Abstract
Over the last several decades the idea that adenosine (Ado) plays a role in sleep control was postulated due in large part to pharmacological studies that showed the ability of Ado agonists to induce sleep and Ado antagonists to decrease sleep. A second wave of research involving in vitro cellular analytic approaches and subsequently, the use of neurochemical tools such as microdialysis, identified a population of cells within the brainstem and basal forebrain arousal centers, with activity that is both tightly coupled to thalamocortical activation and under tonic inhibitory control by Ado. Most recently, genetic tools have been used to show that Ado receptors regulate a key aspect of sleep, the slow wave activity expressed during slow wave sleep. This review will briefly introduce some of the phenomenology of sleep and then summarize the effect of Ado levels on sleep, the effect of sleep on Ado levels, and recent experiments using mutant mouse models to characterize the role for Ado in sleep control and end with a discussion of which Ado receptors are involved in such control. When taken together, these various experiments suggest that while Ado does play a role in sleep control, it is a specific role with specific functional implications and it is one of many neurotransmitters and neuromodulators affecting the complex behavior of sleep. Finally, since the majority of adenosine-related experiments in the sleep field have focused on SWS, this review will focus largely on SWS; however, the role of adenosine in REM sleep behavior will be addressed.
PHENOMENOLOGY OF SLEEP
Sleep may be phenomenologically characterized by four criteria: a species specific posture, characteristic changes in the electroencephalogram (EEG), an increase in response threshold to environmental stimuli, and quick reversibility [76]. Using these criteria, sleep can be further divided into two main stages, slow wave sleep (SWS) and rapid eye movement sleep (REM). In mammals, EEG and electromyogram (EMG) activity is sufficient to determine sleep/waking state. During waking, the EEG is dominated by low amplitude, high frequency activity, with high muscle activity apparent in the EMG. During SWS, EEG activity is dominated by high amplitude, low frequency activity known as slow wave activity (SWA, 0.5-4.5 Hz oscillation in membrane potential), with little EMG modulation. During REM sleep, EEG activity is dominated by low amplitude, high frequency activity similar to that of waking, with EMG modulation absent except for the occasional muscle twitch. Sleep behavior is controlled by circadian and homeostatic mechanisms, with circadian rhythms influencing the time of day in which sleep expression is likely to occur and homeostatic mechanisms providing a cumulative ‘time awake’ input which results in increasing sleep drive with increasing amounts of waking. Examples of EEG traces for each of these states are shown in Fig. (1). The remainder of this review will focus in large part on SWS because Ado has been most closely linked, both theoretically and experimentally, to this sleep stage and to the EEG activity that characterizes it, SWA.
Fig. (1).
Raw electroencephalograph and electromyography examples of waking, SWS, and REM sleep from a C57/BL6 mouse. EEG signals were recorded from screw electrodes overlying the cortex, while EMG signals were recorded from panel electrodes from the dorsal neck muscle. Each trace shows a 10 sec epoch. The y-axis is set at 250 µv.
BRAIN REGIONS INVOLVED IN SLEEP BEHAVIOR
The hunt to identify the brain regions sufficient and necessary for the induction and maintenance of sleep behavior has been ongoing for many years. This search has been met with limited success because while there are many brain areas that show sleep modulated activity, no single brain area is necessary for sleep. Numerous brain areas implicated in sleep control have been lesioned, however, if an animal survives, it sleeps, though sometimes sleep returns only after several weeks of recovery [38, 73]. Currently there are several brain areas that are thought to be important for sleep control, including: mesopontine tegmentum [62], hypothalamus [66], and basal forebrain [27]. In vitro and in vivo studies have determined the effect of Ado agonists and antagonists on activity within these sleep centers. In the mesopontine tegmentum, Ado agonists inhibit cholinergic neurons [50] and reduce evoked glutamatergic EPSCs and GABAergic IPSCs [2]. The mesopontine tegmentum is part of the cholinergic arousal system so decreasing activity in these cholinergic, glutamatergic, and GABAergic cells would serve to facilitate sleep and the thalamocortical neural activity of burst firing that underlies the slow wave activity in the EEG during slow wave sleep [45, 50]. In the hypothalamus, Ado agonists inhibit the wake-active hypocretin/ orexin neurons [33] and disinhibit or excite the sleep-active neurons in the preoptic/anterior hypothalamic area and ventrolateral preoptic area [11, 19, 39, 40]. Finally, in the basal forebrain, Ado agonists inhibit wake-on neurons [1, 67]. The basal forebrain has been the focus of the hypothesis that Ado is involved in sleep homeostasis due to the finding that the basal forebrain was the only cholinergic arousal center to show sustained, elevated levels of Ado after 6 hrs of sleep deprivation [44]. Nonetheless, the failure to show a similar elevation in the cholinergic brainstem center may have resulted from the small size of nuclei within the brainstem and the relatively large size of the microdialysis probe. Together, direct inhibition of wake-active neurons and their inhibition through the excitation of sleep-active neurons may increase the probability that sleep with high slow wave activity will occur. Ado may contribute to this process through inhibition of arousal centers, as well as, through inhibition of thalamocoritical systems providing excitatory drive to these same centers.
