Abstract
Study Objectives:
Sleep is crucial to memory consolidation in humans and other animals; however, the effect of insufficient sleep on subsequent learning and memory remains largely elusive.
Design:
Learning and memory after 1-day sleep deprivation (slpD) was evaluated using Pavlovian olfactory conditioning in Drosophila, and locomotor activity was measured using the Drosophila Activity Monitoring System in a 12:12 light-dark cycle.
Results:
We found that slpD specifically impaired 1-h memory in wild type Canton-S flies, and this effect could persist for at least 2 h. However, alternative stresses (heat stress, oxidative stress, starvation, and rotation stress) did not result in a similar effect and left the flies’ memory intact. Mechanistic studies demonstrated that flies with either silenced transmission of the mushroom body (MB) during slpD or down-regulated cAMP levels in the MB demonstrated no slpD-induced 1-h memory impairment.
Conclusion:
We found that slpD specifically impaired 1-h memory in Drosophila, and either silencing of MB transmission during slpD or down-regulation of the cAMP level in the MB protected the flies from slpD-induced impairment.
Citation:
Li X; Yu F; Guo A. Sleep deprivation specifically impairs short-term olfactory memory in drosophila. SLEEP 2009;32(11):1417-1424.
Keywords: Drosophila, sleep, learning and memory, cAMP, slpD, mushroom body
SLEEP PLAYS A KEY ROLE IN LEARNING AND MEMORY.1,2 A LARGE BODY OF MOLECULAR, CELLULAR, SYSTEMIC, AND BEHAVIORAL EVIDENCE HAS demonstrated the important role of sleep in memory consolidation, but the issue remains controversial.3–9 However, few studies have touched on the equally critical issue of whether sleep is also essential for subsequent learning and memory.
In humans and other animals, sleep deprivation (slpD) causes a significant deficit in hippocampal plasticity10–13 during episodic memory encoding, and results in impaired subsequent retention.13 In Drosophila, sleep loss caused learning impairment14 and short sleep mutants showed memory impairment.15 Moreover, waking experience affected sleep needs in Drosophila.16 All these studies demonstrated that sufficient sleep is important in learning and memory. Genetic studies of olfactory memory formation in Drosophila have identified several genes that function at distinct temporal phases of memory17 including rutabaga and dunce in short-term memory (STM); amnesiac in middle-term memory (MTM), which can be erased by cold shock; and radish in anesthesia-resistant memory (ARM), which relates to long-term memory.17 However, the types of memory impaired by slpD are unknown.
As in mammals, rest in Drosophila can be characterized by a long period of immobility and an increased arousal threshold at a particular time during the circadian day.18,19 Young flies sleep extensively, but the amount of sleep decreases in older flies, and is modulated by stimulants and hypnotics.19 It has been previously reported that the brain activity of Drosophila is reliably correlated with activity state, and local field potential fluctuations can be reliably recorded from the brains of awake, moving fruit flies.20 Moreover, sleep homeostasis is regulated by cAMP response element-binding protein,21 heat-shock genes,22 the amnesiac gene,23 the dopamine level in the brain,24 the GABAA receptor,25 serotonin receptor 1A26 and the MB.27,28 Cirelli et al. reported that sleep was reduced in Drosophila shaker mutants.29 In sum, Drosophila is an ideal model system to study the relationship between sleep and memory.
Here, we demonstrated that 1-day slpD, but not stress, impairs 1-h memory in Drosophila, and this effect can persist for at least 2 h in the Pavlovian olfactory conditioning paradigm. Our results also illustrated that alteration in the MB state during slpD is responsible for the 1-h memory impairment resulting from slpD, suggesting that the MB could be a key area where cross-talk between sleep and memory occurs.
EXPERIMENTAL PROCEDURES
Fly Stocks and Rearing Conditions
Flies were cultured on standard medium as described previously (Wurzburg recipe)30 at 25°C and 60% relative humidity with a 12 h light/dark cycle. Approximately 500 flies were reared in one food vial and were transferred to fresh food vials every day after eclosion. The siblings were divided into subgroups of 100 flies on the third day. Some of the subgroups were designated as control groups and others, housed in a similar social environment but deprived of sleep for 1 day starting on the second day, were designated as the slpD groups (Figure 2A). Rut2080, Rut2769, dnc1, and UAS-rut were kindly provided by Prof. R. Davis. UAS-rutRNAi came from the Vienna Drosophila RNAi Center (stock number: 5569). The Canton-S (CS) strain was used for the wild type flies (Figures 1–3).
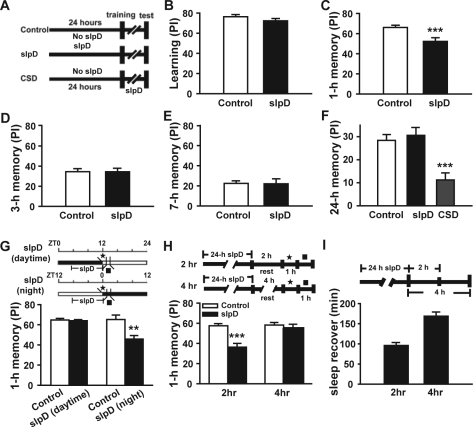
Figure 2.
