Summary
An 11-year-old boy with terminal hepatic failure due to Wilson’s disease was treated 18 months ago with orthotopic liver transplantation. Postoperatively, there has been evidence of clearance of body copper stores but without accumulation of copper in biopsy specimens of the transplanted liver after 6 and 17 months. Further follow-up will be necessary before deciding whether the disorder has been cured by liver replacement and in turn whether this constitutes proof that Wilson’s disease is an inborn error of hepatic metabolism. The observations so far are consistent with these conclusions.
Introduction
D-Penicillamine is recognised as the treatment of choice in Wilson’s disease.1–3 However, even with therapy some patients are left with post-necrotic cirrhosis and portal hypertension. In addition, a small group of patients has been labelled as having “acute Wilson’s disease”4–6—an entity which has the distinctive hepatic histopathological feature of Mallory bodies, and an extremely high mortality-rate despite treatment with specific copper-chelating agents. Moreover, even the more chronic form of Wilson’s disease may lead to severe hepatic insufficiency, particularly in children, before the appearance of any diagnostic neurological or ocular signs.7–9
We report here a case of Wilson’s disease which was treated successfully by total host hepatectomy and orthotopic homotransplantation of a cadaveric liver. There has been no clinical or biochemical evidence of recurrence of Wilson’s disease in the one and a half years of postoperative follow-up.
Case-report
An 11-year-old boy was admitted on July 14, 1969, in hepatic pre-coma. He was said to have had a protuberant abdomen since birth. Although he had developed generalised pruritus at 8 months of age, the diagnosis of a liver disorder was not definitely established until admission to another hospital in April, 1966. At that time the cephalin flocculation was 4+, bromsulphalein retention was 30% at 45 minutes, and serum-alkaline-phosphatase was 16.4 Bodansky units (normal less than 5). An open liver biopsy was reported as “post-necrotic cirrhosis”. In April, 1969, he was admitted to a second hospital with obvious advanced liver disease. There were no Kayser-Fleischer rings on slit-lamp examination. A second liver biopsy again showed cirrhosis, with the additional finding of the alcoholic hyaline of Mallory. This biopsy was interpreted as being consistent with Wilson’s disease. The diagnosis of Wilson’s disease was supported by the eventual histopathological study of the excised liver by members of our staffs and several consulting pathologists, including Dr. Hans Popper (New York) and Prof. K. A. Porter (London).
The patient was transferred to the University of Colorado Medical Center in hepatic pre-coma. He had anasarca and massive ascites. There were prominent venous collaterals on the abdomen, spider angiomas on the chest and upper extremities, and liver palms. Gynæcomastia was not present. He was somnolent, disoriented, and incontinent with fetor hepaticus. He had marked clonus and was hyper-reflexic, but the Babinski responses were equivocal. He had no evidence of asterixis. The serum-bilirubin was 20.6 mg. per 100 ml., the serum-alkaline-phosphatase 35 I.U. per litre (normal 57–258), S.G.O.T. 49 I.U. per litre (normal 3–27), S.G.P.T. 14 I.U. per litre (normal 1–30), serum-total-protein 4.5 g. per 100 ml., serum-albumin 1.5 g. per 100 ml., γ-globulin 2.2 g. per 100 ml., prothrombin-time 31.5 seconds (control 13 seconds) or 13%. The hæmatocrit was 27%; white blood-cells 15,000 per c.mm.; platelets 30,000 per c.mm. The blood-ammonia was 432 mg. nitrogen per 100 ml. and the electroencephalogram was markedly abnormal, showing diffuse slow waves, compatible with a metabolic encephalopathy.
Within a few hours of his arrival the patient developed upper-gastrointestinal hæmorrhage and became agonal. Although the preoperative diagnosis was Wilson’s disease, no consideration was given to the possibility of D-penicillamine treatment, since death was imminent.
Transplantation
When the abdomen had been entered through a bilateral subcostal incision, several litres of ascitic fluid were aspirated. The liver was dark brown, firm, and the site of finely nodular cirrhosis. The organ was removed in its entirety and replaced10 with an orthotopic hepatic homograft from a cadaveric donor of approximately the same age as the recipient. The donor had no stigmata or family history suggestive of Wilson’s disease and the serum copper and cæsruloplasmin were within normal limits.