ORIGIN OF EXTRACELLULAR ADO
Ado levels are influenced by neuronal activity. Ado is a secondary by-product of the breakdown of ATP and cAMP. When ATP is co-released with neurotransmitters, ecto-nucleotidases in the extracellular space can rapidly dephosphorylate ATP, ADP, and AMP into Ado [15]. Ado can also be released into the extracellular space by two equilibrative nucleoside transporters.
ATP release from astrocytes also contributes to extracellular levels of Ado that have a powerful modulatory effect on synaptic transmission [43]. The role of this astrocyte-derived Ado in sleep/waking homeostasis was recently investigated. Astrocytic transmitter release was prevented in a mutant mouse using a conditional knockout of the synaptobrevin II protein involved in exocytosis that was expressed only in astrocytes [22]. These mutant mice spent the same amount of time in waking, SWS, and REM sleep as wildtype mice, however mutant mice showed reduced SWA and a decrease in recovery sleep following sleep deprivation.
INFLUENCE OF ADO LEVELS ON SLEEP AND WAKEFULNESS
In the mid to late 1900s it was found that Ado agonists decrease wakefulness and increase sleep [10, 17, 23, 46]. Furthermore, such agonists also tend to increase deeper stages of SWS at the expense of lighter SWS [49], with deep and light stages defined on the basis of amount of slow waves, greater than 50% per epoch versus less than 50% per epoch, respectively. Additionally, Ado agonists increase SWA or delta power [6, 61] as assessed by Fast Fourier Transform (FFT) analysis. SWA power reflects the relative amount of the EEG signal that falls within the SWA band (0.5-4.5 Hz).
Conversely, Ado receptor antagonists increase wakefulness and decrease sleep [61, 72, 75]. One of the most commonly used pharmacological agents, caffeine, is a nonselective Ado antagonist which primarily acts at two of the four Ado receptor subtypes, the A1R and A2aR to influence sleep/waking behavior. The estimated daily intake of caffeine in American citizens is about 280 milligrams, which is above the functional dose for decreasing sleep [29, 31]. Furthermore, caffeine and other antagonists decrease SWA within sleep as well [31, 32], an effect which is modulated by caffeine-sensitivity in humans [52]. Both agonists and antagonists affect sleep and SWA when given systemically [49, 61, 75] or within the brain [6, 39, 41, 46, 70]. Some of the biggest effects are seen when Ado is injected directly into the basal forebrain [4, 46]. This point will be discussed in detail in the next section.
In addition to agonists and antagonists, other compounds that alter endogenous Ado levels have been shown to modify sleep and SWA within sleep. These compounds include an Ado kinase inhibitor that increases Ado levels by inhibiting the phosphorylation of Ado to AMP, Ado deaminase inhibitors, which increase Ado levels by inhibiting the breakdown of Ado into inosine, and transport inhibitors, which blocks the transport of Ado into the cell. Ado kinase, deaminase, and transport inhibitors decrease wakefulness and increase sleep [45, 47, 48]. The Ado kinase inhibitor also increases SWA within SWS [47]. Furthermore, recent genetic screening experiments have shown that a genetic variant of Ado deaminase that decreases metabolism of Ado to inosine, results in an increase in deep SWS and SWA [53].
Evidence from extracellular injection of antisense mRNA is consistent with the idea that the expression of Ado receptor levels influence sleep/waking behavior. Injection of antisense deoxyoligonucleotides against Ado receptor mRNA into the basal forebrain, increases waking, while decreasing SWS and SWA [69]. Under conditions of recovery from 6 hr sleep deprivation, changes in waking, sleep, and SWA were even more pronounced. However some caution in the interpretation of these results is needed because of the well documented non-specific and “off-target” effects of antisense injections. We did not observe any change in SWS duration with localized knockout of the A1R [technique described in 58] in either the forebrain or the brainstem cholinergic arousal centers using an AAV-vector mediated localized expression of Cre recombinase together with a floxed A1R gene [unpublished observations, Ronan, P. and Greene, R.W., 2002]. Based on these observations we concluded that A1Rs on pre-synaptic terminals are especially important for the SWS effects of localized injections of either A1R agonists [46] or blockers of Ado re-uptake [45] since these pre-synaptic receptors would not be affected by localized knockout of the A1R gene (Ronin and Greene unpublished observations). In fact, these findings led us to use a conditional knockout of the A1R that would affect most of the pre-synaptic terminals that synapse onto neurons of the cholinergic arousal centers [7] as described below.
