Abstract
Adenosine is a ubiquitous signaling molecule, with widespread activity across all organ systems. There is evidence that adenosine regulation is a significant factor in traumatic brain injury (TBI) onset, recovery, and outcome, and a growing body of experimental work examining the therapeutic potential of adenosine neuromodulation in the treatment of TBI. In the central nervous system (CNS), adenosine (dys)regulation has been demonstrated following TBI, and correlated to several TBI pathologies, including impaired cerebral hemodynamics, anaerobic metabolism, and inflammation. In addition to acute pathologies, adenosine function has been implicated in TBI comorbidities, such as cognitive deficits, psychiatric function, and post-traumatic epilepsy. This review presents studies in TBI as well as adenosine-related mechanisms in co-morbidities of and unfavorable outcomes resulting from TBI. While the exact role of the adenosine system following TBI remains unclear, there is increasing evidence that a thorough understanding of adenosine signaling will be critical to the development of diagnostic and therapeutic tools for the treatment of TBI.
Key Words: Adenosine deaminase, adenosine kinase, nucleotidase, nucleoside transport, caffeine, comorbidity.
INTRODUCTION – ADENOSINE IN TBI
Adenosine (Ado) is a signaling molecule with widespread actions throughout the body. A Pubmed search for adenosine reviews restricted to 2008 revealed over 50 articles reviewing adenosine physiology, pathology, modulation, and therapeutic targets for subjects ranging from cardiovascular, renal, enteric, and sleep function to asthma, inflammation, and dermal wound healing. In the central nervous system (CNS), adenosine (dys)regulation is implicated in cognition, psychiatric function, Parkinson’s disease, Alzheimer’s disease, epilepsy, and hypoxia/ischemia. This review is intended to highlight the role of adenosine acutely following TBI as well as the potential adenosine-related mechanisms in co-morbidities of and unfavorable outcomes resulting from TBI, building on earlier studies [113].
The distribution of adenosine receptors makes Ado both an attractive target for continuing study and provides a potentially daunting complication for adenosine-based treatment strategies. There is an extensive set of tools for the study of adenosine, including agonists and antagonists of varying selectivity and specificity, transgenic mice, and functional assays. Several adenosine-related therapeutic candidates are either currently available or in clinical trials [11, 52], potentially easing the regulatory burden for subsequent applications. However, the ubiquitous nature and diverse effects of adenosine signaling requires particular attention to drug delivery and activation, and the potentially significant side effect profile.
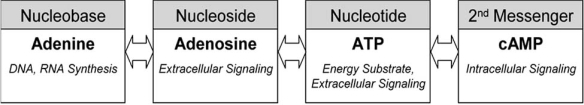
Adenosine is a potent neuromodulator, acting at CNS synapses to restrict synaptic activity via four known g-protein coupled receptor subtypes, reviewed in [11]. Adenosine receptors are found throughout the brain [15], and are implicated in diverse neurological functions and pathologies [48]. In addition to its role as a signaling molecule, the nucleoside Ado is an intermediary in a metabolic pathway that includes the nucleobase adenine, the nucleotide ATP (a primary energy substrate), and the second messenger cyclic adenosine monophosphate (cAMP) (Fig. (1)), which further highlights the varied consquences of Ado dysregulation. The neuroprotective role of Ado is well established in inflammation, ischemia/reperfusion injury, and asthma [90] as well as in diverse CNS diseases [20]. Unlike excitatory and inhibitory amino acids with an “all or none” effect, Ado acts in the CNS as a modulator [119], which may be a key factor reducing negative side effects such as those found with NMDA receptor antagonists [57].
Fig. (1).
Adenosine and its metabolites are active at all levels of cellular function.
Traumatic brain injury is a significant health burden in the United States; the US Centers for Disease Control estimated 1.4 million TBIs per year in 2001 [81]. In a recent survey of Iraq war veterans, 15% of returning soldiers reported a mild TBI; of those, 48% had symptoms of post-traumatic stress disorder [65]. Additional lasting effects significantly associated with a brain injury are chronic pain [102], fatigue and other sleep disturbances [141], cognitive problems [78], anxiety [98], and epilepsy [23, 122]. While these symptoms subside for many patients, they can persist for a lifetime of disability [66, 105].
NEUROPHYSIOLOGY OF THE ADENOSINE SYSTEM
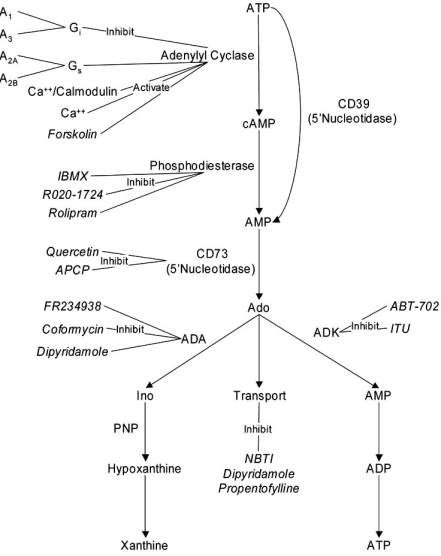
The A1 and A2A receptors are widely expressed in brain, with high adenosine affinity (~100nM [38]), and complementary actions. The A1 receptor is a Gi/Go coupled metabotropic receptor, acting to inhibit adenylyl cyclase and cAMP production, with uniform expression throughout the CNS [16, 37]. It is generally inhibitory at synapses, activating K+ and Cl- channels and inhibiting P- and N-type voltage gated calcium channels. The A2A receptor is a Gs coupled metabotropic receptor, activating adenylyl cyclase and cAMP production (Fig. (2)). While RT-PCR studies show expression throughout the brain [37], it is preferentially expressed in the striatum, nucleus accumbens, and thalamus [118]. A2A receptors interact with A1 receptors, forming functional heteromers [31], as well as with several excitatory receptors, notably the dopamine [8, 44] and glutamate systems [121, 135]. Free adenosine in the brain (the “tone”) is typically in the nanomolar range [11, 83]. Adenosine is increased locally to millimolar levels during low frequency synaptic activity [43], acting primarily via the A1 receptor as a presynaptic inhibitor of excitatory amino acid release and postsynaptically to maintain hyperpolarization [34]. Adenosine appears to act as the unifying signaling molecule in studies of the molecular basis of learning [34]. It acts as an autocrine signaling molecule at the tetanized synapse, enhancing synapse strength via A2A receptor activation [4]. It acts as a paracrin signal via a calcium wave in the astrocytic syncitium, acting distant from the tetanized synapse to achieve heterosynaptic depression by A1 receptor activation [58]. In addition to their role at the synapse, astrocytes release Ado at endothelial cells, causing vasodilation via A2A receptor activation, which enhances local circulation and provides the additional metabolic support rquired during intense synaptic stimulation [61].
Fig. (2).
Adenosine and metabolites regulated in response to TBI. Compounds in italics are exogenous drugs discussed in the text.
