Abstract
Study Objective:
To assess night shift improvements in mood, fatigue, and performance when the misalignment between circadian rhythms and a night shift, day sleep schedule is reduced.
Design:
Blocks of simulated night shifts alternated with days off. Experimental subjects had interventions to delay their circadian clocks to partially align with a night shift schedule. Control subjects had no interventions. Subjects were categorized according to the degree of circadian realignment independent of whether they were in the experimental or control groups. Twelve subjects were categorized as not re-entrained, 21 as partially re-entrained, and 6 as completely re-entrained.
Setting:
Home sleep and laboratory night shifts.
Participants:
Young healthy adults.
Interventions:
Experimental subjects had intermittent bright light pulses during night shifts, wore dark sunglasses outside, and had scheduled sleep episodes in darkness.
Measurements and Results:
A computerized test battery was administered every 2 hours during day and night shifts. After about one week on the night shift schedule, which included a weekend off, the partially and completely re-entrained groups had markedly improved mood, fatigue, and performance compared to the group that was not re-entrained. The completely and partially re-entrained groups were similar to each other and had levels of mood, fatigue, and performance that were close to daytime levels.
Conclusions:
Partial re-entrainment to a permanent night shift schedule, which can be produced by feasible, inexpensive interventions, is associated with greatly reduced impairments during night shifts.
Citation:
Smith MR; Fogg LF Eastman CI. A compromise circadian phase position for permanent night work improves mood, fatigue, and performance. SLEEP 2009;32(11):1481-1489.
Keywords: Shift work, performance, alertness, mood, human, circadian rhythms, bright light, melatonin
ALERTNESS AND PERFORMANCE DURING NIGHT WORK CAN BE SERIOUSLY IMPAIRED.1,2 THIS OCCURS BECAUSE THE MASTER CIRCADIAN CLOCK OF MOST night workers, which controls the body's circadian rhythms (e.g., alertness, temperature, melatonin), does not shift to realign with a night work, day sleep schedule.3 A sharp increase in sleepiness and decrease in performance occurs around the minimum of the circadian rhythm of body temperature (Tmin), which is usually during the night shift.4,5 Even with adequate daytime sleep, night shift decrements remain if the circadian clock is not shifted (e.g., Sharkey et al.6). Consequently, night work is associated with safety risks for both the individual worker as well as society.1,2
Alertness and performance during night shifts can be improved by stimulants such as caffeine7 and modafinil.8,9 Bright light exposure during night shifts can also improve alertness via its direct alerting effect.10 Short naps may also be useful for reducing the decrements in night shift alertness.7 However, none of these interventions can overcome the nadir in the circadian rhythm of alertness.7,11 These countermeasures do not address the underlying cause of the problem, which is misalignment between circadian rhythms and the sleep and work schedule.
Laboratory and field studies of night work have shown that scheduled exposure to bright light and darkness (sleep) can be used to shift the circadian clock to completely align with a night work, day sleep schedule.12–16 Complete re-entrainment greatly improves alertness and performance during night shifts.12,17 Despite the appeal of complete re-entrainment from an alertness and safety perspective, few night workers are likely to adopt it because the slowness with which the circadian clock adjusts precludes shifting back to a diurnal schedule on days off. We have thus assessed the feasibility of a compromise sleep schedule combined with interventions to delay the circadian clock to only partially entrain to the night work, day sleep schedule. The goal of partial re-entrainment is to delay the sleepiest circadian time out of the night work period, into the first portion of the daytime sleep episodes after work, as well as to maintain it near the end of late nighttime sleep episodes on days off. When a compromise phase position is maintained throughout alternations between night shifts and days off, it is conducive of afternoon and evening alertness on days off, as well as alertness during night shifts.
In a series of 4 studies with alternating blocks of night shifts and days off,18–21 we defined the target compromise circadian phase position as a dim light melatonin onset (DLMO) of 3:00. At this phase, the Tmin, an estimate of the sleepiest circadian time, which occurs about 7 h after the DLMO,22–24 will fall at ∼10:00. The sleepiest circadian time would thus be early in the daytime sleep episodes after night work (daytime sleep started at 8:30) and late in the sleep episodes on days off (sleep started at 3:00). The last 2 studies of this series showed that scheduled exposure to bright light and darkness (sleep) delayed the circadian rhythms of most of the subjects to a point near the compromise phase position after two blocks of night shifts separated by an intervening weekend off.20,21 Here we present mood, fatigue, and performance data from these 2 studies. Our primary objective was to compare mood, fatigue, and performance for subjects who were completely, partially, or not re-entrained to the night work, day sleep schedule. We hypothesized that subjects who achieved partial re-entrainment (circadian phase close to the target compromise phase position) would show improvements during the night shifts relative to subjects that were not re-entrained, and would be similar to those who achieved complete re-entrainment to the night work, day sleep schedule. A secondary objective was to compare mood, fatigue, and performance measured during night shifts to daytime levels. We hypothesized that subjects achieving either partial or complete re-entrainment would rate themselves and perform at close to daytime levels, while not re-entrained subjects would show more night shift impairment, relative to daytime levels.
METHODS & DESIGN
The last 2 studies in this series were named #320 and #421 in their titles. For clarity, here we refer to them as studies A20 and B,21 respectively. Each was a between-subjects design with a control and experimental group. In all groups there were large individual differences in the final circadian phase position at the end of the series of night shifts and days off. The present analyses pool data from these 2 studies and are based on final circadian phase position independent of group assignment (experimental or control) or study. Subjects were divided into 3 groups (not re-entrained, partially re-entrained, completely re-entrained), as explained below.
