figure 2. Effect of Increasing Concentrations of Ca2+, or Other Ions, on Moss HSR.
Supplemental Figure 3. Thapsigargin Concentrations Below 50 μM Could Not Release Ca2+ from Intracellular Stores.
Supplemental Figure 4. BA Pretreatment Enhances Thermotolerance in P. patens.
Supplemental Figure 5. Radicicol Induces a Transient Elevation of [Ca2+]cyt.
Supplemental Figure 6. Effect of HSR Inhibitors on Plant-Acquired Thermotolerance.
Supplemental Figure 7. Comparison of Two Independent UBI-AEQ Lines.
Supplemental Figure 8. Molecular Characterization of UBI-AEQ Transgenic Line.
Supplemental Table 1. Calcium Blockers, Inhibitors of Kinases, or Phospholipase C Tested in the P. patens HSP-GUS Line for an Inhibitory Effect on the HSR.
Supplemental Table 2. Primers Used to Synthesize the Probes for the Molecular Analysis of the UBI-AEQ Transgenic Line.
Supplemental Methods. DNA Gel Blot Analysis of the UBI-AEQ Line.
Supplementary Material
Acknowledgments
We thank A. Cuming (University of Leeds) for kindly providing the LEA-GUS line and P. Maechler (University of Geneva) for the aequorin cDNA. We also thank P. De Los Rios (Ecole Polytechnique Fédérale de Lausanne) for the valuable discussions and C. Cicekli and P. Koenig (University of Lausanne) for technical assistance. This research was financed in part by grants from the State Secretariat for Education and Research of Switzerland, from Cost Action 859, and from the Alzheimer's Drug Discovery Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Pierre Goloubinoff (pierre.goloubinoff@unil.ch).
Some figures in this article are displayed in color online but in black and white in the print edition.
Online version contains Web-only data.
References
- Abravaya, K., Myers, M.P., Murphy, S.P., and Morimoto, R.I. (1992). The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 6 1153–1164. [DOI] [PubMed] [Google Scholar]
- Ananthan, J., Goldberg, A.L., and Voellmy, R. (1986). Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science 232 522–524. [DOI] [PubMed] [Google Scholar]
- Aviezer-Hagai, K., Skovorodnikova, J., Galigniana, M., Farchi-Pisanty, O., Maayan, E., Bocovza, S., Efrat, Y., von Koskull-Doring, P., Ohad, N., and Breiman, A. (2007). Arabidopsis immunophilins ROF1 (AtFKBP62) and ROF2 (AtFKBP65) exhibit tissue specificity, are heat-stress induced, and bind HSP90. Plant Mol. Biol. 63 237–255. [DOI] [PubMed] [Google Scholar]
- Baniwal, S.K., et al. (2004). Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 29 471–487. [DOI] [PubMed] [Google Scholar]
- Carratu, L., Franceschelli, S., Pardini, C.L., Kobayashi, G.S., Horvath, I., Vigh, L., and Maresca, B. (1996). Membrane lipid perturbation modifies the set point of the temperature of heat shock response in yeast. Proc. Natl. Acad. Sci. USA 93 3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M.J., Schumacher, M.A., Tominaga, M., Rosen, T.A., Levine, J.D., and Julius, D. (1997). The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389 816–824. [DOI] [PubMed] [Google Scholar]
- Christensen, A.H., and Quail, P.H. (1996). Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 5 213–218. [DOI] [PubMed] [Google Scholar]
- Collins, A.C., Wehner, J.M., and Wilson, W.R. (1993). Animal models of alcoholism: Genetic strategies and neurochemical mechanisms. Biochem. Soc. Symp. 59 173–191. [PubMed] [Google Scholar]
- Cuming, A.C., Cho, S.H., Kamisugi, Y., Graham, H., and Quatrano, R.S. (2007). Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol. 176 275–287. [DOI] [PubMed] [Google Scholar]
- de Marco, A., Vigh, L., Diamant, S., and Goloubinoff, P. (2005). Native folding of aggregation-prone recombinant proteins in Escherichia coli by osmolytes, plasmid- or benzyl alcohol-overexpressed molecular chaperones. Cell Stress Chaperones 10 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik, V., and Maathuis, F.J.M. (2007). Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 175 387–404. [DOI] [PubMed] [Google Scholar]