The low-affinity (micromolar [38]) A2B and A3 receptors are also widely expressed in brain, though at low levels [37]. Their low affinity for adenosine makes them likely mediators of excessive adenosine signaling, such as occurs in trauma, but there is little research on their specific roles. Like the A1 and A2A receptors, the A2B and A3 receptors have complementary actions; the A2B receptor is Gs coupled, and the A3 Gi/Gq coupled (Fig. (2)). Unlike the A1 and A2A receptors, their expression seems to be mainly astrocytic. Stimulation of the A2B receptor rapidly triggers interleukin-6 production, making this a likely step in the inflammatory response following trauma [140]. A2B receptors are upregulated following ischemic preconditioning, again suggesting a primary role in endogenous neuroprotective mechanisms [149]. The role of the A3 receptor is more controversial [10]. Studies have shown that A3 receptor activation is protective in astrocytes [17]. In neurons, a more complicated response has been revealed, with A3 receptor activation protecting CA1 neurons during short duration oxygen-glucose deprivation, yet causing damage during long-duration depriviation [116].
PATHOLOGY OF THE ADENOSINE SYSTEM AFTER TBI
The study of TBI is unusual in that the invasive procedures required to stabilize and monitor a severely injured patient have facilitated clinical studies that would be impossible in other injuries or chronic diseases. Intracranial pressure (ICP) has long been recognized as an indicator of TBI severity [56, 97]. ICP monitoring is an important component of a multi-modal monitoring procedure in the neurological intensive care unit [145]. As it requires insertion of a catheter into the ventricle, ICP monitoring allows regular monitoring of CSF. Consequently, we have an extensive understanding of changes to adenosine and its metabolites following human TBI.
Cyclic AMP
3'-5'-cyclic adenosine monophosphate (cAMP) is a key component of the adenosine metabolic pathway (Fig. (2)). cAMP is a second messenger regulated by G-protein coupled receptors which serve to rapidly couple extracellular signals to intracellular responses. cAMP is is modulated by diverse extracellular signals, including hormones, dopamine, glutmate, and adenosine. Current understanding of cAMP demonstrates the functional diversity of cAMP signaling [5] and highlights its role in synaptic plasticity [2, 39]. Specificity in cAMP signaling may be due to cellular compartmentalization [110, 148], though this is not yet well understood in brain cells.
Early studies of the second messenger cAMP were based on the hypothesis that cAMP is a metabolic regulator. Clinical studies show that depth of coma is correlated to reduced cAMP in CSF, falling as low as 1.5 nM during grade V coma, with a steady improvement correlated to coma depth, to nearly normal levels (20 nM) upon reaching grade I [45, 124]. Of note, in these studies plasma levels of cAMP remained normal (9-19 nM), even when CSF levels were < 6 nM, suggesting that the CSF cAMP measures reflect changes specific to the brain, and not a systemic reduction [45]. Regulation of CSF cAMP levels has been demonstrated in many neurological diseases. Eight-to-twelve hours following cerebral infarction, increased cAMP has been measured [100], but levels are depressed by 3 days [24]. Following epileptic seizure, depressed cAMP levels were measured for 3 days [100], and are chronically low in patients with multiple sclerosis [94]. These measurements of cAMP provide a valuable diagnostic tool, as well as insight into the evolution of brain injury.
Cerebral Blood Flow
Oxygen and glucose are critical to brain function; continuous cerebral blood flow (CBF) is necessary as there are no energy stores in the brain. The mechanisms of CBF and metabolism have been described in detail [142]. Autoregulatory mechanisms in the brain circulation can compensate for minor or short term disruptions in blood flow, pressure, and/or volume. Brain swelling is a common consequence of TBI; as the volume within the intact skull is fixed, such swelling results results in compression of the ventricles (reduction in CSF volume) and vasoconstriction (reduction in blood flow). While clinical monitoring of patients with severe head injury has revealed wide fluxuations in CBF, oxygen demand remains consistently low [108], even during hyperperfusion [33]. Kochanek et al. measured CSF levels of adenosine and cerebral oxygen use to test the hypothesis that adenosine could account for this uncoupling, as adenosine causes vasodilation via A2A receptors on endothelial cells, and represses synaptic activity through the A1 receptor on neurons. They found that increased adenosine was associated with depressed arterio-jugular venous oxygen difference and increased risk of death [32, 73]. Using microdialysis, Bell et al. demonstrated increased adenosine and cAMP levels in the cortex after TBI during secondary oxygen desaturation, correlating these increases to increased glutamate and lactate [14], further supporting a role for adenosine release as a critical mediator of the cerebral response to TBI.
Cellular Metabolism
Closely related to cerebral blood flow is cellular metabolism, the means by which brain cells produce their primary energy substrate ATP. The first step is glycolysis, in which glucose is converted to pyruvate:
Glucose + 2NAD+ + 2ADP + 2Pi >> Pyruvate + 2NADH + 2H+ + 2ATP + 2H2O.
Under physiologic conditions, pyruvate and NADH become the substrates for aerobic metabolism by the citric acid cycle, oxidizing NADH to NAD+ as well as generating ATP. Under anaerobic conditions, NAD+ is replenished by the enzyme lactate dehydrogenase:
Lactic acid is readily measured in the CSF, and a clear indicator of hypoxic conditions in the brain.
Hypoxia and ischemia are common co-morbidities of TBI [29, 55, 67], and their treatment is the focus of significant clinical literature [28, 51, 63, 64, 95, 150]. Not all cerebral metabolic crisis is due to ischemia however; combined PET scans and microdialysis showed significant increases in the lactate/pyruvate ratio in the absence of ischemia [143], suggesting increased anaerobic metabolism.
Breakdown of ATP in metabolically stressed tissue is a potentially significant source of adenosine; clinical measurements following TBI demonstrate lasting alterations in the adenosine metabolic pathway. Cerebralspinal fluid (CSF) samples taken following severe TBI show significant elevations in adenosine in both adults [32] and children [120, 129]. Further studies have shown that components of the adenosine metabolic pathway including cAMP, adenosine, inosine, hypoxanthine, and xanthine are all transiently upregulated during secondary hypoxic periods in both clinical [14], and experimental [13] settings. Clinically, Ado levels are typically highest at early time points, though late elevations (> 72 hours following TBI) have been noted in children [120], and likely reflect the progression of secondary injury. Using microdialysis, Bell et al. demonstrated increased adenosine and cAMP levels in the cortex after TBI during secondary oxygen desaturation, correlating these increases to increased glutamate and lactate [14]. In rodent models of fluid percussion injury, transient decreases in ATP (with recovery by 24 hours) followed similar kinetics of adenosine increase; however, when FPI was combined with secondary ischemia, ATP remained depressed at 24 hours [6]. While these studies make it clear that the adenosine system is altered by TBI, it is not yet clear whether this increased adenosine reflects endogenous neuroprotection mechanisms or is a byproduct of ATP breakdown.
MODULATION OF ADENOSINE RECEPTOR ACTIVITY
Direct modulation of adenosine (up to 30-fold) in response to stimuli has been measured in multiple model systems [83]. Neuroprotection by adenosine receptor modulation has been demonstrated in several model systems, including hypoxia/ischemia [123, 133], epilepsy [18, 101], Parkinson’s disease [27, 69, 130], inflammation [26, 60] among others [27, 38, 119]. They play a key role in many organ systems, however, and have been the target of several drug treatments for respiratory disease [137, 139, 147], cardiovascular disease [40, 111], and hepatic injury [12]. While no clinically effective neuroprotective strategies based on adenosine receptor modulation have yet emerged, modulation of adenosine receptor activity has provided insight into the diverse roles of adenosine in neural function and dysfunction.