1-day slpD specifically impairs STM memory in Drosophila. Canton-S flies were used. (A) Schematic representation of the experimental protocol for different groups. The control group was not subjected to slpD before or after training. The slpD group underwent 24-h slpD before training, but no slpD after training. The CSD group was not subjected to slpD before training, but was subjected to slpD after training and before testing. (B) The performance index (PI) of the flies that were or were not subjected to 1-day slpD (n = 22, P > 0.05). (C)- (E) The 1-, 3-, and 7-h memory of replicate control and slpD groups (n = 8–15). slpD specifically impairs 1-h memory in Drosophila (P < 0.001). In contrast, no significant changes in 3- or 7-h memory were observed in slpD groups compared with controls (P > 0.05). (F) LTM was measured using space training in replicate control, slpD and CSD groups (n = 8–10). (G) Schematic representation of the experimental protocol for 1-h memory of daytime and nighttime slpD (top). The flies underwent slpD from zeitgeber time (ZT) 16 to ZT0 for the nighttime slpD group and from ZT4 to ZT12 for the daytime slpD group. For investigation of the contribution of nighttime sleep, training started at ZT0 and testing started at ZT1; for investigation of the contribution of daytime sleep, training started at ZT12, and testing started at ZT13. The controls without slpD were trained and tested at same time to slpD groups to exclude circadian effects. The flies of the nighttime slpD group showed obvious 1-h memory impairment (P < 0.01) but the flies of the daytime slpD group did not (n = 10–12) (bottom). (H) We trained the flies at 2 and 4 h following 1-day slpD and then tested memory 1 h later (top). The impairment of 1-h memory remained even at 2 h (n ≥ 8, P < 0.001), but could not be detected at 4 h after the end of slpD (n ≥ 8, P > 0.05) (bottom). (I) Sleep restore during the 2-h and 4-h interval following the end of 1-d slpD (n = 28). □, training period; ▪, testing period.
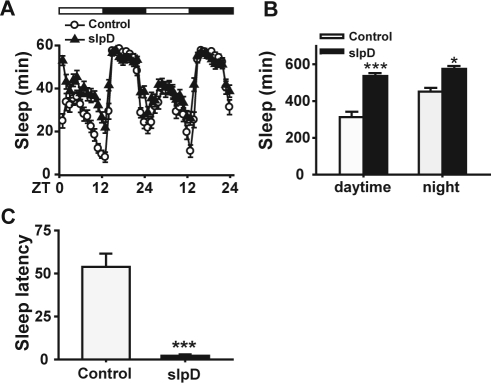
Figure 1.
Sleep during daytime increased significantly after 1-day slpD in Drosophila. Canton-S flies were used. (A) The daily sleep curve of the flies after 1-day automated slpD stimulus (n = 28). The sum of the periods of sleep for every hour is plotted on the Y-axis. The light-dark cycle is plotted on the X-axis. (B) Quantification of sleep on the following day for flies that were or were not subjected to 1-day slpD. (C) The control flies took over 50 min to fall asleep for the first time, but flies in the slpD group required only a few minutes after 1-day slpD.
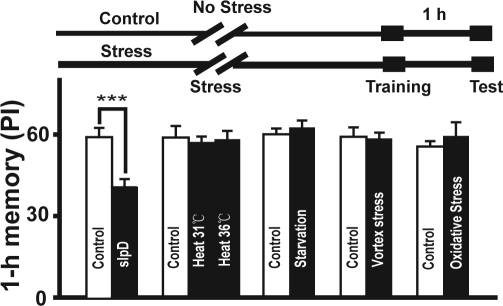
Figure 3.
Stress did not affect 1-h memory. Canton-S flies were used. The effect of heat, starvation, rotation, and oxidative stress on 1-h memory in Drosophila was tested (n ≥ 8). The results indicated that these stresses did not affect 1-h memory (P < 0.05). Heat tests were performed at 31°C for 24 h or at 36°C for 2 h. For starvation, the flies were maintained in 1% agar for 24 h. Oxidative stress was produced by maintaining the flies in vials containing 20 mM paraquat dissolved in 1% agar and 5% sucrose for 18 h. Under the rotation stress conditions, the flies received the rotation stimulus for 45 min, and then were given 45 min to recover, and this procedure was repeated for 24 h.
Automated Sleep Deprivation Paradigm
We constructed a novel slpD apparatus that could prevent a group of flies from sleeping. Vials (1.8 cm in radius and 10 cm in height) each containing approximately 100 flies were rotated at a speed of 5 to 6 rpm along their major axis in a motor-controlled apparatus for 1 min and then were given 1 min to recover (one circle), this procedure was repeated. The direction of rotation (clockwise or counter-clockwise) of the vials was randomly selected at the start point of each circle. When vertical position is reached during every rotation, the vials dropped a distance of 3 cm,18,29 and most flies (∼80%) fell to the bottom of the vials. The flies were examined every 3 h to determine their sleep states, and the results demonstrated that the rotation and 3-cm drop of the vials was effective in stopping the Drosophila from sleeping. However, the exact extent of sleep loss could not be determined. Except where otherwise indicated, the duration of slpD was 24 h. In the experiment of 8-9 h slpD, flies were kept awake from zeitgeber time 16 (ZT16) to ZT0 for nighttime slpD groups, and from ZT4 to ZT12 for daytime slpD groups (Figure 2G, top). To investigate the contribution of nighttime slpD, training started at ZT0, and testing started at ZT1 for nighttime slpD groups and their controls; to investigate the contribution of daytime slpD, training started at ZT12, and testing at ZT 13 for the daytime slpD groups and controls. The two controls were trained and tested at same time as the slpD groups to exclude circadian effects.
