Abstract
For many years the neuromodulator adenosine has been recognized as an endogenous anticonvulsant molecule and termed a “retaliatory metabolite.” As the core molecule of ATP, adenosine forms a unique link between cell energy and neuronal excitability. In parallel, a ketogenic (high-fat, low-carbohydrate) diet is a metabolic therapy that influences neuronal activity significantly, and ketogenic diets have been used successfully to treat medically-refractory epilepsy, particularly in children, for decades. To date the key neural mechanisms underlying the success of dietary therapy are unclear, hindering development of analogous pharmacological solutions. Similarly, adenosine receptor–based therapies for epilepsy and myriad other disorders remain elusive. In this review we explore the physiological regulation of adenosine as an anticonvulsant strategy and suggest a critical role for adenosine in the success of ketogenic diet therapy for epilepsy. While the current focus is on the regulation of adenosine, ketogenic metabolism and epilepsy, the therapeutic implications extend to acute and chronic neurological disorders as diverse as brain injury, inflammatory and neuropathic pain, autism and hyperdopaminergic disorders. Emerging evidence for broad clinical relevance of the metabolic regulation of adenosine will be discussed.
Key Words: Metabolism, neuroprotection, neurodegeneration, sleep, pain, autism, addiction, dopamine.
INTRODUCTION
Adenosine is a potent neuromodulatory purine present throughout the extracellular space of the central nervous system. Adenosine acts at pre- and postsynaptic G protein-coupled receptor subtypes (A1, A2A, A2B, and A3) [58], and the influence of A1 and A2A receptor subtypes predominates due to their higher affinity for adenosine (~100 nM) [100] and their activation by ongoing levels of adenosine. In brain, A1 receptors are distributed widely [73] and A2A receptors are located preferentially in the basal ganglia and olfactory tubercle [169]. Both the A1 and A2 receptor subtypes are antagonized by caffeine, the most widely used psychoactive substance worldwide, and caffeine’s actions at adenosine A1 and A2A receptors in the central nervous system underlie its alerting, locomotor and cognitive effects [136].
Adenosine exerts a tonic inhibition of neuronal excitability via A1 receptors in many brain regions, including hippocampus and cerebral cortex, and this baseline inhibition influences both baseline synaptic activity [132, 175] and neuronal plasticity [36]. Adenosine’s inhibitory influence also alters seizure threshold directly, and increased extracellular adenosine during a seizure plays a key role in postictal depression [193] and in keeping a seizure focus localized [140] via adenosine A1 receptors [74]. Large additional amounts of adenosine are mobilized during metabolically stressful cellular conditions such as low oxygen or glucose [57], and increased extracellular adenosine acting at the adenosine A1 receptor has been shown to be neuroprotective during conditions of metabolic stress [45]. Overall, adenosine holds well established and profound therapeutic potential for conditions such as stroke, brain injury, pain and epilepsy, among others [17, 79].
Whereas adenosine’s role as an endogenous neuroprotective molecule during pathology such as stroke, hypoxia and brain injury is of paramount clinical importance, and has long been the focus on adenosine-based therapies, the ongoing effects of adenosine are critical to baseline neuronal excitability and sleep behaviors. In addition, adenosine influences the risk for an epileptic seizure, and can control significantly the expression and progression of a broad range of acute and chronic neurological conditions. With an unparalleled long-term epidemiological database based on manipulating the influence of endogenous adenosine, i.e. the worldwide consumption of caffeine [60, 85], a strategy focused on regulating endogenous adenosine is likely to be well tolerated and non-toxic.
To date, receptor-based strategies to augment the inhibitory influence of adenosine by targeting A1 receptors have been unable to harness its clinical potential, primarily due to peripheral side effects [43, 51]. Accordingly, interest has intensified in the regulation of adenosine directly by physiological stimulation [42, 45, 76, 77], metabolism [125] and adenosine kinase [75], an astrocytic intracellular enzyme that, together with equilibrative adenosine transport, controls extracellular adenosine levels. We now appreciate the active role of astrocytes in regulating extracellular adenosine [156], underscoring the multi-faceted and direct impact that glia have on neuronal activity and signaling. Together, these recent findings highlight the dynamic regulation of adenosine by cellular and metabolic stimuli, and thus expose new clinically-relevant strategies for augmenting adenosine.
ADENOSINE: A KEY LINK BETWEEN METABOLISM AND NEURONAL SIGNALING
As both the core of ATP and a prevalent neuromodulator, adenosine is poised to link changes in cell metabolism with changes in neuronal activity [109]. Indeed, adenosine levels rise dramatically in the extracellular space during all types of metabolic stress and earned adenosine the apt title of “retaliatory metabolite” [138] - its profound inhibitory influence at both pre- and postsynaptic receptors serves to limit energy demand and excitotoxicity when energy availability is compromised [59]. The direct release of adenosine via nucleoside transporters can increase extracellular adenosine under physiologically stressful conditions [114], and typically adenosine’s role as a retaliatory metabolite is thought to be mobilized when intracellular ATP dephosphorylation outstrips ATP production [145, 170]. However, the regulation of adenosine by ongoing physiological stimuli and under non-pathological conditions of adequate or even high intracellular ATP is becoming more appreciated [45, 96, 128]. In addition, degradation of extracellular ATP is a major source of extracellular adenosine [29, 44, 82], so manipulations that increase extracellular ATP have a net effect on neuromodulation by adenosine [44].
Ketogenic strategies such as fasting or adhering to a ketogenic (high-fat, low-carbohydrate) diet increase ATP and other energy molecules in brain [20, 37, 126, 134]. These metabolic manipulations are known to reduce seizures significantly [194], and have been shown to offer neuroprotection in animal models of brain injury [70, 125]. Emerging evidence suggests that mimicking key cellular aspects of ketogenic metabolism increases extracellular adenosine [96], and furthermore, that an increased influence of adenosine at the A1 subtype plays a key role in the anticonvulsant success of ketogenic strategies [127] via its combined presynaptic inhibition of glutamatergic terminals and its postsynaptic hyperpolarization via K+ channels. Due to the functional coupling and inverse relationship between adenosine and dopamine receptors (A2A/D2 and A1/D1) [66], a general increase in extracellular adenosine could also decrease dopaminergic transmission. A diverse set of physiological and pathological stimuli that modulate extracellular adenosine are outlined in Table 1.
Table 1.
Conditions that Increase Adenosine in the CNS
| Manipulation | Reference |
|---|---|
| Hypoxia | Fowler 1989 [55] Dale & Frenguelli 2000 [34] Saransaari & Oja 2003 [172] Martín, Fernández, Perea, Pascual, Haydon, Araque & Ceña 2007 [123] |
| Ischemia | Fowler 1990 [56] Latini, Corsi, Pedata & Pepeu 1995 [108] Frenguelli, Llaudet & Dale 2003 [63] Parkinson, Xiong & Zamzow 2005 [155] Frenguelli, Wigmore, Llaudet & Dale 2007 [64] |
| NMDA receptor activation | Manzoni, Manabe & Nicoll 1994 [119] Semba & White 1997 [176] Melani, Corsi, Giménez-Llort, Martínez, Ogren, Pedata & Ferré 1999 [130] Brambilla, Chapman & Greene 2005 [21] |
| H2O2 | Masino, Mesches, Bickford & Dunwiddie 1999 [129] Saransaari & Oja 2003 [172] |
| Hypoglycemia or impaired glycolysis | Fowler 1993 [57] Zhu & Krnjević 1993 [201] Calabresi, Centonze, Pisani & Bernardi 1997 [22] Zhao, Tekkök & Krnjević [200] Minor, Rowe, Soames Job, Ferguson [131] |
| Increased temperature | Gabriel, Klussman & Igelmund 1998 [67] Masino & Dunwiddie 1999 [124] |
| Hypercapnia/acidification | Dulla, Dobelis, Pearson, Frenguelli, Staley & Masino 2005 [42] Gourine, Llaudet, Dale & Spyer 2005 [77] Otsuguro, Yamaji, Ban, Ohta & Ito 2006 [146] |
| Depolarization | Pedata, Pazzagli, Tilli & Pepeu 1990 [157] Latini, Corsi, Pedata & Pepeu 1995 [108] |
| Metabolic poisons | Doolette1997 [40] Zhu & Krnjević 1997 [202] Saransaari & Oja 2003 [172] |
| Astrocyte activation | Zhang, Wang, Ye, Ge, Chen, Jiang, Wu, Poo & Duan 2003 [199] Parkinson & Xiong 2004 [154] Pascual, Casper, Kubera, Zhang, Revilla-Sanchez, Sul, Takano, Moss, McCarthy & Haydon 2005 [156] |
| Seizures | Whitcomb, Lupica, Rosen & Berman 1990 [193] During & Spencer 1993 [46] Berman, Fredholm, Aden & O’Connor 2000 [11] Kaku, Jiang, Hada, Morimoto & Hayashi 2001 [93] Etherington, Patterson, Meechan, Boison, Irving, Dale & Frenguelli 2008 [52] |
| Intense exercise | Dworak, Diel, Voss, Hollman & Strüder 2007 [47] |
| Sleep deprivation | Porkka-Heiskanen, Strecker, Thakkar, Bjorkum, Greene & McCarley 1997 [162] Porkka-Heiskanen, Strecker & McCarley 2000 [161] Kalinchuk, McCarley, Stenberg, Porkka-Heiskanen & Basheer 2008 [94] Murillo-Rodriguez, Liu, Blanco-Centurion & Shiromani, 2008 [133] |
An overview of both physiological and pathological conditions of altered metabolism and cellular activity that can increase extracellular adenosine. Due to the rapid dephosphorylation of extracellular ATP to adenosine, increased extracellular ATP yields a net increase in adenosine. This table is not meant to be exhaustive of the literature, but to highlight the ubiquitous and rapid nature of the adenosine response and thus its broad and dynamic influence in the nervous system.