Together the evidence from Ado agonists, antagonists, an Ado kinase inhibitor, Ado deaminase inhibitors, and an Ado transport blocker all suggest that increasing Ado levels increases sleep, while decreasing Ado levels increases wakefulness.
INFLUENCE OF BEHAVIORAL STATE (WAKEFULNESS AND SLEEP) ON ADO LEVELS WITH AN EMPHASIS ON THE BASAL FOREBRAIN
As mentioned in a previous section, injection of Ado agonists and antagonists into the brain, and specifically into the basal forebrain [46, 65], modify neural activity and ultimately influence behavioral state, arguing that modification of Ado levels is sufficient to alter sleep/waking state. This raises the question of whether sleep/waking state influences Ado levels. If Ado is, in fact, involved in controlling sleep/waking state, then Ado levels should rise during waking and decrease during subsequent sleep.
During sleep Ado levels decrease in the cortex, basal forebrain, hypothalamus, and brainstem [44, 45]. When animals are kept awake for extended periods of time (sleep deprivation), however, levels of Ado continue to rise or are stable only in one of these brain regions, the basal forebrain [4, 44]. This piece of evidence lead to a modification of the hypothesis that Ado is involved in sleep/waking control, to the hypothesis that Ado acts specifically within the basal forebrain to influence sleep/waking state. However, as previously mentioned this lack of evidence may, at least in part reflect the technical limitations of the microdialysis technique used to assess these changes. Evidence from in vitro studies suggests that in the brainstem cholinergic arousal center, persistent, increased synaptic glutamate release within the range of what may be expected during waking will gradually increase Ado levels in the microenvironment of the synapse and this in turn, will feedback to decrease glutamate through activation of pre-synaptic A1Rs [9]. This same kind of effect is likely to occur in other brain regions involved in sleep induction and maintenance and their target regions as well. Ado levels progressively rise during waking, inhibiting cholinergic and noncholinergic neurons, thereby decreasing acetylcholine release which underlies the cholinergic arousal system [50]. Furthermore, decreased acetylcholine release facilitates the transition of fast spiking to burst pause firing in thalamocortical neurons [36], which serves to synchronize neural firing, resulting in the high amplitude, low frequency activity that underlies SWA during sleep.
A recent controversy developed when Blanco-Centurion and colleagues tested the hypothesis of behavioral state modification by Ado’s action on cholinergic neurons in the basal forebrain. They selectively lesioned cholinergic basal forebrain neurons using the cholinergic-selective toxin 192 immunoglobulin-saporin (IgG-saporin) [8]. However, they found that although Ado levels did not increase in these lesioned animals, they still had normal sleep homeostasis. This finding challenged the belief that Ado modulated behavioral state by acting on cholinergic neurons in the basal forebrain. However, subsequent research using IgG-saporin has reinstated the idea that cholinergic neurons in the basal forebrain are involved in the Ado regulation of sleep homeostasis. Two groups (McCarley and Semba) directly injected IgG-saporin into the basal forebrain to destroy cholinergic neurons and found that lesioned animals displayed significantly less rebound sleep in response to acute sleep deprivation, indicating abnormal sleep homeostasis [28, 30]. Additionally, the McCarley group also used i.c.v. IgG-saporin injections to decrease basal forebrain cholinergic cell numbers and found that two weeks after injection, animals showed normal sleep homeostasis in response to sleep deprivation, while three weeks after injection, animals displayed abnormal sleep homeostasis [28]. Therefore, it is likely that Blanco-Centurion may not have found an effect of cholinergic cell lesions on sleep homeostasis because they measured the effect of sleep deprivation on homeostasis two weeks following i.c.v. injection.
ADO RECEPTORS INVOLVED IN MODULATING SLEEP HOMEOSTASIS
One question that has fueled several recent experiments is: which Ado receptor(s) underlie the effects of Ado on sleep/waking homeostasis? There are currently 4 known Ado receptors, A1R, A2aR, A2bR and A3R. For sleep/waking homeostasis the A1Rs and A2aRs have received the most attention due to their expression pattern in the nervous system, the availability of selective agonists and antagonists and selective molecular lesions of genes encoding the receptor subtypes. A1R are expressed at high levels throughout the brain, particularly in the cortex, hippocampus, thalamus and cerebellum [14, 51]. A2aRs are expressed most strongly in the striatum [18]. Using RT-PCR, Dixon and colleagues found low levels of A2aR , A2bR, and A3R ’s expressed throughout the brain [14].