Agonist/Antagonist Studies
In vivo studies of adenosine receptor modulation in TBI have demonstrated modest therapeutic improvements. The non-selective Ado agonist 2-chloro-adenosine (2CA), active at A2B > A1 > A2A receptors (IUPHAR database [59]), has been used in several experimental models. Kochanek et al. have demonstrated that intraparenchymal injection of 2CA increases CBF in the rat brain in a broad, dose-dependent and persistent manner [75]. Administration of 2CA by intrahippocampal injection after CCI restored CBF, more effectively in moderate injury than in severe injury [74]. By other measures, however, the effects of 2CA treatment are mixed. Pre-treatment with 2CA by intra-cerebral ventricular (ICV) injection demonstrated partial restoration of [Mg2+] levels, and a small, but non-significant, improvement in neuroscore at 1 and 7 days following injury [62]. Post-CCI, intrahippocampal injection of 2CA resulted in improved wire-grip scores but no improvement in memory tasks or hippocampal cell survival [138]. Post injury treatment with the A1 receptor agonist CCPA improved the CA3 cell count, but had no significant effects on learning and behavior measures [138], while the A1 receptor antagonist DPCPX and its solvent DMSO both resulted in increased lesion volume and reduced balance beam activity [138]. Together, these results reinforce the role of adenosine receptors as neuromodulators, with effects specific to the selected outcome measure. Additional studies with longer survival times are also likely to demonstrate more definitive effects of adenosine receptor antagonism and antagonism.
Interpretation of drug studies is complicated by our evolving understanding of receptor pharmacology as well as different methods of assessing agonist/antagonist kinetics. Within these limits, relative adenosine affinity must also be considered; A1 and A2A receptors have a much higher Ado affinity (10 – 100 times greater [38]) than A2B and A3 receptors, so modulation of the A2B and A3 receptors may only influence the most extreme adenosine response, and may define the difference between beneficial and harmful adenosine activity. The presence of A1/A2A heteromers further complicates the interpretation of activation/inhibition studies. The use of adenosine receptor knockout mice allows the examination of the specific receptor effects without the confounding effects of invasive drug delivery methods and non-specific vehicle effects (eg, DMSO). Adenosine receptor knockouts have a relatively normal phenotype, making them an attractive tool for the study of adenosine regulation following TBI [49]. Knockout of the A1 receptor leads to lethal status epilepticus following cortical contusion injury [76], reinforcing the neuroprotective role of the A1 receptor as a synaptic inhibitor. Concurrent measurement of cAMP in this model would be interesting, as A1 receptor activation inhibits adenylyl cyclase and A1 knockout would be expected to enhance cAMP production (Fig. (2)), giving some additional insight into the causality of the cAMP-adenosine cycle. Knockout of the A2A receptor is neuroprotective in the CCI model [87], with improved neuromuscular behavior, reduced tunel staining, and reduced glutmate release. Perhaps most interestingly, the pro-inflammatory cytokines TNF-α and IL-1β were significantly decreased by 24 hours following injury in the A2A knockout mice when compared to wild-type mice. Though the specificity of these effects is still unclear, these results further illustrate the complexity of adenosine signaling and the interdepencence of adenosine signaling, neural tissue, the immune response, and the cerebrovascular system; any therapy affecting one parameter is likely to have an affect on the others.
Caffeine
Caffeine is a well-known stimulant with a primary inhibitory activity at the A1 and A2A receptors. Coffee, tea, and soda are common sources of dietary caffeine, containing 40-120 mg/serving, and in excess of 200 mg in “energy” drinks. Elevated caffeine in CSF has been correlated to improved outcome in clinical studies; at 6 months following TBI, elevated caffeine was a stronger predictor of outcome than Glasgow Coma Score or alcohol on admission [126]. In modeled TBI, acute pre-treatment with caffeine (50 – 150 mg/kg) worsens outcome by all measures studied, including mortality, behavior, edema and blood-brain barrier breakdown, and peroxidase activity [3, 86]. In contrast, chronic pre-treatment with caffeine resulted in improvements in neuroscore, edema, apoptosis, CSF glutamate, and markers of the inflammatory response (CD45, TNF-α, and IL-1) [86]. Of note, chronic caffeine treatment resulted in increased A1 receptor mRNA expression [86]; a corresponding increase in A1 receptor expression is likely a significant contributor to the protective effects of chronic caffeine.
The effects of caffeine are not limited to the adenosine receptors. Caffeine stimulates IP-3 receptor mediated intracellular calcium release; calcium activates adenylyl cyclase, which catalyzes ATP into cAMP. Mechanical deformation of neural cells causes a significant acute rise in cytosolic [Ca2+] [82, 91, 125]; however, following stretch, it is not possible to to elicit this calcium response [146], suggesting that acute pre-treatment with caffeine may cause rapid and sustained increase in neuronal calcium, disrupting ionic homeostasis, and sensitizing the brain to a subsequent injury. The combined actions of caffeine to enhance cAMP production via adenylyl cyclase activation may have a role in the effects of acute caffeine treatment; however, evidence in the literature is contradictory. Clinically, cAMP increases during oxygen desaturation after TBI [14], but does not change during secondary swelling [73]. In a rodent model of CCI, no acute changes were measured in cAMP [13]. In contrast, following lateral fluid percussion injury, not only were significant reductions in cAMP measured, but phosphodiesterase inhibition with rolipram restored cAMP levels, reduced negative histopathological findings, and decreased the inflammatory response [7]. While the precise role of caffeine in the severity and recovery from TBI remains unknown, its wide use and clear effects in modeled TBI make it a potential confounding factor in clinical evaluation and treatment.
ADENOSINE REGULATION
Adenosine levels in the brain are typically in the range of 30-300 nM. Key Ado modulators include 5’-nucleotidases, adenosine deaminase (ADA), and adenosine kinase (ADK) (Fig. (2)). While neurons and glia both contribute to the maintainence of adenosinergic tone, expressing many similar enzymes, there is evidence that they respond to stimuli by different pathways [110]. Further, regionally distinct expression and activity of each of these enzymes suggests that the adenosine tone may differ by region [112]. Adenosine involvement in the sleep/wake cycle is well established (recent reviews include [80, 96, 132]), and activity of these adenosine modulators in the sleep regions of the brain follow similar diurnal patterns [92]. Further studies have demonstrated the effects of age on adenosine modulator activity, and shown that, while ADA does not vary with age, 5’nucleotidases and ADK both increase significantly with age [93]. While there is little direct study of these adenosine modulators in the context of TBI, their rapid and direct influence on the levels of adenosine in the brain make them a likely influence on outcome after TBI.