ADENOSINE, METABOLISM AND EPILEPSY
The inhibitory adenosine A1 receptor is functionally dominant in hippocampus and cerebral cortex, and underlies adenosine’s role as an endogenous anticonvulsant [43]. Accordingly, any manipulation which increases extracellular adenosine offers significant potential for both preventing and halting epileptic seizures, the vast majority of which initiate and propagate in these forebrain regions. Unlike the point-to-point and activity-dependent changes in synaptic transmission effected by classical neurotransmitters, adenosine exerts a tonic modulatory influence on neuronal activity. Thus the anticonvulsant effects of adenosine are dissimilar mechanistically to classical actions of glutamate and GABA, the neurotransmitters targeted most often for the treatment of epilepsy [168]. Adenosine itself is not packaged in vesicles, and its non-synaptic release, such as from astrocytes and through nucleoside transporters, can influence a neural “neighborhood.” Akin to a broad-based influence of altered metabolism, such as via ketogenic strategies, the presence of a tonic level of adenosine in the extracellular space makes adenosine a major player in the dynamics of nervous system activity and even in determining the set point of normal physiology versus pathology in brain function – such as by determining seizure threshold. Indeed, local release of adenosine itself [89] or regulating local metabolism of adenosine by reducing its intracellular rephosphorylation by adenosine kinase [18] both offer significant emerging potential for treating epilepsy. Importantly, adenosine is effective in stopping seizures which are pharmacoresistant [16], thus opening new therapeutic opportunities for intractable epilepsy.
Physiological conditions which offer both high ATP levels and increased extracellular adenosine are particularly ideal for epilepsy and for many acute and chronic neurological disorders characterized by metabolic dysfunction and neuronal vulnerability or frank progressive neurodegeneration. Enhanced ATP levels provide energy reserves for a neuron to continue functioning under stress, essential for maintaining cell calcium and other ion gradients across the cell and mitochondrial membrane. Increased extracellular adenosine, at levels permitting normal synaptic transmission, plasticity and cognition, offers a neuroprotective buffer against insults, reduces excitation, and averts excessive ATP demand, expressly critical in cells with compromised energy capacity. Ideally, metabolic strategies enhance ATP, increase extracellular adenosine, and boost on-demand adenosine within a neural neighborhood to provide local seizure control and neuroprotection. As such, metabolic and dietary strategies such as ketogenic diet therapy may increase regional or global adenosine and increase overall seizure threshold as described below.
THE REGULATION OF ADENOSINE AND BIOENERGETICS BY KETOGENIC METABOLISM
Multiple lines of evidence suggest that adenosine, ATP, and general cellular energy are upregulated by ketogenic metabolism. To highlight these effects, it is necessary to review the underlying biochemistry of ketosis. Due to restricted carbohydrate intake, blood glucose is very low during chronic consumption of a ketogenic diet (or prolonged fasting). During conditions of limited glucose the liver maintains energy homeostasis by converting fatty acids and some amino acids to ketone bodies (β-hydroxybutyrate, acetoacetate, acetone) which are then transported by the blood to other tissues for use as fuel. The brain is particularly dependent on this process, since it is poor both at using fatty acids directly for fuel and at converting fatty acids to ketone bodies; the brain is a unique tissue in this regard [65, 147]. While the enzymes involved in ketone body synthesis are detectable in brain, they are present at far lower levels than in liver [4, 27, 28].
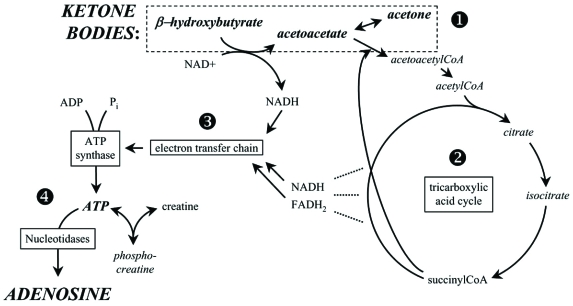
Ketone bodies lead to energy production by conversion to acetyl-CoA which then enters the mitochondrial tricarboxylic acid cycle, replacing pyruvate (derived from glycolysis) as an acetyl-CoA source. The tricarboxylic acid cycle then leads as usual to proton flow out of the mitochondria matrix; this gradient in turn powers ATP production by ATP synthase in the mitochondrial inner membrane. Not only can ketone bodies substitute for glucose, metabolism of ketone bodies is more efficient than that of glucose, leading to more available energy for ATP synthesis. This effect derives from the higher heat of combustion of ketone bodies compared to pyruvate [187]; ketone body metabolism leads to reduction of the mitochondrial NAD couple (NAD+/NADH) and oxidation of the mitochondrial co-enzyme Q couple (Q/QH2). The difference between the redox potentials of these couples determines the magnitude of the proton gradient which in turn determines the free energy (ΔG’) of ATP hydrolysis – the increased difference with ketone body metabolism leads to increased ΔG’ for ATP production [187]. Key aspects of this energy cycle and its relationship to adenosine are summarized in Fig. (1).
Fig. (1).
The metabolic relationship between ketones and adenosine. Compounds upregulated by a ketogenic diet or exogenous ketones are italicized. (1) During ketolytic metabolism, the ketone bodies β-hydroxybutyrate (and its breakdown products acetone and acetoacetate) are either generated locally or hepatically and transported via the blood to other tissues (such as brain). Ketones are converted intracellularly into acetyl-CoA which enters the tricarboxylic acid cycle. (2) This mitochondrial energy cycle generates, at multiple steps (----), protons and electrons that are channeled to the electron transport chain by NADH and FADH2 (β-hydroxybutyrate conversion to acetoacetate also contributes). Many steps of the tricarboxylic acid cycle are omitted for simplicity. (3) The electron transport chain drives an electrochemical gradient across the mitochondrial outer membrane and ultimately oxidative phosphorylation which forms ATP from ADP and phosphate (Pi) by ATP synthase. (4) Enhanced ATP can be converted to phosphocreatine for energy storage, or broken down to its core molecule, adenosine. Adenosine levels inside and outside of the cell membrane are influenced concurrently by an equilibrative transporter. Due to the very large ATP / adenosine ratio inside the cell, a small increase in intracellular ATP could translate into a large relative increase in intracellular, and thus extracellular, adenosine.
Experimental application of ketone bodies in vitro leads to increased ATP/ADP and phosphocreatine/creatine ratios and increased levels of the tricarboxylic acid cycle substrates citrate and isocitrate [95, 173]. Overall, studies of ketogenic metabolism in the brain demonstrate an increased energetic status using measures ranging from respiration to mitochondrial density to levels of energy-storing substances such as ATP and phosphocreatine. Importantly, the brain’s bioenergetic response to ketosis appears to differ from peripheral tissues so far examined – increases in cell energy predominate in brain (Table 2).
Table 2.
Influence of Ketone Metabolism on Cellular Energy
| Energetic Molecules | Expression of Mitochondrial Genes or Proteins | Mitochondria | Respiration | |
|---|---|---|---|---|
| Brain |
Increased ATP [37, 99, 127, 134, 143] Increased phosphocreatine [20, 151] |
Upregulated ATP synthase [20, 141] Upregulated uncoupling protein [182] Unchanged succinate dehydrogenase [167] |
Increased number [20, 143] Increased or reduced number (region-dependent) [6] |
Increased respiration [182] |
| Peripheral tissues | Unchanged ATP production [158] | Unchanged citrate synthase [158] Unchanged succinate dehydrogenase [137, 167] |
Moderately increased size [48] Unchanged [6] |
Evidence for changes in cellular energy in brain and peripheral tissues after ketogenic metabolism in vivo or in vitro. An increase or upregulation is indicated by italics throughout. “Expression of genes or proteins” includes mRNA expression and protein expression via immunochemical or activity-based assays; here we include both cell and mitochondriarelated genes/proteins. Peripheral tissues include skeletal muscle and liver.