The excised native liver weighed 540 g., compared to a predicted 840 g. for a child of this age and weight. Biliary drainage was with cholecystoduodenostomy. The recipient’s greatly enlarged spleen was also removed. Grossly and microscopically there were multiple splenic infarcts. Because of extensive venous collaterals, the operation was a difficult one, necessitating transfusion of 12 litres blood.
Post-transplantation Course
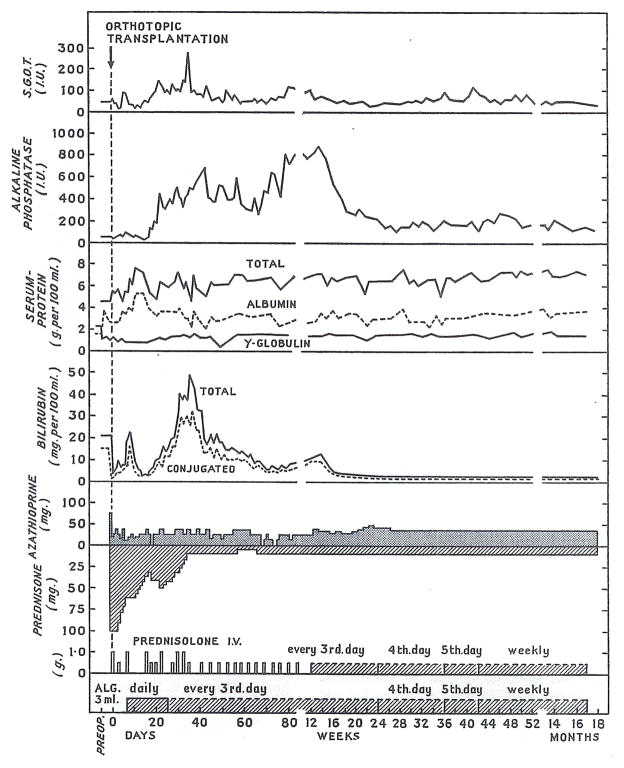
There was an immediate clearing of the sensorium, a fall in the serum-bilirubin and blood-ammonia levels, an increase in the total-serum-protein, and a reduction in the prothrombin-time. The patient was treated with a triple-drug immunosuppressive regimen that included azathioprine, prednisone, and heterologous antilymphocyte globulin (A.L.G.).10 Two severe rejection episodes occurred—one after a few days and the other after 3 weeks. The most prominent biochemical findings at these times were changes suggestive of biliary-tract obstruction, with an extreme degree of hyperbilirubinæmia (maximum 43 mg. per 100 ml.), of which most was glucuronide, and raised alkaline phosphatase (maximum 990 I.U. per litre) (fig. 1). With reversal of the second rejection episode, these and the other adverse findings of rejection receded completely. The patient has had essentially normal hepatic function since 6 months post-transplantation (fig. 1).
Fig. 1. Course after liver replacement.
A.L.G. = horse antilymphocyte globulin.
Open biopsies of the homograft, obtained after 6 and 17 months, were studied for evidence of rejection by Prof. K. A. Porter, of St. Mary’s Hospital and Medical School, London. The initial biopsy, apart from some cellular infiltration of the portal tracts and prominence of the Kupffer cells, was essentially normal. The second biopsy showed some excess reticulin and collagen in and around the portal tracts and also around some of the liver lobules. A few of the small hepatic-artery branches now showed some intimal thickening.
The patient is receiving 37.5 mg. azathioprine and 15 mg. of prednisone daily. He now has no demonstrable neurological impairment or evidence of Kayser-Fleischer rings on slit-lamp examination. No dietary limitations have been imposed, and D-penicillamine or potassium sulphide have never been administered except during the tests to be described below.
Studies of Copper Metabolism
The diagnosis of Wilson’s disease was confirmed by determining the copper content of the frozen native liver. As measured in the Denver laboratory,11 the excised organ had 216 μg. copper per gramme wet weight. In the New York laboratory,12 the copper per gramme dry weight was 1313 μg. Except for Wilson’s disease, hepatic copper values approaching these levels have been reported only in primary biliary cirrhosis or biliary atresia.13,14 As controls in the Colorado laboratory, the copper concentration was determined in 11 livers that were affected by biliary atresia (2 examples), hepatoma (2 examples), chronic active hepatitis (5 examples), and cirrhosis (2 examples). The values averaged 17.4 μg. of copper per gramme wet liver (range 9–45), the highest content being in the liver of an 11-year-old girl with intrahepatic biliary atresia.