Sleep Test
Due to anti-geotaxis (negative gravitaxis),31 the flies crawled up and entered the individual glass tubes one by one. Then, individual 3-4 days old female flies in 65 mm-long glass tubes were loaded into sleep monitors and locomotor activities were monitored with the Drosophila Activity Monitor System (DAMS; Trikinetics, Waltham, MA) under 12 h light/dark cycling conditions at 25°C. Locomotor activities were acquired in 1-min bins and analyzed with MATLAB-based software (Actimetrics, Evanston, IL). Sleep was defined as a period of ≥ 5 min of behavioral immobility26 (0 counts/min).
In the experiment of measuring sleep quality after the group was subjected to mechanically induced slpD, the flies crawled up the individual glass tubes one by one from ZT23:40 to ZT24 and were loaded into sleep monitors immediately. All flies were subjected to moderate shaking several times before the sleep recording began. Persistent deprivation was achieved by shaking the vials manually during the transfer procedure. Another group of flies that did not undergo 1-day slpD were loaded into sleep monitors at the same time as a control.
Stress Test
All stress tests were conducted on 3-day-old flies. For the heat stress test, the flies crawled very slowly and some died after exposure to a temperature of 36°C for 4 h, making memory testing difficult. Therefore, the heat stress test was performed at 31°C for 24 h or at 36°C for 2 h with normal feeding.22 For the starvation stress test, flies were maintained in 1% plain agar for 24 h. Oxidative stress was evaluated in flies that had been maintained in vials with 1% agar containing 20 mM dissolved paraquat and 5% sucrose at 25°C for 18 h. Under the rotation stress conditions, the flies received the rotation stimulus for 45 min, and then were given 45 min to recover accompanied with 3-cm drop when the vials rotated to the vertical direction, and this procedure was repeated for 24 h.
Pavlovian Olfactory Conditioning
All behavioral assays were carried out in a conditioned environmental room in which flies ∼3-4 days old underwent olfactory conditioning at 25 ± 0.5°C and 70% relative humidity under red light. Standard single-cycle training was performed as documented previously.32 Briefly, approximately 100 flies were exposed sequentially to 2 aversive odors (3-octanol, OCT or 4-methylcyclohexanol, MCH) for 60 s with 45-s rest intervals after presentation of each odor. During exposure to the first conditioned stimulus (CS+) odor (either OCT or MCH), the flies received the unconditioned stimulus (US) synchronously, i.e., 1.5-s pulses of 60-V DC electric shocks, every 5 s. To measure “learning,” flies were transferred immediately after training to the choice point of a T-maze and forced to choose between the 2 odors. To test memory retention, the trained flies were tapped into empty vials for 1, 3, or 7 h, and then put into the choice point of a T-maze in which they were exposed simultaneously to CS+ and CS−. For long-term memory (LTM) testing, flies were subjected to multiple spaced training sessions (10 training sessions with a 15-min rest between each). After training, flies were transferred to food vials and stored at 18°C for 24 h before observation of their distribution in the T-maze arms.33 The performance index (PI) was calculated according to a standard protocol,32 so that a 50:50 distribution (no memory) yielded a PI of zero, and a 0:100 distribution away from the CS+ yielded a PI of 100. In our experiments, training for control and slpD groups was carried out by turns.
Olfactory acuity was assayed by exposing naive flies to odor (at the same concentrations used for Pavlovian training) versus air in the T-maze during a 2-min test trial. Shock reactivity was quantified by placing metallic grids in each arm of the T-maze and then exposing the naive flies to electrical shock versus no shock (at intensities used for Pavlovian training) during a 2-min test trial. For both olfactory acuity and shock reactivity, the PIs were calculated as above (n = 8 PIs for each group).34 Flies of both sexes were used in all Pavlovian olfactory conditioning experiments.
Data are presented as mean ± standard error of the mean (SEM). For data with a Gaussian distribution, statistical significance was tested using Student's t-test. For data with a non-Gaussian distribution, statistical significance was tested with a rank-sum test instead. One-way ANOVA was used for sleep analysis.
RESULTS
Automated Rotation and Drop of Vials is Effective for Sleep Deprivation
Because we employed a rotation stimulus for the sleep deprivation paradigm, we observed the state of the flies every 3 h during slpD to make sure that the paradigm works as designed. First, when the vertical position was reached during every rotation, the vials would drop 3 cm, and most flies (∼80%) fell to the bottom of the vials. Second, the sleep state of flies was tested after 1 day of the automated rotation stimulus. The results showed that control flies were active in the daytime and slept most of the time during the night. However, the slpD groups showed little activity during the daytime and significant sleep rebound on the following day compared with the controls without slpD (Figure 1A and 1B), in agreement with previous reports that slpD can cause an obvious sleep rebound in Drosophila.18,19 Moreover, compared with the control flies, which took over 50 min to fall asleep for the first time, flies in the slpD group required only a few min to fall asleep after 1-day slpD (Figure 1C); therefore, the rotation and 3-cm drop was effective in awakening the Drosophila in vials and the flies were actually deprived of sleep although the extent of the sleep loss could not be calculated with this method. These results indicated that the automated rotation stimulus is an effective method for inducing deprivation of sleep in Drosophila.
