Abstract
Laboratory research involving berries is a promising example of food-based cancer prevention. Berries contain many known chemopreventive agents, such as anthocyanins and ellagitannins, that can be greatly concentrated in freeze-dried berry powders. Based on our program of berry research, this commentary presents the first reported stepwise scheme for the preclinical and clinical development of foodstuffs for cancer prevention. Our preclinical work within this scheme includes promising approaches for assessing the chemopreventive potential of berry powder and berry extracts in preclinical model systems; for determining these compounds’ mechanisms of action; and for identifying the active constituents in berries. The commentary also presents preliminary results of clinical trials in the oral cavity, esophagus and colon using various formulations of freeze-dried berries. The relative merits of berry powders, extracts or individual constituents (anthocyanins) for cancer prevention are also discussed.
Keywords: Chemoprevention, berries, esophagus, colon, animal, human
Introduction
Chemoprevention is the administration of one or more chemical entities, either as individual drugs or as dietary supplements, to prevent the initiation of premalignant lesions or their progression to cancer or cancer recurrence (1). Most chemoprevention studies have been conducted with individual compounds, including various nutrients and non-nutrient phytochemicals. Our laboratory has devoted considerable effort in the past toward developing individual compounds for cancer prevention, especially the non-nutrient phytochemicals ellagic acid and phenylethyl isothiocyanate (2, 3). Recently, however, we have devoted most of our effort to developing and applying a “food-based” approach to cancer prevention using freeze-dried, commercially available, edible berries. Our approach to evaluating the efficacy of whole berries (containing numerous compounds) for cancer prevention is nearly identical to that used by chemoprevention scientists working with individual compounds.
Our interest in berries stemmed from early studies with ellagic acid, which is found in the pulp and seeds but not the juice of berries (4). Because water accounts for about 85%–90% of the wet weight of berries, we reasoned that the removal of water from berries would result in an approximately 10-fold concentration of the ellagic acid. Therefore, we began to freeze-dry berries under anoxic conditions to insure the integrity of their components, and to grind the dried berries into powder. Chemical analysis of different berry powders revealed that berries contain multiple chemopreventive agents in addition to ellagic acid (5). Table 1 presents a list of some potential chemopreventive agents in black raspberries (BRBs). Blackberries, strawberries, blueberries and others contain chemopreventive agents similar to those in BRBs (Table 1) but differing in quality and/or quantity (data not published). Therefore, berry powders contain a combination of chemoprotective agents that might be expected to act at multiple stages in the carcinogenesis process. This is undoubtedly the case for other foodstuffs as well. Indeed, we were encouraged to test berry powder by early reports on the chemopreventive potential of other foodstuffs such as tea (6, 7), broccoli (8), tomato juice (9), and soybeans (10).
Table 1.
Some potential chemopreventive agents in powder made from black raspberries harvested in 1997, 2001 and 2006
|
Crop year* |
||||
|---|---|---|---|---|
| Component | 1997 | 2001 | 2006 | References |
| Minerals | ||||
| calcium | 215.00 | 175.00 | 188.00 | 21 |
| selenium* | <5.00 | <5.00 | <5.00 | 22 |
| zinc | 2.69 | 2.34 | 2.16 | 23 |
| Vitamins | ||||
| α-carotene | <0.02 | <0.02 | <0.03 | 24 |
| β-carotene | <0.02 | 0.06 | <0.07 | 25 |
| α-tocopherol | n.d. | n.d. | 10.40 | 26 |
| γ-tocopherol | n.d. | n.d. | 11.20 | 27 |
| folate | 0.06 | 0.08 | 0.14 | 28 |
| Sterols | ||||
| β-sitosterol | 80.10 | 88.80 | 110.00 | 29 |
| campesterol | 3.40 | 5.90 | 5.50 | 30 |
| Simple phenols | ||||
| ellagic acid | 166.30 | 185.00 | 225.00 | 2 |
| ferulic acid | 17.60 | <5.00 | 47.10 | 31 |
| ρ-coumaric acid | 9.23 | 6.82 | 6.92 | 32 |
| chlorogenic acid | n.d. | n.d. | 0.14 | 32, 33 |
| quercetin | n.d. | 43.60 | 36.50 | 32, 34 |
| Anthocyanins (complex phenols) | ||||
| cyanidin-3-O-glucoside | n.d. | 250.00 | 278.50 | 13, 35 |
| cyanidin-3-O-sambubioside | n.d. | 220.00 | 56.00 | 13, 35 |
| cyanidin-3-O-rutinoside | n.d. | 2002.00 | 1790.00 | 13, 35 |
| cyanidin-3-O-xylosylrutinoside | n.d. | 510.00 | 853.50 | 13, 35 |
Abbreviation: n.d., not determined.
All measures in the crop-year columns are mg/100 g dry weight, except for that of selenium, which is μg/100 g dry weight.
