Homochirality is a ubiquitous feature in living systems and plays a crucial role in biological processes. In the biosphere, many naturally occurring biochemical molecules such as proteins, nucleic acids, and carbohydrates are homochiral.[1] In comparison, homochirality in the lithosphere and the inorganic world is a quite rare phenomenon. For example, even though quartz is chiral, it is found in nature in both right- and left-handed forms.[2] Also, unlike homochiral organic molecules,[3] chirality in the inorganic system often depends on the crystallization process which allows the generation of chirality through the organization of achiral inorganic units into the enantiomorphous space group. This crystallization process, however, almost always generates a mixture of right- and left-handed crystals, even though statistical fluctuations may occasionally result in enantioenriched bulk samples.[4]
There has long been a strong interest in creating crystalline homochiral porous materials that may be utilized for heterogeneous enantioselective catalysis, separation etc.[5–8] With few exceptions, homochiral porous solids prepared so far acquire homochirality through the incorporation of enantiopure organic ligands that are bonded to the crystalline framework either as a crosslinking ligand or pendant ligand. Induction by chiral additives or solvents has also been found to generate homochirality in crystals of a few metal-organic coordination polymers in which metal-ligand interactions may be responsible for the chiral induction.[9, 10] Clearly, these methods are not suitable for the synthesis of homochiral zeolite-type materials that are constructed entirely from inorganic building blocks.11
The bulk asymmetric crystallization of inorganic open-frameworks from achiral precursors into 3-D open-framework materials is so far an exceptional and yet highly desirable process because of its implication in heterogeneous asymmetric catalysis.[12, 13] Crystallization of some simple salts such as sodium chlorate under the influence of chiral co-solute (e.g., D-glucose) was reported a long time ago, albeit its enantioselectivity was disputed recently.[4] Such chiral mineral-biomolecule interactions have also been linked to the origin of biological homochirality.
In the absence of chiral induction agents, homochiral crystallization of inorganic salts from achiral precursors (e.g., NaClO3) have also been reported.[14] These experiments can lead to bulk homochiral crystals on the basis of statistical fluctuation of crystallization events. However, absolute chirality from run to run is generally not predictable. In addition, these structures (e.g., NaClO3) bear no similarity to materials with 3-D zeolite-type topology.
We are interested in developing synthetic methods that can lead to the bulk asymmetric crystallization of inorganic zeolite-type frameworks because of the potential applications of such materials in heterogeneous enantioselective catalysis. For inorganic crystals, the generation of chirality through crystallization is not uncommon, considering that many crystals with enantiomorphous space groups are known. What is uncommon and difficult to do is the controlled generation of homochiral or enantioenriched bulk samples. Since one of the most prominent families of inorganic zeolite-type materials are metal phosphates,[15, 16] we begin our work by investigating methods to create asymmetric crystallization in the zeolite-type metal phosphate system. For this work, we select one zeolite-type topology designated as CZP (CZP stands for chiral zincophosphate) that has an intrinsically chiral topology. It is based on a hydrated sodium zincophosphate (1, formula: NaZnPO4˙H2O, space group: P6122 or P6522).[17] It has a large-pore 12-memberedring channels along the hexagonal c axis. Even though being chiral, CZP has never been known in enantiopure or enantioenriched forms.
In devising synthetic strategies to create homochiral or enantioenriched CZP crystals, we hypothesize that if a nucleotide is employed as achiral induction agent, it may undergo enantioselective interaction with the crystal nuclei through its phosphate group. Such enantioselective interactions between the enantiopure nucleotide molecules and the racemic CZP crystals should offer an opportunity to achieve the asymmetric crystallization of zeolitic CZP crystals. Furthermore, the chiral interactions between biomolecules and inorganic crystals have always been of interest because of their relevance to the origin of biological homochirality. Here, we report the chirality induction effect of a ribonucleotide (uridine-5’-monophosphate disodium, denoted as ump) on the catalytic asymmetric crystallization of 1.
Upon mixing ZnO (0.052 g), H3PO4 (0.092 g, 85% aqueous solution), and H2O (2 mL) followed by the adjustment of pH to 11 using 6M NaOH (about 17 drops), a polycrystalline white powder was obtained and subsequently identified as 1 by X-ray powder diffraction. The same sample showed no solid state CD (circular dichroism) signal, suggesting the bulk was racemic. When a small amount of ump (0.0472 g) was added into the above fresh heterogeneous mixture, followed by hydrothermal treatment in a vial at 80°C for 4 days, crystals suitable for single crystal X-ray diffraction were obtained. All available evidences suggest that the asymmetric crystallization of CZP crystals (or more specifically, conversion of racemic crystals into enantioenriched crystals) occurred. Such asymmetric crystallization was catalyzed by the enantiopure nucleotide and could be repeated many times.