slpD Specifically Impairs Short-term Memory
In humans and other animals, slpD causes a significant deficit in hippocampal plasticity10–13 during episodic memory encoding, resulting in impairment of subsequent retention.13 We examined learning and 1-, 3-, and 7-h memory after 1-day slpD in CS files using Pavlovian olfactory conditioning (Figure 2A). No significant difference was observed in learning ability between the slpD group and the control group (Figure 2B). However, the 1-h memory of the slpD group was significantly impaired (Figure 2C), whereas, the subsequent 3-h and 7-h memory was unaffected after 1-day slpD compared to the control group (Figure 2D and E). We also tested the long-term memory (LTM) of the flies after 1-day slpD, which can be formed through space training. The result demonstrated that the LTM of the flies remained normal when 1-day slpD occurred before training, but was severely impaired when the flies were subjected to slpD after training (Figure 2F). This is consistent with a report that 4-h slpD immediately after training abolishes courtship memory.16 Since flies sleep mostly at night, so we further tested the 1-h memory after 8-9 h slpD during daytime and nighttime, respectively. We found that only the flies deprived of sleep during the night showed obvious 1-h memory impairment compared with the controls that were trained and tested at the same time without sleep deprivation (Figure 2G). Because no differences were observed in 1-h memory between the daytime and nighttime slpD controls, the circadian effect can be excluded, and therefore loss of memory was likely to be caused by sleep deprivation (at night) rather than merely mechanical stimulation.
The above results demonstrated that slpD could impair 1-h memory in Drosophila. Next, we addressed the interesting question of how long the effect of 1-day slpD would persist. We trained the flies at 2 and 4 h following the termination of 24 h slpD, and then tested memory 1 h later (Figure 2H, top). The results showed that the defect of 1-h memory still persisted even at 2 h, but could not be detected at 4 h after the termination of slpD. Meanwhile, we analyzed sleep recovery at 2 and 4 h after 1-day slpD. We found that flies slept for 1.5 and 3 h during the 2-h and 4-h interval, respectively (Figure 2I), following the termination of 24-h slpD. These results indicate that the 1-h memory impairment resulting from 1-day slpD can persist for at least 2 h.
From another perspective, if flies are defective for task-related skills, including olfactory acuity and shock reactivity,17 they would also exhibit defects in learning and memory in the olfactory conditioning procedure. Compared to the control group, the avoidance of the 2 odors by the slpD group was not significantly different just after slpD or 1 h after slpD at the concentrations used for training or testing (Table 1). Meanwhile, given the electrical stimulus at 60 V, the reactivity to shock (Table 1) and the learning ability (Figure 2B) of the slpD group were not influenced by slpD immediately after slpD or 1 h after slpD. Thus, the severe reduction of 1-h memory originated exclusively from slpD-induced impairment in memory processing, and did not result from changes in olfactory acuity or sensitivity to electric shock.
Table 1.
slpD Has No Effect on Olfactory Activity or Shock Reactivity in Drosophila
Aversive Olfactory Avoidance and Shock Reactivity
| Group | SR | OA (MCH) | OA (OCT) |
|---|---|---|---|
| Control | 75.0 ± 2.7 | 41.5 ± 4.4 | 42.8 ± 1.9 |
| slpD | 77.6 ± 2.3 | 42.5 ± 1.4 | 40.4 ± 3.9 |
| slpD (after 1 h) | 74.5 ± 2.8 | 46.7 ± 3.8 | 48.8 ± 4.9 |
1-day slpD does not affect the “task-relevant” sensorimotor responses (olfactory acuity, OA, or shock reactivity, SR) required for proper performance in Pavlovian assays. The “task-relevant” abilities to sense and escape from the odors (olfactory acuity) or foot shock (shock reactivity) were quantified in the T-maze (see Methods). No significant differences were detected between control and slpD groups immediately after slpD, and the flies showed normal sensorimotor responses 1 h after slpD. n = 8–10 flies per group. All scores are expressed as the mean performance index (PI) ± SEM.
Stress Does Not Impair 1-h Memory
Stress may also be induced by slpD. To determine whether the reduction of 1-h memory is specifically due to slpD or is caused nonspecifically by stress, Canton-S flies were subjected to several stressors including heat stress, oxidative stress, starvation, and rotation stress. As shown in Figure 3, the flies exhibited intact 1-h memory after various stress treatments. The rotation stimulus was 1 min on and 1 min off during slpD, whereas, during the rotation stress condition flies received 45 min of rotation stimulus and then were given 45 min to recover, and this procedure was repeated for 24 h; hence, the total stimulus duration and intensity of these two treatments over 1 day was the same. So the reduction of 1-h memory is specifically due to slpD.