A1R agonists and antagonists modify sleep/waking when given peripherally [49, 72] and centrally [34, 39, 68]. A2AR agonists and antagonists also modify sleep/waking when given peripherally [61, 75] and centrally [12, 24, 34, 39, 56, 59]. Additionally, in humans, A1Rs are upregulated following sleep deprivation [16], while in rodents A1Rs are upregulated in the basal forebrain following sleep deprivation [3].
A1Rs are Gi-coupled and generally considered inhibitory, while A2aR s are Gs coupled and considered excitatory. A1Rs may modify sleep/waking by direct and indirect mechanisms including: inhibition of cholinergic neurons in the mesopontine tegmentum that are part of the cholinergic arousal system [50], inhibition wake-active hypocretin/orexin neurons in the lateral hypothalamus [33] and wake-active neurons in the basal forebrain [1, 67], and disinhibition of sleep-active neurons in the preoptic/anterior hypothalamic area and ventrolateral preoptic area [11, 40].
Mechanisms of sleep/waking homeostasis for A2ARs include: inhibition of histaminergic neurons [24], excitation of sleep-active neurons in the ventrolateral preoptic nucleus [19], and modulation of acetylcholine release in the pontine reticular formation [12], resulting in increased time in SWS and REM sleep. Additionally, A2AR agonists increase SWS and REM sleep when injected into the prostaglandin D2 sensitive zone under the basal forebrain, while injection of A2AR antagonists into the same zone decreases prostaglandin D2-induced SWS [57]. The most dominant A2aR effect in rodents may be indirect through activation of inhibitory motor circuits in the striatum, where most of the A2aR s are expressed. This may explain the robust stimulant effects of the non-specific adonesine receptor antagonist, caffeine in A1R knock out animals, while A2aR knock out animals showed much subtler effects at higher doses of caffeine [25]. Locomotor activity is increased in these animals and locomotion is a powerful arousing stimulus in rodents. In contrast to the reported adenosine antagonists arousing effects being mediated through A2aR, injection of an A2aR antagonist into the ventral striatum increased time in SWS and REM sleep, as well as, decreased FOS expression within wake-active orexin neurons in the hypothalamus [55] which provides a mechanism to control sleep behavior that may not be locomotion-specific. Alternatively, this effect may have been mediated through a non-specific action of the A2aR antagonist to cause phosphodiesterase inhibition.
Recent experiments have used genetic manipulation to determine whether A1Rs or A2aR s are responsible for the effect of Ado on sleep/waking homeostasis. Stenberg and colleagues used constitutive A1R knockout mice and found normal sleep/homeostasis and SWA in response to sleep deprivation by gentle handling [63]. In contrast, our lab, using conditional A1R knockout mice (a Cre-loxP based system using a CAMKII promoter for the Cre recombinase gene), found a significant decrease in SWA in response to both acute and chronic sleep restriction [7]. For acute sleep restriction, we used a slowly moving treadmill (~ 3 cm/sec) to enforce waking for 4 hrs followed by 2 hrs of unrestricted behavior. For chronic sleep restriction, this 6 hr cycle (4 hrs treadmill on, 2 hrs treadmill off) was repeated 8 times across 48 hrs. The use of the treadmill to enforce waking is a potentially less variable method of sleep restriction since, unlike gentle handling, in which animals are given novel objects on an “as needed” basis in which arousal state could fluctuate, the treadmill is constantly “on” and the animals are forced to move at a relatively constant rate. Interestingly, the mice with intact A1Rs, compensated for the sleep restriction with increased SWA but unchanged SWS duration (SWS duration was compared to the same time period under non-sleep restricted conditions). The compensatory SWA response in the A1R knockout mice was largely missing. These conditional knockout mice were able to express SWA during SWS but were unable to increase SWA when sleep was restricted. Thus, the A1R receptor is involved in the homeostatic modulation of SWA, but is not necessary for SWA expression, in other words, while the A1R is not necessary for the expression of SWA (knockout mice still show EEG activity in the SWA band), the A1R does play a role in the modulation of SWA (knockout mice show significantly less sleep-deprivation induced SWA).