Subjects
Twenty-four subjects completed study A, and 19 subjects completed study B. Four subjects completed both studies, and the data for these subjects' participation was included only once, leaving 39 subjects in the analyses. The decision of which data to include for these 4 subjects was made by the corresponding author before data analyses began on the basis of the final DLMO for each subject, in an attempt to make the sample sizes of the 3 groups more similar. The age, sex, and morningness-eveningness score25 for the 3 groups were similar (Table 1). Subjects had a BMI < 30 kg/m2, were nonsmokers, habitually drank < 300 mg caffeine/day, and did not take prescription medication, except for 9 female subjects who used hormonal contraceptives. A urine toxicology screen when beginning the study verified that subjects did not use recreational drugs. In the month preceding the study, subjects had not worked night shifts or crossed more than 3 time zones. These studies were approved by the Rush University Medical Center Institutional Review Board. All subjects provided written informed consent.
Table 1.
Subject Demographics
| n | M:F | Age (Mean ± SD) | M/Ea (Mean ± SD) | |
|---|---|---|---|---|
| Not Re-Entrained | 12 | 5:7 | 28.1 ± 1.7 | 53.3 ± 5.1 |
| Partially Re-Entrained | 21 | 9:12 | 24.0 ± 1.1 | 54.5 ± 1.6 |
| Completely Re-Entrained | 6 | 2:4 | 25.2 ± 2.7 | 54.7 ± 4.5 |
Morningness-Eveningness score25
Baseline Sleep and Morning Light Schedule
During a 15 day baseline period, all subjects maintained a regular sleep/wake schedule. Subjects remained in bed from 23:00-7:00 on weeknights, while on weekends bedtime was between 23:00-00:00, with wake time between 7:00-8:00. Subjects were required to go outside for ≥ 15 minutes of light exposure every day between 8:00-9:00. A baseline circadian phase assessment (described below) was conducted on days 15-16. After this phase assessment subjects returned to the baseline schedule of sleep/wake and light exposure for an additional 6 days before coming to the lab for the first night shift.
STUDY INTERVENTIONS
Beginning on study day 23, subjects came to the lab for a series of night shifts (23:00 to 07:00). Subjects in study A underwent 3 night shifts, 2 days off, and 4 more night shifts (Figure 1). Subjects in study B underwent 3 night shifts, 2 days off, 5 more night shifts, and 2 more days off. On each night shift the experimental subjects were exposed to 15-min intermittent bright light pulses from light boxes containing fluorescent lamps (5095K, Sun Ray, Sun Box Company, Inc), timed to delay circadian rhythms. In study A, the experimental group received 5 light pulses. The first pulse began at 00:45 and the last pulse ended at 5:00. In study B, the experimental group received 4 light pulses. The first pulse began at 00:45 and the last pulse ended at 4:00. The light pulse from 4:45 to 5:00 during each night shift in study B was omitted because the final phase assessment in study A showed that some experimental subjects delayed slightly more than desired, into the complete re-entrainment category. At a typical distance and angle of gaze, the illuminance of the bright light pulses was ∼ 4100 lux, the irradiance was ∼ 1200 μW/cm2, and the photon density was ∼ 3.1 x 1015 photons/cm2/second. Light pulses were separated by 45 minutes of room light ( < 50 Lux, 4100K). Subjects in the control groups remained in this room light throughout the night shifts.
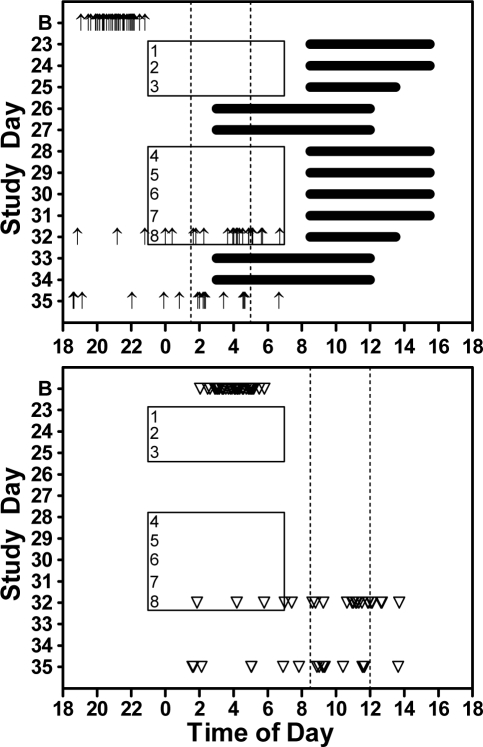
Figure 1.
Night shifts from 23:00-7:00 (rectangles) are numbered (1-8). Dark horizontal lines in the top panel indicate scheduled sleep episodes for the experimental groups. Sleep episodes for the control groups were self-selected. Arrows show the times of the dim light melatonin onset (DLMO, top panel) and triangles show the estimated sleepiest circadian times (DLMO + 7 h, bottom panel) for individual subjects during the baseline phase assessment (B) and during the final phase assessment for study A (day 32) and study B (day 35). Subjects were assigned to one of 3 re-entrainment categories based on the time of their final DLMO, and was independent of whether they were in an experimental or control group. Dashed vertical lines in the top panel indicate the criteria used to define the 3 re-entrainment groups, while in the bottom panel 7 h was added to these DLMO criteria to facilitate visualization of the sleepiest circadian time. Subjects with a final DLMO earlier than 1:30 were classified as not re-entrained, with a final DLMO between 1:30 and 5:00 as partially re-entrained, and with a final DLMO later than 5:00 as completely re-entrained. Note that the triangles falling in the left-most category (not re-entrained) correspond to the time of the night shifts or commute home. The triangles falling in between the 2 vertical lines (the partially re-entrained category) correspond to times of sleep for experimental subjects on both work days and on days off.