STM Impairment is Blocked by Silencing of the MB during slpD
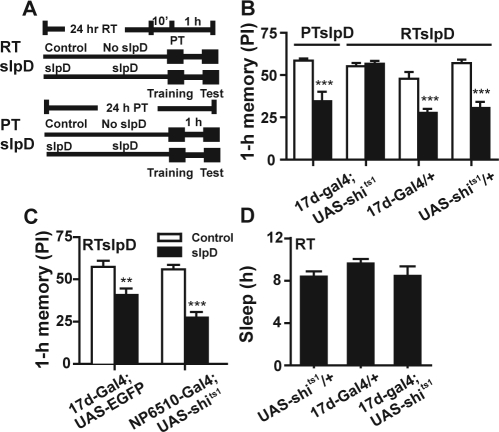
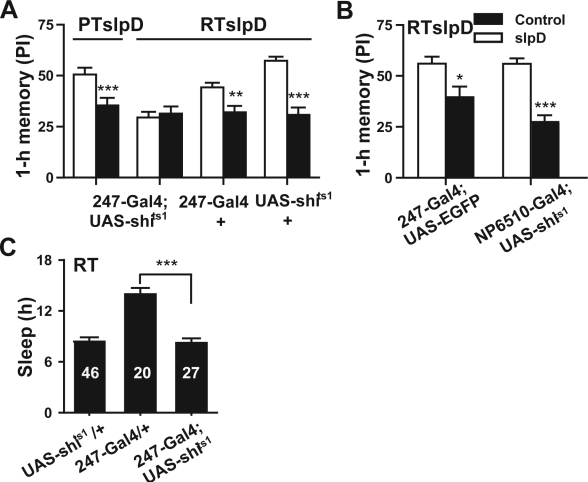
MB is an important neural locus for olfactory learning and memory 34 and also a key area for the regulation of sleep.27,28 Therefore, if slpD changes the state of the MB, memory may also be affected. We suspected that memory retention might be protected from impairment if the MB is silenced during slpD. To test this hypothesis, we expressed the shits1 to α/β lobes in MB under the driver of 17d-Gal (17d-Gal4;UAS-shits1) and to MB under the driver of 247-Gal4 (247-Gal4;UAS-shits), which function normally at permissive temperature (PT, < 29°C) and α/β lobes or MB neural transmission is completely blocked at restrictive temperature (RT, > 29°C).34,35 We set the environmental temperature at RT during slpD and at PT during training and memory testing (Figure 4A). As expected, the 17d-Gal4;UAS-shits1 flies did not show 1-h memory impairment after slpD at RT (Figure 4B). Flies of the same strain undergoing slpD at PT exhibited a weak performance similar to that of Canton-S flies in the 1-h olfactory memory retrieval task (Figure 4B). Moreover, the RT during slpD also did not improve performance in the 1-h olfactory memory retrieval task for the control strains of 17d-Gal4 and UAS-shits1 (Figure 4B) and the flies with enhanced green fluorescence protein (EGFP) expression in the same brain area (Figure 4C). Likewise, the blocking of synaptic transmission in the fan-shaped body (NP6510-Gal4;UAS-shits1), which inhibits visual-task related memory retrieval for contour orientation,36 did not ameliorate the 1-h memory impairment caused by slpD (Figure 4C). Moreover, the 247-Gal4;UAS-shits1 flies showed similar results: 1-h memory was not impaired when the flies were given slpD at RT (Figure S1A). The control groups 247-Gal4 and 247-Gal4;UAS-EGFP, which were subjected to slpD at RT, and 247-Gal4;UAS-shits1, which was maintained at PT during slpD, did not show a similar improvement of the performance in 1-h memory after 1-day slpD (Figure S1A and Figure S1B, Supplemental Figures S1 and S2 appear online only at www.journalsleep.org).
Figure 4.
The impairment of 1-h memory was blocked by silencing of α/β neurons during slpD. (A) Schematic representing the experimental protocol for the RT slpD (RTslpD, top) and PT slpD (PTslpD, bottom). We set the environment temperature at 31°C (RT) during slpD and at 25°C (PT) during training and memory testing (top) for the RTslpD, and maintained PT constantly for the PTslpD. (B) the impairment of 1-h memory resulting from slpD was unchanged in the 17d-Gal4;UAS-shits1 flies when subjected to slpD at PT (n = 10–11, P < 0.001), but disappeared when these flies were subjected to RT during slpD (n = 10–11, P > 0.05). Moreover, RT during slpD did not improve the performance of the no-function strain 17d-Gal4 and UAS-shits1 in the 1-h olfactory memory retrieval task (n = 9–11, P < 0.01). (C) Expression of EGFP in α/β neurons under the 17d-Gal4 driver did not ameliorate the 1-h memory impairment suffered from slpD at RT (n = 8–9, P < 0.05). Likewise, synaptic transmission silencing of the fan-shaped body (NP6510-Gal4;UAS-shits1), which inhibits visual task-related memory retrieval involving contour orientation,,36 did not improve performance following slpD in the 1-h olfactory memory retrieval task (n = 8, P < 0.001). (D) The sleep of flies with different genotypes (n = 20–30). We found that the sleep of 17d-Gal4;UAS-shits1 was not significantly reduced compared with 2 controls (P > 0.05).
To rule out that MB silencing decreases sleep, we compared the sleep status of each genotype at RT and found that there was no significant sleep reduction in flies with the MB silenced (17d-Gal4;UAS-shits1 and 247-Gal4;UAS-shits) compared to control flies (Figure 4D and Figure S1C). The results are in agreement with the finding that 247-Gal4;UAS-shits1 flies exhibited reduced sleep only during the early morning at RT.27 In contrast, the 17d-Gal4 flies, along with other MB-silenced lines, exhibited similar or even increased levels of sleep at RT.27 This indicated that the 17d-Gal4;UAS-shits1 and 247-Gal4;UAS-shits1 flies required roughly the same amount of sleep as the controls. Taken together, the abolition of 1-h memory impairment resulting from slpD in 17d-Gal4;UAS-shits1 flies is specifically due to the block of neural transmission in the MB during slpD. We speculate that 1-day slpD may change the state of the MB gradually and consequently cause 1-h memory impairment. Silencing the MB during slpD can block slpD-induced transmission in the MB, which, in turn, eliminates the 1-h memory defect.