As for the effect of A2aRs on sleep homeostasis, Urade and collegues used A2aR knockout mice and found a lack of modulation of sleep/waking in response to injection of A1R agonists into the lateral ventricle [71], suggesting that A2aRs underlie the ability of Ado to modulate sleep/waking. Negative results are difficult to interpret in this case and may have resulted from an insufficient concentration of A1R agonist at the most relevant receptor sites, including pre-synaptic excitatory terminals onto basal forebrain neurons. In a separate report, this group showed that A2aR knockout mice have abnormal autonomic control during REM sleep compared to wildtype controls [54].
A1R ACTIVATION AND THE FUNCTION OF SWA OF SLEEP
The loss of A1Rs in the CNS resulted in a remarkably specific sleep phenotype, characterized by the absence of the compensatory increase in SWA in response to sleep restriction. Although there was a significant decrease in SWA expression in A1R knockout mutants under baseline conditions, the effect size was small. Under baseline conditions, the mutants could perform a working memory task equally well compared to their matched wild phenotype littermates. When both groups of mice were challenged with sleep restriction, only the mutants showed a reduced performance in association with a loss of compensatory SWA response [7].
The working memory task places particular demands on prefrontal cortical circuits that involve sustained neuronal activity, thought to reflect active, short term encoding of the working memory [20]. The selective loss of compensatory SWA may have compromised the ability of the prefrontal cortical circuits to generate and sustain the needed neuronal activity for effective working memory dependent performance. This is a different kind of deficit than that involving consolidation of memory as working memory is short lasting (less than 10’s of seconds) and does not involve consolidation at all. Our findings suggest that with mild sleep restriction, working memory function may be more sensitive to compensatory SWA loss than hippocampal dependent memory and its consolidation [7].
A1R ACTIVATION LEADS TO INCREASED SWA AT THE CELLULAR LEVEL IN THALAMOCORTICAL NEURONS
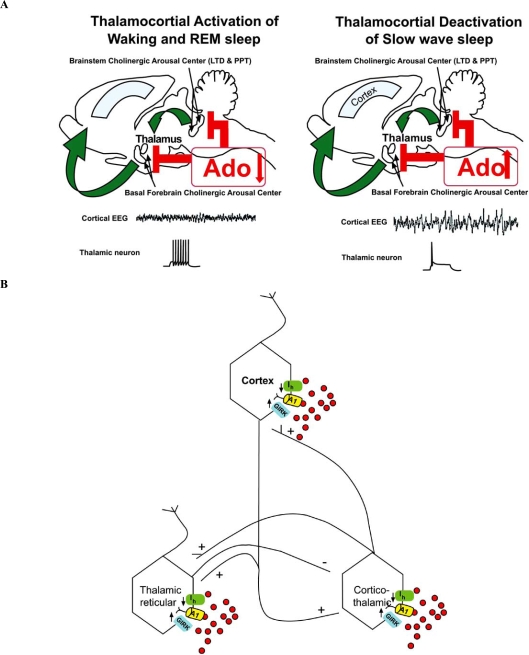
Several investigators have shown that modulation of A1Rs influence SWA without modifying sleep time. Activation of A1Rs can enhance SWA through two, additive mechanisms; one indirect that involves the brainstem cholinergic arousal system and the other through direct actions on thalamic and cortical neurons. Fig. (2) shows an illustration of both the indirect and direct sites of action and effects of Ado on SWA. The indirect Ado action results from high waking (and REM sleep) cholinergic tone in thalamocortical neurons, relative to the low tone during SWS [35, 74]. This tone is in turn modulated in the brainstem and basal forebrain cholinergic arousal centers in a state specific manner by A1Rs [21, 45, 50]. Acetylcholine inhibits slow frequency activity in thalamocortical neurons [13, 37, 64], so inhibition of brainstem cholinergic neurons via A1Rs during SWS allows the expression of slow wave activity.
Fig. (2).
A. Indirect A1R -mediated modulation SWA. Projections from the brainstem reticular formation cholinergic arousal center to the thalamus and from basal forebrain cholinergic arousal center to the cortex and thalamus mediate the cholinergic tone. During waking, cholinergic tone is high, resulting in a desynchronized, high frequency cortical EEG (middle trace), as is monoaminergic tone (not shown). During REM sleep only cholinergic tone is high in association with a desynchronized EEG, similar to that observed during waking. The desynchronized activity is due in part to the cholinergically induced depolarization of thalamic and cortical neurons facilitating synaptically responsive non-bursting spiking patterns (bottom trace). During SWS, cholinergic tone is decreased due, in part to increased adenosine mediated inhibition of cholinergic center arousal neurons. The emergence of synchronized, low frequency cortical EEG (middle trace) requires this decreased activity in the cholinergic arousal centers of the forebrain and brainstem, allowing hyperpolarization of thalamic and cortical neurons. This is a necessary condition for burst-pause oscillatory firing of thalamic and cortical neurons (bottom trace). The thalamic neuron recordings were made in vitro, under control, waking-like conditions (bottom left) and in the presence of adenosine to approximate SWS-like conditions (modified with permission from [42]).