Experimental subjects were required to remain in bed during scheduled times after night shifts and on weekends off (black bars in top panel of Figure 1). Scheduled sleep was from 8:30 to 15:30 on days 23-24 and 28-31, from 8:30-13:30 after the last night shift before a weekend off (days 25 and 32), and from 3:00-12:00 on days off (days 26, 27, 33, 34). Experimental subjects were required to go outside for ≥ 15 minutes of light exposure within the first 2 h after awakening starting on day 23. The purpose of this “light brake” was to keep their circadian clocks from delaying past the target compromise phase position. Sleep and light exposure for control subjects were unrestricted.
Subjects wore sunglasses at all times when outside during daylight hours. Control subjects wore light sunglasses (ranging from 0% transmission at 400 nm to about 55% at 650 nm). Experimental subjects wore darker sunglasses (ranging from 0% at 400 nm to about 25% at 650 nm) that more strongly attenuated short wavelength light. The spectral transmission of both lenses have been published.18 The primary purpose of the sunglasses was to attenuate phase-advancing outdoor light exposure during the travel home time after night shifts for the experimental groups.
Circadian Phase Assessments
Detailed procedures for the phase assessments have been previously described.18 For both studies, the baseline phase assessment lasted from 15:30 on day 15 until 12:00 on day 16. In study A, a final 24-h phase assessment began at 18:00 on day 32. In study B, the final phase assessment began after the second weekend off, at 18:00 on day 35. During phase assessments saliva was sampled every 30 min under dim light ( < 5 lux) using a salivette (Sarstedt, Newton, NC, USA). Samples were frozen and shipped on dry ice to Pharmasan Labs (Osceola, WI), where they were radioimmunoassayed for melatonin. The sensitivity of the assay was 0.7 pg/mL. The intra-assay variability was 12.1%, and the inter-assay variability was 13.2%.
Mood, Fatigue, and Performance Testing
A test battery was administered on desktop computers 4 times during three day shifts (days 17, 18, and 21) and each night shift. During day shifts, the test battery was administered beginning at 10:05, 12:05, 14:05, and 16:05. During night shifts, it was administered beginning at 00:05, 2:05, 4:05, and 6:05 [see Figure 2 in Smith et al.19]. In our results we report data at 00:30, 2:30, 4:30, and 6:30 because the test battery lasted ∼ 25 minutes. As part of each test battery, subjects completed the Profile of Mood States (POMS),26 three 10-point scales assessing tiredness and mental and physical exhaustion, and the Automated Neuropsychological Assessment Metrics (ANAM).27 For the POMS, data for the fatigue-inertia subscale and total mood disturbance were analyzed. Endpoints of the scales assessing tiredness and exhaustion were (1) “fresh as a daisy” versus “tired to death,” (2) “physically exhausted” versus “energetic”, and (3) “mentally exhausted” versus “sharp.”
Figure 2.
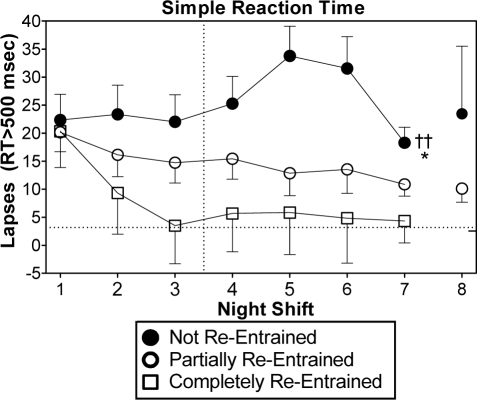
Lapses (reaction time > 500 milliseconds) during the 6:30 test bout of the simple reaction time task. The vertical line between night shifts 3 and 4 indicates that these shifts were separated by 2 days off. Scores are difference-from-baseline. The dotted horizontal lines indicate the baseline mean for all subjects. Symbols denoting statistical significance next to night shift 7 indicate a main effect of group during night shifts 1-7. †† indicates a difference (P < 0.01) between the not re-entrained and completely re-entrained groups. * indicates a difference (P < 0.05) between the not re-entrained and partially re-entrained groups. The tick mark on the right y-axis indicates average baseline scores during the last test bout at the end of the day shifts (16:30). Error bars show SEM. The other ANAM tasks showed a similar progression across night shifts.
The ANAM tasks included simple reaction time, procedural reaction time, mathematical processing, delayed matching to sample, code substitution, and the Stanford Sleepiness Scale.28 The simple reaction time task is similar to the psychomotor vigilance task (PVT).29 In the ANAM version, an asterisk appeared in the middle of the computer screen at variable intervals, and the subjects pressed the left mouse button which recorded reaction time (RT). Lapses were defined as RT > 500 msec. The procedural reaction time task assessed processing efficiency and reaction time when following a defined set of mapping rules. The basic block version of this test was administered. In it a single digit number between 2 and 5 was displayed within a box on the screen. Subjects indicated whether the number was 2 or 3 (left mouse click), or 4 or 5 (right mouse click). The mathematical processing task measured computational skills and working memory. This task entailed adding and subtracting 3 digits between 1 and 9, and indicating whether the answer was greater than 5 (right mouse click) or less than 5 (left mouse click). The delayed matching to sample task measured visuospatial working memory and spatial processing. Subjects viewed a sample pattern produced by a 4 × 4 grid of light and dark squares. After a 5-sec delay in which the screen was blank, 2 comparison grids were displayed side by side on the screen, and the subject indicated which of the 2 grids matched the previously shown grid (left or right mouse click). The code substitution task measured sustained attention and visual search capacity and is similar to the Digit Symbol Substitution Test (DSST).30 Subjects viewed a “key” across the top of the screen pairing 9 digits with 9 symbols. A single digit-symbol pair was presented at the bottom of the screen and the subject indicated whether the pair matched (left mouse click) or didn't match (right mouse click) the pair in the key above. Subjects received immediate feedback after each response for incorrect responses on the code substitution task. Further details on these tests,31 including their use in clinical32 and non-clinical populations,33 as well their construct validation,34 have been published.