5’-Nucleotidases
Located on the cell surface (ecto-) and in the cytosol (cytosolic, endo-), 5’-nucleotidases act to hydrolyze ATP, AMP, and ADP to Ado [22]. Two commonly studied nucleotidases are CD39 (also known as apyrase and nucleotide triphosphate diphosphohydrolase) and CD73. CD39 hydrolyzes ATP to ADP and AMP, while CD73 hydrolyzes AMP to Ado. In particular, increases in CD73 function, mRNA, and protein have been shown in endothelial monolayers, as early as 8 hours following the onset of hypoxia or reperfusion [88, 90], suggesting that it may be a component of the endogenous response leading to ischemic preconditioning [47]. Models of pilocarpine-induced epilepsy suggest that enhanced ecto-nucleotidase activity may have a significant role in the “silent” phase of epileptogenesis [144]. In vivo studies examining the cellular response to a cortical stab injury (CSI) show a biphasic regulation of CD73: by 4 hours following the CSI, AMP hydrolysis is significantly reduced in the region of the stab wound [104], but by 15 days it has increased significantly in all regions of the brain considered [103]. In contrast, CD39 activity changed little, and only in the region of the CSI [104].
Adenosine Deaminase
Adenosine Deaminase (ADA) catalyzes the conversion of Ado to inosine (reviewed in [46]), and can be located either in the cytosol or on the extracellular surface of the plasma membrane of neurons, glia, and endothelial cells [30, 99, 117]. ADA acts rapidly during ischemic events to decrease local Ado [109]. As Ado has anti-inflammatory properties [26, 60], it has been hypothesized that inhibition of ADA might increase abient Ado, reducing inflammation. The ADA inhibitor FR234938 reduced plasma levels of the pro-inflammatory cytokine TNF-α and elevated the anti-inflammatory cytokine IL-10 in response to lipopolysaccharide treatment [77]. Further, treatment with the ADA inhibitor deoxycoformycin was protective following permanent focal ischemia, with a notable reduction in swelling [89]. Of interest, colocalization of ADA, the A1 receptor, and the cytokine CD26 (also known as dipeptidyl peptidase IV, DPP-IV) has been noted in many organs and across species [1, 16]. CD26 interacts directly with ADA on T-cells [70] as an active component of the immune response, and the colocalization of this trio of proteins on endothelial cells [79] may have a role in the regulation of the blood-brain barrier following trauma. While the mechanisms of these interactions are not clear, this colocalization and conservation across species suggest a causal mechanism.
Adenosine Kinase
In the developing brain, adenosine kinase (ADK) expression is primarily in the cytosol of neurons [134]. As the brain develops, however, ADK expression shifts to the cytosol of astrocytes; extracellular Ado is taken up by astrocytes via passive and facilitated diffusion, where it is phosphorylated into AMP by ADK. While it has not been studied directly in TBI, ADK regulation of adenosine has been implicated in many neurological disorders [20]. In acute injury such as ischemia, ADK upregulation is associated with poor outcome [115], while reduced ADK improves outcome [114], reviewed in [19]. Chronic diseases (such as epilepsy) have been associated with astrogliosis and ADK upregulation across multiple models, including kainic acid induced [42], kindled [85], and astrogliotic [84], reviewed in [21]. The additive effects of ADA and ADK inhibition [128] may provide an additional avenue for therapies targeting adenosine modulators.
Adenosine Transport
The third major system for adenosine clearance is nucleoside transporters, reviewed in [72]. In microdialysis studies, larger increases in ambient adenosine were measured in vivo in response to treatment with uptake inhibitors than were measured with ADA inhibitors, without the concurrent rise in adenosine metabolites inosine and hypoxanthine [9]. In a model of cerebral contusion, treatment with the transport inhibitor propentofylline increased expression of the neuroprotective peptide basic fibroblast growth factor [25]. As ethanol is a common factor in TBI, a potential additive role of adenosine in the cardiovascular response was measured in a swine model of TBI; however, no significant protection was measured with adenosine transport inhibition [41]. Adenosine uptake inhibition remains an untapped source of potential neurprotective strategies [106].
ADENOSINE REGULATION AND TBI CO-MORBIDITIES
While many TBI survivors regain gross motor and mental functions, comorbidities often remain throughout life. Studies following mild and moderate TBI survivors show improvements in community integration, cognitive and neuropsychiatric measures over 6-12 months [107], 5 years [136], and 22 years [105]. However, impairments persist, and there is significant opportunity for therapeutic intervention. Chronic pain is a common result of TBI, even after mild injury [102]. Adenosine modulation has been widely implicated in inflammation [26, 53, 60] and analgesia [38, 72, 127]. Anxiety is one of many neuropsychiatric comorbidities of TBI [98], with clear correlates to adenosine dysregulation [35, 54, 68]. Post traumatic epilepsy has a long latency, often decades, and is particularly common following severe injury in both pediatric [131] and adult [50] populations. Even in individuals without clinical seizure manifestations, epileptiform activity has been reported [122]. In an A1 receptor knockout mouse, TBI led to lethal status epilepticus [76], suggesting a clear role for the A1 receptor in seizure suppression. To date, two models of spontaneous seizure development following lateral fluid percussion injury (LFPI) have been published, with different characteristics. D’Ambrosio et al. reported rapid onset of high frequency, short duration cortical seizure activity, accompanied by generally mild behavior manifestations within the first 8 weeks of (1-2 on a the 1-5 Racine scale)[36]. Kharatishvili et al. report a latency of 2-12 months before seizure onset of seizures, similar to the latency observed clinically following TBI, with seizure activity lasting nearly two minutes [71]. Further studies implicate adenosine dysregulation in epileptogenesis and epilepsy, reviewed in [21]. Taken together, these results suggest that adenosine modulation a source of therapeutic potential across the spectrum of traumatic brain injury and recovery.
CONCLUSION
There is strong evidence that adenosine (dys)regulation plays a role in the brain following TBI. It is still not clear whether Ado is an endognous neuroprotective measure or a byproduct of stressed cellular metabolism; the truth is likely in between, and linked to injury severity. Current studies highlight the potential of adenosine to act in both protective and detrimental pathways; the multi-factoral pathologies of TBI and effects of adenosine signaling may mean that adenosine is not a practical target for neuroprotective therapies following TBI. Whether the adenosine system emerges as a neuroprotective target, it is clear that adenosine has a role in the evolution of neural recovery after traumatic brain injury, and a thorough understanding of its influences in the CNS will be critical in the development of diagnostic and therapeutic tools.
ACKNOWLEDGEMENTS
The author would like to thank Citizens United for Research in Epilepsy (CURE, CureEpilepsy.org) and the National Institutes of Health (NIH R21 NS057475) for their generous support.