Supplementary Figure 1.
(A) The 1-hr memory impairment resulting from slpD was conserved in the flies of 247-Gal4;UAS-shits when subjected to slpD at PT(n = 10-11, P < 0.001), but disappeared when subjected to RT during slpD (n = 10–11, P > 0.05), Moreover, restrictive temperature (RT, 31°C) during slpD also did not help the none function strain 247-Gal4 and UAS-shits1 get a good score in the 1-hr olfactory memory retrieval task (n = 9–11, P < 0.01). (B) The expression of EGFP in MB under the driver of 247-Gal4 did not ameliorate the 1-hr memory impairment suffered from slpD at RT (n = 8–9, P < 0.05). Likewise, synaptic transmission silence of fan-shaped body (NP6510-Gal4; UAS-shits1), which inhibits the visual task related memory retrieval about contour orientation [34], did not get a good score in the 1-hr olfactory memory retrieval task(n = 8, P < 0.001). (C) The sleep of different genotype (n = 20–30). We found that the sleep of 247-Gal4;UAS-shits1 significantly reduced compared with the line of 247-Gal4/+, but without no reduction compared with the line of UAS-shits1/+ (P > 0.05).
Down-regulation of cAMP in the MB Abolished slpD-induced STM Impairment
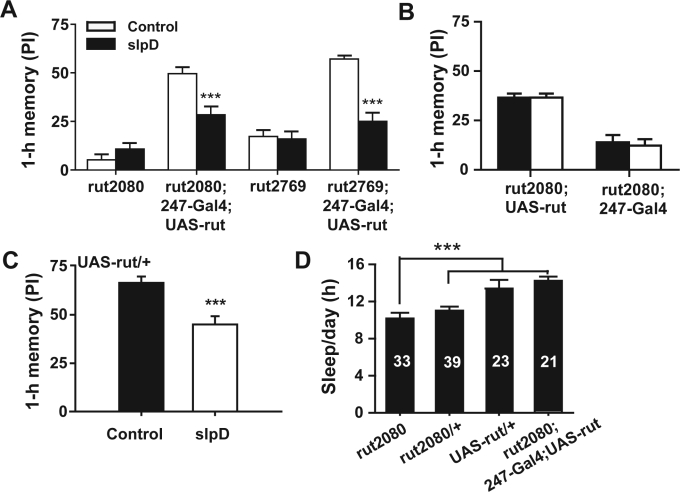
After we found that the MB is involved slpD-induced 1-h memory impairment, we further investigated the role of cAMP in this process. In Drosophila, cAMP is one of the most important molecules involved in memory formation and retrieval. The most important genes regulating the cAMP level are rut and dnc,17 both highly expressed in MB. The rut mutant with no functional Ca2+/CaM-dependent adenylyl cyclase,37 has significantly lower levels of cAMP in the brain compared with control flies. On the contrary, the dnc mutant which encodes non-functional cAMP-specific phosphodiesterase,38 has higher cAMP level in the MB.17 Both mutants cause an obvious impairment in the learning and memory of flies,17 suggesting that homeostasis, rather than the absolute level of cAMP, is more important in Drosophila memory. We tested whether 1-h memory impairment resulting from slpD is mediated by the regulation of the cAMP level in the MB. Although 1-h memory impairment from slpD was absent in the rut mutants (rut2769 and rut2080) (Figure S2A), and could be restored by rescuing rut expression in the MB of both mutants (Figure S2A–C), the performance index of the rut mutants is very low, so we cannot reach any firm conclusion as to whether the slpD-induced 1-h memory impairment is absent in rut mutants.
Supplementary Figure 2.
(A) The 1-h memory of the rut2080 and rut2769 mutants that were or were not subjected to 1-day slpD treatment was plotted. No significant impairment was observed in the 1-hr memory of these 2 mutants (n = 13–19, P > 0.05). However, the impairment of 1-h memory in the 2 rut mutants was recovered by rescuing the expression of rut in MB (n = 7–12, P < 0.001). (B) Both the flies of rut2080;uas-rut and rut2080;247-Gal4 showed no slpD-reduced 1-h memory impairment (n = 8–10, P > 0.05). (C) The memory of UAS-rut/+ also showed slpD-reduced 1-hr memory impairment. (D) The sleep time decreased significantly in rut2080 mutant.
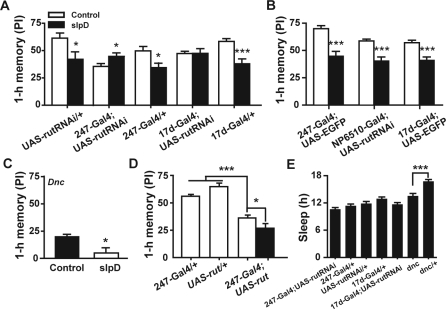
When we used restricted expression of rutRNAi to down-regulate the cAMP level throughout the MB or in the α/β lobes of the MB, slpD-induced 1-h memory impairment was also eliminated (Figure 5A). Furthermore, the 247-Gal4;UAS-rutRNAi flies showed memory improvement after 1-day slpD (Figure 5A) compared with the controls which still exhibited slpD-induced 1-h memory impairment (Figure 5A and 5B). In contrast, 1-h memory after 1-day slpD was also impaired in the dnc mutants (Figure 5C) and in the flies overexpressing rut in the MB in wild-type background, although these flies showed memory impairment compared to control flies with normal rut expression (Figure 5D, Figure S2C). We also measured the sleep of flies with each genotypes, and found that there was no reduction of sleep when cAMP levels were down-regulated by restricted expressing of rutRNAi to throughout the MB or in the α/β lobes of the MB (Figure 5E). Both rut2080and dnc mutants slept less than the heterozygotes (Figure 5E and S2C). However, the dnc mutants also showed slpD-induced 1-h memory impairment. Thus, these results suggest that slpD can up-regulate the cAMP level in the MB through the action of the rut gene and consequently affect STM.