- Finka, A., Saidi, Y., Goloubinoff, P., Neuhaus, J.M., Zryd, J.P., and Schaefer, D.G. (2008). The knock-out of ARP3a gene affects F-actin cytoskeleton organization altering cellular tip growth, morphology and development in moss Physcomitrella patens. Cell Motil. Cytoskeleton 65 769–784. [DOI] [PubMed] [Google Scholar]
- Finka, A., Schaefer, D.G., Saidi, Y., Goloubinoff, P., and Zryd, J.P. (2007). In vivo visualization of F-actin structures during the development of the moss Physcomitrella patens. New Phytol. 174 63–76. [DOI] [PubMed] [Google Scholar]
- Forreiter, C., Kirschner, M., and Nover, L. (1997). Stable transformation of an Arabidopsis cell suspension culture with firefly luciferase providing a cellular system for analysis of chaperone activity in vivo. Plant Cell 9 2171–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, W., Ratnadewi, D., and Reski, R. (2005). Physcomitrella patens is highly tolerant against drought, salt and osmotic stress. Planta 220 384–394. [DOI] [PubMed] [Google Scholar]
- Gong, M., van der Luit, A.H., Knight, M.R., and Trewavas, A.J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116 429–437. [Google Scholar]
- Hille, B. (2001). Ion Channels of Excitable Membranes. (Sunderland, MA: Sinauer Associates).
- Hinault, M.P., Ben-Zvi, A., and Goloubinoff, P. (2006). Chaperones and proteases: Cellular fold-controlling factors of proteins in neurodegenerative diseases and aging. J. Mol. Neurosci. 30 249–265. [DOI] [PubMed] [Google Scholar]
- Horváth, I., Glatz, A., Varvasovszki, V., Török, Z., Pali, T., Balogh, G., Kovacs, E., Nadasdi, L., Benko, S., Joo, F., and Vígh, L. (1998). Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: Identification of hsp17 as a “fluidity gene”. Proc. Natl. Acad. Sci. USA 95 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes, E., Ermolayeva, E., and Sanders, D. (1997). Red light-induced membrane potential transients in the moss Physcomitrella patens: Ion channel interaction in phytochrome signalling. J. Exp. Bot. 48 599–608. [DOI] [PubMed] [Google Scholar]
- Kamisugi, Y., and Cuming, A.C. (2005). The evolution of the abscisic acid-response in land plants: Comparative analysis of group 1 LEA gene expression in moss and cereals. Plant Mol. Biol. 59 723–737. [DOI] [PubMed] [Google Scholar]
- Kaplan, B., Sherman, T., and Fromm, H. (2007). Cyclic nucleotide-gated channels in plants. FEBS Lett. 581 2237–2246. [DOI] [PubMed] [Google Scholar]
- Kim, B.H., and Schöffl, F. (2002). Interaction between Arabidopsis heat shock transcription factor 1 and 70 kDa heat shock proteins. J. Exp. Bot. 53 371–375. [DOI] [PubMed] [Google Scholar]
- Larkindale, J., and Vierling, E. (2008). Core genome responses involved in acclimation to high temperature. Plant Physiol. 146 748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, A.G. (2003). Lipid-protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta 1612 1–40. [DOI] [PubMed] [Google Scholar]
- Lee, J.H., and Schöffl, F. (1996). An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF, and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol. Gen. Genet. 252 11–19. [DOI] [PubMed] [Google Scholar]
- Li, B., Liu, H.T., Sun, D.Y., and Zhou, R.G. (2004). Ca2+ and calmodulin modulate DNA-binding activity of maize heat shock transcription factor in vitro. Plant Cell Physiol. 45 627–634. [DOI] [PubMed] [Google Scholar]
- Liao, Y., Erxleben, C., Abramowitz, J., Flockerzi, V., Zhu, M.X., Armstrong, D.L., and Birnbaumer, L. (2008). Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci. USA 105 2895–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H.T., Gao, F., Li, G.L., Han, J.L., Liu, D.L., Sun, D.Y., and Zhou, R.G. (2008). The calmodulin-binding protein kinase 3 is part of heat-shock signal transduction in Arabidopsis thaliana. Plant J. 55 760–773. [DOI] [PubMed] [Google Scholar]