In addition to published neurochemical and biochemical evidence, recent neurochemical evidence suggests a regional upregulation of energy molecules in rats after maintenance on a ketogenic diet. Preliminary results demonstrate increased ATP or adenosine in specific brain regions [127, 143], including a significant increase in ATP and a strong trend toward increased adenosine in cerebral cortex [127], and upregulated ATP synthase gene expression in hippocampus [20, 141]. Field recordings from the CA1 region of hippocampal slices show a reduced electrophysiological response to hypoxia [20] and exogenous adenosine [127] after ketogenic diet therapy, consistent with increased endogenous adenosine. Hippocampal CA3 pyramidal neurons autoregulate their activity based on acute changes in intracellular ATP and extracellular glucose consistent with the metabolic consequences of ketogenic diet therapy [96]; detailed cellular mechanisms are described below.
As noted above, ketogenic metabolism occurs during restricted glucose and is characterized by increased ATP. During single-cell patch clamp recordings, when extracellular glucose is reduced but not eliminated, and the level of intracellular ATP is sufficient or high in the patch pipette, CA3 pyramidal neurons regulate their own excitability by releasing ATP, activating adenosine A1 receptors and opening postsynaptic K+ channels [96]. In contrast, during single cell recordings of CA3 pyramidal neurons, when extracellular glucose is reduced and intracellular ATP is low, CA3 pyramidal cells depolarize significantly [96]. These low ATP/low glucose conditions are similar to an ischemic stroke, where both oxidative phosphorylation and glucose availability are interrupted. Thus, an autocrine regulation of CA3 neuron excitability during low glucose depends critically on intracellular ATP; the subsequently increased extracellular adenosine likely influences nearby neurons, particularly important in CA3, a region with recurring excitatory collaterals. Overall this concurrent intracellular ATP / extracellular glucose manipulation mimicking metabolic aspects of ketogenic diet therapy provides a clear example of a situation where intracellular ATP is high yet extracellular adenosine increases – ideal for autocrine regulation by increasing seizure threshold and promoting neuronal survival.
Evidence for autocrine neuronal regulation is consistent with adenosine’s role as a neuroprotective agent, mobilized proactively under these conditions by non-pathological changes in altered metabolism. Similarly, ketone-based metabolism [35] or a ketogenic diet [70] has already shown neuroprotective properties in injury models in multiple brain regions. While it seems counterintuitive for brain cells to release ATP when extracellular glucose is low, ultimately this process can save cell energy: under conditions of sufficient ATP, there is a large ratio of ATP:adenosine (10,000:1) inside the cell. Upon releasing a relatively small amount of ATP, a coherent set of mechanisms degrade extracellular ATP to adenosine, activate adenosine A1 receptors and reduce excitability. Thus, releasing a relatively small amount of ATP during low glucose is a powerful preemptive strike against a condition of increased excitability – potentially placing energy demands which could exceed energy supply and compromise neuronal function.
CLINICAL IMPLICATIONS
The clinical efficacy of a ketogenic diet is well-established for pediatric epilepsy. It has been validated retrospectively by multiple clinical sites [38, 62, 153, 188] and confirmed recently in a randomized controlled trial [135]. Similar to adenosine, the ketogenic diet is effective in medically-refractory epilepsy, and thus reduces seizures via mechanisms other than those targeted by antiepileptic drugs available currently [168]. A significant subset of pediatric epilepsy patients become and remain seizure-free, and can reduce or eliminate their medication even after discontinuing diet therapy. Most common side effects are short term, and include hunger, constipation, and lethargy [135, 188]. However, kidney stones can develop in 5-7% of children on the diet [171].
The ketogenic diet may also offer benefits for adult epilepsy [15], and recently has been shown to improve dramatically or even reverse the metabolic syndrome which characterizes type II diabetes [198]. Notably, a ketogenic diet was more effective than a calorically restricted diet, and virtually reversed metabolic syndrome in all persons who were able to comply with the restrictions of dietary therapy [192]. Basic research has also shown dramatic results in slowing tumor growth in brain cancer [177], and synergistic effects of the simultaneous use of a ketogenic diet and 2-deoxyglucose [121], a metabolic manipulation which inhibits glycolysis, increases adenosine [200] and decrease seizures [69, 181]. Thus, the therapeutic applications of a ketogenic diet and metabolism-based therapies are broadening rapidly beyond pediatric epilepsy to other chronic and prevalent conditions.
As a whole, the capacity for a ketogenic diet to increase specifically the influence of endogenous adenosine offers testable and clinically-relevant predictions; many of these predictions are validated by published clinical and basic research, lending further conceptual support [125, 126]. Below we highlight briefly some of the central nervous system implications of a relationship between a ketogenic diet and adenosine. A subset is supported strongly by published research, in particular neuroprotection and sleep. Data are emerging for neurodegeneration, hyperdopaminergic disorders and autism. In addition, we speculate upon major clinical implications for pain.
Brain Injury
The attractive features of adenosine as an endogenous anticonvulsant are recapitulated in its role as a neuroprotective molecule. Increasing adenosine’s inhibitory influence via the adenosine A1 receptor protects neurons in virtually any model of brain injury [45], and the adenosine A1 receptor has long been a major focus of and goal for adenosine-based neuroprotective therapies. Increasing the influence of adenosine at A1 receptors offers neuroprotection either before [90] or after [190] the injury. Unfortunately, and akin to epilepsy, the peripheral side effects of receptor-based strategies have stymied drug development and adenosine A1 receptors have lost momentum as a primary neuroprotective target.
Importantly, and as predicted based on a relationship between ketone metabolism and adenosine, the ketogenic diet or analogous metabolic strategies have shown neuroprotective properties in multiple brain injury models and brain regions, including spinal cord injury [160]. Some of these effects have been counterintuitive – neuroprotection was observed after a ketogenic strategy such as fasting [35], or after strategies that interrupted metabolism [102] or inhibited glycolysis [111]. Importantly, neuroprotection was observed even if altered metabolism was initiated after the injury [152], making this a particularly promising strategy. Based on differential consequences and responses in the central nervous system versus the periphery (Table 2), a metabolic strategy could lend new therapies and recommendations for the treatment of brain injuries, and open a new and exciting chapter for adenosine and neuroprotection.
Neurodegeneration
The neuroprotective effects of adenosine via altered metabolism may extend to neurodegenerative diseases. Metabolic dysregulation at the cellular level is a hallmark of many chronic and neurodegenerative disorders [7], and often it is unclear if it causes and/or results from the progressive neuronal dysfunction. The brain has an extremely high fat content, and as noted in Table 2 offers some differential responses to metabolic manipulations as compared to peripheral tissues. These dual and unique features of brain composition and brain metabolism enhance therapeutic opportunities for chronic and progressive disorders [1], and suggest the potential for limited or reduced peripheral side effects.
As one example, metabolic therapy may offer symptomatic delay or improvement even in a genetic, neurodegenerative disorder such as Huntington’s disease [41, 186]. Drug treatments targeting type II diabetes, which, similar to a ketogenic diet, offer improved glycemic control, can reduce symptoms and increase survival in mouse models of Huntington’s disease [116, 122]. With gene therapy still beyond the near-term horizon, metabolic treatment strategies which delay or reduce symptoms of disease could offer significant improvements in quality of life for current patients with Huntington’s disease and a host of neurodegenerative conditions. In addition, at least some neurodegenerative diseases are associated with an increased risk of seizures, such as Alzheimer’s disease [3]. It is unknown whether subclinical seizures may occur and contribute to the progression of Alzheimer’s disease or other neurodegenerative diseases in humans. Ultimately, similar to epilepsy, adenosine-based metabolic therapies could limit acute dysfunction and offer ongoing neuroprotection against progressive neuronal decline and degeneration.
Sleep
Adenosine promotes sleep [165, 189], and treating sleep disorders and enhancing the quality of sleep is a major concern for public health [23]. The specific adenosine-based mechanism established initially as sleep-promoting was increased activity at the A1 receptor in the basal forebrain [166]. More recently the adenosine A2A receptor has been recognized for an important role in sleep [88, 174]. The alerting effects of caffeine are attributed to its actions at adenosine receptors in forebrain areas important for cognition as well as arousal centers, and it may be that endogenous adenosine acting at both of the receptor subtypes in brain that are antagonized by caffeine – A1 and A2A adenosine receptors – play a role in sleep behaviors [8].