Figure 5.
Down-regulation of the cAMP level in MBs abolished slpD-induced 1-h memory impairment. (A) Down-regulation of cAMP level using rutRNAi under the 247-Gal4 and 17d-Gal4 drivers abolished the 1-h memory impairment resulting from slpD (n = 8–13, P > 0.05), but the Gal4- or UAS-only flies exhibited slpD-induced 1-h memory impairment (n = 8–10, P < 0.05). (B) Flies with EGFP expression in the MB and in α/β neurons suffered 1-h memory impairment after 1-day slpD (n = 8–10, P < 0.001). Furthermore, NP6510-Gal4;UAS-rutRNAi flies also showed 1-h memory impairment after 1-day slpD (n = 8, P < 0.001). (C), (D) Up-regulation of the cAMP level in Dnc mutants or in flies overexpressing rut in the MB did not abolish 1-h memory impairment resulting from slpD (n = 8–15, P < 0.05). (E) Amount of sleep in flies with different genotypes. Sleep was not significantly reduced by expression of rutRNAi in the MB compared with 2 controls; however, dnc double mutants showed significant sleep loss compared with heterozygotes (P < 0.05).
DISCUSSION
Although Drosophila has been established as an excellent model for genetic studies for a hundred years, the use of Drosophila for the study of sleep patterns,18,19,22,24 especially the relationship between sleep and memory,14–16 has only emerged recently. In this study, we found that 1-day slpD specifically impaired the 1-h memory in Drosophila with Pavlovian olfactory conditioning. This effect could not be mimicked by other stressors, and persisted for several hours after the deprivation ceased. The silencing of MB during slpD only, or down-regulation of the cAMP levels in the MB, was sufficient to prevent slpD-induced 1-h memory impairment. These studies extend the understanding of the function of sleep and support the perspective that sufficient sleep is important for cognitive activities.14,15,39 Moreover, they provide clues to the possible pathway responsible for the interaction between sleep and cognition.
Interestingly, our data suggests that 1-day slpD as shown in our paradigm specifically affects STM retention following slpD, but leaves learning ability intact. However, we believe that the extent of slpD-induced impairment of subsequent cognitive ability is task type- and intensity-dependent. In a light and gustation (quinine or sugar) stimulus-coupling conditioning task,14 6-h or 12-h slpD led to a decrease in learning, which indicated that learning capacity can also be disrupted without adequate sleep. In our experiment, a 60-V electric stimulus applied as the US was strong enough to force the flies to be alert, and was above the threshold for establishing the connection between CS and US32 in flies undergoing 1-day slpD. In fact, the flies were hyper-aroused immediately at the initiation of training, even if they had been deprived sleep for 24 h. This also suggests that strong stimuli may help animals to overcome the effects of sleep deprivation on learning ability, and here we showed that learning performance after 1-day slpD was not impaired.
However, an inherent problem is that stress may cause memory impairment in some conditions and must be induced in slpD studies. A possible interpretation for the slpD-induced 1-h memory impairment is that slpD caused severe stress in flies, and the stress led to 1-h memory impairment. Previous studies have revealed that stress can both improve40 and impair memory,41 depending on the extent or intensity of the stress. Our results provided evidence that the flies did not show 1-h memory impairment after various stress treatments (Figure 3). Although the rotation stress is equal in intensity to the slpD stimulus,22 the flies showed no 1-h memory impairment after rotation stress. Furthermore, the 8-9 h nighttime slpD resulted in slpD-induced 1-h memory impairment, but the daytime slpD did not. The controls without slpD were trained and tested at same time, and the performance index did not differ between ZT13 and ZT1, so nighttime slpD-induced 1-h memory impairment is not due to circadian effects. As we know, the flies sleep most at night, so loss of memory was likely to be caused by sleep deprivation (at night) rather than merely mechanical stimulation. Therefore, the 1-h memory impairment resulting from 1-day slpD cannot be attributed to stress, but must be caused by other changes in specific regions of the Drosophila brain.
MB is an important neural locus in Drosophila that is essential to olfactory memory,34,42,43 and disruption of neurotransmission in the MB blocks retrieval, but not acquisition 34,35,43,44 In our studies, we found that the flies did not show slpD-induced 1-h memory impairment after the MB was silenced during slpD (Figure 4B and Figure S1). This is consistent with previous studies showing that 1-day slpD specifically impaired 1-h memory, but not 3-h and 7-h memory, which are stored in dorsal paired medial (DPM) neurons.45 Consequently, we suggest that 1-h memory impairment resulting from slpD is due to impaired retrieval 1 h after slpD. Moreover, we found that 1-h memory impairment resulting from 1-day slpD was absent in flies in which the MB and α/β neurons were silenced during slpD (247-Gal4;UAS-shits1 and 17d-Gal4;UAS-shits1) (Figure 4B), indicating that the α/β lobe in MB is the part affected during slpD, and therefore may be responsible for slpD-induced STM loss. If we shut down the transmission of the MB in response to the environment, the flies could be protected from impairment of memory after slpD. So the MB appears to be the key brain structure in the response to the slpD stimulus.