- Liu, H.T., Un, D.Y., and Zhou, R.G. (2005). Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant Cell Environ. 28 1276–1284. [Google Scholar]
- Lohmann, C., Eggers-Schumacher, G., Wunderlich, M., and Schöffl, F. (2004). Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol. Genet. Genomics 271 11–21. [DOI] [PubMed] [Google Scholar]
- Marom, M., Sebag, A., and Atlas, D. (2007). Cations residing at the selectivity filter of the voltage-gated Ca2+-channel modify fusion-pore kinetics. Channels 1 377–386. [DOI] [PubMed] [Google Scholar]
- Mittler, R. (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11 15–19. [DOI] [PubMed] [Google Scholar]
- Mogk, A., Deuerling, E., Vorderwulbecke, S., Vierling, E., and Bukau, B. (2003). Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol. Microbiol. 50 585–595. [DOI] [PubMed] [Google Scholar]
- Morgan, A.J., and Jacob, R. (1994). Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem. J. 300 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, R.I. (1998). Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12 3788–3796. [DOI] [PubMed] [Google Scholar]
- Mosser, D.D., Duchaine, J., and Massie, B. (1993). The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol. Cell. Biol. 13 5427–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto, H., and Vigh, L. (2007). The small heat shock proteins and their clients. Cell. Mol. Life Sci. 64 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth, C. (2006). Aequorin as a reporter gene. Methods in Molecular Biology. Arabidopsis Protocols, 2nd ed, J. Salinas and J.J. Sanchez-Serrano (Totowa, NJ: Humana Press), pp. 307–328. [DOI] [PubMed]
- Qudeimat, E., Faltusz, A.M.C., Wheeler, G., Lang, D., Brownlee, C., Reski, R., and Frank, W. (2008). A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc. Natl. Acad. Sci. USA 105 19555–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing, S.A., et al. (2008). The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319 64–69. [DOI] [PubMed] [Google Scholar]
- Rizzuto, R., Simpson, A.W., Brini, M., and Pozzan, T. (1992). Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358 325–327. [DOI] [PubMed] [Google Scholar]
- Saidi, Y., Domini, D., Choy, F., Zryd, J.P., Schwitzguebel, J.P., and Goloubinoff, P. (2007). Activation of the heat shock response in plants by chlorophenols: Transgenic Physcomitrella patens as a sensitive biosensor for organic pollutants. Plant Cell Environ. 30 753–763. [DOI] [PubMed] [Google Scholar]
- Saidi, Y., Finka, A., Chakhporanian, M., Zryd, J.P., Schaefer, D.G., and Goloubinoff, P. (2005). Controlled expression of recombinant proteins in Physcomitrella patens by a conditional heat-shock promoter: A tool for plant research and biotechnology. Plant Mol. Biol. 59 697–711. [DOI] [PubMed] [Google Scholar]
- Sandoval, A.J., Riquelme, J.P., Carretta, M.D., Hancke, J.L., Hidalgo, M.A., and Burgos, R.A. (2007). Store-operated calcium entry mediates intracellular alkalinization, ERK1/2, and Akt/PKB phosphorylation in bovine neutrophils. J. Leukoc. Biol. 82 1266–1277. [DOI] [PubMed] [Google Scholar]
- Sangwan, V., Orvar, B.L., Beyerly, J., Hirt, H., and Dhindsa, R.S. (2002). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 31 629–638. [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan, P.V., and Poovaiah, B.W. (2004). Decoding Ca2+ signals in plants. CRC Crit Rev Plant Sci 23 1–11. [DOI] [PubMed] [Google Scholar]
- Sattelmacher, B. (2001). Tansley review no. 22 - The apoplast and its significance for plant mineral nutrition. New Phytol. 149 167–192. [DOI] [PubMed] [Google Scholar]
- Schöffl, F., Prandl, R., and Reindl, A. (1998). Regulation of the heat-shock response. Plant Physiol. 117 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., Mosser, D.D., and Morimoto, R.I. (1998). Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri, S.S., and Dhindsa, R.S. (2008). A heat-activated MAP kinase (HAMK) as a mediator of heat shock response in tobacco cells. Plant Cell Environ. 31 218–226. [DOI] [PubMed] [Google Scholar]