ABBREVIATIONS
- 2CA
= 2-Chloroadenosine
- Ado
= Adenosine
- ADA
= Adenosine Deaminase
- ADK
= Adenosine Kinase
- ADP
= Adenosine Diphosphate
- AMP
= Adenosine Monophosphate
- ATP
= Adenosine Triphosphate
- cAMP
= Cyclic Adenosine Monophosphate
- CCI
= Cortical Contusion Injury
- CCPA
= 2-Chloro-N(6)-Cyclopentyladenosine
- CNS
= Central Nervous System
- CSF
= Cerebral Spinal Fluid
- CSI
= Cortical Stab Injury
- DMSO
= Dimethyl Sulfoxide
- DPP-IV
= Dipeptidyl Peptidase IV
- ICV
= Intra-Cerebroventricular
- LFPI
= Lateral Fluid Percussion Injury
- NMDA
= N-methyl-D-aspartic acid
- TBI
= Traumatic Brain Injury
REFERENCES
- 1.Abbott CA, Baker E, Sutherland GR, McCaughan GW. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics. 1994;40:331–338. doi: 10.1007/BF01246674. [DOI] [PubMed] [Google Scholar]
- 2.Abrams TW. Cyclic AMP (cAMP) Role in Learning and Memory. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 265–277. [Google Scholar]
- 3.Al Moutaery K, Al Deeb S, Ahmad Khan H, Tariq M. Caffeine impairs short-term neurological outcome after concussive head injury in rats. Neurosurgery. 2003;53:704–711. doi: 10.1227/01.neu.0000079487.66013.6f. discussion 711-702. [DOI] [PubMed] [Google Scholar]
- 4.Almeida T, Rodrigues RJ, de Mendonca A, Ribeiro JA, Cunha RA. Purinergic P2 receptors trigger adenosine release leading to adenosine A2A receptor activation and facilitation of long-term potentiation in rat hippocampal slices. Neuroscience. 2003;122:111–121. doi: 10.1016/s0306-4522(03)00523-2. [DOI] [PubMed] [Google Scholar]
- 5.Antoni FA. Molecular diversity of cyclic AMP signalling. Front. Neuroendocrinol. 2000;21:103–132. doi: 10.1006/frne.1999.0193. [DOI] [PubMed] [Google Scholar]
- 6.Aoyama N, Lee SM, Moro N, Hovda DA, Sutton RL. Duration of ATP reduction affects extent of CA1 cell death in rat models of fluid percussion injury combined with secondary ischemia. Brain Res. 2008;1230:310–319. doi: 10.1016/j.brainres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins CM, Oliva AA Jr, Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp. Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azdad K, Gall D, Woods AS, Ledent C, Ferre S, Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballarin M, Fredholm BB, Ambrosio S, Mahy N. Extracellular levels of adenosine and its metabolites in the striatum of awake rats: inhibition of uptake and metabolism. Acta Physiol. Scand. 1991;142:97–103. doi: 10.1111/j.1748-1716.1991.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 10.Baraldi PG, Cacciari B, Romagnoli R, Merighi S, Varani K, Borea PA, Spalluto G. A(3) adenosine receptor ligands: history and perspectives. Med. Res. Rev. 2000;20:103–128. doi: 10.1002/(sici)1098-1128(200003)20:2<103::aid-med1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Baraldi PG, Tabrizi MA, Gessi S, Borea PA. Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem. Rev. 2008;108:238–263. doi: 10.1021/cr0682195. [DOI] [PubMed] [Google Scholar]
- 12.Beldi G, Enjyoji K, Wu Y, Miller L, Banz Y, Sun X, Robson SC. The role of purinergic signaling in the liver and in transplantation: effects of extracellular nucleotides on hepatic graft vascular injury, rejection and metabolism. Front. Biosci. 2008;13:2588–2603. doi: 10.2741/2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell MJ, Kochanek PM, Carcillo JA, Mi Z, Schiding JK, Wisniewski SR, Clark RS, Dixon CE, Marion DW, Jackson E. Interstitial adenosine, inosine, and hypoxanthine are increased after experimental traumatic brain injury in the rat. J. Neurotauma. 1998;15:163–170. doi: 10.1089/neu.1998.15.163. [DOI] [PubMed] [Google Scholar]
- 14.Bell MJ, Robertson CS, Kochanek PM, Goodman JC, Gopinath SP, Carcillo JA, Clark RS, Marion DW, Mi Z, Jackson EK. Interstitial brain adenosine and xanthine increase during jugular venous oxygen desaturations in humans after traumatic brain injury. Crit. Care Med. 2001;29:399–404. doi: 10.1097/00003246-200102000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Benarroch E. EAdenosine and its receptors: multiple modulatory functions and potential therapeutic targets for neurologic disease. Neurology. 2008;70:231–236. doi: 10.1212/01.wnl.0000297939.18236.ec. [DOI] [PubMed] [Google Scholar]
- 16.Beraudi A, Traversa U, Villani L, Sekino Y, Nagy JI, Poli A. Distribution and expression of A1 adenosine receptors, adenosine deaminase and adenosine deaminase-binding protein (CD26) in goldfish brain. Neurochem. Int. 2003;42:455–464. doi: 10.1016/s0197-0186(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 17.Bjorklund O, Shang M, Tonazzini I, Dare E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur. J. Pharmacol. 2008;596:6–13. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 19.Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol. Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog. Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borowiec A, Lechward K, Tkacz-Stachowska K, Skladanowski AC. Adenosine as a metabolic regulator of tissue function: production of adenosine by cytoplasmic 5'-nucleotidases. Acta Biochim. Pol. 2006;53:269–278. [PubMed] [Google Scholar]
- 23.Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 24.Buttner T, Hornig CR, Busse O, Dorndorf W. CSF cyclic AMP and CSF adenylate kinase in cerebral ischaemic infarction. J. Neurol. 1986;233:297–303. doi: 10.1007/BF00314162. [DOI] [PubMed] [Google Scholar]
- 25.Buytaert-Hoefen KA, Kreber LA, Millar CJ, Walsh UT, Brannigan C, Hernandez TD. Propentofylline after focal cortical lesion in the rat: impact on functional recovery and basic fibroblast growth factor expression. Neurosci. Lett. 2002;331:188–192. doi: 10.1016/s0304-3940(02)00873-x. [DOI] [PubMed] [Google Scholar]
- 26.Chen JF, Pedata F. Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr. Pharm. Des. 2008;14:1490–1499. doi: 10.2174/138161208784480126. [DOI] [PubMed] [Google Scholar]
- 27.Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de Mendonca A. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and "fine tuning" modulation. Prog. Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Chesnut RM. Management of brain and spine injuries. Crit. Care Clin. 2004;20:25–55. doi: 10.1016/s0749-0704(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 29.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D'Onofrio M, Caciagli F, Di Iorio P. Involvement of astrocytes in purine-mediated reparative processes in the brain. Int. J. Dev. Neurosci. 2001;19:395–414. doi: 10.1016/s0736-5748(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 31.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark RS, Carcillo JA, Kochanek PM, Obrist WD, Jackson EK, Mi Z, Wisneiwski SR, Bell MJ, Marion DW. Cerebrospinal fluid adenosine concentration and uncoupling of cerebral blood flow and oxidative metabolism after severe head injury in humans. Neurosurgery. 1997;41:1284–1292. doi: 10.1097/00006123-199712000-00010. discussion 1292-1283. [DOI] [PubMed] [Google Scholar]