This commentary presents a concise summary of current laboratory work with BRBs and discusses several important topics not detailed in previous reviews as well (11–13). It details a stepwise scheme for assessing the chemopreventive potential of berries and other foodstuffs in preclinical models and clinical trials. It is important to mention that this approach has involved the integrative efforts of numerous basic scientists, physician and dental scientists and practitioners, statisticians, laboratory and clinical trial managers and technicians, postdoctoral trainees, and graduate students. I also discuss the potential advantages and disadvantages of powders, extracts and individual compounds, including related issues of different formulations and routes of administration; the updated status of clinical BRB trials, including the final polyp-regression results of our trial in familial adenomatous polyposis (FAP) patients and a list of all pilot clinical trials (to my knowledge) of BRBs and their specific biomarker endpoints; and initial batch-to-batch consistency and/or variation of BRB powders from a single source farm.
Scheme for Evaluating the Chemopreventive Potential of Berry Powder
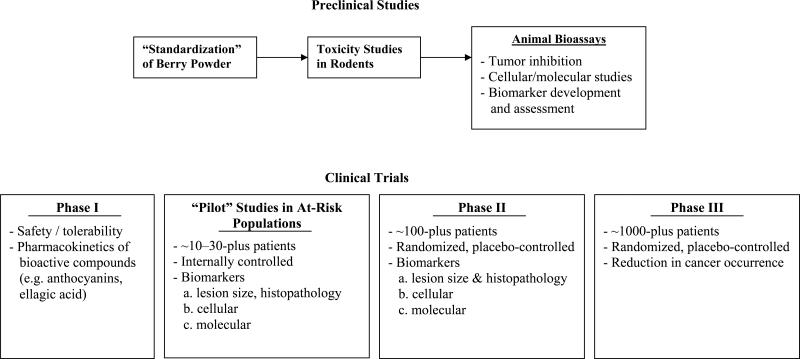
We and others have suggested the following stepwise approach for evaluating the chemopreventive potential of berry powders (Fig. 1): 1) develop “standardized” powders using chemical analyses; 2) evaluate toxicity in rodents; 3) determine anti-tumorigenic effects and the mechanism(s) for these effects in rodents; 4) conduct phase I clinical trials in humans; 5) conduct “pilot” trials of different berry powder formulations for effects on precancerous lesions and biomarkers in humans; 6) conduct randomized, placebo-controlled phase II biomarker trials; and 7) conduct phase III trials to determine cancer prevention efficacy. This approach could easily be applied to the assessment of powders from other foodstuffs and is similar to that described by Kelloff et al. (14) for the preclinical (in vitro and animal) and clinical development of individual compounds. The scheme of Kelloff et al. differs from ours principally in their proposed initial step, which is to either synthesize an individual compound or isolate one from naturally occurring sources; a standardized berry powder in our approach contains multiple compounds. Indeed, we and our collaborators also have used the individual-agent approach, isolating anthocyanins (from BRBs) and identifying those with chemopreventive potential in animals (see below; ref. 15, 16). The specific steps of our approach for developing berries and berry components for cancer prevention are summarized in the following sections.
Fig. 1.
Scheme for assessing berries (or other foodstuffs) for chemopreventive efficacy.
“Standardizing” berry powders for chemoprevention studies
Early studies revealed that the ellagic acid and anthocyanin contents in berries obtained from different farms in Ohio varied as much as 2 to 4 fold (4, 17). Therefore, to minimize this inherent variability, we obtain all berries from a single farm in Southern Ohio. Most studies have been conducted with BRBs (Rubus occidentalis) of a single variety (Jewel) because they have among the highest levels of anthocyanins and ellagitannins (18) and exhibit higher antioxidant activity (19) compared with most other commercially available berry types.
Ripe BRBs are picked mechanically, washed with water, and frozen at −20° C on the farm within 2−3 hours of picking. The berries are then shipped frozen to Van Drunen Farms in Momence, Illinois, where they are freeze-dried under anoxic conditions to protect the integrity of berry components. Next, seeds are removed by forcing the freeze-dried berries through a small sieve, and the dried pulp is ground into a powder. The berry powder is shipped at a low temperature to Ohio State University, where it is stored at −20° C until used in experimental studies. For standardization purposes, each batch of powder undergoes a quantitative chemical analysis of 26 randomly selected nutrients and non-nutrient components, including some agents with chemopreventive potential (5, 20). The levels of the 26 components remain within 10%–20% of the initial analyses for at least two years in powder stored at −20° C (20).
Table 1 shows some of the potential chemopreventive agent content (5, 21–35) of powders that were prepared from BRBs obtained in 1997, 2001 and 2006; relatively high levels of calcium, ß-sitosterol, ellagic acid, quercetin and the anthocyanins are notable. The amounts of calcium, zinc, ß-sitosterol, α-carotene, ellagic acid, ρ-coumaric acid, quercetin, cyanidin-3-O-glucoside, cyanidin-3-O-rutninoside and cyanidin-3-O-xylosylrutinoside in the yearly powders varied from 10%–40%, whereas the amounts of other constituents (ß-carotene, folate, ferulic acid and cyanidin-3-O-sambubioside) varied from 60%–90%. The relatively high variability in levels of ß-carotene and folate is likely due to difficulties in accurately measuring the low levels of these agents in the powder. Selenium is present in microgram quantities in BRBs; therefore, values for selenium are reported as <5.00 μg/100 g dry weight. Because we routinely analyze only a small percentage of the overall number of compounds in BRBs, it is likely that BRBs contain known (and perhaps unknown) chemopreventive agents in addition to those listed in Table 1. Therefore, berries, like other foodstuffs, represent combinations of agents that may exhibit chemopreventive potential, particularly when concentrated by freeze-drying.