- Török, Z., Goloubinoff, P., Horvath, I., Tsvetkova, N.M., Glatz, A., Balogh, G., Varvasovszki, V., Los, D.A., Vierling, E., Crowe, J.H., and Vígh, L. (2001). Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. USA 98 3098–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova, N.M., Horvath, I., Török, Z., Wolkers, W.F., Balogi, Z., Shigapova, N., Crowe, L.M., Tablin, F., Vierling, E., Crowe, J.H., and Vígh, L. (2002). Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. USA 99 13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinger, L., Diamant, S., Buchner, J., and Goloubinoff, P. (1998). The small heat-shock protein IbpB from Escherichia coli stabilizes stress-denatured proteins for subsequent refolding by a multichaperone network. J. Biol. Chem. 273 11032–11037. [DOI] [PubMed] [Google Scholar]
- Venkatachalam, K., and Montell, C. (2007). TRP channels. Annu. Rev. Biochem. 76 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling, E. (1991). The role of heat shock proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42 579–620. [Google Scholar]
- Vígh, L., Horvath, I., Maresca, B., and Harwood, J.L. (2007). Can the stress protein response be controlled by 'membrane-lipid therapy'? Trends Biochem. Sci. 32 357–363. [DOI] [PubMed] [Google Scholar]
- Vígh, L., Maresca, B., and Harwood, J.L. (1998). Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 23 369–374. [DOI] [PubMed] [Google Scholar]
- Voellmy, R., and Boellmann, F. (2007). Chaperone regulation of the heat shock protein response. Adv. Exp. Med. Biol. 594 89–99. [DOI] [PubMed] [Google Scholar]
- Volkov, R.A., Panchuk, I.I., Mullineaux, P.M., and Schöffl, F. (2006). Heat stress-induced H(2)O(2) is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 61 733–746. [DOI] [PubMed] [Google Scholar]
- Wang, Q., Downey, G.P., Bajenova, E., Abreu, M., Kapus, A., and McCulloch, C.A. (2005). Mitochondrial function is a critical determinant of IL-1-induced ERK activation. FASEB J. 19 837–839. [DOI] [PubMed] [Google Scholar]
- Wang, W., Vinocur, B., Shoseyov, O., and Altman, A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9 244–252. [DOI] [PubMed] [Google Scholar]
- Wheeler, G.L., and Brownlee, C. (2008). Ca2+ signalling in plants and green algae - Changing channels. Trends Plant Sci. 13 506–514. [DOI] [PubMed] [Google Scholar]
- Yamada, K., Fukao, Y., Hayashi, M., Fukazawa, M., Suzuki, I., and Nishimura, M. (2007). Cytosolic HSP90 regulates the heat shock response that is responsible for heat acclimation in Arabidopsis thaliana. J. Biol. Chem. 282 37794–37804. [DOI] [PubMed] [Google Scholar]
- Yasumura, Y., Crumpton-Taylor, M., Fuentes, S., and Harberd, N.P. (2007). Step-by-step acquisition of the gibberellin-DELLA growth-regulatory mechanism during land-plant evolution. Curr. Biol. 17 1225–1230. [DOI] [PubMed] [Google Scholar]
- Yi, Z., and Hong-Bo, S. (2008). The responding relationship between plants and environment is the essential principle for agricultural sustainable development on the globe. C. R. Biol. 331 321–328. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., and Plant, S. (1992). Mechanism of release of Ca2+ from intracellular stores in response to ionomycin in oocytes of the frog Xenopus laevis. J. Physiol. 458 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., Zhou, R.-G., Gao, Y.-J., Zheng, S.-Z., Xu, P., Zhang, S.-Q., and Sun, D.-Y. (2009). Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 149 1773–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M.X. (2005). Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 451 105–115. [DOI] [PubMed] [Google Scholar]
- Zou, J., Guo, Y., Guettouche, T., Smith, D.F., and Voellmy, R. (1998). Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94 471–480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.