The first evidence of the asymmetric crystallization was provided by single crystal structure refinement. Crystal structures of 15 randomly selected crystals were refined using single-crystal X-ray diffraction data. The Flack parameter of each refinement indicates 14 crystals belong to the P6122 space group while only one crystal adopts the opposite P6522 space group (Table S1 in the supporting file). This suggests that the enantiomeric excess (ee) is approximately 85% in favor of the P6122 form when ump is used as the catalyst.
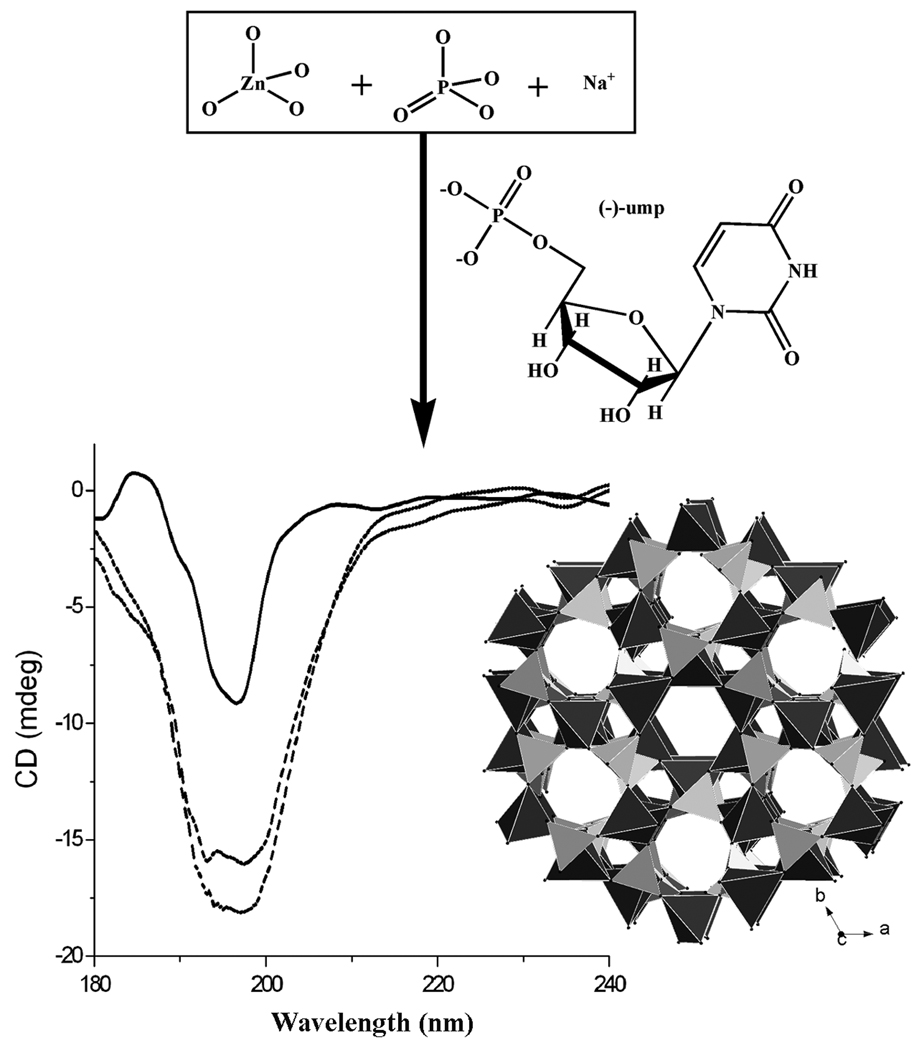
The second evidence for the asymmetric crystallization comes from the solid-state CD spectroscopy. The CD spectra for samples obtained from three separate synthetic batches catalyzed with ump show that the bulk samples of (−)-1 consistently exhibit negative CD signals at ~197 nm (Figure 1).
Figure 1.
An illustration of asymmetric crystallization of CZP zeolite-type crystals (crystal structure shown in polyhedral style) induced by ump catalyst, and the solid-state CD spectra of the enantioenriched bulk samples (three curves are for three different samples prepared in three separate syntheses).