Toxicity studies in rodents
One of the most desirable features of a chemopreventive agent is little or no toxicity at concentrations producing chemopreventive efficacy. We have evaluated the toxicity of BRBs in rats fed a synthetic diet (AIN-76A diet) plus either 5% or 10% BRB powder by weight (w/w) for up to nine months. These percentages of BRB powder in a rat diet would be equivalent to approximately 0.9 to 1.8 oz of BRB powder in the daily human diet, as calculated on a body surface area basis (36). Since one ounce of berry powder is equivalent in content to about 10 ounces of fresh berries, 0.9 to 1.8 oz of powder averages out to about 0.8 lb of fresh whole BRBs per day overall.
Histopathologic studies indicated that these BRB diets did not produce toxic effects in any major organs of the animals, and there were no significant differences in either body weight or food consumption between rats on either of the BRB-supplemented diets versus control rats on the AIN-76A-alone diet during the nine-month treatment. An unexpected benefit of the berry diets in rats was a 10% reduction in total blood cholesterol.
Inhibition of carcinogen-induced tumors and mechanistic studies in vivo
Diets containing 5% and 10% BRB powder inhibit carcinogen-induced tumors in the rat esophagus, colon and mammary gland and the hamster cheek pouch (5, 37–39). The most reliable measure of tumor inhibition in these studies is tumor multiplicity; in general and depending on the temporal sequence of administration of the carcinogen and the berry diet, the extent of inhibition of tumor multiplicity ranges from about 30%–70%. Optimal tumor inhibition occurs when the BRBs are added to the diet before, during and after treatment with carcinogens, suggesting that consumption of berries throughout life may maximize their chemopreventive effectiveness in humans. That berry diets do not inhibit 100% of tumorigenesis suggests that the inhibitory components of BRBs are not completely absorbed and/or that berry compounds do not affect certain critical signaling pathways of carcinogenesis. It should be mentioned that diets containing 5% and 10% strawberry and blackberry powders were nearly as effective as BRB powders in inhibiting tumors induced in the rat esophagus by the carcinogen N-nitrosomethylbenzylamine (NMBA; ref. 13). In contrast, diet with 5% or 10% blueberry powder was ineffective (13), and studies are underway to determine the basis for this result.
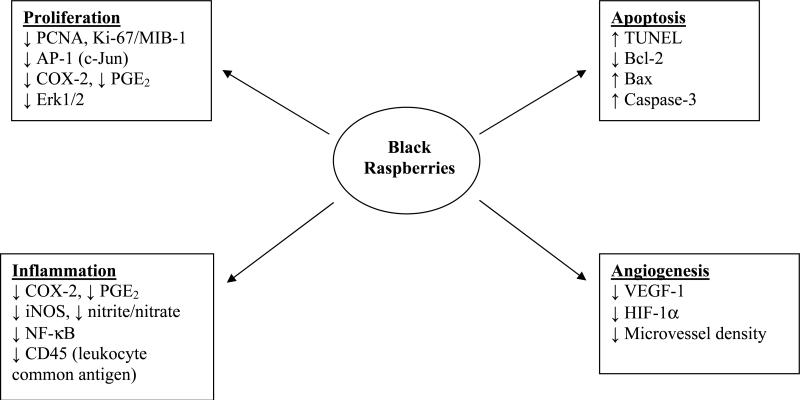
Cellular and molecular mechanisms of chemoprevention by berries have been studied most often in vivo with BRBs in the NMBA model of rat esophageal carcinogenesis. BRBs influence cellular and molecular events associated with proliferation, apoptosis, inflammation and angiogenesis (ref. 13, 20; Fig. 2). A recent investigation involving DNA microarray identified NMBA-dysregulated genes in the initiation stage of rat esophageal carcinogenesis that were restored to near normal levels of expression by BRBs (40). These restored genes were associated with multiple cellular functions indicating that the active components of BRBs elicit a genome-wide effect in modulating genes involved in the early events of esophageal carcinogenesis. Perhaps this is not surprising in view of the array of chemopreventive agents in berries that potentially act on different signaling pathways. Mechanistic data from in vitro studies with berry extracts (presented below) confirm the wide range of effects of berry components on cellular and molecular events associated with carcinogenesis.
Fig. 2.
Effects of black raspberries on cellular events and associated genes in the N-nitrosomethylbenzylamine (NMBA)-treated rat esophagus. PCNA, proliferating cell nuclear antigen; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa B; VEGF-1, vascular endothelial growth factor-1; HIF-1α, hypoxia-inducible factor-1α.
