Abstract
The mechanism by which the recreational drug (±)-3,4-methylenedioxymethamphetamine (MDMA) destroys brain serotonin (5-HT) axon terminals is not understood. Recent studies have implicated MDMA metabolites, but their precise role remains unclear. To further evaluate the relative importance of metabolites versus the parent compound in neurotoxicity, we explored the relationship between pharmacokinetic parameters of MDMA, 3,4-methylenedioxyamphetamine (MDA), 3,4-dihydroxymethamphetamine (HHMA), and 4-hydroxy-3-methoxymethamphetamine (HMMA) and indexes of serotonergic neurotoxicity in the same animals. We also further evaluated the neurotoxic potential of 5-(N-acetylcystein-S-yl)-HHMA (5-NAC-HHMA), an MDMA metabolite recently implicated in 5-HT neurotoxicity. Lasting serotonergic deficits correlated strongly with pharmacokinetic parameters of MDMA (Cmax and area under the concentration-time curve), more weakly with those of MDA, and not at all with those of HHMA or HMMA (total amounts of the free analytes obtained after conjugate cleavage). HHMA and HMMA could not be detected in the brains of animals with high brain MDMA concentrations and high plasma HHMA and HMMA concentrations, suggesting that HHMA and HMMA do not readily penetrate the blood-brain barrier (either in their free form or as sulfate or glucuronic conjugates) and that little or no MDMA is metabolized to HHMA or HMMA in the brain. Repeated intraparenchymal administration of 5-NAC-HHMA did not produce significant lasting serotonergic deficits in the rat brain. Taken together, these results indicate that MDMA and, possibly, MDA are more important determinants of brain 5-HT neurotoxicity in the rat than HHMA and HMMA and bring into question the role of metabolites (including 5-NAC-HHMA) in MDMA neurotoxicity.
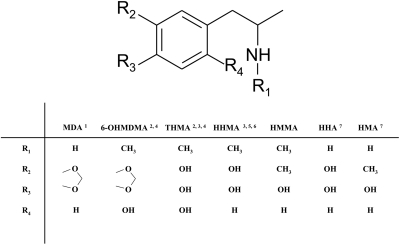
Despite much research, the mechanism by which (±)-3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) destroys brain serotonin (5-HT) axon terminals remains unknown. One hypothesis that has recently drawn considerable attention is that a drug metabolite is involved (Capela et al., 2009; Perfetti et al., 2009). Over the years, various metabolites of MDMA and related drugs have been evaluated for possible 5-HT neurotoxic activity (Fig. 1). Steele et al. (1991), for example, assessed the neurotoxic potential of 3,4-dihydroxymethamphetamine (HHMA; which they designated α-methylepinine) and concluded that it alone was not responsible for MDMA neurotoxicity. Likewise, McCann and Ricaurte (1991) evaluated the neurotoxic properties of α-methyldopamine and 3-O-methyl-α-methyldopamine [alternatively designated as 3,4-hydroxyamphetamine (HHA) and 4-hydroxy-3-methoxyamphetamine, respectively] and reached similar conclusions.
Fig. 1.
Metabolites of MDMA and related drugs that have been evaluated for 5-HT neurotoxic potential: 1Ricaurte et al. (1985), 2Zhao et al. (1992), 3Johnson et al. (1992), 4Elayan et al. (1992), 5Steele et al. (1991), 6Escobedo et al. (2005), and 7McCann and Ricaurte (1991). With the exception of MDA, the only other MDMA metabolite of the various metabolites shown known to have 5-HT neurotoxic potential is THMA. However, recent efforts in our laboratory to identify THMA in the brain of rats given neurotoxic doses of MDMA (20–60 mg/kg p.o.) have been unsuccessful, even though THMA given intracerebroventricularly can be readily measured in the rat brain for up to 3 h after administration using LC-MS methods (M. Mueller and G. Ricaurte, unpublished observations). 6-OHMDMA, 6-hydroxy-3,4-methylenedioxymethamphetamine;HMA, hydroxymethamphetamine.
Based on theoretical considerations and the identification of MDMA metabolites that are analogs of the well established neurotoxin 6-hydroxydopamine (Lim and Foltz, 1991a,b), the neurotoxicological properties of 6-hydroxy-3,4-methylenedioxymethamphetamine and 2,4,5-trihydroxymethamphetamine (THMA) were investigated (Johnson et al., 1992; Zhao et al., 1992). 6-Hydroxy-3,4-methylenedioxymethamphetamine, administered intraventricularly and intraparenchymally, was found to be without effect. In contrast, THMA (same routes of administration) produced substantial depletions of 5-HT and dopamine that lasted for at least 5 to 7 days beyond drug administration (Johnson et al., 1992; Zhao et al., 1992). Given the known selectivity of MDMA for 5-HT neurons, results with THMA were deemed to be inconclusive but suggestive of the possibility that THMA may play a role in MDMA neurotoxicity, because the effect on dopamine may have been related to the route of THMA administration (Zhao et al., 1992).