The third evidence for the asymmetric crystallization is provided by a comparative study of the crystallization processes performed in the absence of ump. In such a case, the resulting bulk sample is a conglomerate. The single crystal X-ray diffraction study of 6 randomly selected crystals shows that right- and left-handed CZP crystals are present in the same amount (Table S2). Furthermore, no CD signal was detected for the bulk sample prepared in the absence of ump.
To probe the nature of this unusual chiral induction effect, we also experimented with both uridine and D-ribose (chiral parts of ump) as the chiral induction agent. The purpose is to determine whether the chiral sugar unit can exert a direct influence on the crystal growth. We found that neither uridine nor D-ribose exhibits the chiral induction effect (Figure S1 in the supporting file). This suggests that there is probably a cooperative effect between the binding of the phosphate group in ump to CZP crystal nuclei and the generation of the absolute chirality of CZP crystals.
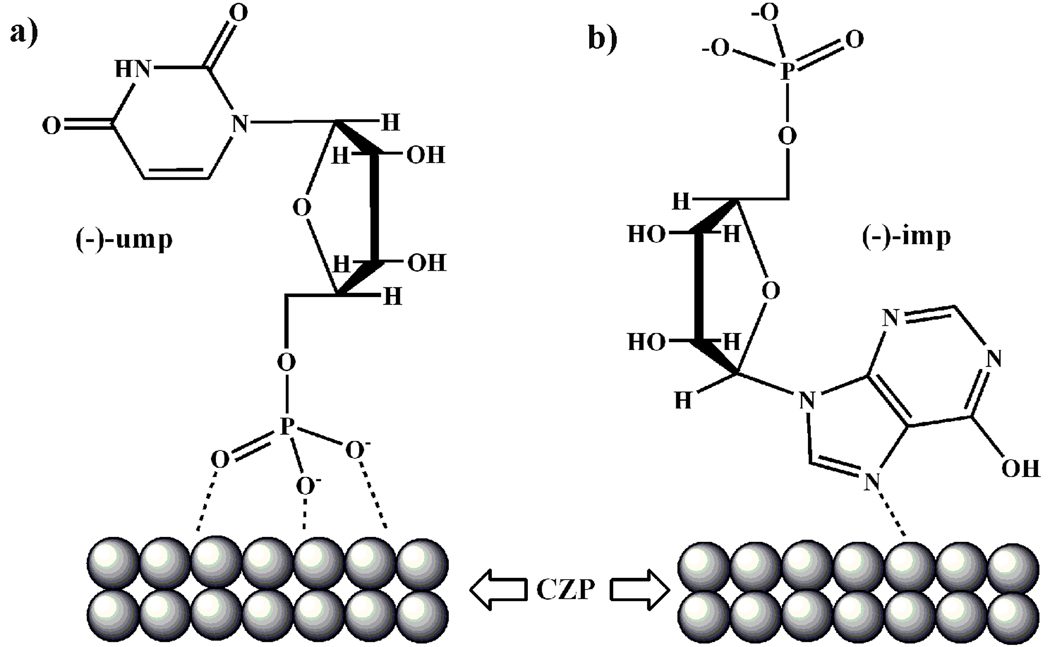
The use of a different nucleotide provides additional insight into the mechanism of the chirality induction. We find that the nucleotide, inosine-5’-monophosphate disodium (imp), is ineffective in the chirality induction. We believe that the difference between ump and imp in their catalytic induction ability is related to their base groups. The literature shows that imp has a strong tendency to use the base group (hypoxanthine, related to purine with a fused ring structure) to bond to the metal cation,[18] while ump tends to use its phosphate group for metal binding.[19] Therefore, we suggest that the enantioselective interaction occurs between the CZP crystal nuclei and ump at the solid-solution interface (Figure 2a) and that for imp, the interaction between the base group and metal cations could disrupt the metal-phosphate interaction and leads to the loss of enantioselectivity on CZP crystals (Figure 2b).
Figure 2.
An illustration of the possible interactions between (a) ump and CZP crystal nuclei and (b) imp and CZP crystal nuclei.
The mechanism of the chirality induction can be further probed by studying the effect of the reaction temperature. When the synthesis is performed at 120 °C under otherwise same synthetic conditions, the resulting bulk sample becomes racemic. Here the loss of chiral induction effect is likely due to the hydrolysis of ump into phosphate groups and uridine. This is supported by the fact that at 120°C, crystals 1 can also be grown by using ump as the sole phosphate source. In this case, by reacting a mixture of ump, Zn(NO3)2˙6H2O and 1,3-bis(4-piperidinyl)propane (pH = 11.3) at 120 °C, crystals 1 are obtained. This confirms that ump hydrolyzes at 120 °C to provide the PO43− source for the crystal growth of 1.