B. Activation of A1Rs can directly facilitate SWA. Ado hyperpolarizes thalamic and cortical neurons by increasing potassium conductance through the GIRK channel and by decreasing the Ih current, which facilitates burst-pause firing at the expense of single spikes [42]. Both these changes also reduce cell conductance at the threshold for burst generation providing an additional facilatory effect on bursting. These effects may be synchronized by the thalamocortical- thalamo-reticular circuits to generate the high amplitude SWA observed during sleep.
The direct effect of A1R activation to facilitate SWA of thalamic and cortical neurons is due to a combination of increased GIRK channel conductance and decreased hyperpolarization activated current and a relative functional deafferentation resulting from pre-synaptic inhibition (as shown in Fig. 3). In vitro circuit analysis has shown that Ado will enhance slow oscillations of single neurons in the absence of other modulatory input [42]. Localized increase of Ado can induce these post-synaptic effects and when combined with local pre-synaptic A1R activation to reduce afferent input, and can increase SWA measured with local field recordings as demonstrated by localized blockade of Ado uptake with nitrobenzylthioinoside [45]. It is reasonable to suggest that, just as with brainstem arousal neurons [9], increased synaptic activation of NMDARs during waking in the thalamocortical system will slowly increase local Ado concentration to directly enhance SWA in localized thalamocortical circuits. In fact asymmetric increases in SWA activity have been observed in association with asymmetric use-dependent activity during waking [26].
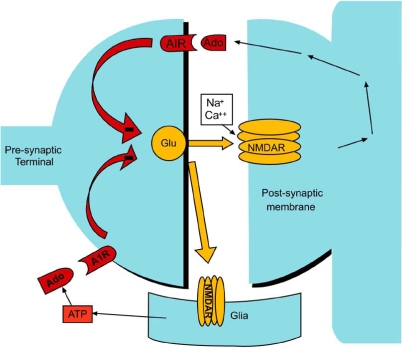
Fig. (3).
Pre-synaptic effect of adenosine on glutamatergic activity. During cellular activity, adenosine is released from neurons and glia. This adenosine feeds back onto pre-synaptic neurons to inhibit glutamate release via A1Rs. This inhibition of an excitatory compound reduces neural activity.
The modulation of SWA in a use-dependent fashion is a key prediction of the hypothesis that sleep serves as a restorative function for the brain. Benington and Heller posited that the increased release of adenosine that occurs due to waking activity, works through A1Rs to facilitate the expression of SWA, which allows for the glycogen stores, depleted during waking, to be replenished during sleep [5]. Recently Pack and colleagues reviewed research that supports the overall hypothesis and have included additional metabolic-factors, besides Ado, that may play a role in energy restoration during sleep [60]. Finally, the use of A1R knockout animals that show reduced SWA following sleep deprivation [7] and the use of a mutant mouse with inhibited astrocytic-release of adenosine [22] indicate that adenosine is an important factor controlling SWA expression. Additionally, these studies show that increased SWA expression during recovery sleep following sleep deprivation is important for cognitive performance [7], but increased SWA pressure during waking impairs recognition memory [22].
CONCLUDING REMARKS
There is ample evidence that Ado plays a role in regulating sleep/waking behavior, as well as, SWA. Together this evidence suggests that both A1Rs and A2aRs act in various areas of the brain to decrease neural activity and facilitate sleep, although the role of the A2aR may be indirect via the striatal locomotor systems. A1Rs primarily act to both reduce cholinergic tone and, in thalamocortical systems, to facilitate cellular oscillations at SWA frequency in response to sleep loss. Gene deletion of CNS A1Rs prevents the compensatory SWA enhancement when sleep is restricted, in association with reduced cognitive performance. This suggests a functional role for A1R mediated compensatory SWA activity to maintain normal cognitive performance.
ACKNOWLEDGEMENTS
This work is funded by NIH grant R01 MH067777-05 and the Department of Veterans Affairs.