As mentioned above, there has recently been a resurgence of interest in the possibility that MDMA metabolites might play a role in MDMA neurotoxicity. A role for systemically formed MDMA metabolites is often inferred from the observation that direct injection of MDMA into brain fails to reproduce the 5-HT neurotoxic effects of peripherally administered MDMA (Schmidt and Taylor, 1988; Esteban et al., 2001). A report that cytochrome P450 modulators (SKF-525A and phenobarbital) influence MDMA-induced 5-HT depletions (Gollamudi et al., 1989) is also often cited to support the role of a drug metabolite. However, in that study, SKF-525A and phenobarbital altered acute (3 h) but not lasting effects of MDMA on brain 5-HT neurons.
Hiramatsu et al. (1990) were the first to report metabolism of MDMA to a reactive quinone, which formed a glutathione adduct that might be responsible for MDMA neurotoxicity. More recently, other glutathione and N-acetylcysteine conjugates of catechol metabolites of MDMA and MDA have been identified and implicated in MDMA neurotoxicity (Miller et al., 1997; Bai et al., 1999; Jones et al., 2005; Capela et al., 2007; Pizarro et al., 2008). Of these, 5-(N-acetylcystein-S-yl)-N-methyl-α-methyldopamine [here designated as 5-(N-acetylcystein-S-yl)-HHMA (5-NAC-HHMA)] has been the metabolite most strongly implicated (Jones et al., 2005; Erives et al., 2008).
MDMA metabolism proceeds mainly through two pathways at different rates in different species (Meyer et al., 2008). The first pathway involves O-demethylenation of MDMA to HHMA, followed by O-methylation to 4-hydroxy-3-methoxymethamphetamine (HMMA), with subsequent O-conjugation with sulfate or glucuronic acid. The second pathway involves initial N-demethylation to MDA, followed by deamination and oxidation to the corresponding benzoic acid derivatives conjugated with glycine. As mentioned above, catechol metabolites of MDMA and MDA (HHMA and HHA) can be further oxidized to their corresponding quinones, which can then form adducts with glutathione and other thiol-containing compounds (Hiramatsu et al., 1990; Monks et al., 2004).
The purpose of the present study was severalfold: 1) to assess the relative importance of the parent compound (MDMA) versus its major metabolites (HHMA, HMMA, and MDA) in MDMA neurotoxicity; 2) to determine which pharmacokinetic parameter of MDMA or its metabolites best predicts subsequent 5-HT neurotoxicity; and 3) to further assess the 5-HT neurotoxic potential and selectivity of the catechol thioether, 5-NAC-HHMA.
Materials and Methods
Animals.
Male Sprague-Dawley rats (Harlan, Indianapolis, IN) that were 49 to 69 days of age and weighed 200 to 299 g were used for all experiments. Animals were housed three per cage (except during drug treatment and after surgical cannula implantation, when they were housed singly) in standard polypropylene cages (17 inches × 10 inches × 8 inches) at 22 ± 2°C ambient temperature (except during drug treatment, when the ambient temperature was 25°C), with free access to food and water. Animals were maintained on a 12:12-h light/dark cycle. The facilities for housing and care of the animals are accredited by the American Association for the Assessment and Accreditation of Laboratory Animal Care. Animal care and experimental manipulations were approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Study Design.
To assess the relationship between MDMA and its major metabolites (MDA, HHMA, and HMMA) and brain 5-HT neurotoxicity, pharmacokinetic parameters of MDMA, HHMA, HMMA, and MDA were measured during the period of drug exposure and related to indexes of brain 5-HT neurotoxicity (depletions of 5-HT and 5-HIAA) measured 1 week later in the same animals. The reason for the 1-week delay was that MDMA and metabolites are known to alter 5-HT and 5-HIAA levels acutely (by inducing 5-HT release, by blocking its reuptake and, possibly, by blocking metabolism of 5-HT by monoamine oxidase). Thus, “acute” depletions of 5-HT and 5-HIAA may not necessarily reflect neurotoxicity. Indeed, Chu et al. (1996) have already shown that there is no relationship between acute depletions of 5-HT and brain MDMA and metabolite levels, probably because at least some of the perturbations seen in 5-HT levels while MDMA is still in the tissue are related to pharmacological (rather than toxic) effects of MDMA on the 5-HT neuron. In contrast, depletions of 5-HT and 5-HIAA documented at least 1 week after drug exposure (when drug and metabolites are no longer on board) are known to be related to 5-HT axon loss (Molliver et al., 1990; Ricaurte et al., 1992). To further assess the 5-HT neurotoxic potential of 5-NAC-HHMA, the compound was administered directly into the striatum at a dose and frequency previously reported to produce lasting 5-HT deficits. Possible involvement of the 5-HT transporter in the anticipated 5-HT deficits was assessed with fluoxetine, a 5-HT uptake blocker that is known to protect against MDMA neurotoxicity (Schmidt, 1987).