The cAMP molecule is a common element responsible for memory formation, consolidation and retention in various animals including Drosophila. In Drosophila, cAMP level regulation is vital during the establishment of STM in classical olfactory conditioning, and disturbance of the cAMP pathway causes a severe deficiency in learning and memory.17,37 Our results showed that down-regulation of the cAMP level in the MB blocked slpD-induced 1-h memory impairment (Figure 5A and Figure S2). Moreover, the 247-Gal4;UAS-rutRNAi flies showed improvement in 1-h memory after 1-day slpD (Figure 5A). However, up-regulation of the cAMP level in the dnc mutants and over-expression of rut in the MB does not block slpD-induced 1-h memory impairment (Figure 5D and Figure 5E). Two recent studies proposed that sleep could decrease synaptic connection46 and social experience could increase the sleep and the number of synaptic terminals.47 Therefore, sleep is involved in maintaining synaptic homeostasis.46,47 On this basis, we speculate that 1-day slpD may increase some protein levels in synapses, resulting in alteration of synaptic plasticity. This conjecture is consistent with the results showing that 1-h memory was impaired following slpD. Moreover, memory was protected from impairment following slpD when we blocked synaptic transmission or the changes of the state in some brain areas.
In summary, we propose that 1-day slpD may up-regulate the level of cAMP in the MB by activation of Ca2+P/calmodulin-responsive adenylyl cyclase, changing the state of the MB accordingly, and hence, specifically impairing STM in Drosophila.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation of China Grants 30270341, 30630028, and 30621004 (A.G.), the Multidisciplinary Research Program (Brain and Mind) of the Chinese Academy of Sciences, Major State Basic Research Program Grants G20000 77800, 2006CB806600, and 2006CB911003 (A.G.), and the Precedent Project of Important Intersectional Disciplines in the Knowledge Innovation Engineering Department of the Chinese Academy of Sciences Grants KJCX1-09-03 and KSCX2-YW-R-28 (A.G.). We thank Zuo-ren Wang for his helpful comments, Yue-qing Peng for the data analysis, and Ke Zhang for constructive suggestions.
REFERENCES
- 1.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 2.Oniani TN. Role of sleep in the regulation of learning and memory. Hum Physiol. 1982;8:381–91. [PubMed] [Google Scholar]
- 3.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–76. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilley AJ, Empson JA. REM sleep and memory consolidation. Biol Psychol. 1978;6:293–300. doi: 10.1016/0301-0511(78)90031-5. [DOI] [PubMed] [Google Scholar]
- 5.Vertes RP. Memory consolidation in sleep; dream or reality. Neuron. 2004;44:135–48. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–63. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–33. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–50. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Talamini LM, Nieuwenhuis IL, Takashima A, Jensen O. Sleep directly following learning benefits consolidation of spatial associative memory. Learn Mem. 2008;15:233–7. doi: 10.1101/lm.771608. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa A, Kanayama Y, Matsumura H, Tsuchimochi H, Ishida Y, Nakamura S. Selective rapid eye movement sleep deprivation impairs the maintenance of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2006;24:243–8. doi: 10.1111/j.1460-9568.2006.04874.x. [DOI] [PubMed] [Google Scholar]
- 11.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–65. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–65. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 14.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27:5384–93. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–81. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 17.Tamura T, Chiang AS, Ito N, et al. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 2003;40:1003–11. doi: 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- 18.Hendricks JC, Finn SM, Panckeri KA, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–38. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 19.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 20.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12:1934–40. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks JC, Williams JA, Panckeri K, et al. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–15. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 22.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–91. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Guo F, Lu B, Guo A. amnesiac regulates sleep onset and maintenance in Drosophila melanogaster. Biochem Biophys Res Commun. 2008;372:798–803. doi: 10.1016/j.bbrc.2008.05.119. [DOI] [PubMed] [Google Scholar]
- 24.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–75. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–9. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–62. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–6. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 28.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–60. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 29.Cirelli C, Bushey D, Hill S, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 30.Guo A, Li L, Xia SZ, Feng CH, Wolf R, Heisenberg M. Conditioned visual flight orientation in Drosophila: dependence on age, practice, and diet. Learn Mem. 1996;3:49–59. doi: 10.1101/lm.3.1.49. [DOI] [PubMed] [Google Scholar]
- 31.Kamikouchi A, Inagaki HK, Effertz T, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–71. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 32.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–77. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 33.Ge X, Hannan F, Xie Z, et al. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci U S A. 2004;101:10172–6. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–80. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- 35.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–3. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Seiler H, Wen A, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–6. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 37.Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–89. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 38.Chen CN, Denome S, Davis RL. Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci U S A. 1986;83:9313–7. doi: 10.1073/pnas.83.24.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers NL, Dorrian J, Dinges DF. Sleep, waking and neurobehavioural performance. Front Biosci. 2003;8:s1056–1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 40.Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learn Mem. 2007;14:329–35. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci. 2001;928:168–75. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–55. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 43.Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem. 2006;13:659–68. doi: 10.1101/lm.221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride SM, Giuliani G, Choi C, et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–77. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 45.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–57. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 46.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–12. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–8. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]