During both the previous admissions to other hospitals, several other relevant studies were obtained. In April, 1966, the 24-hour urinary copper excretion was 50 μg. on two occasions, a normal value in that laboratory. The serum-copper was 178 μg. per 100 ml. (normal stated to be 80–280). In April, 1969, during the second admission to hospital elsewhere, serum-cæruloplasmin was reported as 26 mg. per 100 ml. (normal 20–40). Preoperative cæruloplasmin values in the Denver laboratories in three different preoperative specimens after freeze storage for 6 months were 26, 27, and 31 mg. per 100 ml., using an enzymatic assay.11 The concentration in the first sample was found to be 28 mg. per 100 ml. by Dr. George Cartwright, of the University of Utah, using a similar analytical method. However, in the New York laboratory, cæruloplasmin concentration of this specimen was 15 mg. per 100 ml. by enzymatic assay12 as well as with a calibrated immunochemical method.15
Following liver transplantation, serial 24-hour urinary copper excretion was determined repeatedly. There was a massive cupriuresis which declined to normal (less than 30 μg. per 24 hours) after several months (fig. 2). At the end of 7 months a 3-day course of D-penicillamine (1.5 g. per day) was given and there was a 20-fold increase in urine copper excretion, which then rapidly returned to normal. Copper excretion remained normal until 15 months post-transplantation, at which time it became slightly elevated (fig. 2). During the 18 months of follow-up, 15 serial serum-cæruloplasmins varied from 29.5 to 55 mg. per 100 ml. (normal 22–43) and coincidentally measured serum-coppers ranged from 80 to 171 μg. per 100ml. (normal 70–118).
Fig. 2.
Urinary excretion of copper after orthotopic liver transplantation for Wilson’s disease.
The homograft biopsies obtained at 6 months and 17 months after transplantation contained 15 and 13.6 μg. copper per gramme wet weight in the Denver laboratory (normal less than 20). The dry weight per gramme liver in the New York laboratories at 6 and 17 months was 54 and 21.3 μg.
As controls, cases were studied in which orthotopic hepatic transplantation was carried out for indications other than Wilson’s disease. 24-hour urine-copper excretion was estimated in three patients 40–665 days after liver replacement for biliary atresia; this ranged from 18 to 26 μg. per 24 hours. In addition, copper was measured in twelve livers that had been transplanted 35–400 days previously to patients with biliary atresia or hepatoma. The grafts contained an average of 10.5 μg. copper per gramme wet weight liver tissue (range 7–19).
Genetic Studies
Both parents and all three siblings of the patient had 24-hour urinary copper excretion and serum copper and cæruloplasmin estimations. In addition, these family members had 64Cu-incorporation studies performed16 (see table). There was no clear evidence that either parent or any of the other children were heterozygous. The mother was not taking contraceptive pills at the time of testing, nor has she ever done so in the past.
FINDINGS IN PATIENT’S RELATIVES
| Case | Cæruloplasmin (mg./100ml.)* | Serum-copper (μg./100 ml.)† | 24 hr. urine-copper (μg.)‡ | 64Cu: ratio 48hr./1–2hr.§ |
|---|---|---|---|---|
| Father. . | 31.5 | 135 | 17 | 1.606 |
| Mother. . | 58.9 | 183 | 6 | 1.354 |
| Sibling 1 | 24.5 | 81 | 4 | 0.858 |
| Sibling 2 | 29.0 | 113 | 5 | 1.104 |
| Sibling 3 | 67.9 | 198 | 5 | 2.030 |
Normal = 22–43.
Normal = 70–118.
Normal = < 30.
Heterozygotes = < 0.599.