Drugs and Reagents.
Racemic MDMA hydrochloride was obtained through the National Institute on Drug Abuse (Rockville, MD). Racemic HHMA hydrochloride and methanolic solutions (1000 mg/l) of racemic MDMA hydrochloride and racemic MDA hydrochloride were purchased from Lipomed (Cambridge, MA). Methanolic solutions (1000 mg/l) of racemic HMMA and methanolic solutions (100 mg/l) of racemic MDMA-d5 and MDA-d5 were obtained from Cerilliant Corporation (Round Rock, TX). Fluoxetine, 4-hydroxymethamphetamine (pholedrine), 4-methylcatechol, EDTA, 5,7-dihydroxytryptamine (5,7-DHT), and glucuronidase type HP-2 from Helix pomatia (glucuronidase activity ≥100,000 units/ml and sulfatase activity <7500 units/ml) were obtained from Sigma-Aldrich (St. Louis, MO). Sodium metabisulfite (SMBS) was obtained from Merck (Darmstadt, Germany). Perchloric acid was obtained from Mallinckrodt Baker, Inc. (Phillipsburg, NJ). Xylazine was obtained from Butler Animal Health Supply (Dublin, OH). Ketamine was supplied by Phoenix Pharmaceuticals (St. Joseph, MO). 5-NAC-HHMA was synthesized as described recently (Felim et al., 2007). The authenticity of the MDMA, HHMA, HMMA, MDA, and 5-NAC-HHMA samples used in the present studies was confirmed using liquid chromatography-mass spectrometry (LC-MS) methods to determine the corresponding pseudomolecular ions and at least one fragment ion for each compound. Analysis was performed in full scan (mass range from 100 to 1000) to check for presence of possible impurities.
Drug Treatment.
MDMA was given orally (by gavage) at a dose of 20 mg/kg at an ambient temperature of 25°C. For studies involving intrastriatal administration of 5-NAC-HHMA, rats received four consecutive doses of either 21 or 42 nmol of the compound, with a 12-h interval between each dose. This particular dose regimen was selected because it is the same one that was used by Bai et al. (1999), who first reported on the neurotoxic potential of 5-NAC-HHMA. In an additional experiment involving 5-NAC-HHMA, rats were pretreated with 10 mg/kg fluoxetine (i.p.) 15 min before each intrastriatal injection of 21 nmol of 5-NAC-HHMA. In the latter experiment, the well established selective 5-HT neurotoxin, 5,7-dihydroxytryptamine (5,7-DHT) was used as a positive control and was also given intrastriatally, at a dose of 52 nmol. Doses refer to the base form of all drugs.
Blood Sampling and Plasma Preparation.
For determination of plasma concentrations of MDMA (and its metabolites) and their pharmacokinetic profiles, blood was sampled at various times after MDMA administration. Blood collection times were selected to allow for accurate determinations of drug pharmacokinetic parameters. For logistic reasons, blood was sampled at 0.75, 1.5, 3, 6, and 12 h after MDMA administration in one group of animals (n = 15); in a second group of rats (n = 9), blood was collected at 1, 3, 6, 8, 9, and 24 h after MDMA treatment. At each time point, approximately 0.2 ml of blood was collected by means of retro-orbital bleeding. One week after MDMA treatment, all animals were sacrificed for regional brain 5-HT and 5-HIAA determinations, as detailed below. A third group of animals (n = 8 at each time point) was used for determination of plasma and brain concentrations of MDMA (and its metabolites). In this group of animals, the major point of interest was the relationship between plasma and brain MDMA and metabolite concentrations. Blood sampling in this experiment occurred at 1, 3, 6, 8, and 24 h MDMA treatment. Blood samples were dispensed into 2-ml BD Vacutainer hematology tubes, containing 4 mg of K3 EDTA solution (BD Biosciences, Franklin Lakes, NJ) and stored on ice for up to 30 min until centrifuged. Samples were centrifuged at 1100g for 10 min. Plasma was withdrawn using a 5-ml 3/4 Pasteur pipette and decanted into a 1.5-ml polypropylene tube, and SMBS (250 mM) was added at a volume of 30 μl/ml plasma to minimize oxidation of the compounds of interest. Plasma samples were stored at −20°C until assay.