In keeping with adenosine’s role as a sleep-promoter, and the potential for a ketogenic diet to increase ATP and extracellular adenosine, the ketogenic diet has been shown to improve sleep quality and quantity in children with epilepsy, especially normalizing the ultradian cycling between slow-wave and paradoxical sleep [83]. Sleep electroencephalograph (EEG) changes were observed consistently with ketogenic diet therapy, and normalization or improvement in sleep EEG was correlated with an improvement in seizures [153]. In control subjects without a diagnosis of epilepsy, effects are typically moderate and include reports of an increase in the latency to enter rapid eye movement (REM) sleep [106] and an increase in slow-wave sleep [2]. Whereas a high-fat, low-carbohydrate ketogenic diet increased slow-wave sleep, a high-carbohydrate, low-fat isocaloric diet decreased slow-wave sleep [159].
Recent evidence implicates the role of extracellular astrocyte-derived ATP as critical for the sleep-promoting influence of adenosine [82]. Furthermore, there is a well established association between poor sleep and epilepsy [105]. With such a high prevalence and cost associated with these co-morbidities, metabolic therapy appears to offer significant promise. More research is needed in this area to determine the therapeutic relationship among adenosine, a ketogenic diet and sleep.
Autism
Multiple lines of evidence suggest that the influence of adenosine may be insufficient in autism spectrum disorders (ASD), and that increasing adenosine would reduce both physiological and behavioral hallmarks of ASD. Preliminary studies have shown that the ketogenic diet improves symptoms of autism [53], and Rett syndrome [80]. To our knowledge, dysregulation of adenosine in brain has not been tested directly with respect to symptoms of autism, although these studies are underway. Nevertheless, adenosine is a purine molecule with roles in both metabolism and neuronal signaling, and abnormalities in purine metabolism have been documented [19, 120, 142, 149, 150] in a subset of ASD.
Along with dysregulation of purine metabolism, ASD are characterized by several behavioral and physiological hallmarks of disordered adenosine: sleep disruption [25, 72, 184], markedly increased incidence of seizures [118, 195], immune dysfunction [87, 91], and reports - including self-reports - of increased anxiety and “sensitivity” and “hyperactivity” of the nervous system [24, 78, 178]. Often there is a dual diagnosis, with psychiatric comorbidities present in least 70% of individuals diagnosed with ASD. The most common comorbidities are social anxiety disorder, attention-deficit/hyperactivity disorder, and oppositional defiant disorder [178].
Many of the behaviors exhibited by persons with autism and/or reported by high-functioning persons with autism involve stimuli that would be predicted to release ATP and/or adenosine based on associated mechanical pressure, increased neuronal activity, decreased pH or increased temperature (ref. [45] and Table 1). For example, rocking, spinning and Grandin’s well-known “Hug Machine” [49] all exert mechanical pressure or sudden changes in acceleration, intense intellectual activity and focus can reduce anxiety associated with ASD [78], and intense neuronal activity increases extracellular adenosine [114]. Intense exercise causes a metabolic decrease in pH [84], decreased pH has been shown to increase adenosine [42, 146], and intense exercise has been shown to increase brain adenosine [47] and improve symptoms of autism [98]. Reports show improved behavior, language and social function associated with a fever in persons with autism [30], and basic research demonstrates that a similar temperature increase in hippocampal slices increases extracellular adenosine and inhibits neuronal activity significantly [124].
Certainly many symptoms and behaviors of ASD are due to aberrant neurochemistry, neuroanatomical development and genetically-determined substrates. However, increasing the inhibitory influence of adenosine could help significantly with multiple behavioral and physiological sequelae. Importantly, increasing adenosine improves sleep, decreases seizures and reduces anxiety – all physiological effects that could be achieved by a metabolic strategy such as a ketogenic diet and would improve quality of life significantly in persons and families affected by ASD. Ketogenic diet therapy and/or increasing adenosine could improve social and behavioral symptoms and reduce or alleviate serious chronic physiological symptoms which impact the ability to learn and remember (sleep disruption/inadequate REM sleep/seizures [86, 118]) and ultimately cause permanent brain dysfunction and cognitive impairment (recurrent seizures) [25, 26]. Given adenosine’s profound effects on neuronal activity, sleep and seizures, the relationship among metabolism, autism and adenosine, including the efficacy of a ketogenic diet in reducing symptoms of autism, needs to be explored directly.
Hyperdopaminergic Disorders
Dopamine and adenosine have long been known to be opposing at numerous levels. Behaviorally, whereas adenosine receptor antagonists (e.g. caffeine) are stimulants, such properties belong to agonists of the dopamine system (e.g. amphetamine). Biochemically, the two high affinity adenosine receptors in brain each have an antagonistic dopaminergic counterpart. Specifically, A1 and D1 receptors, and A2A and D2 receptors, have opposing effects on 2nd messenger pathways, most notably the production of cyclic AMP through heterotrimeric G-protein regulation of adenylate cyclase [61, 97]. More recently, it has become apparent that such interactions can involve direct receptor/receptor cross-talk via A1/D1 and A2A/D2 receptor heteromers [54]. Therefore, it is logical to explore modulation of adenosinergic activity as a possible treatment for disorders of dopaminergic function.
Thus far there is compelling evidence that adenosine antagonists are useful in treating a hypodopaminergic disease, namely Parkinson’s disease [197]. Conversely, the ketogenic diet and an associated augmentation of adenosine may be of use in hyperdopaminergic states. The list of clinical conditions that are hypothesized to involve overactivity in the dopamine system is impressive, and includes conditions as diverse as schizophrenia, tardive dyskinesia, attention deficit/hyperactivity disorder, Tourette’s syndrome, Huntington’s disease, and drug addiction/relapse. Research in animal models of some of these disorders suggests the beneficial effects of promoting adenosine [12, 14, 39, 71, 92, 101, 113, 191]. Adenosine augmentation via ketogenic diet or analogous metabolic strategies may be particularly useful in those hyperdopaminergic disorders involving neurodegeneration, offering the combined benefits of treating symptoms as well as retarding the underlying degeneration.
Preliminary human data exist on the benefits of ketogenic diet therapy and schizophrenia [148]. Ketogenic diet therapy was tried because the physiological effects of other treatments – such as electroconvulsive shock – result in decreased blood pH, and ketone metabolism increases acid production. In addition, physicians noted carbohydrate cravings and increased intake prior to a relapse and hypothesized that persons with schizophrenia may have problematic or altered carbohydrate metabolism. Despite the small sample size and incomplete control over dietary therapy, promising results were noted [148].
Like epilepsy, schizophrenia is a chronic condition characterized by recurring episodes as well as significant proportion of cases that remain intractable to therapies available currently [107]. Finally, given the high coincident rates of epilepsy and schizophrenia [164] and type II diabetes and schizophrenia [39, 139, 144, 179], and the success of a variety of low carbohydrate and ketogenic diets in treating type II diabetes [198] and adult epilepsy [5, 103, 104], dietary therapy would seem like an optimal primary or adjuvant therapy to reduce all of these medical conditions.
Pain
Control of chronic pain remains a major clinical problem. For example, the strongest analgesics, the opiates, have been viewed historically as poorly effective against neuropathic pain; only a subset of patients respond well to opiates [163]. Anticonvulsant drugs are a non non-opiate alternative used with success against neuropathic pain, yet they have their own set of undesirable side effects. Pain relief using anticonvulsants demonstrates that an overall inhibition of neuronal activity is a strategy that can alleviate pain, and so it is not surprising that adenosine persists as a prized, but problematic, target for pain relief [180]. Adenosine A1 receptor agonists given systemically reduce chronic pain effectively in experimental animals [31, 112], and conversely, genetic knockout of the A1 receptor enhances pain sensitivity [90, 196]. Clinically, systemic adenosine alleviates neuropathic pain [10], yet the presence of adenosine A1 receptors in the heart and other peripheral tissues, and the short biological half-life of adenosine in the blood, have stymied this type of therapy. Side effects can be avoided with intrathecal adenosine [9, 50], but this route of administration is obviously invasive.
Based on evidence that ketogenic strategies increase adenosine in the central nervous system, they will also be likely to alleviate pain, although research on this topic has only begun [203]. The parallels between ketogenic diets and adenosine A1 receptor activation include their efficacy in pharmocoresistant epilepsy, and neuronal inhibition via anticonvulsants appears to be one mechanism for alleviating at least a subset of neuropathic pain. We suggest that adenosine-mediated central nervous system inhibition via metabolic strategies such as ketogenic diet therapy will be effective against neuropathic pain.
Beyond these possible effects on the neural substrates of pain generally, a ketogenic metabolism may have beneficial effects with inflammatory pain by reducing inflammation itself through several mechanisms. 1) adenosine A1 receptors have been shown to be anti-inflammatory in a number of tissues, including brain [68, 110, 183, 185]. 2) Compared to glycolytic metabolism, ketolytic metabolism produces fewer reactive oxygen species, which are known to contribute to inflammation [81, 117, 187]; 3) the long-chain polyunsaturated fatty acids in the ketogenic diet activate peroxisome proliferator-activated receptors, an effect which will reduce inflammation by inhibiting nuclear factor κB and other pro-inflammatory pathways [13, 32, 33, 115]. Altogether, multiple consequences of ketone metabolism, including increased adenosine, appear to have much clinical promise as safe, effective, non-addictive treatments for chronic neuropathic and inflammatory pain conditions.