Phase I clinical trial of BRBs in humans
Clinical trials of BRBs were based on promising preclinical data. A phase I trial evaluated the safety and tolerability of BRB powder (45 g as a slurry in water daily for 7 days) and measured anthocyanins and ellagic acid in the plasma and urine of 11 healthy participants (41). This dose of BRB powder is equivalent to the human consumption of about 16 ounces (1 lb) by weight of fresh whole BRBs daily. BRBs were administered in powder form rather than fresh for two reasons: a) 1 lb of fresh BRBs is a substantial, problematic quantity to consume on a daily basis, particularly for humans who cannot tolerate berry seed; b) fresh BRBs are available in stores only 1−2 months of each year, whereas high-quality BRB powder is available during the entire year. For chemoprevention, therefore, berry powder is more feasible. The berry powder was well tolerated, with a low incidence of mild or moderate constipation in 4 of the 11 subjects. Maximum concentrations of anthocyanins and ellagic acid occurred at 1−2 hours in plasma and at 1/2−4 hours in urine. The overall uptake of anthocyanins and ellagic acid was <1% of the administered dose as determined by measurement of free anthocyanins and ellagic acid in plasma. It is likely, however, that the uptake of these compounds was underestimated since their metabolites and protein-bound forms were not measured in plasma (41, 42). In a subsequent pilot study of oral BRB powder (32 or 45 g/day for 6 months) in Barrett's esophagus patients (43), about 15% of patients reported symptoms of occasional diarrhea, constipation or epigastric pain, but the symptoms were not severe and all patients continued berry powder consumption throughout the study. The collective human and animal data suggest that BRB powder is well tolerated in humans at doses of up to 45 g/day for at least 6 months and in animals at effective chemopreventive concentrations in the diet.
“Pilot” intervention trials in humans
A series of “pilot” clinical trials are being conducted in individuals at higher-than-normal risk for cancer to determine if BRBs have potential for chemoprevention in humans (Table 2). These trials are internally controlled (i.e., each patient serves as his/her own control), involve few patients (15 to 30), and determine the effects of BRBs on dysplastic lesions and relevant biomarkers after relatively short-term (1 to 9 months) treatment. We view these trials as a time- and cost-effective means of assessing whether berries exhibit effects in specific cohorts with desirable characteristics for further examination in randomized, placebo-controlled, phase II and III clinical trials. Results from pilot trials in patients with Barrett's esophagus or oral dysplasia (43–45) clearly show that topical BRB in a 10% bioadhesive gel was more effective against oral dysplasia than oral BRB powder was against Barrett's esophagus, presumably because the topical treatment facilitated the absorption of berry anthocyanins and other compounds into the oral lesions (44). Ongoing trials are also examining the effects of BRB lozenges on the expression of nuclear factor kappa B (NF-кB) in tumor tissues from patients with oral squamous cell carcinoma and on recurrence in clinically treated patients with oral squamous cell carcinoma (46, 47).
Table 2.
“Pilot” clinical trials of black raspberries in at-risk populations
| Trial | Delivery route | No. Patients | Biomarkers | Status | References |
|---|---|---|---|---|---|
| Barrett's esophagus | Oral | 20 | - lesion size | Complete | 43 |
| - histopathology | |||||
| - cell proliferation | |||||
| - oxidative stress | |||||
| - phase II enzymes | |||||
| Esophageal dysplasia | Oral | 60 | - lesion size | Ongoing | - |
| - histopathology | |||||
| - cell proliferation | |||||
| - COX-2, iNOS | |||||
| Oral dysplasia | Berry gel | 27 | - lesion size | Complete | 44, 45 |
| - histopathology | |||||
| - loss of heterozygosity (LOH) | |||||
| - COX-2, iNOS | |||||
| - gene modulation (microarray) | |||||
| Colon cancer | Oral | 30 | - cell proliferation | Complete | 48 |
| - apoptosis | |||||
| - angiogenesis | |||||
| - β-catenin | |||||
| - E-cadherin | |||||
| - c-myc | |||||
| - cyclin D1 | |||||
| Rectal polyps | Oral and rectal suppository | 14 | - polyp number | Complete | 49 |
| - polyp size | |||||
| Prostate cancer | Oral | 20 | - cell proliferation | Ongoing | - |
| - prostate specific antigen | |||||
| Oral cancer | Lozenge | 35 | - NFκB and other genes | Ongoing | 46, 47 |
Abbreviations: COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase; NFκB, nuclear factor kappa B.
Recent results from two pilot trials in colorectal cancer or FAP suggest that berries may be useful for chemoprevention of colon cancer. BRB powder (20g/3x/day) administered orally in a slurry of water for a short term (2-to-4 weeks). produced a positive trend for changes in the expression of Ki-67 (marking cell proliferation), TUNEL (apoptosis), CD105 (angiogenesis) and genes associated with the Wnt pathway (ß-catenin, E-cadherin, c-Myc, cyclin D1) in colorectal tumors (and not in normal colon; ref. 48). Only the reduction in Ki-67 cell proliferation rates, however, was significant (P<0.05). The positive modulation of a key biomarker such as Ki-67 of tumor development after short-term treatment with BRBs is encouraging. The recovery of BRB anthocyanins from normal colon tissues obtained from berry-treated patients indicated that the anthocyanins reached the target tissue and were absorbed locally.