Measurement of Plasma MDMA and Metabolite Concentrations.
Plasma MDMA, MDA, HHMA, and HMMA concentrations were determined as described recently (Mueller et al., 2007). In brief, aliquots (100 μl) of rat plasma were preserved with 20 μl of SMBS (250 mM) and 10 μl of EDTA (250 mM). After addition of 100 μl of an aqueous solution of the racemic internal standards MDMA-d5, MDA-d5, and pholedrine (1.0 μg/ml each) and 10 μl of glucuronidase solution, samples were mixed (15 s) on a rotary shaker and left at 50°C for 90 min to perform conjugate cleavage. After cooling to room temperature, 20 μl of 4-methylcatechol (1 mg/ml) was added, and samples were briefly vortexed. Perchloric acid (10 μl) was then added, and the samples were mixed again on a rotary shaker for 15 s to perform protein precipitation. The samples were centrifuged (16,000g for 5 min), and the supernatant was transferred to autosampler vials. Aliquots (5 μl) were injected into an LC-MS system and amounts of MDMA and metabolites were determined. The linear range for each analyte was 20 to 1000 ng/ml MDMA, HHMA, HMMA, and 10 to 500 ng/ml MDA. Method accuracy was greater than 80%. The lowest point of the calibration curve was the limit of quantification of the method (20 ng/ml for MDMA, HHMA, and HMMA each and 10 ng/ml for MDA).
Measurement of Brain MDMA and Metabolite Concentrations.
For determination of brain concentrations of MDMA, HHMA, HMMA, and MDA, samples were prepared and analyzed according to a recently published LC-MS method (Mueller et al., 2008). Values for HHMA and HMMA represent total amounts (i.e., amounts measured after cleavage of sulfate and glucuronic acid conjugates). In particular, aliquots of rat cortices (approximately 100 mg) were weighed and for each microgram of tissue, 10 μl of internal standards solution were added. After homogenization with a Polytron homogenization unit (model PT 10-35, 15 s, setting 6; Kinematica Inc., Bohemia, NY), 10 μl of glucuronidase solution were added, and the samples were briefly mixed (15 s) on a rotary shaker and left at 50°C on a waterbath for 90 min to perform conjugate cleavage. After cooling to room temperature, the samples were centrifuged (16,000g for 10 min), and the supernatant was transferred to autosampler vials. Aliquots (5 μl) were injected into the LC-MS system. The linear range for each analyte was 2 to 100 μg/g MDMA, 1 to 50 μg/g MDA, and 0.1 to 5 μg/g HHMA and HMMA. Method accuracy was greater than 90%. The lowest point of the calibration curve was defined as the limits of quantitation of the method (2 μg/g for MDMA, 1 μg/g for MDA, and 0.1 μg/g for HHMA and HMMA). Values for HHMA and HMMA represent free amounts (i.e., amounts measured after cleavage of sulfate and glucuronic acid conjugates).
Calculation of Pharmacokinetic Parameters.
Peak plasma concentrations (Cmax), times of peak plasma concentration (Tmax), area under the concentration-time curve (AUC), and the elimination half-lives (t1/2) were obtained using the pharmacokinetic functions for Microsoft Excel (developed by J. L. Usansky, A. Desai, and D. Tang-Liu, http://www.boomer.org/pkin/xcel/pkf/pkf.doc).
Surgical Cannula Implantation.
Animals were anesthetized with xylazine (25 mg/kg, i.p.) and ketamine (35 mg/kg i.p.). Guide cannulae (20 gauge; Plastic One, Roanoke, VA) were surgically implanted into the right striatum [anteroposterior, 0.4 mm; mediolateral, −3.0 mm; dorsoventral, 4.0 mm (Paxinos and Watson, 1986)]. Cannulae were fixed to the skull with dental acrylic (Ortho-Jet, Lang Dental, Wheeling, IL) and two stainless steel screws. Dummy cannulae were placed in the guide cannulae, and animals were individually housed and allowed a 7-day recovery period.
Intrastriatal Administration of 5-NAC-HHMA.