Renal Function
Before operation the blood-urea-nitrogen was 17 mg. per 100 ml. and the serum-creatinine 1.0 mg. per 100 ml. Unfortunately, no other evaluation of renal function could be obtained. Afterwards, serial phosphorus and creatinine clearances were performed. Aminoacids were measured by ion-exchange chromatography on a ‘Beckman-Spinco’ aminoacid analyser and phenylalanine, tyrosine, alanine, glycine, glutamine, and serine clearances were determined.11 The measures were within normal limits at all times post-transplantation, thereby ruling out the possibility that the renal injury sometimes associated with Wilson’s disease17,18 had developed.
Discussion
The characteristic findings in Wilson’s disease5,19,20 can include a low serum-cæruloplasmin, an increased urinary excretion of copper, an elevation of non-cæruloplasmin copper in the serum, Kayser-Fleischer rings, and an elevated copper content of the liver and other organs. Any or all of these diagnostic criteria, except the last one, may be absent8,9,19,20; and this may lead to the tragedy of not providing treatment with the chelating agent, penicillamine. Moreover, since cæruloplasmin levels are depressed in only about 10% of heterozygous carriers of the abnormal gene, and the 64Cu-incorporation test is of only fair reliability, the findings in the siblings and parents of our patient cannot be regarded as proof that some of them do not possess a single “Wilson’s disease gene”.
In our patient, Wilson’s disease was suspected intermittently in the 3 years preceding transplantation, but the results of the biochemical tests of serum and/or urine were either normal or equivocal. For example, all the preoperative cæruloplasmin determinations were within normal limits except one result from the New York laboratories. These generally negative findings plus the absence of the Kayser-Fleischer rings delayed recognition of the disease until there was no hope of conservative medicinal treatment. Nevertheless, the firm diagnosis of Wilson’s disease was ultimately established from the high copper concentration in the native liver as well as from the presence of Mallory bodies in this specimen. The first of these abnormalities can be found in primary biliary cirrhosis or atresia,13,14 and the second may be present in alcoholic cirrhosis21 or Indian childhood cirrhosis,22 but obviously these diagnoses did not pertain to this 11-year-old white American boy.
Despite the absence of several of the common criteria for the diagnosis of Wilson’s disease, evidence of extensive copper deposition in extrahepatic tissues was provided indirectly by the massive cupriuresis which followed operation and which continued for several months before the urinary copper level returned to normal. Even after 7 months, the magnitude of urinary copper excretion in response to a 3-day penicillamine course was somewhat greater than is usually observed in normal people.1,23 At all times in the past year, urine excretion of copper has been slightly elevated, either at high normal or above.
It has been suggested by Walshe20 and by Sternlieb and Scheinberg19 and others that in Wilson’s disease the primary defect is hepatic. This hypothesis is based upon the fact that in all clinical permutations of the disease the highest concentrations of copper are in the liver. The extrahepatic organ systems are presumably not affected until the buffering capacity of the liver for copper is exceeded. Thereafter toxic levels presumably develop in other parts of the body.
If the essential defect in Wilson’s disease were hepatic, provision of a new liver would be expected to be curative, since liver homografts and the proteins they synthesise retain their donor specificity in the new host for a long time and probably permanently.10,24,27 Consequently, the studies in our case are consistent with the hypothesis that Wilson’s disease is a liver-based inborn error of metabolism, although they do not prove it. In the 18 months since the liver was transplanted the total body-copper has declined steadily, while the copper concentration in the new liver has remained normal. In so far as can be determined within the limits of the follow-up, this patient now has no clinical or biochemical evidence of Wilson’s disease.
Acknowledgments
This work was supported by United States Public Health Service grants. AT-AM-08898, AM-01059, AM-12148, AM-06344, AM-07772, RR-00051, RR-00069, HE-09110, and special project 252.