REFERENCES
- 1.Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J. Physiol. 1999;521(Pt 3):679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigoni E, Rainnie DG, McCarley RW, Greene RW. Adenosine-mediated presynaptic modulation of glutamatergic transmission in the laterodorsal tegmentum. J. Neurosci. 2001;21:1076–1085. doi: 10.1523/JNEUROSCI.21-03-01076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–1899. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- 4.Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain Res. Mol. Brain Res. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- 5.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog. Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 6.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 7.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J. Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the Basal forebrain. J. Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambilla D, Chapman D, Greene R. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron. 2005;46:275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Buday P, Carr CJ, Miya TS. A pharmacological study of some nucleosides and nucleotides. J. Pharm. Pharmacol. 1961;13:290–299. [Google Scholar]
- 11.Chamberlin NL, Arrigoni E, Chou TC, Scammell TE, Greene RW, Saper CB. Effects of adenosine on gabaergic synaptic inputs to identified ventrolateral preoptic neurons. Neuroscience. 2003;119:913–918. doi: 10.1016/s0306-4522(03)00246-x. [DOI] [PubMed] [Google Scholar]
- 12.Coleman CG, Baghdoyan HA, Lydic R. Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J. Neurochem. 2006;96:1750–1759. doi: 10.1111/j.1471-4159.2006.03700.x. [DOI] [PubMed] [Google Scholar]
- 13.Curro Dossi R, Pare D, Steriade M. Short-lasting nicotinic and long-lasting muscarinic depolarizing responses of thalamocortical neurons to stimulation of mesopontine cholinergic nuclei. J. Neurophysiol. 1991;65:393–406. doi: 10.1152/jn.1991.65.3.393. [DOI] [PubMed] [Google Scholar]
- 14.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, Basheer R, Haas HL, Zilles K, Bauer A. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J. Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldberg W, Sherwood SL. Infections of drugs into the lateral ventricle of the cat. J. Physiol. 1954;23:148–167. doi: 10.1113/jphysiol.1954.sp005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. Mol. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 19.Gallopin T, Luppi PH, Cauli B, Urade Y, Rossier J, Hayaishi O, Lambolez B, Fort P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A re-ceptors in the ventrolateral preoptic nucleus. Neuroscience. 2005;34:1377–1390. doi: 10.1016/j.neuroscience.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 21.Greene RW, Rainnie DG. Mechanisms affecting neuronal excitability in brainstem cholinergic centers and their impact on behavioral state. In: Lydic R, Baghdoyan H.A, editors. Handbook of behavioral state control: cellular and molecular mechanisms. Boca Raton: CRC Press; 1999. pp. 277–296. [Google Scholar]
- 22.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haulica I, Ababei L, Branisteanu D, Topoliceanu F. Letter: Preliminary data on the possible hypnogenic role of adenosine. J. Neurochem. 1973;21:1019–1020. doi: 10.1111/j.1471-4159.1973.tb07549.x. [DOI] [PubMed] [Google Scholar]
- 24.Hong ZY, Huang ZL, Qu WM, Eguchi N, Urade Y, Hayaishi O. An adenosine A receptor agonist induces sleep by increasing GABA release in the tuberomammillary nucleus to inhibit histaminergic systems in rats. J. Neurochem. 2005;92:1542–1549. doi: 10.1111/j.1471-4159.2004.02991.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 26.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 27.Jones BE, Kryger MH, Roth T, Dement WC. Principles and Practice of Sleep Medicine. Philadelphia: W.B. Saunders; 2000. Basic Mechanisms of Sleep-Wake States; pp. 134–154. [Google Scholar]
- 28.Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157:238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karacan I, Thornby JI, Anch M, Booth GH, Williams RL, Salis PJ. Dose-related sleep disturbances induced by coffee and caffeine. Clin. Pharmacol. Ther. 1976;20:682–689. doi: 10.1002/cpt1976206682. [DOI] [PubMed] [Google Scholar]
- 30.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J. Neurosci. 2008;28:491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;2:229–238. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- 32.Landolt HP, Retey JV, Tonz K, Gottselig JM, Khatami R, Buckelmuller I, Achermann P. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology. 2004;29:1933–1939. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- 33.Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J. Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marks GA, Shaffery JP, Speciale SG, Birabil CG. Enhancement of rapid eye movement sleep in the rat by actions at A1 and A2a adenosine receptor subtypes with a differential sensitivity to atropine. Neuroscience. 2003;116:913–920. doi: 10.1016/s0306-4522(02)00561-4. [DOI] [PubMed] [Google Scholar]
- 35.Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, Giagheddu M, Imperato A, Gessa GL. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 36.McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. TINS. 1989;12:215–222. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 37.McCormick DA. Actions of acetylcholine in the cerebral cortex and thalamus and implications for function. Prog. Brain Res. 1993;98:303–308. doi: 10.1016/s0079-6123(08)62412-7. [DOI] [PubMed] [Google Scholar]