The dummy cannulae were replaced with internal cannulae (24 gauge; Plastic One) connected to PE 20 tubing that in turn were connected to a 1-μl Hamilton 7000 series glass syringe (Hamilton Co., Reno, NV) containing the various injection solutions. Artificial cerebrospinal fluid (aCSF) served as a vehicle control and was prepared as described previously by Miller et al. (1997). In a first experiment, rats received either 1 μl of aCSF (control group, n = 8), 21 nmol of 5-NAC-HHMA (n = 10), 42 nmol of 5-NAC-HHMA (n = 5), or 52 nmol of 5,7-DHT (positive control group, n = 4). In a second experiment, animals were pretreated with either saline (0.3 ml) or fluoxetine (15 mg/kg i.p.) 15 min before intrastriatal injections. After pretreatment, either 1 μl of aCSF or 21 nmol of 5-NAC-HHMA were injected into the striatum (total four groups, n = 6 in each group). One microliter of the drug solution was injected manually into the striatum [anteroposterior, 0.4 mm; mediolateral, −3.0 mm; dorsoventral, 5.0 mm (Paxinos and Watson, 1986)] at a rate of 0.2 μl over 5 min for a total of four consecutive doses, with each dose administered 12 h apart. After the injection was completed, the internal cannulae were left in the striatum for an additional 2 min. Animals were awake but gently restrained during the injections. After injection, the dummy cannulae were replaced. Animals were sacrificed 2 weeks later for determination of 5-HT and 5-HIAA levels, as described below.
Determination of Brain 5-HT and 5-HIAA Concentrations.
Samples of cortex and striatum were analyzed for their content of 5-HT and 5-HIAA 1 or 2 weeks after drug treatment, as described previously (Mechan et al., 2006).
Statistics.
The significance of differences between means was determined using a two-tailed Student's t test or analysis of variance followed by Tukey's multiple comparison test. Correlations were explored using Pearson's product moment correlation. Statistical analyses were performed using Prism (version 3.02; GraphPad Software Inc., San Diego, CA). Differences and correlations were considered significant if p < 0.05.
Results
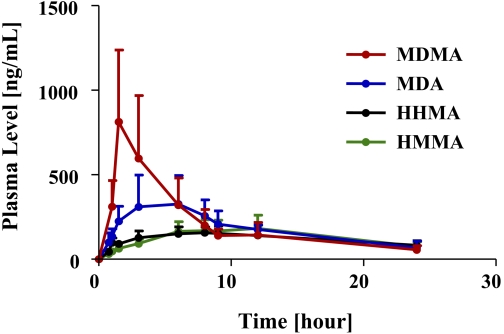
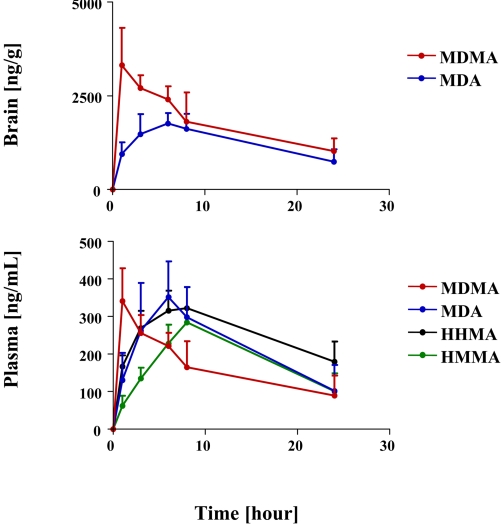
Plasma profiles of MDMA and its major metabolites after a single neurotoxic dose of MDMA (20 mg/kg p.o.) are shown in Fig. 2 and pharmacokinetic parameters are specified in Table 1 . As shown in Fig. 2 and Table 1, MDMA had the highest peak plasma concentrations (Cmax), followed by MDA and then HHMA and HMMA. In particular, the Cmax of MDMA was approximately 2-fold higher than that of MDA and approximately 4 times and 3 times higher than that of HHMA and HMMA, respectively.
Fig. 2.
Plasma profile of MDMA and its metabolites (MDA, HHMA, and HMMA) in rats (n = 24) given a single oral dose of MDMA (20 mg/kg). Concentrations of HHMA and HMMA represent total amounts of free HHMA and HMMA obtained after conjugate cleavage, as detailed under Materials and Methods.
TABLE 1.
Pharmacokinetic parameters of MDMA and its metabolites in plasma of rats given a single oral dose of 20 mg/kg MDMA
Values represent the mean ± S.D. (n = 24).
| Analyte | Cmax | AUC | Tmax | t1/2 |
|---|---|---|---|---|
| ng/ml | ng/ml · h | h | ||
| MDMA | 652 ± 368.7 | 4469 ± 1694.2 | 2.5 ± 1.7 | 5.8 ± 3.5 |
| MDA | 361 ± 174.1 | 3926 ± 1348.1 | 5.7 ± 2.0 | N.D. |
| HHMA | 170 ± 46.9 | 2383 ± 897.8 | 7.2 ± 2.4 | N.D. |
| HMMA | 201 ± 67.6 | 2409 ± 846.2 | 7.7 ± 2.1 | N.D. |
N.D., not determined.