Contributor Information
R. S. DuBois, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
G. Giles, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
D. O. Rodgerson, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
J. Lilly, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
G. Martineau, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
C. G. Halgrimson, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
G. Schroter, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
T. E. Starzl, Departments of Surgery and Pediatrics, University of Colorado School of Medicine, and Denver Veterans Administration Hospital, Denver, Colorado 80220
I. Sternlieb, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York 10461
I. H. Scheinberg, Department of Medicine, Albert Einstein College of Medicine, Bronx, New York 10461
DR. DUBOIS AND OTHERS: REFERENCES
- 1.Walshe JM. Am J Med. 1956;21:487. doi: 10.1016/0002-9343(56)90066-3. [DOI] [PubMed] [Google Scholar]
- 2.Walshe JM. Lancet. 1960;i:188. doi: 10.1016/s0140-6736(60)90109-4. [DOI] [PubMed] [Google Scholar]
- 3.Sternlieb I, Scheinberg IH. J Am med Ass. 1964;189:748. doi: 10.1001/jama.1964.03070100042008. [DOI] [PubMed] [Google Scholar]
- 4.Walshe JM. Archs Dis Childh. 1962;37:253. doi: 10.1136/adc.37.193.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi AJ, Sherlock S, Scheuer PJ, Cummings JN. Lancet. 1967;ii:575. doi: 10.1016/s0140-6736(67)90735-0. [DOI] [PubMed] [Google Scholar]
- 6.Popper J. Birth Defects. Original Article Series: Wilson’s Disease. 1968;4:103. [Google Scholar]
- 7.Chalmer TC, Iber FL, Uzman LL. New Engl J Med. 1957;256:235. doi: 10.1056/NEJM195702072560601. [DOI] [PubMed] [Google Scholar]
- 8.Fisher MM, Sherlock S. Archs Dis Childh. 1964;39:14. doi: 10.1136/adc.39.203.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danks DM, Stevens BJ. Lancet. 1969;i:22. doi: 10.1016/s0140-6736(69)90987-8. [DOI] [PubMed] [Google Scholar]
- 10.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: 1969. [Google Scholar]
- 11.O’Brien D, Ibbott FA, Rodgerson DO. Laboratory Manual of Pediatric Micro-BioChemical Techniques. New York: 1968. [Google Scholar]
- 12.Morell AG, Windsor J, Sternlieb I, Scheinberg IH. In: Laboratory Diagnosis of Liver Disease. Sunderman FW, Sunderman FW Jr, editors. St. Louis: 1968. [Google Scholar]
- 13.Sternlieb I, Harris RC, Scheinberg IH. Revue int Hépat. 1966;16:1105. [PubMed] [Google Scholar]
- 14.Worwood M, Taylor DM, Hunt AH. Br med J. 1968;iii:344. doi: 10.1136/bmj.3.5614.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheinberg IH, Gitlin D. Science. 1952;116:484. doi: 10.1126/science.116.3018.484. [DOI] [PubMed] [Google Scholar]
- 16.Sternlieb I, Morell AG, Bauer CD, Combes B, DeBobes-Sternberg S, Scheinberg IH. J clin Invest. 1961;40:707. doi: 10.1172/JCI104304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bearn AG, Yu TF, Gutman AB. ibid. 1957:36, 1107. doi: 10.1172/JCI103506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff SM. Lancet. 1964;i:843. doi: 10.1016/s0140-6736(64)91573-9. [DOI] [PubMed] [Google Scholar]
- 19.Sternlieb I, Scheinberg IH. New Engl J Med. 1968;278:352. doi: 10.1056/NEJM196802152780702. [DOI] [PubMed] [Google Scholar]
- 20.Walshe JM. Brain. 1967;90:149. doi: 10.1093/brain/90.1.149. [DOI] [PubMed] [Google Scholar]
- 21.Mallory FB. Bull Johns Hopkins Hosp. 1911;22:69. [Google Scholar]
- 22.Sherlock S. Diseases of the Liver and Biliary System. Oxford: 1968. p. 534. [Google Scholar]
- 23.Tu JB, Blackwell RQ, Fresh JW, Watten RH. Birth Defects. Original Article Series: Wilson’s Disease. 1968;4:114. [Google Scholar]
- 24.Starzl TE, Marchioro TL, Rowlands DT, Jr, Kirkpatrick GH, Wilson WEC, Rifkind D, Waddell WR. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuster G, Shorter RG, Dawson MB, Hallenbeck GA. Surg Forum. 1967;18:360. [Google Scholar]
- 26.Kashiwagi N, Groth CG, Starzl TE. Proc Soc exp Biol Med. 1968;128:248. doi: 10.3181/00379727-128-32988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alper CA, Johnson AM, Birtch AG, Moore FD. Science. 1969;153:286. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]