- 38.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 39.Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D. Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am. J. Physiol. Regul. Integr. Comp. Phy. 2005;289:R1715–1723. doi: 10.1152/ajpregu.00247.2005. [DOI] [PubMed] [Google Scholar]
- 40.Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 41.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. USA. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pape HC. Adenosine promotes burst activity in guinea-pig geniculocortical neurones through two different ionic mechanisms. J. Physiol. 1992;447:729–753. doi: 10.1113/jphysiol.1992.sp019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 44.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 45.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- 47.Radek RJ, Decker MW, Jarvis MF. The adenosine kinase inhibitor ABT-702 augments EEG slow waves in rats. Brain Res. 2004;1026:74–83. doi: 10.1016/j.brainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Hypnotic effects of deoxycorformycin in rats. Brain Res. 1983;271:392–395. doi: 10.1016/0006-8993(83)90309-8. [DOI] [PubMed] [Google Scholar]
- 49.Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J. Pharmacol. Exp. Ther. 1984;228:268–274. [PubMed] [Google Scholar]
- 50.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol. Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- 52.Retey JV, Adam M, Gottselig JM, Khatami R, Durr R, Achermann P, Landolt HP. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. J. Neurosci. 2006;26:10472–10479. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, Berger W, Landolt HP. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc. Natl. Acad. Sci. USA. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakata M, Sei H, Eguchi N, Morita Y, Urade Y. Arterial pressure and heart rate increase during REM sleep in adenosine A2A-receptor knockout mice, but not in wild-type mice. Neuropsychopharmacology. 2005;30:1856–1860. doi: 10.1038/sj.npp.1300727. [DOI] [PubMed] [Google Scholar]
- 55.Satoh S, Matsumura H, Kanbayashi T, Yoshida Y, Urakami T, Nakajima T, Kimura N, Nishino S, Yoneda H. Expression pattern of FOS in orexin neurons during sleep induced by an adenosine A2A receptor agonist. Behav. Brain Res. 2006;170:277–286. doi: 10.1016/j.bbr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 56.Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur. J. Neurosci. 1999;11:1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- 57.Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc. Natl. Acad. Sci. USA. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scammell TE, Arrigoni E, Thompson MA, Ronan PJ, Saper CB, Greene RW. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. J. Neurosci. 2003;23:5762–5770. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayai-shi O. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 60.Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog. Neurobiol. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwierin B, Borbely AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur. J. Pharmcol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 62.Siegel JM. Brain mechanisms of sleep and wakefulness. In: Chase M, editor. Basics of Sleep Behavior. Sleep Research Society. 1991. [Google Scholar]
- 63.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J. Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 64.Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J. Neurosci. 1991;11:3200–3217. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav. Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- 66.Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann. N. Y. Acad. Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- 67.Thakkar MM, Delgiacco RA, Strecker RE, McCarley RW. Adenosinergic inhibition of basal forebrain wakefulness-active neurons: a simultaneous unit recording and microdialysis study in freely behaving cats. Neuroscience. 2003;122:1107–1113. doi: 10.1016/j.neuroscience.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Thakkar MM, Engemann SC, Walsh KM, Sahota PK. Adenosine and the homeostatic control of sleep: effects of A1 receptor blockade in the perifornical lateral hypothalamus on sleep-wakefulness. Neuroscience. 2008;153:875–880. doi: 10.1016/j.neuroscience.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 69.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J. Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ticho SR, Radulovacki M. Role of adenosine in sleep and tem-perature regulation in the preoptic area of rats. Pharmacol. Biochem.Behav. 1991;40:33–40. doi: 10.1016/0091-3057(91)90317-u. [DOI] [PubMed] [Google Scholar]
- 71.Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, Schwarzschild MA, Fink JS, Hayaishi O. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology. 2003;61:S94–96. doi: 10.1212/01.wnl.0000095222.41066.5e. [DOI] [PubMed] [Google Scholar]
- 72.Virus RM, Ticho S, Pilditch M, Radulovacki M. A comparison of the effects of caffeine, 8-cyclopentyltheophylline, and alloxazine on sleep in rats.Possible roles of central nervous system adenosine receptors. Neuropsychopharmacology. 1990;3:243–249. [PubMed] [Google Scholar]
- 73.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat.II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 74.Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J. Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanik G, Glaum S, Radulovacki M. The dose-response effects of caffeine on sleep in rats. Brain Res. 1987;403:177–180. doi: 10.1016/0006-8993(87)90141-7. [DOI] [PubMed] [Google Scholar]
- 76.Zepelin H. Mammalian sleep. In: Kryger M.H, Roth T, Dement W, editors. Principles and practice of sleep medicine. Philadelphia: W.B. Saunders company; 2004. [Google Scholar]