SUMMARY
Herein we highlight major implications of the emerging relationship among adenosine, a ketogenic diet and epilepsy and provide a broad and provocative overview of a subset of the therapeutic predictions of this metabolic relationship. The clinical implications discussed in detail are supported by preliminary or historical data, or by infrequent publication in disparate fields of research, and deserve further examination and integration. An evidence–based metabolic treatment as an adjuvant or alternate strategy is particularly attractive and important as we seek cost-effective solutions for the diverse conditions highlighted herein. Together these conditions exact an enormous toll on health care and quality of life as they are chronic, prevalent, increasing (particularly diabetes and autism), and often comorbid (sleep disorders and epilepsy, for example).
In the central nervous system, adenosine offers unparalleled yet untapped opportunities for seizure protection, neuroprotection, sleep, and pain relief, among others. Understanding the regulation of adenosine helps achieve these long-standing clinical goals and extends beyond them to alleviating both acute and chronic conditions in adults and children. In summary, the metabolic relationship among adenosine, a ketogenic diet, and epilepsy could open major new therapeutic applications and avoid peripheral side effects in a way that has eluded receptor-based strategies.
ACKNOWLEDGEMENTS
We acknowledge the support of NIH (NINDS), the National Science Foundation and Trinity College.
REFERENCES
- 1.Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell. Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afaghi A, O'Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosci. 2008;11:146–154. doi: 10.1179/147683008X301540. [DOI] [PubMed] [Google Scholar]
- 3.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47:867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J. Neurochem. 1991;56:1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 5.Baborka CJ. Epilepsy in adults: results of treatment by ketogenic diet in one hundred cases. Arch. Neurol. 1930;6:904–914. [Google Scholar]
- 6.Balietti M, Giorgetti B, Fattoretti P, Grossi Y, Di Stefano G, Casoli T, Platano D, Solazzi M, Orlando F, Aicardi G, Bertoni-Freddari C. Ketogenic diets cause opposing changes in synaptic morphology in CA1 hippocampus and dentate gyrus of late-adult rats. Rejuvenation Res. 2008;11:631–640. doi: 10.1089/rej.2007.0650. [DOI] [PubMed] [Google Scholar]
- 7.Barañano KW, Hartman AL. The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr. Treat. Options Neurol. 2008;10:410–419. doi: 10.1007/s11940-008-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog. Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Belfrage M, Segerdahl M, Arnér S, Sollevi A. The safety and efficacy of intrathecal adenosine in patients with chronic neuropathic pain. Anesth. Analg. 1999;89:136–142. doi: 10.1097/00000539-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Belfrage M, Sollevi A, Segerdahl M, Sjölund KF, Hansson P. Systemic adenosine infusion alleviates spontaneous and stimulus evoked pain in patients with peripheral neuropathic pain. Anesth. Analg. 1995;81:713–717. doi: 10.1097/00000539-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Berman RF, Fredholm BB, Aden U, O'Connor WT. Evidence for increased dorsal hippocampal adenosine release and metabolism during pharmacologically induced seizures in rats. Brain Res. 2000;872:44–53. doi: 10.1016/s0006-8993(00)02441-0. [DOI] [PubMed] [Google Scholar]
- 12.Bishnoi M, Chopra K, Kulkarni SK. Protective effect of adenosine reuptake inhibitors in haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes. Pharmacology. 2007;79:171–183. doi: 10.1159/000100924. [DOI] [PubMed] [Google Scholar]
- 13.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. J. Steroid Biochem. Mol. Biol. 2003;85:267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 14.Blum D, Gall D, Galas MC, d'Alcantara P, Bantubungi K, Schiffmann SN. The adenosine A1 receptor agonist adenosine amine congener exerts a neuroprotective effect against the development of striatal lesions and motor impairments in the 3-nitropropionic acid model of neurotoxicity. J. Neurosci. 2002;22:9122–9133. doi: 10.1523/JNEUROSCI.22-20-09122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodenant M, Moreau C, Sejourné C, Auvin S, Delval A, Cuisset JM, Derambure P, Destée A, Defebvre L. Interest of the ketogenic diet in a refractory status epilepticus in adults. Rev. Neurol. (Paris) 2008;164:194–199. doi: 10.1016/j.neurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener. Dis. 2007;4:28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- 17.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog. Neurobiol. 2008;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bottini N, De Luca D, Saccucci P, Fiumara A, Elia M, Porfirio MC, Lucarelli P, Curatolo P. Autism: evidence of association with adenosine deaminase genetic polymorphism. Neurogenetics. 2001;3:111–113. doi: 10.1007/s100480000104. [DOI] [PubMed] [Google Scholar]
- 20.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 21.Brambilla D, Chapman D, Greene R. Adenosine mediation of presynaptic feedback inhibition of glutamate release. Neuron. 2005;46:275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Calabresi P, Centonze D, Pisani A, Bernardi G. Endogenous adenosine mediates the presynaptic inhibition induced by aglycemia at corticostriatal synapses. J. Neurosci. 1997;17:4509–4516. doi: 10.1523/JNEUROSCI.17-12-04509.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruso CC. Possible broad impacts of long work hours. Ind. Health. 2009;44:531–536. doi: 10.2486/indhealth.44.531. [DOI] [PubMed] [Google Scholar]
- 24.Chalfant AM, Rapee R, Carroll L. Treating anxiety disorders in children with high functioning autism spectrum disorders: a controlled trial. J. Autism Dev. Disord. 2007;37:1842–1857. doi: 10.1007/s10803-006-0318-4. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Lemonnier E, Lazartigues A, Planche P. Sleep problems and information processing, a "disconnection effect" in autism? Med. Hypotheses. 2006;66:1245–1246. doi: 10.1016/j.mehy.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Chez MG, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A. Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav. 2006;8:267–271. doi: 10.1016/j.yebeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Cullingford TE, Dolphin CT, Sato H. The peroxisome proliferator-activated receptor α-selective activator ciprofibrate upregulates expression of genes encoding fatty acid oxidation and ketogenesis enzymes in rat brain. Neuropharmacology. 2002;42:724–730. doi: 10.1016/s0028-3908(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 28.Cullingford TE, Eagles DA, Sato H. The ketogenic diet upregulates expression of the gene encoding the key ketogenic enzyme mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in rat brain. Epilepsy Res. 2002;49:99–107. doi: 10.1016/s0920-1211(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 29.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 30.Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120:e1386–e1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- 31.Curros-Criado MM, Herrero JF. The antinociceptive effects of the systemic adenosine A1 receptor agonist CPA in the absence and in the presence of spinal cord sensitization. Pharmacol. Biochem. Behav. 2005;82:721–726. doi: 10.1016/j.pbb.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Cuzzocrea S, Mazzon E, Dugo L, Patel NS, Serraino I, Di Paolo R, Genovese T, Britti D, De Maio M, Caputi AP, Thiemermann C. Reduction in the evolution of murine type II collagen-induced arthritis by treatment with rosiglitazone, a ligand of the peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2003;48:3544–3556. doi: 10.1002/art.11351. [DOI] [PubMed] [Google Scholar]
- 33.Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur. J. Pharmacol. 2004;483:79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J. Physiol. 2000;526:143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J. Neurosci. Res. 2008;86:1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- 36.de Mendonça A, Almeida T, Bashir ZI, Ribeiro JA. Endogenous adenosine attenuates long-term depression and depotentiation in the CA1 region of the rat hippocampus. Neuropharmacology. 1997;36:161–167. doi: 10.1016/s0028-3908(96)00173-6. [DOI] [PubMed] [Google Scholar]
- 37.DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB Jr. Chronic ketosis and cerebral metabolism. Ann. Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 38.DiMario FJ Jr, Holland J. The ketogenic diet: a review of the experience at Connecticut Children's Medical Center. Pediatr. Neurol. 2002;26:288–292. doi: 10.1016/s0887-8994(01)00405-2. [DOI] [PubMed] [Google Scholar]
- 39.Dionyssopoulos T, Hope W, Coupar IM. Effect of adenosine analogues on the expression of opiate withdrawal in rats. Pharmacol. Biochem. Behav. 1992;42:201–206. doi: 10.1016/0091-3057(92)90516-i. [DOI] [PubMed] [Google Scholar]
- 40.Doolette DJ. Mechanism of adenosine accumulation in the hippocampal slice during energy deprivation. Neurochem. Int. 1997;30:211–223. doi: 10.1016/s0197-0186(96)00055-1. [DOI] [PubMed] [Google Scholar]