Many theories exist on the origin of homochirality in biological systems. One such theory suggests that the enantioselective adsorption of small organic molecules on the chiral surface of minerals such as quartz may play a role in the initial enantiomeric excess of some biomolecules. In this aspect, it is of interest to note that in the chiral induction effect reported here, it is the chirality of enantiopure biomolecules that control the absolute chirality of mineral-like chiral inorganic crystals. Such process is the reverse process of the mineral induction effect in the "origin-of-homochirality" theory. This raises an interesting question about whether there is a mutual chirality amplification effect during the generation of homochirality in biomolecules in the prebiotic period.
In conclusion, we report here an unusual example of the asymmetric crystallization of an inorganic zeolite-type material. The asymmetric crystallization is achieved by using a nucleotide as the chirality induction agent, based on a strategy that matches the functional groups of the chirality induction agent with the bonding features of the chiral crystals. Additional evidences such as effects of other nucleotides, nucleosides, D-sugars, and reaction temperature further support that the mechanism of the chirality induction is through the cooperative effect between the phosphate binding to the crystal nuclei and absolute chirality control from the sugar unit. We believe the method reported here represents a new paradigm in the development of homochiral crystalline materials and may help to provide new insights into the chiral mineral-biomolecule interactions.
Experimental Section
Single-crystal structure analysis
Each crystal was glued to a glass fiber with epoxy resin and mounted on a Bruker APEX II diffractometer equipped with a fine focus, 2.0 kW sealed tube X-ray source (MoKα radiation, γ = 0.71073Å) operating at 50kV and 30mA. Each structure was solved by direct methods followed by successive difference Fourier methods.
Measurement of solid CD spectra
The mixture of about 3-mg sample and 40 mg dried KBr powder was well grounded and then pressed into a disk for use in the CD measurement, using a J-810 spectropolarimeter.
Supplementary Material
Acknowledgments
We thank the support of this work by NIH (X. B. 2 S06 GM063119-05) and X. B is a Henry Dreyfus Teacher Scholar.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Mason SF. Nature. 1984;311:19–23. doi: 10.1038/311019a0. [DOI] [PubMed] [Google Scholar]
- 2.Hazen RM, Sholl DS. Nat. Mater. 2003;6:367–374. doi: 10.1038/nmat879. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PJ, Kozlowski MC. Fundamentals of Asymmetric Catalysis. Sausalito, California: University Science Books; 2009. [Google Scholar]
- 4.Alexander AJ. Cryst. Growth Des. 2008;8:2630–2632. [Google Scholar]
- 5.a) Férey G. Chem. Soc. Rev. 2008;37:191. doi: 10.1039/b618320b. [DOI] [PubMed] [Google Scholar]; b) Férey G, Mellot-Draznieks C, Serre C, Millange F. Acc. Chem. Res. 2005;38:217. doi: 10.1021/ar040163i. [DOI] [PubMed] [Google Scholar]; c) Guillou N, Livage C, Drillon M, Ferey G. Angew. Chem. 2003;115:5472. doi: 10.1002/anie.200352520. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:5314. [Google Scholar]
- 6.a) Kesanli B, Lin W. Coord. Chem. Rev. 2003;246:305. [Google Scholar]; b) Ma L, Abney C, Lin W. Chem Soc. Rev. 2009;38:1248–1256. doi: 10.1039/b807083k. [DOI] [PubMed] [Google Scholar]; c) Wu C-D, Hu A, Zhang L, Lin W. J. Am. Chem. Soc. 2005;127:8940. doi: 10.1021/ja052431t. [DOI] [PubMed] [Google Scholar]