For the last 4 ANAM tests described above, the percent correct, median reaction time (RT), and the number of slow responses for correct answers were analyzed. Slow responses are akin to lapses, but were defined as responses which exceeded the 90th percentile of the cumulative distribution of each subject's daytime responses. The threshold for slow responses is thus based on each individual's baseline performance, rather than applying an arbitrary threshold to define a lapse in all subjects on tasks for which a commonly accepted lapse threshold has not been established. This method has been used in previous simulated night shift studies.35,36
Technical difficulties with data retrieval rendered the Stanford Sleepiness Scale data unavailable.
Additional Procedures
Subjects completed daily event logs to record consumption of caffeine, alcohol, and over-the-counter medication. During the baseline portion of the study ≤ 2 alcoholic drinks per day were allowed. Alcohol was prohibited the day before a night shift and in the 24 h prior to and during each phase assessment. Caffeine ( ≤ 300mg) was permitted before 17:00 on baseline days, but was prohibited during both day and night shifts and in the 6 h before and during both phase assessments. On daily sleep logs subjects recorded bedtime, sleep onset, wake time, and nighttime awakenings > 5 min.
Data Analysis
Circadian Phase
A locally weighted least squares (LOWESS) curve was fit to each melatonin profile (GraphPad Prism). To determine the DLMO, a threshold was calculated by taking the average of the 5 lowest consecutive raw data points plus 15% of the average of the 5 highest consecutive raw data points.20,21 The DLMO was the time the fitted curve exceeded and remained above the threshold.
Subjects were divided into 3 groups according to their DLMO at the time of the final phase assessments (Figure 1). The classification for these groups was the same as in our previous study,17 and was based on where the sleepiest circadian time would occur relative to the night work periods and the day sleep episodes. We estimate the sleepiest circadian time as being near the Tmin, which occurs ∼ 7 h after the DLMO.22–24 Subjects that had a final DLMO earlier than 1:30 were classified as not re-entrained (n = 12; 2 experimental and 10 control subjects). This means their sleepiest circadian time (DLMO + 7 h) was earlier than 8:30, and likely occurred either during the night work period or the commute home. Subjects that had a final DLMO between 1:30–5:00 were classified as partially re-entrained (n = 21; 12 experimental and 9 control subjects). For experimental subjects this put the estimated sleepiest circadian time in the first half of daytime sleep and into the end of the sleep episode on days off. Subjects that had a final DLMO later than 5:00 were classified as completely re-entrained (n = 6; all experimental subjects). For experimental subjects, this put the sleepiest circadian time in the second half of the daytime sleep episodes after night shifts.
Mood, Fatigue, and Performance Testing
To account for large individual differences during day shifts (baseline), all data were transformed into difference-from-baseline scores. The data from the first day shift was excluded as practice. Scores on the second and third day shifts were averaged to form a baseline value. This baseline value was subtracted from scores on each night shift test bout to obtain difference-from-baseline scores.
Improvements in mood, fatigue, and performance were expected to occur when subjects' circadian clocks had delayed far enough so that the sleepiest circadian time moved out of the night work period and commute time home (i.e. partial or complete re-entrainment). Based on the final DLMOs from the entire series of studies,18–21 we estimate that for most experimental subjects this occurred in the middle of the second block of night shifts (see Figure 1). We thus focused our analyses on night shifts 5–8. All subjects participated in night shifts 5–7, and the scores for these night shifts were averaged for each of the 4 test bouts. Group sizes for the night 5–7 analyses are shown in Table 1. Only subjects in study B participated in night shift 8, so the results for this night are presented separately. There were 17 subjects in study B, and only 1 fell into the completely re-entrained group, so this group was not included, leaving 2 groups and 16 subjects (9 partially re-entrained, 7 not re-entrained) for night 8 analyses.
A repeated measures ANOVA was used to analyze each dependent variable. For the average of nights 5–7, the between subjects factor of group had 3 levels (not re-entrained, partially re-entrained, completely re-entrained), and the within subjects factor of time-of-night had 4 levels (00:30, 2:30, 4:60, and 6:30 test bouts). For night 8, the between subjects factor had only 2 levels (not re-entrained and partially re-entrained). Significant main effects of group were followed by least significant difference post hoc tests, and significant group × time-of-night interactions were elucidated with simple main effects.37
Where the data was skewed, statistics were performed on data that was transformed using the formula (x+c)y, where c was a constant added to all difference-from-baseline scores so that they were positive numbers, and y was a decimal that normalized the distribution. However, all figures depict the untransformed data to facilitate its interpretation.
Data for one dependent variable had an outlier that was excluded. For one subject, data on the mathematical processing task on nights 5–7 was excluded because this subject's median RT during the 6:30 test bout was 5 SDs above the group mean.
Many of the dependent variables that were analyzed with ANOVAs showed the expected significant main effects of time-of-night, such as a gradual deterioration in performance as each night shift progressed. These changes can be seen clearly in the figures that will be presented. However, because we are most interested in how circadian phase affected this change across a night shift, and for brevity, here we only report statistics for main effects of group or group × time-of-night interactions.
To facilitate interpretation of these data, we also present total sleep time (TST) during the days before night shifts 5–8. TST was determined from sleep logs, and was calculated by taking the difference between the sleep onset and waking time, minus awakenings > 5 min. For control subjects, who could sleep whenever they chose, TST was all the sleep that occurred after the end of one night shift and the start of the next night shift. Because there was not homogeneity of variance, TST for the 3 re-entrainment groups on each of days before night shifts 5–8 was compared with Kruskal-Wallis tests.
Summary statistics for all data are means and standard deviations unless otherwise indicated. A 2-tailed significance level of 0.05 was used.