- 33.Cruz J, Jaggi JL, Hoffstad OJ. Cerebral blood flow, vascular resistance, and oxygen metabolism in acute brain trauma: redefining the role of cerebral perfusion pressure? Crit. Care Med. 1995;23:1412–1417. doi: 10.1097/00003246-199508000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem. Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr. Pharmaceut. Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 39.Eckel-Mahan KL, Storm DR. Second Messengers: Calcium and cAMP Signaling. In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. Oxford: Academic Press; 2008. pp. 427–448. [Google Scholar]
- 40.Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Exp. Opin. Investig. Drugs. 2008;17:1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- 41.Fabian MJ, Proctor KG. Hemodynamic actions of acute ethanol after resuscitation from traumatic brain injury. J. Trauma. 2002;53:864–875. doi: 10.1097/00005373-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 43.Ferre S, Borycz J, Goldberg SR, Hope BT, Morales M, Lluis C, Franco R, Ciruela F, Cunha R. Role of adenosine in the control of homosynaptic plasticity in striatal excitatory synapses. J. Integr. Neurosci. 2005;4:445–464. doi: 10.1142/s0219635205000987. [DOI] [PubMed] [Google Scholar]
- 44.Ferre S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr. Pharm. Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleischer AS, Rudman DR, Fresh CB, Tindall GT. Concentration of 3',5' cyclic adenosine monophosphate in ventricular CSF of patients following severe head trauma. J. Neurosurg. 1977;47:517–524. doi: 10.3171/jns.1977.47.4.0517. [DOI] [PubMed] [Google Scholar]
- 46.Franco R, Casado V, Ciruela F, Saura C, Mallol J, Canela EI, Lluis C. Cell surface adenosine deaminase: much more than an ectoenzyme. Prog. Neurobiol. 1997;52:283–294. doi: 10.1016/s0301-0082(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 47.Frassetto SS, Schetinger MR, Schierholt R, Webber A, Bonan CD, Wyse AT, Dias RD, Netto CA, Sarkis JJ. Brain ischemia alters platelet ATP diphosphohydrolase and 5'-nucleotidase activities in naive and preconditioned rats. Braz. J. Med. Biol. Res. 2000;33:1369–1377. doi: 10.1590/s0100-879x2000001100017. [DOI] [PubMed] [Google Scholar]
- 48.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int. Rev. Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 49.Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 50.Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- 51.Gaetz M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004;115:4–18. doi: 10.1016/s1388-2457(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 52.Gao ZG, Jacobson KA. Emerging adenosine receptor agonists. Exp. Opin. Emerg. Drugs. 2007;12:479–492. doi: 10.1517/14728214.12.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol. Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, Johansson B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur. J. Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- 55.Graham DI, Adams JH, Doyle D. Ischaemic brain damage in fatal non-missile head injuries. J. Neurol. Sci. 1978;39:213–234. doi: 10.1016/0022-510x(78)90124-7. [DOI] [PubMed] [Google Scholar]
- 56.Graham DI, Lawrence AE, Adams JH, Doyle D, McLellan DR. Brain damage in non-missile head injury secondary to high intracranial pressure. Neuropathol. Appl. Neurobiol. 1987;13:209–217. doi: 10.1111/j.1365-2990.1987.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 57.Gunduz-Bruce H. The acute effects of NMDA antagonism: From the rodent to the human brain. Brain Res. Rev. 2009;60:279–286. doi: 10.1016/j.brainresrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR, Greenhill SD, Hale VA, Sharman JL, Bonner TI, Catterall WA, Davenport AP, Delagrange P, Dollery CT, Foord SM, Gutman GA, Laudet V, Neubig RR, Ohlstein EH, Olsen RW, Peters J, Pin JP, Ruffolo RR, Searls DB, Wright MW, Spedding M. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res. 2009;37:D680–685. doi: 10.1093/nar/gkn728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J. Leukoc. Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 62.Headrick JP, Bendall MR, Faden AI, Vink R. Dissociation of adenosine levels from bioenergetic state in experimental brain trauma: potential role in secondary injury. J. Cereb. Blood Flow Metab. 1994;14:853–861. doi: 10.1038/jcbfm.1994.107. [DOI] [PubMed] [Google Scholar]
- 63.Heath DL, Vink R. Secondary mechanisms in traumatic brain injury: a nurse's perspective. J. Neurosci. Nurs. 1999;31:97–105. doi: 10.1097/01376517-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Hilton G. Cerebral oxygenation in the traumatically brain-injured patient: are ICP and CPP enough? J. Neurosci. Nurs. 2000;32:278–282. doi: 10.1097/01376517-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. New Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 66.Jacobsson LJ, Westerberg M, Lexell J. Demographics, injury characteristics and outcome of traumatic brain injuries in northern Sweden. Acta Neurol. Scand. 2007;116:300–306. doi: 10.1111/j.1600-0404.2007.00896.x. [DOI] [PubMed] [Google Scholar]
- 67.Jeremitsky E, Omert L, Dunham CM, Protetch J, Rodriguez A. Harbingers of poor outcome the day after severe brain injury: hypothermia, hypoxia, and hypoperfusion. J. Trauma. 2003;54:312–319. doi: 10.1097/01.TA.0000037876.37236.D6. [DOI] [PubMed] [Google Scholar]
- 68.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalda A, Yu L, Oztas E, Chen JF. Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson's disease. J. Neurol. Sci. 2006;248:9–15. doi: 10.1016/j.jns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 71.Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 72.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol. Sci. 2006;27:416–425. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Kochanek PM, Clark RS, Obrist WD, Carcillo JA, Jackson EK, Mi Z, Wisniewski SR, Bell MJ, Marion DW. The role of adenosine during the period of delayed cerebral swelling after severe traumatic brain injury in humans. Acta Neurochir. Suppl. 1997;70:109–111. doi: 10.1007/978-3-7091-6837-0_34. [DOI] [PubMed] [Google Scholar]
- 74.Kochanek PM, Hendrich KS, Jackson EK, Wisniewski SR, Melick JA, Shore PM, Janesko KL, Zacharia L, Ho C. Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spin-labeled magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2005;25:1596–1612. doi: 10.1038/sj.jcbfm.9600154. [DOI] [PubMed] [Google Scholar]
- 75.Kochanek PM, Hendrich KS, Robertson CL, Williams DS, Melick JA, Ho C, Marion DW, Jackson EK. Assessment of the effect of 2-chloroadenosine in normal rat brain using spin-labeled MRI measurement of perfusion. Magn. Reson. Med. 2001;45:924–929. doi: 10.1002/mrm.1123. [DOI] [PubMed] [Google Scholar]
- 76.Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 77.Kuno M, Seki N, Tsujimoto S, Nakanishi I, Kinoshita T, Nakamura K, Terasaka T, Nishio N, Sato A, Fujii T. Anti-inflammatory activity of non-nucleoside adenosine deaminase inhibitor FR234938. Eur. J. Pharmacol. 2006;534:241–249. doi: 10.1016/j.ejphar.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 78.Kwok FY, Lee TM, Leung CH, Poon WS. Changes of cognitive functioning following mild traumatic brain injury over a 3-month period. Brain Inj. 2008;22:740–751. doi: 10.1080/02699050802336989. [DOI] [PubMed] [Google Scholar]