- 41.Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc. Natl. Acad. Sci. USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunwiddie TV. Adenosine and suppression of seizures. Adv. Neurol. 1999;79:1001–1010. [PubMed] [Google Scholar]
- 44.Dunwiddie TV, Diao LH, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 46.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann. Neurol. 1993;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 47.Dworak M, Diel P, Voss S, Hollmann W, Strüder HK. Intense exercise increases adenosine concentrations in rat brain: implications for a homeostatic sleep drive. Neuroscience. 2007;150:789–795. doi: 10.1016/j.neuroscience.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 48.Eagles DA, Chapman GB. A light- and electron-microscope study of hepatocytes of rats fed different diets. C. R. Biol. 2007;330:62–70. doi: 10.1016/j.crvi.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Edelson SM, Edelson MG, Kerr DC, Grandin T. Behavioral and physiological effects of deep pressure on children with autism: a pilot study evaluating the efficacy of Grandin's Hug Machine. Am. J. Occup. Ther. 1999;53:145–152. doi: 10.5014/ajot.53.2.145. [DOI] [PubMed] [Google Scholar]
- 50.Eisenach JC, Rauck RL, Curry R. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 51.Elzein E, Zablocki J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Investig. Drugs. 2008;17:1901–1910. doi: 10.1517/13543780802497284. [DOI] [PubMed] [Google Scholar]
- 52.Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2008;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, Prokopiou A, Christodoulou P, Liapi-Adamidou G, Helidonis E, Sbyrakis S, Smeitink J. Application of a ketogenic diet in children with autistic behavior: pilot study. J. Child Neurol. 2003;18:113–118. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- 54.Ferré S, Agnati LF, Ciruela F, Lluis C, Woods AS, Fuxe K, Franco R. Neurotransmitter receptor heteromers and their integrative role in 'local modules': The striatal spine module. Brain Res. Rev. 2007;55:55–67. doi: 10.1016/j.brainresrev.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fowler JC. Adenosine antagonists delay hypoxia-induced depression of neuronal activity in hippocampal brain slice. Brain Res. 1989;490:378–384. doi: 10.1016/0006-8993(89)90258-8. [DOI] [PubMed] [Google Scholar]
- 56.Fowler JC. Adenosine antagonists alter the synaptic response to in vitro ischemia in the rat hippocampus. Brain Res. 1990;509:331–334. doi: 10.1016/0006-8993(90)90560-x. [DOI] [PubMed] [Google Scholar]
- 57.Fowler JC. Purine release and inhibition of synaptic transmission during hypoxia and hypoglycemia in rat hippocampal slices. Neurosci. Lett. 1993;157:83–86. doi: 10.1016/0304-3940(93)90648-5. [DOI] [PubMed] [Google Scholar]
- 58.Fredholm BB. Purinoceptors in the nervous system. Pharmacol. Toxicol. 1995;76:228–239. doi: 10.1111/j.1600-0773.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 59.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 60.Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 61.Fredholm BB, Jonzon B, Lindstrom K. Adenosine receptor mediated increases and decreases in cyclic AMP in hippocampal slices treated with forskolin. Acta Physiol. Scand. 1983;117:461–463. doi: 10.1111/j.1748-1716.1983.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 62.Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102:1358–1363. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- 63.Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J. Neurochem. 2003;86:1506–1515. doi: 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- 64.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J. Neurochem. 2007;101:1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:243–251. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 67.Gabriel A, Klussmann FW, Igelmund P. Rapid temperature changes induce adenosine-mediated depression of synaptic transmission in hippocampal slices from rats (non-hibernators) but not in slices from golden hamsters (hibernators) Neuroscience. 1998;86:67–77. doi: 10.1016/s0306-4522(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 68.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am. J. Physiol. Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 69.Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat. Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 70.Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gianfriddo M, Corsi C, Melani A, Pèzzola A, Reggio R, Popoli P, Pedata F. Adenosine A(2A) antagonism increases striatal glutamate outflow in the quinolinic acid rat model of Huntington's disease. Brain Res. 2003;979:225–229. doi: 10.1016/s0006-8993(03)02942-1. [DOI] [PubMed] [Google Scholar]
- 72.Godbout R, Bergeron C, Limoges E, Stip E, Mottron L. A laboratory study of sleep in Asperger's syndrome. Neuroreport. 2000;11:127–130. doi: 10.1097/00001756-200001170-00025. [DOI] [PubMed] [Google Scholar]
- 73.Goodman RR, Snyder SH. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J. Neurosci. 1982;2:1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gouder N, Fritschy J-M, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44:877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- 75.Gouder N, Scheurer L, Fritschy JM, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J. Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gourine A, Bondar SI, Spyer KM, Gourine AV. Release of ATP and glutamate in the nucleus tractus solitarii mediate pulmonary stretch receptor (Breuer-Hering) reflex pathway. J. Physiol. 2008;586:3963–3978. doi: 10.1113/jphysiol.2008.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 78.Grandin T, Scariano MM. Emergence: Labeled Autistic. New York: Warner Books; 1996. [Google Scholar]
- 79.Guieu R, Couraud F, Pouget J, Sampieri F, Bechis G, Rochat H. Adenosine and the nervous system: clinical implications. Clin. Neuropharmacol. 1996;19:459–474. doi: 10.1097/00002826-199619060-00001. [DOI] [PubMed] [Google Scholar]
- 80.Haas RH, Rice MA, Tauner DA, Merritt TA. Therapeutic effects of a ketogenic diet in Rett syndrome. Am. J. Med. Genet. Suppl. 1986;1:225–246. doi: 10.1002/ajmg.1320250525. [DOI] [PubMed] [Google Scholar]
- 81.Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008;211:85–96. doi: 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 82.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hallböök T, Lundgren J, Rosén I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia. 2007;48:59–65. doi: 10.1111/j.1528-1167.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 84.Herrero Matteo LM, Ros Die E, Sanchez Fernandes-Bravo J. [Changes in the muscular pH and other parameters of tissue hypoxia during prolonged and intense exercise] Angiologia. 1977;29:118–134. [PubMed] [Google Scholar]
- 85.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit. Rev. Food Sci. Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 86.Hill CM, Hogan AM, Karmiloff-Smith A. To sleep, perchance to enrich learning? Arch. Dis. Child. 2007;92:637–643. doi: 10.1136/adc.2006.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hornig M, Lipkin WI. Infectious and immune factors in the pathogenesis of neurodevelopmental disorders: epidemiology, hypotheses, and animal models. Ment. Retard. Dev. Disabil. Res. Rev. 2001;7:200–210. doi: 10.1002/mrdd.1028. [DOI] [PubMed] [Google Scholar]
- 88.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 89.Huber A, Padrun V, Déglon N, Aebischer P, Möhler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc. Natl. Acad. Sci. USA. 2001;98:7611–7616. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela LM, Fernéndez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hårdemark A, Betscholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 92.Kafka SH, Corbett R. Selective adenosine A 2A receptor dopamine D 2 receptor interactions in animal models of schizophrenia. Eur. J. Pharmacol. 1996;295:147–154. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- 93.Kaku T, Jiang MH, Hada J, Morimoto K, Hayashi Y. Sodium nitroprusside-induced seizures and adenosine release in rat hippocampus. Eur. J. Pharmacol. 2001;413:199–205. doi: 10.1016/s0014-2999(01)00763-4. [DOI] [PubMed] [Google Scholar]
- 94.Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157:238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. Control of glucose utilization in working perfused rat heart. J. Biol. Chem. 1994;269:25502–25514. [PubMed] [Google Scholar]
- 96.Kawamura M, Jr., Masino SA. Purinergic autocrine regulation of CA3 pyramidal neurons: activation of adenosine A1 receptors in the rat hippocampus. Soc. Neurosci. Abstr. 2008;825:26. [Google Scholar]
- 97.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 98.Kern L, Koegel RL, Dunlap G. The influence of vigorous versus mild exercise on autistic stereotyped behaviors. J. Autism Dev. Disord. 1984;14:57–67. doi: 10.1007/BF02408555. [DOI] [PubMed] [Google Scholar]
- 99.Kim D, Rho J. Ketone bodies potect against impairment of synaptic transmission in rat hippocampal slices by mitochondrial respiratory inhibitors. Soc. Neurosci. Abstr. 2007;786:713. [Google Scholar]