FAP is a dominantly inherited disease characterized by the early onset of colonic polyposis and a nearly 100% risk of colon cancer by the age of 40. The traditional management of FAP is colectomy followed by lifelong endoscopic surveillance of the rectum and removal of rectal polyps. In a pilot study involving FAP patients who had undergone a colectomy (49), 7 patients received BRB powder (20g/3x/day) in a slurry of water plus two rectal suppositories (700 mg BRBs each) inserted one hour before bedtime; the other 7 patients received an oral powder placebo in a slurry of water plus the two active BRB suppositories; treatment lasted 9 months. The number of polyps was reduced by a median of 38% overall after 9 months (compared with polyp counts at baseline), including a median reduction of 53% in patients receiving both routes of berry treatment and 25% in patients treated with suppositories only. Studies are underway to determine the molecular mechanism(s) for BRB-induced polyp regression in these patients. The pilot results suggest that BRBs may be as or more effective than non-steroidal anti-inflammatory drugs in regressing rectal polyps in FAP patients. Four other patients in this trial in 18 total patients, however, dropped out early because of rectal fissures caused by the suppositories. Therefore, the use of BRB suppositories for future trials to prevent rectal cancers is questionable.
Phase II and III clinical trials
To date, a single phase II clinical trial of BRBs in the oral cavity has been undertaken and is ongoing (C. Weghorst, personal communication, January 7, 2009); no phase III trials have been initiated. The pilot trial results suggest that there are sufficient positive data to initiate the first phase II clinical trials of BRBs in the colon and more studies in the oral cavity.
Berry Extracts and Bioactive Constituents
Water- and/or solvent-soluble extracts obtained from foods such as tea, grape seed and pomegranate have been studied extensively for chemoprevention (50–52). Although they contain mixtures of compounds, extracts are thought to be more easily “standardized” than are whole foodstuffs, and they usually can be prepared with minimal difficulty. Extracts from different berry types, including BRBs, produce in vitro effects associated with chemoprevention including inhibition of cell transformation, proliferation and carcinogen-induced gene expression and stimulation of apoptosis and differentiation (13). Huang et al. (53) have shown that an alcohol extract of BRB powder reduces the activities of multiple carcinogen-induced genes in JB-6 mouse epidermal cells, including genes associated with the signal transduction pathways of phosphoinositide-3 kinase (PI-3K)/Akt, activator protein (AP-1), ERKs/p38K, and NFкB. An ethanol/H2O extract of BRBs was fractionated using high-performance liquid chromatography, and the subfractions were tested for their ability to down-regulate carcinogen-induced AP-1 and NF-кB activities in JB-6 cells; the major constituents of the most active subfractions were 3 (of the 4) anthocyanins in BRBs: cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside and cyanidin 3-O-(2G)-xylosylrutinoside (15). We recently assessed these anthocyanins for in vivo activity, finding that a diet containing an anthocyanin-rich fraction of BRBs was as effective in inhibiting NMBA-induced esophageal tumorigenesis in rats as was a diet containing 5% whole (not fractionated) BRB powder (16). Both diets contained the same, relatively small amount of anthocyanins (3.8 μmol/g diet), suggesting that relatively small doses of anthocyanins have important chemopreventive effects and that an anthocyanin-rich fraction of BRBs might be useful for cancer chemoprevention.
Pure anthocyanins, including cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside in BRBs, exhibit multiple anti-carcinogenic effects in vitro, as summarized by Wang and Stoner (54). In vivo, cyanidin-3-O-glucoside (at 0.3% of the diet) inhibits adenoma development in APCmin mice (55), and the anthocyanin delphinidin (found in pomegranates) inhibits biomarkers of skin tumorigenesis in CD-1 mice (52). These data suggest that additional studies of pure anthocyanins as potential chemopreventive agents are warranted.
Berry Powders versus Extracts versus Anthocyanins
Table 3 lists some advantages and disadvantages of using berry powders, berry extracts (water- and solvent-soluble), and pure anthocyanins (as examples of individual berry compounds) for cancer chemoprevention. Similar advantages and disadvantages undoubtedly apply to other foodstuffs and their derivatives. The choice of berry formulation (powder, extract, anthocyanin) for specific chemoprevention studies depends, in part, on the target tissue. For example, BRB gels applied topically to oral lesions optimize the delivery of anthocyanins to target tissues (44), and the topical application of an alcohol/water extract of BRB powder to mouse skin inhibited UVB-induced skin tumorigenesis (unpublished data). The oral consumption of berry powders may prove to be effective for colon cancer prevention (48, 49). A major advantage of berries and their component anthocyanins and of other foodstuffs and their components—e.g., tea/epigallocatechin-3-gallate, grapes/resveratrol, tumeric/curcumin, and tomatoes/lycopene—for chemoprevention is their apparent lack of toxicity in animals and in humans in comparison with toxicity of certain retinoids, selenium compounds, non-steroidal anti-inflammatory drugs, and ß-carotene (56–59). The various berry formulations, however, have not been administered to humans in multi-year trials, where toxicity is more likely and has occurred with many other preventive agents. Therefore, it may be premature to assume that berry formulations will be non-toxic in multi-year trials.
Table 3.