- 79.Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 80.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem. Pharmacol. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 81.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2006 [Google Scholar]
- 82.LaPlaca MC, Lee VM, Thibault LE. An in vitro model of traumatic neuronal injury: loading rate-dependent changes in acute cytosolic calcium and lactate dehydrogenase release. J. Neurotrauma. 1997;14:355–368. doi: 10.1089/neu.1997.14.355. [DOI] [PubMed] [Google Scholar]
- 83.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 84.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J. Clin. Invest. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- 86.Li W, Dai S, An J, Li P, Chen X, Xiong R, Liu P, Wang H, Zhao Y, Zhu M, Liu X, Zhu P, Chen JF, Zhou Y. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151:1198–1207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 87.Li W, Dai S, An J, Xiong R, Li P, Chen X, Zhao Y, Liu P, Wang H, Zhu P, Chen J, Zhou Y. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp. Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Li X, Zhou T, Zhi X, Zhao F, Yin L, Zhou P. Effect of hypoxia/reoxygenation on CD73 (ecto-5'-nucleotidase) in mouse microvessel endothelial cell lines. Microvasc. Res. 2006;72:48–53. doi: 10.1016/j.mvr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 89.Lin Y, Phillis JW. Deoxycoformycin and oxypurinol: protection against focal ischemic brain injury in the rat. Brain Res. 1992;571:272–280. doi: 10.1016/0006-8993(92)90665-v. [DOI] [PubMed] [Google Scholar]
- 90.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 91.Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J. Neurotrauma. 2004;21:61–72. doi: 10.1089/089771504772695959. [DOI] [PubMed] [Google Scholar]
- 92.Mackiewicz M, Nikonova EV, Zimmerman JE, Galante RJ, Zhang L, Cater JR, Geiger JD, Pack AI. Enzymes of adenosine metabolism in the brain: diurnal rhythm and the effect of sleep deprivation. J. Neurochem. 2003;85:348–357. doi: 10.1046/j.1471-4159.2003.01687.x. [DOI] [PubMed] [Google Scholar]
- 93.Mackiewicz M, Nikonova EV, Zimmermann JE, Romer MA, Cater J, Galante RJ, Pack AI. Age-related changes in adenosine metabolic enzymes in sleep/wake regulatory areas of the brain. Neurobiol. Aging. 2006;27:351–360. doi: 10.1016/j.neurobiolaging.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 94.Maida E, Kristoferitsch W. Cyclic adenosine 3',5' monophosphate in cerebrospinal fluid of multiple sclerosis patients. J. Neurol. 1981;225:145–151. doi: 10.1007/BF00313327. [DOI] [PubMed] [Google Scholar]
- 95.Marik PE, Varon J, Trask T. Management of head trauma. Chest. 2002;122:699–711. doi: 10.1378/chest.122.2.699. [DOI] [PubMed] [Google Scholar]
- 96.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 97.Miller JD, Adams JH. The pathophysiology of raised intracranial pressure. In: Adams JH, Duchen LW, editors. Greenfield's Neuropathology. New York: Oxford University Press; 1992. pp. 69–105. [Google Scholar]
- 98.Moore EL, Terryberry-Spohr L, Hope DA. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 2006;20:117–132. doi: 10.1080/02699050500443558. [DOI] [PubMed] [Google Scholar]
- 99.Moriwaki Y, Yamamoto T, Higashino K. Enzymes involved in purine metabolism--a review of histochemical localization and functional implications. Histol. Histopathol. 1999;14:1321–1340. doi: 10.14670/HH-14.1321. [DOI] [PubMed] [Google Scholar]
- 100.Myllyla VV, Heikkinen ER, Vapaatalo H, Hokkanen E. Cyclic amp concentration and enzyme activities of cerebrospinal fluid in patients with epilepsy or central nervous system damage. Eur. Neurol. 1975;3:123–130. doi: 10.1159/000114668. [DOI] [PubMed] [Google Scholar]
- 101.Najm I, Ying Z, Janigro D. Mechanisms of epileptogenesis. Neurol. Clin. 2001;19:237–250. doi: 10.1016/s0733-8619(05)70017-7. [DOI] [PubMed] [Google Scholar]
- 102.Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300:711–719. doi: 10.1001/jama.300.6.711. [DOI] [PubMed] [Google Scholar]
- 103.Nedeljkovic N, Bjelobaba I, Lavrnja I, Stojkov D, Pekovic S, Rakic L, Stojiljkovic M. Early temporal changes in ecto-nucleotidase activity after cortical stab injury in rat. Neurochem. Res. 2008;33:873–879. doi: 10.1007/s11064-007-9529-0. [DOI] [PubMed] [Google Scholar]
- 104.Nedeljkovic N, Bjelobaba I, Subasic S, Lavrnja I, Pekovic S, Stojkov D, Vjestica A, Rakic L, Stojiljkovic M. Up-regulation of ectonucleotidase activity after cortical stab injury in rats. Cell. Biol. Int. 2006;30:541–546. doi: 10.1016/j.cellbi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Nestvold K, Stavem K. Determinants of health-related quality of life 22 years after hospitalization for traumatic brain injury. Brain Inj. 2009;23:15–21. doi: 10.1080/02699050802530540. [DOI] [PubMed] [Google Scholar]
- 106.Noji T, Karasawa A, Kusaka H. Adenosine uptake inhibitors. Eur. J. Pharmacol. 2004;495:1–16. doi: 10.1016/j.ejphar.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Novack TA, Alderson AL, Bush BA, Meythaler JM, Canupp K. Cognitive and functional recovery at 6 and 12 months post-TBI. Brain Inj. 2000;14:987–996. doi: 10.1080/02699050050191922. [DOI] [PubMed] [Google Scholar]
- 108.Obrist WD, Langfitt TW, Jaggi JL, Cruz J, Gennarelli TA. Cerebral blood flow and metabolism in comatose patients with acute head injury. Relationship to intracranial hypertension. J. Neurosurg. 1984;61:241–253. doi: 10.3171/jns.1984.61.2.0241. [DOI] [PubMed] [Google Scholar]
- 109.Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J. Neurochem. 2004;88:1305–1312. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- 110.Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol. Res. 2005;27:153–160. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- 111.Peart JN, Headrick JP. Adenosinergic cardioprotection: multiple receptors, multiple pathways. Pharmacol. Ther. 2007;114:208–221. doi: 10.1016/j.pharmthera.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Phillips E, Newsholme EA. Maximum activities, properties and distribution of 5' nucleotidase, adenosine kinase and adenosine deaminase in rat and human brain. J. Neurochem. 1979;33:553–558. doi: 10.1111/j.1471-4159.1979.tb05187.x. [DOI] [PubMed] [Google Scholar]
- 113.Phillis JW, Goshgarian HG. Adenosine and neurotrauma: therapeutic perspectives. Neurol. Res. 2001;23:183–189. doi: 10.1179/016164101101198316. [DOI] [PubMed] [Google Scholar]
- 114.Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J. Cereb. Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- 115.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J. Cereb. Blood Flow Metab. 2006;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- 116.Pugliese AM, Coppi E, Volpini R, Cristalli G, Corradetti R, Jeong LS, Jacobson KA, Pedata F. Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration. Biochem. Pharmacol. 2007;74:768–779. doi: 10.1016/j.bcp.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rathbone MP, Middlemiss PJ, Gysbers JW, Andrew C, Herman MA, Reed JK, Ciccarelli R, Di Iorio P, Caciagli F. Trophic effects of purines in neurons and glial cells. Prog. Neurobiol. 1999;59:663–690. doi: 10.1016/s0301-0082(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 118.Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 119.Ribeiro JA. What can adenosine neuromodulation do for neuroprotection? Curr. Drug Targets CNS Neurol. Disord. 2005;4:325–329. doi: 10.2174/1568007054546090. [DOI] [PubMed] [Google Scholar]