Relative proportions of MDMA and metabolites were somewhat different when AUC, instead of Cmax, values were considered. In particular, the AUC of MDMA was only 1.14 higher than that of MDA and only approximately 2-fold higher than that of HHMA and HMMA.
The t1/2 of MDMA after oral administration was 5.8 ± 3.5 h. If a biphasic decay process is assumed, the estimated decay rate of the first phase was 3.0 h and the estimated decay rate of the second phase was 10.5 h. The t1/2 of MDA, HHMA, and HMMA could not be computed because, within the time window of measurement (0.75–24 h), there were insufficient data points in the terminal elimination phase of the plasma profiles of MDA, HHMA, and HMMA (Fig. 2).
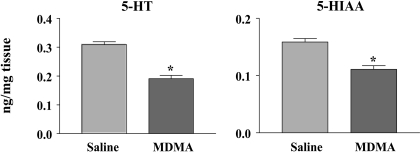
Rats treated with a single 20 mg/kg oral dose of MDMA showed a significant depletion of brain 5-HT 1 week later (Fig. 3). On average, cortical 5-HT was reduced by 38%. There were comparable depletions of 5-HIAA (Fig. 3). The 20 mg/kg dose of MDMA produced a 0.5 to 1°C elevation in core temperature (data not shown).
Fig. 3.
5-HT (left panel) and 5-HIAA (right panel) concentrations in rats treated with saline (n = 17) or a single oral dose of MDMA (20 mg/kg) (n = 24) 1 week previously. ∗, p < 0.05 (two-tailed Student's t test).
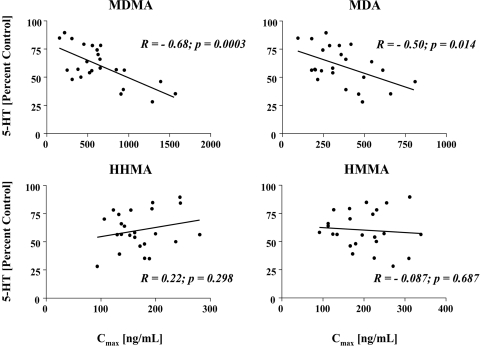
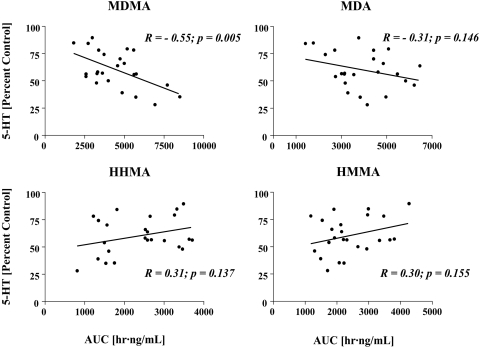
Figure 4 shows results of analyses exploring the relationship between the Cmax of the parent compound and its various metabolites and cortical 5-HT deficits. It is noteworthy that these were within-subject analyses, as plasma drug concentrations and subsequent brain 5-HT deficits were measured in the same animal. Significant relationships were observed between the Cmax of MDMA and MDA and subsequent 5-HT depletions, such that animals with the highest peak plasma concentrations of MDMA and MDA had the largest depletions of brain 5-HT (Fig. 4). In contrast, there were no significant relationships between peak plasma concentrations of HHMA or HMMA and brain 5-HT depletions 1 week later (Fig. 4).
Fig. 4.
Relationship between Cmax of MDMA, MDA, HHMA, or HMMA and cortical 5-HT depletion. Rats received a single oral dose of MDMA (20 mg/kg). One week later, cortical 5-HT levels were determined. The figures reflect within-subject analyses, as drug plasma levels and cortical 5-HT levels were measured in the same animal (n = 24). R (Pearson correlation coefficient) and p values are shown.
Because the relative proportions of MDMA to MDA, HHMA, and HMMA varied depending on whether their respective Cmax or AUC values were considered (see above), we also explored the relationship between the AUC of the parent compound (MDMA) and its various metabolites (MDA, HHMA, and HMMA) and subsequent 5-HT depletions. Only the AUC of MDMA correlated significantly with subsequent cortical 5-HT deficits (Fig. 5).
Fig. 5.