Some advantages and disadvantages of berry powders, extracts and individual anthocyanins for cancer chemoprevention
| Advantages |
Disadvantages |
|
|---|---|---|
| Powders | Contain multiple chemopreventive agents | Difficult to “standardize” |
| Modulate multiple events in carcinogenesis | Stability of berry components influenced by several factors | |
| Appear to cause little or no toxicity | Potential contamination with microbes and chemicals | |
| Can be administered in different formulations | Apparent need for high consumption to be effective | |
| Relatively inexpensive | Seasonal availability of some berry types | |
| Extracts | Potentially contain several chemopreventive agents | More difficult to prepare than powders |
| Modulate multiple carcinogenic events in vitro | Some components unstable | |
| More easily standardized than powders | Efficacy in vitro is variable–high doses usually required | |
| Appear to cause little toxicity | High doses required for in vivo efficacy when given orally | |
| Can be administered in different formulations | May be expensive | |
| Useful for topical application to precancerous lesions | ||
| Anthocyanins | Easily standardized | Unstable at alkaline pH |
| Can be modified to improve bioavailability | Difficult to synthesize | |
| Influence multiple events in carcinogenesis | High doses required for in vitro efficacy | |
| Can be administered in different formulations | Poor bioavailability | |
| Cause little toxicity | Expensive |
A disadvantage of whole berries and other foodstuffs for cancer prevention is the requirement for “standardized” formulations that provide reproducible chemopreventive effects. As indicated above, we found that the contents of ellagic acid and anthocyanins in BRBs from different Ohio farms varied significantly. This variability is likely to be even greater in specific berry types grown throughout the world. Although we have tried to remedy variability by procuring berries from a single farm, this is not a “real-world” solution. Using a single lot of berry powder for an entire animal experiment or human trial, however, should allow a close determination of the amount of a specific chemopreventive agent(s) that will be needed to reproduce potential chemopreventive effects; therefore, we are using single lots to derive values for the amounts of anthocyanins, ellagitannins and other berry components that may be expected to reproduce effects in humans.
Conclusions
A major objective of cancer therapy and prevention investigators is to develop individual therapeutic agents that markedly affect the expression of only one or a very few genes. The objective of this approach is to selectively kill specific types of cancer cells with minimal effects on their normal counterparts. In contrast, berry powders contain a mixture of compounds that appear to affect the expression levels of a wide range of cancer-related genes (to lesser extents than therapeutic agents; ref. 40), thus preventing the conversion of premalignant cells to malignancy at doses that cause minimal or no cytotoxicity. In this regard, berries seem to fulfill the requirement of an “ideal” chemopreventive agent (60). The same is undoubtedly true of many other foodstuffs; e.g., a freeze-dried aqueous extract of broccoli sprouts was effective at dietary levels in inhibiting chemically induced bladder cancer with no observable toxicity in rats (61).
From a practical standpoint, we have found that high-risk individuals are usually willing to participate in clinical trials of berry formulations, and compliance in these trials is excellent. Moreover, the general public is intrigued with food-based approaches for the prevention of diseases including cancer. With potentially lower toxicity and costs, effective food-based approaches not only would be attractive for developed countries but would offer greater portability (versus highly synthesized agents) to underdeveloped countries as well. Therefore, in my opinion, food-based approaches with rational developmental schemes such as the one outlined in this commentary should be an integral part of the overall strategies for the prevention of cancer and other diseases.
The future of food-based chemoprevention will benefit, indeed may rely, on the close collaboration and cooperation of basic scientists, nutritional epidemiologists, and clinical researchers. Mechanistic understandings of foodstuffs can only enhance their prospects for successful interventions in human populations at risk of cancer. Indeed, collaborative research of this nature can even help inform directions for the development of molecular-targeted approaches. As a related example, mechanistic studies indicate that the strong cancer-preventive effects of caloric restriction involve inhibition of the mammalian target of rapamycin (mTOR; ref. 62). This information is potentially valuable to the large enterprise of preclinical and clinical development of mTOR inhibitors.
Acknowledgements
I am deeply indebted to my graduate students, post-doctoral fellows, laboratory technicians, clinical trial manager, and many collaborators at The Ohio State University and at other institutions for their contributions to these studies. Special thanks to Mr. Dale Stokes for his encouragement, enthusiasm and support of this research from the beginning.
Grant support: This research was supported by NIH R01 grants CA096130 and CA103180, and USDA grants 38903-03560 and 38903-19245 to the Ohio Agricultural Research and Development Center (OARDC).
Footnotes
Conflict of interest: The author has no conflicts of interest relative to the information in this article.