RESULTS
The percent correct for several of the ANAM tasks showed a similar pattern to the median RT and slow responses, but for brevity, here we only report the latter 2. Similarly, the 2 mood measurements and the 3 fatigue measurements showed a very similar pattern. Because of the large amount of data they produced with similar results, here we only report results from the total mood disturbance and mental fatigue scales.
Performance
Simple Reaction Time
Figure 2 shows the progression of lapses across all 8 night shifts. Data from the last test bout (6:30) is shown because the worst performance of a night was typically during this test. Although this figure depicts lapses on the simple reaction time task, data from the other ANAM tasks showed a very similar progression. During the first night shift, subjects in all groups had more lapses than during baseline. The partially re-entrained group showed a reduction in the number of lapses across successive night shifts. The completely re-entrained group had lapses near baseline levels starting at night shift 3. In contrast, the not re-entrained group continued to have an elevated number of lapses during all the night shifts. Across night shifts 1–7, there was a main effect of group [F2,36 = 4.70, P = 0.02]. Post hoc tests indicated that the not re-entrained group had significantly more lapses than the partially and completely re-entrained groups. More detailed analyses of the simple reaction time task has been previously reported.20,21
Procedural Reaction Time Task
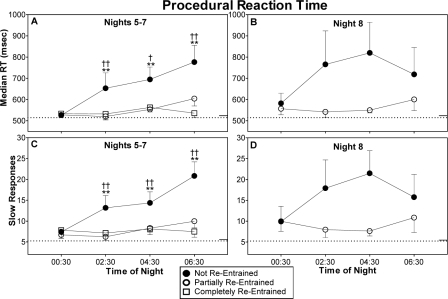
The not re-entrained group had slower reaction times (Figure 3, top row). Median RT showed a significant group × time-of-night interaction for night shifts 5–7 [F6,108 = 3.45, P < 0.01]. The group that was not re-entrained had significantly longer reaction times than either the partially or completely re-entrained groups during the 2:30, 4:30, and 6:30 tests (panel A in Figure 3). On night shift 8, the main effect of group and the group × time-of-night interaction did not reach statistical significance (panel B in Figure 3).
Figure 3.
Median reaction time (top) and slow responses (bottom) on the procedural reaction time task. Remaining aspects of figure as for Fig 2. Statistical symbols in panels A and C denote the time points at which the groups differed, following a significant group x time-of-night interaction. †(P < 0.05) and ††(P < 0.01) indicate differences between the not re-entrained and completely re-entrained groups. **indicates a difference (P < 0.01) between the not re-entrained and partially re-entrained group.
The number of slow responses was also greater for the group that was not re-entrained (Figure 3, bottom row). There was a significant group × time-of-night interaction during night shifts 5–7 [F6,108 = 2.87, P = 0.02]. Simple main effects indicated that the not re-entrained group had significantly more slow responses than both the partially or completely re-entrained groups during the 2:30, 4:30, and 6:30 test bouts (panel C in Figure 3). There were no significant differences between the groups on night shift 8 (panel D in Figure 3).
Mathematical Processing Task
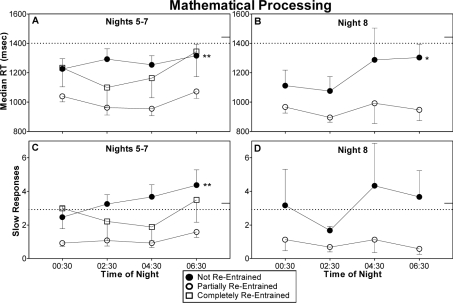
Performance was better than during baseline for all 3 groups, but the partially and completely re-entrained group performed better than the not re-entrained group. Median RT was slower in the not re-entrained group (Figure 4, top row). There was a significant main effect of group during nights 5–7 [F2,34 = 5.65, P < 0.01]. Post hoc tests indicated that the not re-entrained group had significantly slower median RT than the partially re-entrained group (panel A in Figure 4). On night shift 8 there was also a significant main effect of group [F1,12 = 7.79, P = 0.02], indicating that the group that was not re-entrained had significantly slower reaction times than the partially re-entrained group (panel B in Figure 4).
Figure 4.
Median reaction time (top) and slow responses (bottom) on the mathematical processing task. Remaining aspects of figure as for Fig 2. Statistical symbols in panels A, B, and C denote a significant main effect of group. *(P < 0.05) and **(P < 0.01) indicate differences between the not re-entrained and partially re-entrained groups.
The number of slow responses was greater for subjects that did not achieve re-entrainment (Figure 4, bottom row). On nights 5–7 there was a significant main effect of group [F2,35 = 8.25, P < 0.01]. The not re-entrained group had significantly more slow responses than the partially re-entrained group (panel C in Figure 4). Slow responses on night shift 8 showed a similar pattern, but the main effect of group did not achieve statistical significance [F1,13 = 3.92, P = 0.07] (panel D in Figure 4).
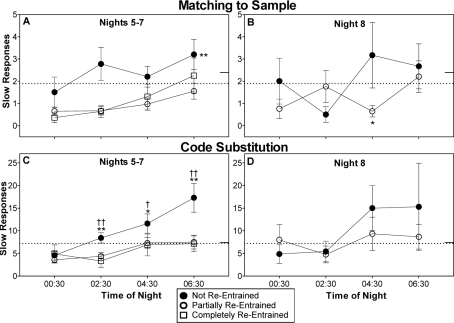
Matching to Sample Task
Median RT was close to baseline levels throughout the night shifts, and there were no significant differences among the groups (data not shown). For the number of slow responses there was a significant main effect of group on night shifts 5–7 [F2,35 = 4.14, P = 0.02]. The group that was not re-entrained had significantly more slow responses than the group that was partially re-entrained, but the difference between the not-entrained and completely re-entrained groups did not reach statistical significance (P = 0.06) (panel A in Figure 5). There was a significant group × time-of-night interaction for the number of slow responses on night shift 8 [F3,39 = 3.48, P = 0.04]. Simple main effects showed that the source of this interaction was significantly more slow responses for the not re-entrained group compared to the partially re-entrained group during the 4:30 test bout (panel B in Figure 5).