- 120.Robertson CL, Bell MJ, Kochanek PM, Adelson PD, Ruppel RA, Carcillo JA, Wisniewski SR, Mi Z, Janesko KL, Clark RS, Marion DW, Graham SH, Jackson EK. Increased adenosine in cerebrospinal fluid after severe traumatic brain injury in infants and children: association with severity of injury and excitotoxicity. Crit. Care Med. 2001;29:2287–2293. doi: 10.1097/00003246-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA. Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J. Neurochem. 2005;92:433–441. doi: 10.1111/j.1471-4159.2004.02887.x. [DOI] [PubMed] [Google Scholar]
- 122.Ronne-Engstrom E, Winkler T. Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurol. Scand. 2006;114:47–53. doi: 10.1111/j.1600-0404.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- 123.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudman D, Fleischer A, Kutner MH. Concentration of 3', 5' cyclic adenosine monophosphate in ventricular cerebrospinal fluid of patients with prolonged coma after head trauma or intracranial hemorrhage. N. Engl. J. Med. 1976;295:635–638. doi: 10.1056/NEJM197609162951202. [DOI] [PubMed] [Google Scholar]
- 125.Rzigalinski BA, Weber JT, Willoughby KA, Ellis EF. Intracellular free calcium dynamics in stretch-injured astrocytes. J. Neurochem. 1998;70:2377–2385. doi: 10.1046/j.1471-4159.1998.70062377.x. [DOI] [PubMed] [Google Scholar]
- 126.Sachse KT, Jackson EK, Wisniewski SR, Gillespie DG, Puccio AM, Clark RS, Dixon CE, Kochanek PM. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 2008;28:395–401. doi: 10.1038/sj.jcbfm.9600539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog. Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 128.Sciotti VM, Van Wylen DG. Increases in interstitial adenosine and cerebral blood flow with inhibition of adenosine kinase and adenosine deaminase. J. Cereb. Blood Flow Metab. 1993;13:201–207. doi: 10.1038/jcbfm.1993.24. [DOI] [PubMed] [Google Scholar]
- 129.Shore PM, Jackson EK, Wisniewski SR, Clark RS, Adelson PD, Kochanek PM. Vascular endothelial growth factor is increased in cerebrospinal fluid after traumatic brain injury in infants and children. Neurosurgery. 2004;54:605–611. doi: 10.1227/01.neu.0000108642.88724.db. discussion 611-602. [DOI] [PubMed] [Google Scholar]
- 130.Simola N, Morelli M, Pinna A. Adenosine A2A receptor antagonists and Parkinson's disease: state of the art and future directions. Curr. Pharm. Des. 2008;14:1475–1489. doi: 10.2174/138161208784480072. [DOI] [PubMed] [Google Scholar]
- 131.Statler KD. Pediatric posttraumatic seizures: epidemiology, putative mechanisms of epileptogenesis and promising investigational progress. Dev. Neurosci. 2006;28:354–363. doi: 10.1159/000094162. [DOI] [PubMed] [Google Scholar]
- 132.Stenberg D. Neuroanatomy and neurochemistry of sleep. Cell Mol. Life Sci. 2007;64:1187–1204. doi: 10.1007/s00018-007-6530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stone TW. Purines and neuroprotection. Adv. Exp. Med. Biol. 2002;513:249–280. doi: 10.1007/978-1-4615-0123-7_9. [DOI] [PubMed] [Google Scholar]
- 134.Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 135.Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Gro MC, Schwarzschild MA, Chen JF, Cunha RA, Popoli P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-D-aspartate effects. J. Neurochem. 2005;95:1188–1200. doi: 10.1111/j.1471-4159.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- 136.Temkin NR, Machamer JE, Dikmen SS. Correlates of functional status 3-5 years after traumatic brain injury with CT abnormalities. J. Neurotrauma. 2003;20:229–241. doi: 10.1089/089771503321532815. [DOI] [PubMed] [Google Scholar]
- 137.Trevethick MA, Mantell SJ, Stuart EF, Barnard A, Wright KN, Yeadon M. Treating lung inflammation with agonists of the adenosine A2A receptor: promises, problems and potential solutions. Br. J. Pharmacol. 2008;155:463–474. doi: 10.1038/bjp.2008.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Varma MR, Dixon CE, Jackson EK, Peters GW, Melick JA, Griffith RP, Vagni VA, Clark RS, Jenkins LW, Kochanek PM. Administration of adenosine receptor agonists or antagonists after controlled cortical impact in mice: effects on function and histopathology. Brain. Res. 2002;951:191–201. doi: 10.1016/s0006-8993(02)03161-x. [DOI] [PubMed] [Google Scholar]
- 139.Vass G, Horvath I. Adenosine and adenosine receptors in the pathomechanism and treatment of respiratory diseases. Curr. Med. Chem. 2008;15:917–922. doi: 10.2174/092986708783955392. [DOI] [PubMed] [Google Scholar]
- 140.Vazquez JF, Clement HW, Sommer O, Schulz E, van Calker D. Local stimulation of the adenosine A2B receptors induces an increased release of IL-6 in mouse striatum: an in vivo microdialysis study. J. Neurochem. 2008;105:904–909. doi: 10.1111/j.1471-4159.2007.05191.x. [DOI] [PubMed] [Google Scholar]
- 141.Verma A, Anand V, Verma NP. Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 2007;3:357–362. [PMC free article] [PubMed] [Google Scholar]
- 142.Verweij BH, Amelink GJ, Muizelaar JP. Current concepts of cerebral oxygen transport and energy metabolism after severe traumatic brain injury. Prog. Brain Res. 2007;161:111–124. doi: 10.1016/S0079-6123(06)61008-X. [DOI] [PubMed] [Google Scholar]
- 143.Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, Glenn TC, McArthur DL, Hovda DA. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vianna EP, Ferreira AT, Dona F, Cavalheiro EA, da Silva Fernandes MJ. Modulation of seizures and synaptic plasticity by adenosinergic receptors in an experimental model of temporal lobe epilepsy induced by pilocarpine in rats. Epilepsia. 2005;46(Suppl 5):166–173. doi: 10.1111/j.1528-1167.2005.01027.x. [DOI] [PubMed] [Google Scholar]
- 145.Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit. Care Clin. 2007;23:507–538. doi: 10.1016/j.ccc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Weber JT. Calcium homeostasis following traumatic neuronal injury. Curr. Neurovasc. Res. 2004;1:151–171. doi: 10.2174/1567202043480134. [DOI] [PubMed] [Google Scholar]
- 147.Wilson CN. Adenosine receptors and asthma in humans. Br. J. Pharmacol. 2008;155:475–486. doi: 10.1038/bjp.2008.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zaccolo M, Magalhaes P, Pozzan T. Compartmentalisation of cAMP and Ca(2+) signals. Curr. Opin. Cell Biol. 2002;14:160–166. doi: 10.1016/s0955-0674(02)00316-2. [DOI] [PubMed] [Google Scholar]
- 149.Zhou AM, Li WB, Li QJ, Liu HQ, Feng RF, Zhao HG. A short cerebral ischemic preconditioning up-regulates adenosine receptors in the hippocampal CA1 region of rats. Neurosci. Res. 2004;48:397–404. doi: 10.1016/j.neures.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 150.Zink BJ. Traumatic brain injury outcome: concepts for emergency care. Ann. Emerg. Med. 2001;37:318–332. doi: 10.1067/mem.2001.113505. [DOI] [PubMed] [Google Scholar]