References
- 1.Morse MA, Stoner GD. Chemoprevention of chemical carcinogenesis and human cancer. In: Warshawsky D, Landolph JR Jr, editors. Molecular Carcinogenesis and the Molecular Biology of Human Cancer. CRC Taylor and Francis; Boca Raton, Florida: 2005. pp. 445–478. Chapter 21. [Google Scholar]
- 2.Mandal S, Stoner GD. Inhibition of N-nitrosobenzylmethylamine induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Stoner GD, Morrissey DT, Heur Y-H, Daniel EM, Galati AJ, Wagner SA. Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1990;51:2063–68. [PubMed] [Google Scholar]
- 4.Daniel EM, Krupnick AD, Heur Y-H, Blinzler JS, Nims RE, Stoner GD. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J.Food Comp. and Anal. 1989;2:338–49. [Google Scholar]
- 5.Kresty LA, Morse MA, Morgan C, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–9. [PubMed] [Google Scholar]
- 6.Khan WA, Wang ZY, Athar M, Mukhtar H. Inhibition of the skin tumorigenicity of (+/−)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene by tannic acid, green tea polyphenols and quercetin in Sencar mice. Cancer Lett. 1988;42:7–12. doi: 10.1016/0304-3835(88)90232-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZY, Huang MT, Ferraro T, et al. Inhibitory effect of green tea in the drinking water on tumorigenesis by ultraviolet light and 12-O-tetradecanoylphorbol-13-acetate in the skin of SKH-1 mice. Cancer Res. 1992;52:1162–70. [PubMed] [Google Scholar]
- 8.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okajima E, Tsutsumi M, Ozono S, et al. Inhibitory effect of tomato juice on rat urinary bladder carcinogenesis after N-butyl-N-(4-hydroxybutyl)nitrosamine initiation. Jpn J Cancer Res. 1998;89:22–6. doi: 10.1111/j.1349-7006.1998.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh T, Yamada K, Yin H, Ito A, Kataoka T, Dohi K. Chemoprevention of N-nitroso-N-methylurea-induced rat mammary carcinogenesis by soy foods or biochanin A. Jpn J Cancer Res. 1998;89:137–42. doi: 10.1111/j.1349-7006.1998.tb00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeram NP. Berries. In: Heber D, Blackburn G, Go V, Milner J, editors. Nutritional Oncology. Elsevier, Inc.; Amsterdam, The Netherlands: 2006. pp. 615–28. Chapter 37. [Google Scholar]
- 12.Stoner GD, Zikri N, Wang L-S, et al. Cancer prevention with freeze-dried berries and berry components. Seminars in Cancer Biol. 2007;17:403–10. doi: 10.1016/j.semcancer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis. 2008;29:1665–74. doi: 10.1093/carcin/bgn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelloff GJ, Boone CW, Malone W, Steele V. Recent results in preclinical and clinical drug development of chemopreventive agents at the National Cancer Institute. In: Wattenberg L, Lipkin M, Boone C, Kelloff G, editors. Cancer Chemoprevention. Plenum Press, Inc.; Boca Raton, Florida: 1991. pp. 41–56. [Google Scholar]
- 15.Hecht SS, Huang C, Stoner GD, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene-7,8-diol-9,10-epoxide induced NFkappaB and AP-1 activity. Carcinogenesis. 2006;27:1617–26. doi: 10.1093/carcin/bgi366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L-S, Hecht S, Carmella S, et al. Anthocyanins in black raspberries prevent esophageal tumors in rats. Cancer Prev Res. 2009;2:84–93. doi: 10.1158/1940-6207.CAPR-08-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tulio AZ, Jr, Reese RN, Wyzgoski FJ, et al. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J Agric Food Chem. 2008;56:1880–8. doi: 10.1021/jf072313k. [DOI] [PubMed] [Google Scholar]
- 18.Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: vaccinium, rubus, and ribes. J Agric Food Chem. 2002;50:519–25. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- 19.Ozgen M, Wyzgoski FJ, Tulio AZ, et al. Antioxidant capacity and phenolic antioxidants of Midwestern black raspberries grown for direct markets are influenced by production site. Hortsci. 2008;43:2039–47. [Google Scholar]
- 20.Stoner GD, Wang L-S, Sardo C, Zikri N, Hecht SS, Mallery SR. Cancer prevention with berries: Role of anthocyanins. In: Milner JA, Romagnolo DF, editors. Bioactive Compounds and Cancer. Humana Press; Totawa, New Jersey: 2009. in press. [Google Scholar]
- 21.Baron JA. Calcium. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention: Volume I. Promising Cancer Chemopreventive Agents. Humana Press; Totawa, New Jersey: 2004. pp. 547–58. Chapter 36. [Google Scholar]
- 22.El-Bayoumy K. Not all chemopreventive selenium compounds are created equal. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention: Volume I. Promising Cancer Chemopreventive Agents. Humana Press; Totawa, New Jersey: 2004. pp. 537–45. Chapter 35. [Google Scholar]
- 23.Dani V, Goel A, Vaiphei K, Dhawan DK. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol Lett. 2007;171:10–18. doi: 10.1016/j.toxlet.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Nishino H. Cancer chemoprevention by natural carotenoids and their related compounds. J Cell Biochem Suppl. 1995;22:231–5. doi: 10.1002/jcb.240590829. [DOI] [PubMed] [Google Scholar]
- 25.Paolini M, Abdel-Rahman SZ, Sapone A, et al. ß-carotene: a cancer chemopreventive agent or a co-carcinogen? Mutat. Res. 2003;543:195–200. doi: 10.1016/s1383-5742(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 26.Huang H-Y, Berndt S, Helzlsouer KJ. Vitamin E as a cancer chemopreventive agent. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention: Volume I. Promising Cancer Chemopreventive Agents. Humana Press; Totawa, New Jersey: 2004. pp. 451–84. Chapter 31. [Google Scholar]
- 27.Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Crit Rev Oncol Hematol. 2003;47:249–59. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 28.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nature Rev. Cancer. 2003;3:601–14. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 29.Ovesna Z, Vachalkova A, Horvathova K. Taraxasterol and beta-sitosterol: new naturally compounds with chemoprotective/chemopreventive effects. Neoplasma. 2004;51:407–414. [PubMed] [Google Scholar]