Figure 5.
Slow responses on the matching to sample and code substitution tasks. Remaining aspects of figure as for Figure 2. Statistical symbol in panel A denotes a significant main effect of group, while symbols in panels B and C denote the time points at which the groups differed, following a significant group x time-of-night interaction. †(P < 0.05) and ††(P < 0.01) indicate differences between the not re-entrained and completely re-entrained groups. *(P < 0.05) and **(P < 0.01) indicate differences between the not re-entrained and partially re-entrained groups.
Code Substitution Task
There were no group differences in median RT (data not shown). However, the group that was not re-entrained had more slow responses (Figure 5, bottom row). There was a significant group × time-of-night interaction on nights 5–7 [F6,108 = 2.78, P = 0.02]. The not re-entrained group had significantly more slow responses than both the partially re-entrained and completely re-entrained groups during the 2:30, 4:30, and 6:30 test bouts (panel C in Figure 5). There were no significant group differences in the number of slow responses on night shift 8 (panel D in Figure 5).
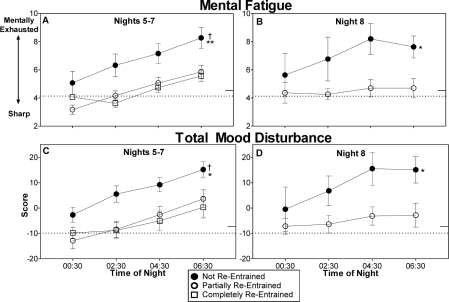
Mental Fatigue and Mood Disturbance
All the groups began the night shifts with ratings of mental fatigue and total mood disturbance that were relatively close to their baseline levels (00:30 time points in Figure 6). During night shifts 5–7 (Figure 6, left panels), mental fatigue and total mood disturbance increased for all groups later in the night shifts, but the partially and completely re-entrained groups remained closer to their baseline ratings late in the nights, while the group that was not re-entrained became more fatigued and had greater mood disturbance. On night shift 8 (Figure 6, right panels), ratings for the partially re-entrained group remained very close to baseline levels, while the not re-entrained group demonstrated increased mental fatigue and mood disturbance, especially later in the night shift.
Figure 6.
Mental fatigue (top) and total mood disturbance from the Profile of Mood States (POMS) (bottom) during night shifts. Higher mood disturbance scores indicate greater levels of mood disturbance. Remaining aspects of figure as for Fig 2. Statistical symbols denote significant main effects of group. † indicates a difference (P < 0.05) between the not re-entrained and completely re-entrained groups. *(P < 0.05) and **(P < 0.01) indicate differences between the not re-entrained and partially re-entrained groups.
On the mental fatigue scale for nights 5–7, there was a significant main effect of group [F2,36 = 5.48, P < 0.01]. Post hoc tests indicated that the not re-entrained group was significantly more mentally fatigued than the partially and completely re-entrained groups (panel A in Figure 6). During night shift 8 there was a main effect of group [F1,14 = 4.84, P = 0.045], indicating that the group that was not re-entrained was more mentally fatigued than the group that achieved partial re-entrainment (panel B in Figure 6).
Total mood disturbance was also higher for the group that was not re-entrained (Figure 6, bottom row). On night shifts 5–7, there was a significant main effect of group [F2,36 = 4.12, P = 0.02], with the group that was not re-entrained having significantly greater mood disturbance than the groups that were partially and completely re-entrained (panel C in Fig, 6). For night shift 8 there was a significant main effect of group [F1,14 = 4.64, P = 0.049], indicating that the group that was not re-entrained had greater mood disturbance than the group that was partially re-entrained (panel D in Figure 6).
Sleep Duration
There was a significant difference in TST on day 28 (occurring before night shift 5) [χ(2) = 9.12, P = 0.01]. TST on day 28 for the not re-entrained group (6.0 ± 1.1 h) was shorter than the partially re-entrained group (6.9 ± 0.5), while the completely re-entrained group was intermediate (6.4 ± 0.4). There were no significant differences in sleep duration on days 29, 30, and 31 among the three groups (mean TST ranged from 5.7 to 6.9 h). More complete analyses of sleep have been previously reported.20,21
DISCUSSION
Reducing circadian misalignment during night shift work markedly reduced ratings of mental fatigue and mood disturbance, while improving measures of performance. On most measurements, the group that achieved partial re-entrainment to the night work, day sleep schedule was better than the group that was not re-entrained, and was comparable to the group that was completely entrained.
These findings are similar to our previous study in which both partial and complete re-entrainment improved mood, alertness, and performance during simulated night shifts.17 However, in the previous study there were no daytime measurements so we could not assess how subjects felt and performed during night shifts relative to their normal daytime functioning. The present study included testing during daytime work hours, and demonstrated that mood, fatigue, and performance during night shifts were at or close to daytime levels for subjects that achieved partial or complete re-entrainment. It is notable that daytime measurements of mood, fatigue, and performance were not obtained from early morning day shifts, but rather from 9 to 5 work days, when self-ratings and performance would be optimal. This degree of night shift improvement in studies that have baseline measures has rarely been demonstrated, and has been shown only in subjects that were completely re-entrained.12 It is notable that the subjects in our studies were not permitted to drink caffeine during the night shifts, a practice that might have completely normalized those measurements that were still slightly above baseline values.