- 100.Klotz KN. Adenosine receptors and their ligands. Naunyn-Schmeidebergs Arch. Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- 101.Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol. Biochem. Behav. 2001;68:797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- 102.Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J. Neurochem. 2005;94:1676–1684. doi: 10.1111/j.1471-4159.2005.03328.x. [DOI] [PubMed] [Google Scholar]
- 103.Kossoff EH, Dorward JL. The modified Atkins diet. Epilepsia. 2008;49(Suppl 8):37–41. doi: 10.1111/j.1528-1167.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 104.Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61:1789–1791. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- 105.Kotagal P, Yardi N. The relationship between sleep and epilepsy. Semin. Pediatr. Neurol. 2008;15:42–49. doi: 10.1016/j.spen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 106.Kwan RM, Thomas S, Mir MA. Effects of a low carbohydrate isoenergetic diet on sleep behavior and pulmonary functions in healthy female adult humans. J. Nutr. 1986;116:2393–2402. doi: 10.1093/jn/116.12.2393. [DOI] [PubMed] [Google Scholar]
- 107.Lara DR, Brunstein MG, Ghisolfi ES, Lobato MI, Bel-monte-de-Abreu P, Souza DO. Allopurinol augmentation for poorly responsive schizophrenia. Int. Clin. Psychopharmacol. 2001;16:235–237. doi: 10.1097/00004850-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Latini S, Corsi C, Pedata F, Pepeu G. The source of brain adenosine outflow during ischemia and electrical stimulation. Neurochem. Int. 1995;27:239–244. doi: 10.1016/0197-0186(95)00042-7. [DOI] [PubMed] [Google Scholar]
- 109.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 110.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am. J. Physiol. Renal Physiol. 2004;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 111.Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J. Neurosci. Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 112.Lee Y-W, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J. Pharmacol. Exp. Ther. 1996;277:1642–1648. [PubMed] [Google Scholar]
- 113.Listos J, Talarek S, Fidecka S. Involvement of adenosine receptor agonists on the development of hypersensitivity to acute dose of morphine during morphine withdrawal period. Pharmacol. Rep. 2008;60:679–685. [PubMed] [Google Scholar]
- 114.Lloyd HG, Lindström K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem. Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- 115.LoVerme J, Fu J, Astarita G, La Rana G, Russo R, Calig-nano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 116.Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR. Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci. Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 117.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malow BA. Sleep disorders, epilepsy, and autism. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:122–125. doi: 10.1002/mrdd.20023. [DOI] [PubMed] [Google Scholar]
- 119.Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- 120.Marie S, Race V, Nassogne MC, Vincent MF, Van den Berghe G. Mutation of a nuclear respiratory factor 2 binding site in the 5' untranslated region of the ADSL gene in three patients with adenylosuccinate lyase deficiency. Am. J. Hum. Genet. 2002;71:14–21. doi: 10.1086/341036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marsh J, Mukherjee P, Seyfried TN. Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet. Nutr. Metab. (Lond.) 2008;5:33. doi: 10.1186/1743-7075-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, Ceña V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- 124.Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J. Neurosci. 1999;19:1932–1939. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008;31:273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Masino SA, Geiger JD. The ketogenic diet and epilepsy: Is adenosine the missing link? Epilepsia. 2009;50:332–333. doi: 10.1111/j.1528-1167.2008.01771.x. [DOI] [PubMed] [Google Scholar]
- 127.Masino SA, Gockel JA, Wasser CA, Pomeroy LT, Wagener JF, Gawryluk JW, Geiger JD. The relationship among ATP, adenosine and a ketogenic diet. Soc. Neurosci. Abstr. 2007;595:512. [Google Scholar]
- 128.Masino SA, Latini S, Bordoni F, Pedata F, Dunwiddie TV. Changes in hippocampal adenosine efflux, ATP levels, and synaptic transmission induced by increased temperature. Synapse. 2001;41:58–64. doi: 10.1002/syn.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Masino SA, Mesches MH, Bickford PC, Dunwiddie TV. Acute peroxide treatment of rat hippocampal slices induced adenosine-mediated inhibition of excitatory transmission in area CA1. Neurosci. Lett. 1999;274:91–94. doi: 10.1016/s0304-3940(99)00693-x. [DOI] [PubMed] [Google Scholar]
- 130.Melani A, Corsi C, Giménez-Llort L, Martínez E, Ogren SO, Pedata F, Ferré S. Effect of N-methyl-D-aspartate on motor activity and in vivo adenosine striatal outflow in the rat. Eur. J. Pharmacol. 1999;385:15–19. doi: 10.1016/s0014-2999(99)00729-3. [DOI] [PubMed] [Google Scholar]
- 131.Minor TR, Rowe MK, Soames Job RF, Ferguson EC. Escape deficits induced by inescapable shock and metabolic stress are reversed by adenosine receptor antagonists. Behav. Brain Res. 2001;120:203–212. doi: 10.1016/s0166-4328(00)00376-4. [DOI] [PubMed] [Google Scholar]
- 132.Mitchell JB, Lupica CR, Dunwiddie TV. Activity-dependent release of endogenous adenosine modulates synaptic responses in the rat hippocampus. J. Neurosci. 1993;13:3439–3447. doi: 10.1523/JNEUROSCI.13-08-03439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murillo-Rodriguez E, Liu M, Blanco-Centurion C, Shiromani PJ. Effects of hypocretin (orexin) neuronal loss on sleep and extracellular adenosine levels in the rat basal forebrain. Eur. J. Neurosci. 2008;28:1191–1198. doi: 10.1111/j.1460-9568.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–380. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- 135.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 136.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Brain Res. Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 137.Nemeth PM, Rosser BW, Choksi RM, Norris BJ, Baker KM. Metabolic response to a high-fat diet in neonatal and adult rat muscle. Am. J. Physiol. Cell Physiol. 1992;262:C282–C286. doi: 10.1152/ajpcell.1992.262.2.C282. [DOI] [PubMed] [Google Scholar]
- 138.Newby AC. Adenosine and the concept of "retaliatory metabolites". Trends Biochem. Sci. 1984;9:42–44. [Google Scholar]
- 139.Newcomer JW. Metabolic risk during antipsychotic treatment. Clin. Ther. 2004;26:1936–1946. doi: 10.1016/j.clinthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 140.Niglio T, Popoli P, Caporali MG, Scotti de Carolis A. Antiepileptic effects of N6-L-phenylisopropyladenosine (L-PIA) on penicillin-induced epileptogenic focus in rabbits. Pharmacol. Res. Commun. 1988;20:561–572. doi: 10.1016/s0031-6989(88)80083-3. [DOI] [PubMed] [Google Scholar]
- 141.Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM, Choi WS. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Mol. Brain Res. 2004;129:80–87. doi: 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 142.Nyhan WL, James JA, Teberg AJ, Sweetman L, Nelson LG. A new disorder of purine metabolism with behavioral manifestations. J. Pediatr. 1969;74:20–27. doi: 10.1016/s0022-3476(69)80004-1. [DOI] [PubMed] [Google Scholar]
- 143.Nylen K, Velazquez JL, Sayed V, Gibson KM, Burnham WM, Snead O.C 3rd. The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(-/-) mice. Biochim. Biophys. Acta. 2009 doi: 10.1016/j.bbagen.2008.12.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O'Neill MF. Study focuses in on potential cause of antipsychotic-induced diabetes. Drug News Perspect. 2005;18:481–482. doi: 10.1358/dnp.2005.18.8.944541. [DOI] [PubMed] [Google Scholar]
- 145.Orford MR, Saggerson ED. A low-Km 5'-nucleotidase from rat brain cytosolic fraction: purification, kinetic properties, and description of regulation by a novel factor that increases sensitivity to inhibition by ATP and ADP. J. Neurochem. 1996;67:795–804. doi: 10.1046/j.1471-4159.1996.67020795.x. [DOI] [PubMed] [Google Scholar]
- 146.Otsuguro K, Yamaji Y, Ban M, Ohta T, Ito S. Involvement of adenosine in depression of synaptic transmission during hypercapnia in isolated spinal cord of neonatal rats. J. Physiol. 2006;574:835–847. doi: 10.1113/jphysiol.2006.109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr. Brain metabolism during fasting. J. Clin. Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pacheco A, Easterling WS, Pryer MW. A pilot study of the ketogenic diet in schizophrenia. Am. J. Psychiatry. 1965;121:1110–1111. doi: 10.1176/ajp.121.11.1110. [DOI] [PubMed] [Google Scholar]
- 149.Page T, Coleman M. Purine metabolism abnormalities in a hyperuricosuric subclass of autism. Biochim. Biophys. Acta. 2000;1500:291–296. doi: 10.1016/s0925-4439(99)00113-1. [DOI] [PubMed] [Google Scholar]