Relationship between AUC of MDMA and its various metabolites and cortical 5-HT depletion in rats given a single oral dose of MDMA (20 mg/kg) and sacrificed 1 week later. The results reflect within-subject analyses, as drug plasma levels and cortical 5-HT levels were measured in the same animal (n = 24). R (Pearson correlation coefficient) and p values are shown.
Given that brain concentrations of MDMA and/or metabolites are, in all likelihood, more proximate causes of brain 5-HT neurotoxicity than plasma concentrations of the various compounds, we next measured brain concentrations of MDMA and its various metabolites (MDA, HHMA, and HMMA) in the brains of rats treated with the same dose of MDMA used in the previous experiment (20 mg/kg p.o.). Brain concentrations of MDMA and metabolites in this study were determined at various times after MDMA administration (1, 3, 6, 8, and 24 h), necessarily in different groups of animals at each time point (n = 8 at each time point). As shown in Fig. 6, top, and Table 2 , only MDMA and MDA were detected in the brain at all time points examined. There was a high correlation between brain and plasma concentrations of MDMA and MDA (r = 0.88 and 0.98, respectively). HHMA and HMMA were not detectable in the brains of animals that had high concentrations of HHMA and HMMA in plasma and high concentrations of MDMA in brain. The limit of detection for HHMA and HMMA in brain tissue was 0.1 μg/g.
Fig. 6.
Plasma and brain profiles of MDMA, MDA, HHMA, and HMMA in rats given a single oral dose of MDMA (20 mg/kg) and sacrificed after 1, 3, 6, 8, and 24 h, respectively (n = 8 at each time point). Determinations were made after conjugate cleavage, as detailed under Materials and Methods. HHMA and HMMA could not be detected in brain tissue. Limit of detection for HHMA and HMMA was 0.1 μg/g.
TABLE 2.
Pharmacokinetic parameters of MDMA and MDA in brain of rats given a single oral dose of 20 mg/kg MDMA
n = 8 at each time point.
| Analyte | Cmax | AUC | Tmax | t1/2 |
|---|---|---|---|---|
| ng/ml | ng/ml · h | h | ||
| MDMA | 3315 | 386,839 | 1.0 | 14.3 |
| MDA | 1761 | 297,299 | 6.0 | 14.3 |
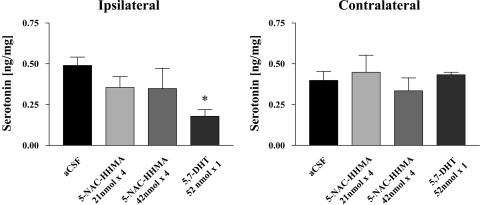
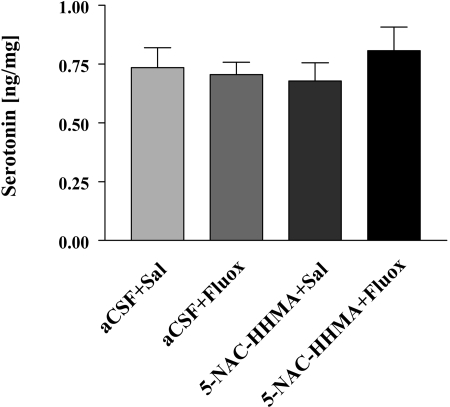
Because previous research has implicated the catechol thioether metabolite of MDMA, 5-NAC-HHMA, in MDMA neurotoxicity (Jones et al., 2005; Erives et al., 2008), we also performed studies to further assess the 5-HT neurotoxic potential of 5-NAC-HHMA and its selectivity. In these studies, we administered 5-NAC-HHMA directly into the striatum, at two different doses (21 and 42 nmol). The established 5-HT neurotoxin, 5,7-DHT (also administered directly into the striatum), served as a positive control. As anticipated, 5,7 DHT produced a sizable depletion of striatal 5-HT 2 weeks later. In contrast, 5-NAC-HHMA produced a modest, nonsignificant decrease in striatal 5-HT content that was neither dose-related (Fig. 7) nor influenced by fluoxetine (Fig. 8). No significant differences were observed in 5-HIAA levels in controls and rats treated with 5-NAC-HHMA groups, with or without fluoxetine pretreatment (data not shown).
Fig. 7.
Concentrations of 5-HT in the ipsilateral and contralateral striatum of rats that received direct unilateral intrastriatal injections of 5-NAC-HHMA at two different concentrations (21 or 42 nmol) 2 weeks previously. Each dose of 5-NAC-HHMA was injected four times, with a 12-h interval between each injection. 5-NAC-HHMA was dissolved in aCSF, at the concentrations shown, shortly before each injection. Control animals received unilateral intrastriatal injections of an equivalent volume of aCSF. Treatment groups were aCSF (n = 8), 21 nmol of 5-NAC-HHMA (n = 10), and 42 nmol of 5-NAC-HMMA (n = 5). A positive control group consisted of animals that received a single intrastriatal injection of 52 nmol of 5,7-DHT (n = 4). Only the effect of 5,7-DHT was significant. ∗, significant relationship.