A few control subjects achieved partial re-entrainment even though they did not receive bright light pulses during night shifts and only wore lightly tinted sunglasses outside. Thus, night shift bright light and dark sunglasses are not necessary to produce partial re-entrainment and the accompanying benefits to performance, fatigue and mood. However, all the control subjects who achieved partial re-entrainment slept late on days off, and in many cases sleep was even later than the 3:00-noon schedule required of experimental subjects (e.g., see Figure 3, panels G-J in ref21). Thus if workers adopt a late enough sleep schedule on days off, then the other manipulations of light exposure may not be necessary. However, since we have shown in Studies A20 and B21 that the experimental interventions almost always produced partial or complete re-entrainment in our experimental subjects, the use of these interventions will reduce circadian misalignment while permitting a more socially acceptable sleep schedule.
In lieu of reducing circadian misalignment by shifting the sleepiest circadian time out of the night shift, alternative approaches for improving night shift alertness include symptomatic relief: caffeine consumption, prophylactic napping and the stimulant modafinil. Caffeine improves night shift alertness and performance in laboratory38,39 and field studies40 of night work, but does not overcome the strong circadian nadir in alertness late in the night shifts that is present when the circadian clock does not shift. An evening nap has also been shown to improve night shift performance.38 The combination of caffeine consumption and an evening nap substantially improve night shift performance and enhance the ability to remain awake,7 and could possibly be one of the best countermeasures for night shift alertness decrements when the circadian clock is not shifted. Modafinil has been shown to improve night shift alertness and performance in healthy volunteers.8 Modafinil also produces improvements in alertness in patients with shiftwork sleep disorder, but in this population of shiftworkers (who experience the most severe sequelae associated with night work) nighttime alertness is still seriously impaired.9 This is a population that could benefit greatly from circadian re-alignment, but whether partial re-entrainment (i.e., the attainment of a compromise circadian phase position) would improve night shift alertness and performance in these patients has not been tested.
The performance and alertness decrements during night of work are not only due to circadian misalignment, but are also due to increased homeostatic sleep pressure. This increase in sleep debt could arise when workers are awake for the entire day before working their first night shift, or may result from the chronic partial sleep deprivation that often accompanies night work. Reducing homeostatic sleep pressure during night shifts by scheduling the sleep episodes to occur before rather than after the night shifts (and advancing rather than delaying the circadian clock)41 can improve alertness and performance during night shifts.36 However, this strategy may be unappealing to most real shift workers, because afternoon/evening sleep episodes would then occur during the hours when most people enjoy leisure and social activities. Regardless of the direction that the circadian clock is shifted, substantial misalignment will still be present during the first few night shifts because the circadian clock adjusts slowly, and so enhancing alertness with caffeine or modafinil during these night shifts may be an option.
A possible criticism of the partial re-entrainment, compromise circadian phase position, approach is that many workers have rapidly rotating shifts rather than permanent night shifts, and thus realigning their circadian clocks with the work/sleep schedule is impossible. However, it may be possible to reduce circadian misalignment with the appropriate control of light and dark if the shifts rotate slowly.42 Furthermore, a United States population survey conducted in 2004 indicates that there are more permanent night workers (3.8 million) than rotating shift workers (3.3 million).43 From a safety perspective, rapidly rotating night shifts, which attempt to attenuate the severity of nighttime alertness impairments by minimizing the sleep deprivation that accompanies night work, do not address a primary cause of those alertness impairments, which is circadian misalignment (i.e. being awake at the sleepiest circadian time). Consequently, a permanent or very slowly rotating shift system that is compatible with partial circadian re-entrainment would be a more reliable way to improve night shift alertness. Studies are needed to test schedules to reduce circadian misalignment within a slowly rotating shift schedule.
One limitation of this study is that we did not know exactly where each subjects' circadian phase was during each night shift. We used circadian phase measurements from the final phase assessment that occurred the day after night shifts 5–7 (study A) or 3 days after night shift 8 (study B). Thus, actual circadian phase during the night shifts could have been different from what we measured during the phase assessment. This could have resulted in subjects at the edges of the not, partial, and complete re-entrainment groups to be placed in the “wrong” category.
Other limitations of these studies provide opportunities for future research. We did not assess mood, fatigue, or performance on days off. Night workers would likely need to feel reasonably well on their days off in order to be satisfied with partial re-entrainment to a compromise circadian phase position. Also, our studies were a hybrid between field and laboratory studies, with night shifts conducted in the laboratory and sleep occurring at home. Subjects were not real shift workers, but rather were young volunteers. This system for producing a compromise circadian phase position should be tested in real night shift workers.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Dr. Stephanie Crowley for her contribution to writing the grant that supported this research; Dr. Helen Burgess, Dr. Victoria Revell, Jillian Canton, Meredith Durkin, Valerie Ellios, Thomas Molina, Vanessa Meyer, Daniel Alderson, Meredith Rathert and Erin Cullnan for assistance with data collection and to our medical directors Dr. Keith Callahan and Dr. Margaret Park. We also thank Uvex Safety and the Sun Box Company. This work was supported by R01 OH003954 from NIOSH and the Centers for Disease Control and Prevention (CDC) to C.I.E. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIOSH or the CDC.