- 7.a) Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. doi: 10.1038/35010088. [DOI] [PubMed] [Google Scholar]; b) Vaidhyanathan R, Bradshaw D, Rebilly J-N, Barrio JP, Gould JA, Berry NG, Rosseinsky MJ. Angew. Chem. 2006;118:6645. doi: 10.1002/anie.200602242. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:6495. [Google Scholar]; c) Cao G, Garcia ME, Alcalá M, Burgess LF, Mallouk TE. J. Am. Chem. Soc. 1992;114:7574. [Google Scholar]; d) Dybtsev DN, Nuzhdin AL, Chun H, Bryliakov KP, Talsi EP, Fedin VP, Kim K. Angew. Chem. Int. Ed. 2006;45:916. doi: 10.1002/anie.200503023. [DOI] [PubMed] [Google Scholar]; e) Xiong R-G, You X-Z, Abrahams B, Xue Z, Che C-M. Angew. Chem. 2001;113:4554. doi: 10.1002/1521-3773(20011203)40:23<4422::aid-anie4422>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2001;40:4422. [Google Scholar]; f) Dybtsev DN, Yutkin MP, Peresypkina EV, Virovets AV, Serre C, Férey G, Fedin VP. Inorg. Chem. 2007;46:6843. doi: 10.1021/ic7009226. [DOI] [PubMed] [Google Scholar]
- 8.a) Zhang J, Bu X. Angew. Chem. 2007;119:6227. [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:6115. [Google Scholar]; b) Zhang J, Chen S, Valle H, Wong M, Austria C, Cruz M, Bu X. J. Am. Chem. Soc. 2007;129:14168. doi: 10.1021/ja076532y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang J, Liu R, Feng P, Bu X. Angew. Chem. 2007;119:8540. doi: 10.1002/anie.200703008. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:8388. [Google Scholar]
- 9.Lin Z, Slawin AMZ, Morris RE. J. Am. Chem. Soc. 2007;129:4880. doi: 10.1021/ja070671y. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Chen S, Wu T, Feng P, Bu X. J. Am. Chem. Soc. 2008;130:12882. doi: 10.1021/ja805272j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Sun J, Bonneau C, Cantín Á, Corma A, Díaz-Cabañas MJ, Moliner M, Zhang D, Li M, Zou X. Nature. 2009;458:1154–1157. doi: 10.1038/nature07957. [DOI] [PubMed] [Google Scholar]; b) Han Y, Zhang DL, Chng LL, Sun JL, Zhao L, Zou XD, Ying JY. Nature Chemistry. 2009;1:123–127. doi: 10.1038/nchem.166. [DOI] [PubMed] [Google Scholar]
- 12.Flanigen EM. In: Introduction to Zeolite Science and Practice. van Bekkum H, Flanigen EM, Jansen JC, editors. New York: Elsevier; 1991. pp. 13–34. [Google Scholar]
- 13.Margelefsky EL, Zeidan RK, Davis ME. Chem Soc. Rev. 2008;37:1118–1126. doi: 10.1039/b710334b. [DOI] [PubMed] [Google Scholar]
- 14.Kondepudi DK, Kaufman RJ, Singh N. Science. 1990;250:975–976. doi: 10.1126/science.250.4983.975. [DOI] [PubMed] [Google Scholar]
- 15.a) Feng PY, Bu XH, Stucky GD. Nature. 1997;388:735–741. [Google Scholar]; b) Lai YL, Lii K-H, Wang SL. J. Am. Chem. Soc. 2007;129:5350. doi: 10.1021/ja070733k. [DOI] [PubMed] [Google Scholar]
- 16.a) Cooper ER, Andrews CD, Wheatley PS, Webb PB, Wormald P, Morris RE. Nature. 2004;430:1012. doi: 10.1038/nature02860. [DOI] [PubMed] [Google Scholar]; b) Parnham ER, Morris RE. Acc. Chem. Res. 2007;40:1005. doi: 10.1021/ar700025k. [DOI] [PubMed] [Google Scholar]; c) Parnham ER, Morris RE. J. Am. Chem. Soc. 2006;128:2204. doi: 10.1021/ja057933l. [DOI] [PubMed] [Google Scholar]; d) Drylie EA, Wragg DS, Parnham ER, Wheatley PS, Slawin AMZ, Warren JE, Morris RE. Angew. Chem. 2007;119:7985. doi: 10.1002/anie.200702239. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2007;46:7839. [Google Scholar]; e) Parnham ER, Drylie EA, Wheatley PS, Slawin AMZ, Morris RE. Angew. Chem. 2006;118:5084. doi: 10.1002/anie.200600290. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:4962. [Google Scholar]
- 17.Harrison WTA, Gier TE, Stucky GD, Broach RW, Bedard RA. Chem. Mater. 1996;8:145–151. [Google Scholar]
- 18.Quiros M, Salas JM, Sanchez MP, Alabart JR, Faure R. Inorg. Chem. 1991;30:2916–2921. [Google Scholar]
- 19.Aoki K, Saenger W. J. Chem. Soc., Dalton Trans. 1984:1401–1409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.