- 150.Page T, Moseley C. Metabolic treatment of hyperuricosuric autism. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:397–400. doi: 10.1016/s0278-5846(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 151.Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40:703–707. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 152.Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J. Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 153.Panico LR, Ríos VG, Demartini MG, Carniello MA. [The electroencephalographic evolution of a group of patients on a ketonic diet] Rev. Neurol. 2000;30:8–15. [PubMed] [Google Scholar]
- 154.Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J. Neurochem. 2004;88:1305–1312. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- 155.Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol. Res. 2005;27:153–160. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- 156.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 157.Pedata F, Pazzagli M, Tilli S, Pepeu G. Regional differences in the electrically stimulated release of endogenous and radioactive adenosine and purine derivatives from rat brain slices. Naunyn Schmeidebergs Arch. Pharmacol. 1990;342:447–453. doi: 10.1007/BF00169463. [DOI] [PubMed] [Google Scholar]
- 158.Peters SJ, St. Amand TA, Howlett RA, Heigenhauser GJF, Spriet LL. Human skeletal muscle pyruvate dehydrogenase kinase activity increases after a low-carbohydrate diet. Am. J. Physiol. Endocrinol. Metab. 1998;275:E980–E986. doi: 10.1152/ajpendo.1998.275.6.E980. [DOI] [PubMed] [Google Scholar]
- 159.Phillips F, Chen CN, Crisp AH, Koval J, McGuinness B, Kalucy RS, Kalucy EC, Lacey JH. Isocaloric diet changes and electroencephalographic sleep. Lancet. 1975;2:723–725. doi: 10.1016/s0140-6736(75)90718-7. [DOI] [PubMed] [Google Scholar]
- 160.Plunet WT, Streijger F, Lam CK, Lee JH, Liu J, Tetzlaff W. Dietary restriction started after spinal cord injury improves functional recovery. Exp. Neurol. 2008;213:28–35. doi: 10.1016/j.expneurol.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 161.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 162.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990;43:273–286. doi: 10.1016/0304-3959(90)90025-9. [DOI] [PubMed] [Google Scholar]
- 164.Qin P, Xu H, Laursen TM, Vestergaard M, Mortensen PB. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005;331:23. doi: 10.1136/bmj.38488.462037.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Radulovacki M, Miletich RS, Green RD. N6 (L-phenylisopropyl) adenosine (L-PHA) increases slow-wave sleep (S2) and decreases wakefulness in rats. Brain Res. 1982;246:178–180. doi: 10.1016/0006-8993(82)90161-5. [DOI] [PubMed] [Google Scholar]
- 166.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rho JM, Sarnat HB, Sullivan PG, Robbins CA, Kim DW. Lack of long-term histopathologic changes in brain and skeletal muscle of mice treated with a ketogenic diet. J. Child Neurol. 2004;19:555–557. [PubMed] [Google Scholar]
- 168.Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res. 2006;69:273–294. doi: 10.1016/j.eplepsyres.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- 170.Sala-Newby GB, Skladanowski AC, Newby AC. The mechanism of adenosine formation in cells. Cloning of cytosolic 5'-nucleotidase-I. J. Biol. Chem. 1999;274:17789–17793. doi: 10.1074/jbc.274.25.17789. [DOI] [PubMed] [Google Scholar]
- 171.Sampath A, Kossoff EH, Furth SL, Pyzik PL, Vining EP. Kidney stones and the ketogenic diet: risk factors and prevention. J. Child. Neurol. 2007;22:375–378. doi: 10.1177/0883073807301926. [DOI] [PubMed] [Google Scholar]
- 172.Saransaari P, Oja SS. Enhanced release of adenosine under cell-damaging conditions in the developing and adult mouse hippocampus. Neurochem. Res. 2003;28:1409–1417. doi: 10.1023/a:1024956701683. [DOI] [PubMed] [Google Scholar]
- 173.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transfer. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 174.Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc. Natl. Acad. Sci. USA. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schubert P. Physiological modulation by adenosine: selective blockade of A1-receptors with DPCPX enhances stimulus train-evoked neuronal Ca influx in rat hippocampal slices. Brain Res. 1988;458:162–165. doi: 10.1016/0006-8993(88)90510-0. [DOI] [PubMed] [Google Scholar]
- 176.Semba K, White TD. M3 muscarinic receptor-mediated enhancement of NMDA-evoked adenosine release in rat cortical slices in vitro. J. Neurochem. 1997;69:1066–1072. doi: 10.1046/j.1471-4159.1997.69031066.x. [DOI] [PubMed] [Google Scholar]
- 177.Seyfried TN, Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr. Metab. 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 179.Smith M, Hopkins D, Peveler RC, Holt RI, Woodward M, Ismail K. First- v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry. 2008;192:406–411. doi: 10.1192/bjp.bp.107.037184. [DOI] [PubMed] [Google Scholar]
- 180.Sollevi A. Adenosine for pain control. Acta Anaesthesiol. Scand. Suppl. 1997;110:135–136. doi: 10.1111/j.1399-6576.1997.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 181.Stafstrom CE, Roopra A, Sutula TP. Seizure suppression via glycolysis inhibition with 2-deoxy-D-glucose (2DG) Epilepsia. 2008;49(Suppl 8):97–100. doi: 10.1111/j.1528-1167.2008.01848.x. [DOI] [PubMed] [Google Scholar]
- 182.Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 183.Sun C-X, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. J. Clin. Invest. 2005;115:35–43. doi: 10.1172/JCI22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tierney E, Nworoko NA, Kelley RI. Behavioral phenotype of RSH/Smith-Lemli-Opitz syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:131–134. doi: 10.1002/1098-2779(2000)6:2<131::AID-MRDD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 185.Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Varma H, Cheng R, Voisine C, Hart AC, Stockwell BR. Inhibitors of metabolism rescue cell death in Huntington's disease models. Proc. Natl. Acad. Sci. USA. 2007;104:14525–14530. doi: 10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 188.Vining EP, Freeman JM, Ballaban-Gil K, Camfield CS, Camfield PR, Holmes GL, Shinnar S, Shuman R, Trevathan E, Wheless JW. A multicenter study of the efficacy of the ketogenic diet. Arch. Neurol. 1998;55:1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 189.Virus RM, Ticho S, Pilditch M, Radulovacki M. A comparison of the effects of caffeine, 8-cyclopentyltheophylline, and alloxazine on sleep in rats. Possible roles of central nervous system adenosine receptors. Neuropsychopharmacology. 1990;3:243–249. [PubMed] [Google Scholar]
- 190.Von Lubitz DKJE, Beenhakker M, Lin RC, Carter MF, Paul IA, Bischofberger N, Jacobson KA. Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist. Eur. J. Pharmacol. 1996;302:43–48. doi: 10.1016/0014-2999(96)00101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weerts EM, Griffiths RR. The adenosine receptor antagonist CGS15943 reinstates cocaine-seeking behavior and maintains self-administration in baboons. Psychopharmacology. 2003;168:155–163. doi: 10.1007/s00213-003-1410-5. [DOI] [PubMed] [Google Scholar]
- 192.Westman EC, Yancy WS, Jr., Mavropoulos JC, Marquart M, McDuffie JR. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. (Lond.) 2008;5:36. doi: 10.1186/1743-7075-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Whitcomb K, Lupica CR, Rosen JB, Berman RF. Adenosine involvement in postictal events in amygdala-kindled rats. Epilepsy Res. 1990;6:171–179. doi: 10.1016/0920-1211(90)90070-c. [DOI] [PubMed] [Google Scholar]
- 194.Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921;2:307–308. [Google Scholar]
- 195.Wong V. Epilepsy in children with autistic spectrum disorder. J. Child Neurol. 1993;8:316–322. doi: 10.1177/088307389300800405. [DOI] [PubMed] [Google Scholar]
- 196.Wu W-P, Hao J-X, Halldner L, Lovdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu X-J. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 197.Xu K, Bastia E, Schwarzschild M. Therapeutic potential of adenosine A(2A) receptor antagonists in Parkinson's disease. Pharmacol. Ther. 2005;105:267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 198.Yancy WS, Jr., Foy M, Chalecki AM, Vernon MC, Westman EC. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. (Lond.) 2005;2:34. doi: 10.1186/1743-7075-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang J-M, Wang H-K, Ye C-Q, Ge W, Chen Y, Jiang Z-l, Wu C-P, Poo M-M, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 200.Zhao YT, Tekkök S, Krnjevic K. 2-Deoxy-D-glucose-induced changes in membrane potential, input resistance, and excitatory postsynaptic potentials of CA1 hippocampal neurons. Can. J. Physiol. Pharmacol. 1997;75:368–374. [PubMed] [Google Scholar]
- 201.Zhu PJ, Krnjevic K. Adenosine release is a major cause of failure of synaptic transmission during hypoglycaemia in rat hippocampal slices. Neurosci. Lett. 1993;155:128–131. doi: 10.1016/0304-3940(93)90689-i. [DOI] [PubMed] [Google Scholar]
- 202.Zhu PJ, Krnjevic K. Adenosine release mediates cyanide-induced suppression of CA1 neuronal activity. J. Neurosci. 1997;17:2355–2364. doi: 10.1523/JNEUROSCI.17-07-02355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ziegler DR, Gamaro GD, Araújo E, Bassani MG, Perry MLS, Dalmaz C, Gonçalves C-A. Nociception and locomotor activity are increased in ketogenic diet fed rats. Physiol. Behav. 2005;84:421–427. doi: 10.1016/j.physbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]