REFERENCES
- 1.Akerstedt T, Czeisler CA, Dinges DF, Horne JA. Accidents and sleepiness: a consensus statement from the international conference on work hours, sleepiness and accidents, Stockholm, 8-10 September 1994. J Sleep Res. 1994;3:195. [Google Scholar]
- 2.Dinges DF, et al. An overview of sleepiness and accidents. J Sleep Res. 1995;4(Suppl. 2):4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 3.Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light treatment for sleep disorders: consensus report. VI. Shift work. J Biol Rhythms. 1995;10:157–64. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 4.Monk TH, Buysse DJ, Reynolds CF, et al. Circadian rhythms in human performance and mood under constant conditions. J Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 5.Akerstedt T, Gillberg M. Displacement of the sleep period and sleep deprivation. Hum Neurobiol. 1982;1:163–71. [PubMed] [Google Scholar]
- 6.Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res. 2001;10:181–92. doi: 10.1046/j.1365-2869.2001.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night work. Sleep. 2006;29:39–50. doi: 10.1093/sleep/29.1.39. [DOI] [PubMed] [Google Scholar]
- 8.Walsh JK, Randazzo AC, Stone KL, Schweitzer PK. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep. 2004;27:434–9. doi: 10.1093/sleep/27.3.434. [DOI] [PubMed] [Google Scholar]
- 9.Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476–86. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SS, Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Terman M. Light treatment for sleep disorders: consensus report. III. Alerting and activating effects. J Biol Rhythms. 1995;10:129–32. doi: 10.1177/074873049501000205. [DOI] [PubMed] [Google Scholar]
- 11.Akerstedt T. Searching for the countermeasure of night-shift sleepiness. Sleep. 2006;29:19–20. doi: 10.1093/sleep/29.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–9. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- 13.Eastman CI. High intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol. 1992;263:R428–R36. doi: 10.1152/ajpregu.1992.263.2.R428. [DOI] [PubMed] [Google Scholar]
- 14.Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- 15.Dawson D, Encel N, Lushington K. Improving adaptation to simulated night shift: Timed exposure to bright light versus daytime melatonin administration. Sleep. 1995;18:11–21. doi: 10.1093/sleep/18.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- 17.Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Complete or partial circadian re-entrainment improves performance, alertness, and mood during night shift work. Sleep. 2004;27:1077–87. doi: 10.1093/sleep/27.6.1077. [DOI] [PubMed] [Google Scholar]
- 18.Lee C, Smith M, Eastman C. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 19.Smith MR, Cullnan EE, Eastman CI. Shaping the light/dark pattern for circadian adaptation to night shift work: Study 2. Physiol Behav. 2008;95:449–56. doi: 10.1016/j.physbeh.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Smith MR, Eastman CI. Night shift performance is improved by a compromise circadian phase position: Study 3. Circadian phase after 7 night shifts with an intervening weekend off. Sleep. 2008;31:1639–45. doi: 10.1093/sleep/31.12.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: Study 4. J Biol Rhythms. 2009;24:161–72. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- 22.Goel N. An arousing, musically enhanced bird song stimulus mediates circadian rhythm phase advances in dim light. Am J Physiol Regul Integr Comp Physiol. 2006;291:R822–7. doi: 10.1152/ajpregu.00550.2005. [DOI] [PubMed] [Google Scholar]
- 23.Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–9. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- 24.Goel N. Late-night presentation of an auditory stimulus phase delays human circadian rhythms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R209–R16. doi: 10.1152/ajpregu.00754.2004. [DOI] [PubMed] [Google Scholar]
- 25.Horne JA, Ostberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 26.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 27.Cernich A, Reeves D, Sun W, Bleiberg J. Automated Neuropsychological assessment metrics sports medicine battery. Arch Clin Neuropsychol. 2007;22(Suppl 1):S101–14. doi: 10.1016/j.acn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 29.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 30.Stone BM. Pencil and paper tests--sensitivity to psychotropic drugs. Br J Clin Pharmacol. 1984;18(Suppl 1):15S–20S. doi: 10.1111/j.1365-2125.1984.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves DL, Winter KP, Bleiberg J, Kane RL. ANAM genogram: historical perspectives, description, and current endeavors. Arch Clin Neuropsychol. 2007;22(Suppl 1):S15–37. doi: 10.1016/j.acn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Kane RL, Roebuck-Spencer T, Short P, Kabat M, Wilken J. Identifying and monitoring cognitive deficits in clinical populations using Automated Neuropsychological Assessment Metrics (ANAM) tests. Arch Clin Neuropsychol. 2007;22(Suppl 1):S115–26. doi: 10.1016/j.acn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Lowe M, Harris W, Kane RL, Banderet L, Levinson D, Reeves D. Neuropsychological assessment in extreme environments. Arch Clin Neuropsychol. 2007;22(Suppl 1):S89–99. doi: 10.1016/j.acn.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Short P, Cernich A, Wilken JA, Kane RL. Initial construct validation of frequently employed ANAM measures through structural equation modeling. Arch Clin Neuropsychol. 2007;22(Suppl 1):S63–77. doi: 10.1016/j.acn.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS ONE. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santhi N, Aeschbach D, Horowitz TS, Czeisler CA. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23:341–52. doi: 10.1177/0748730408319863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winer BJ. Statistical principles in experimental design. New York: McGraw-Hill; 1971. [Google Scholar]
- 38.Schweitzer PK, Muehlbach MJ, Walsh JK. Countermeasures for night work performance deficits: the effect of napping or caffeine on continuous performance at night. Work Stress. 1992;6:355–65. [Google Scholar]
- 39.Muehlbach MJ, Walsh JK. The effects of caffeine on simulated night-shift work and subsequent daytime sleep. Sleep. 1995;18:22–9. doi: 10.1093/sleep/18.1.22. [DOI] [PubMed] [Google Scholar]
- 40.Borland RG, Rogers AS, Nicholson AN, Pascoe PA, Spencer MB. Performance overnight in shiftworkers operating a day-night schedule. Aviat Space Environ Med. 1986;57:241–9. [PubMed] [Google Scholar]
- 41.Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol. 2002;282:R454–R63. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eastman CI. Bright light in work-sleep schedules for shift workers: Application of circadian rhythm principles. In: Rensing L, an der Heiden U, Mackey MC, editors. Temporal disorder in human oscillatory systems. Berlin-Heidelberg-New York: Springer-Verlag; 1987. pp. 176–85. [Google Scholar]
- 43.McMenamin TM. Mon Labor Rev. 2007. Dec 3-15, A time to work: recent trends in shift work and flexible schedules. [Google Scholar]