- 30.Awad AB, Fink CC. Phytosterols as anticancer dietary compounds: evidence and mechanisms of action. J Nutr. 2000;130:2127–30. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 31.Balakrishnan S, Menon VP, Manoharan S. Ferulic acid inhibits 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. J Med Food. 2008;11:693–700. doi: 10.1089/jmf.2007.0103. [DOI] [PubMed] [Google Scholar]
- 32.Stoner GD, Casto BC. Chemoprevention of cancer by fruit phenolic compounds. In: Kelloff GJ, Hawk ET, Sigman CC, editors. Cancer Chemoprevention. Volume I. Promising Cancer Chemopreventive Agents. Humana Press; Totawa, New Jersey: 2004. pp. 419–435. Chapter 29. [Google Scholar]
- 33.Belkaid A, Currie J-C, Desgagnes J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7–19. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang K, Lamprecht SA, Liu Y, et al. Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane-treated mouse colon. Carcinogenesis. 2000;21:1655–60. doi: 10.1093/carcin/21.9.1655. [DOI] [PubMed] [Google Scholar]
- 35.Hou D-X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med. 2003;3:149–59. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- 36.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22:659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 37.Harris GK, Gupta A, Nines RG, et al. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2'-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer. 2001;40:125–33. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 38.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60:227–34. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 39.Casto BC, Kresty LA, Kraly CL, et al. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005–15. [PubMed] [Google Scholar]
- 40.Stoner GD, Reen RK, Dombkowski AA, et al. Carcinogen-altered genes in rat esophagus positively modulated to normal levels of expression by both black raspberries and phenylethyl isothiocyanate. Cancer Res. 2008;68:6460–67. doi: 10.1158/0008-5472.CAN-08-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoner GD, Sardo C, Apseloff G, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–64. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 42.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52:S139–51. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 43.Kresty LA, Frankel WL, Hammond CD, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett's esophagus patients. Nutr Cancer. 2006;54:148–56. doi: 10.1207/s15327914nc5401_15. [DOI] [PubMed] [Google Scholar]
- 44.Shumway BS, Kresty LA, Larsen PE, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin Cancer Res. 2008;14:2421–30. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallery SR, Zwick JC, Pei P, et al. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008;68:4945–57. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson JM, Knobloch TK, Sardo CL, et al. Age-related differences in black raspberry modulated NFкB expression in oral squamous cell carcinoma patients. Proc. Seventh Ann. Amer. Assn. Cancer Res. Int. Conf. on Frontiers in Cancer Prev. Res. 2008;A66:89. [Google Scholar]
- 47.Knobloch TJ, Lee MT, Whitmore GA, et al. Gene expression changes in oral tissues following black raspberry exposure: Interim Affymetrix assay analysis of a Phase 1 clinical trial. Proc. Sixth Ann. Amer. Assn. Cancer Res. Int. Conf. on Frontiers in Cancer Prev. Res. 2007;A129:110. [Google Scholar]
- 48.Wang LS, Sardo C, Henry C, et al. Chemoprevention of human colorectal cancer with freeze-dried black raspberries. Proc. 99th Amer. Assn. Cancer Res. 2008;LB-328:110. [Google Scholar]
- 49.Stoner GD, Hasson H, Sardo CL, et al. Regression of rectal polyps in familial adenomatous polyposis patients with freeze-dried black raspberries. Proc. 6th AACR Frontiers in Cancer Prevention Research. 2008;A63:68. [Google Scholar]
- 50.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 51.Agarwal C, Singh RP, Agarwal R. Grape seed extract induces apoptotic cell death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2002;23:1869–76. doi: 10.1093/carcin/23.11.1869. [DOI] [PubMed] [Google Scholar]
- 52.Afaq F, Saleem M, Kueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolysable tannin-rich pomegranate fruit extract modulates MAPK and NK-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–33. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 53.Huang C, Li J, Song L, et al. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/Akt pathway. Cancer Res. 2006;66:581–7. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 54.Wang L-S, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008;269:281–90. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooke D, Schwarz D, Boocock P, et al. Effect of cyanidin-3-glucoside and an anthocyanin mixture from bilberry on adenoma development in the ApcMin mouse model of intestinal carcinogenesis-relationship with tissue anthocyanin levels. Int J Cancer. 2006;119:2213–2220. doi: 10.1002/ijc.22090. [DOI] [PubMed] [Google Scholar]
- 56.Freemantle SJ, Dragnev KH, Dmitrovsky E. The retinoic acid paradox in cancer chemoprevention. JNCI. 2006;98:426–7. doi: 10.1093/jnci/djj116. [DOI] [PubMed] [Google Scholar]
- 57.Bleys J, Navas-Acien A, Guallar E. Selenium and diabetes: More bad news for supplements. Ann Int Med. 2007;147:271–2. doi: 10.7326/0003-4819-147-4-200708210-00177. [DOI] [PubMed] [Google Scholar]
- 58.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. NEJM. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 59.Albanes D, Heinonen O, Taylor PR, et al. α-tocopherol and ß-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. JNCI. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 60.Alberts DS, Garcia DJ. An overview of clinical cancer chemoprevention studies with emphasis on positive phase III studies. J Nutr. 1995;125:692–7. doi: 10.1093/jn/125.3_Suppl.692S. [DOI] [PubMed] [Google Scholar]
- 61.Munday R, Mhawech-Fauceglia P, Munday CM, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 62.Moore T, Beltran L, Carbajal S, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res. 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]