Fig. 8.
Concentrations of 5-HT in the ipsilateral striatum of rats that received direct unilateral intrastriatal injections of 5-NAC-HHMA (21 nmol) alone or in combination with fluoxetine (Fluox; 10 mg/kg; i.p., 15 min before 5-NAC-HHMA) 2 weeks previously. 5-NAC-HHMA was injected four times, with a 12-h interval between each injection. 5-NAC-HHMA was dissolved in aCSF shortly before each injection. Control animals received unilateral intrastriatal injections of an equivalent volume of aCSF. n = 6 for each treatment group.
Discussion
The potential role of metabolites in MDMA neurotoxicity has been a topic of recent interest (Capela et al., 2009; Perfetti et al., 2009). This is the first study to assess the relationship between pharmacokinetic parameters (Cmax and AUC) of MDMA and its major metabolites (HHMA, HMMA, and MDA) and 5-HT neurotoxic effects in the same animal. Results indicate that MDMA-induced 5-HT neurotoxicity is most closely related to concentrations of MDMA, with a weaker relationship to concentrations of MDA, and no relationship to concentrations of HHMA or HMMA. Indeed, whereas levels of MDMA and MDA in brain were 5- to 10-fold higher than those in plasma, brain HHMA and HMMA could not be detected, despite high plasma HHMA and HMMA concentrations in the same animals. These results, which are consistent with those of Escobedo et al. (2005), suggest that HHMA and HMMA do not readily penetrate the blood-brain barrier (either in their free form or as sulfate or glucuronic conjugates) and indicate that there is little or no brain metabolism of MDMA to HHMA or HMMA. Taken together, these observations and those of others (Steele et al., 1991; Escobedo et al., 2005) cast doubt on the view that HHMA and HMMA are directly involved in MDMA neurotoxicity (Goni-Allo et al., 2008) but leave open the possibility that MDA or a catechol-thioether metabolite of MDMA might be involved.
Although pharmacokinetic parameters of both MDMA and MDA were found to be significantly associated with subsequent 5-HT neurotoxicity, the association with MDMA appeared to be more robust. In particular, both the Cmax and AUC of MDMA were significantly and highly correlated with subsequent 5-HT deficits, whereas only the Cmax of MDA was correlated with 5-HT loss (at a lower significance level). Potential reasons that only MDA Cmax, but not AUC, are related to subsequent 5-HT depletion are as follows: 1) pharmacokinetic parameters for MDA AUC may not be sufficiently precise, because of insufficiently long sampling times; 2) Cmax may be the relevant pharmacokinetic parameter for predicting neurotoxicity; and 3) MDA may not be involved in MDMA neurotoxicity. The current results do not permit definitive conclusions regarding the relative importance of MDMA and MDA in the neurotoxic process as it occurs in rats, as the pharmacokinetic parameter that best predicts 5-HT neurotoxicity is unknown. At least in rats (see below), both MDMA and MDA may contribute in an additive or synergistic fashion to 5-HT neurotoxicity, because they interact with many of the same neuronal systems and elements, and MDA is known to have 5-HT neurotoxic potential (Ricaurte et al., 1985).
Comparisons of the current data, collected in rats, to pharmacokinetic data collected in primates (squirrel monkeys and humans) may also shed light on the relative importance of the parent compound (MDMA) and MDA in 5-HT neurotoxicity. In particular, in squirrel monkeys, MDA is a minor metabolite (3–5%), yet this species also develops MDMA-induced 5-HT neural injury. Although within-subject studies involving pharmacokinetic and neurotoxicity measures have not been conducted in humans, the pharmacokinetics of MDMA in humans are similar to those in squirrel monkeys and demonstrate relatively low levels of MDA production (Kolbrich et al., 2008). A growing body of data indicates that human recreational MDMA users are susceptible to MDMA neurotoxicity (McCann et al., 1998, 2005; Kish et al., 2009) and, taken together with the pharmacokinetic data in humans, argue against a major role for MDA in MDMA-induced neurotoxicity, at least in primates.
As alluded to above, it is not known which pharmacokinetic parameter (Cmax, AUC, or other) of MDMA (or MDA) most influences 5-HT neurotoxicity. However, there are clues in the literature that certain thresholds must be met for neurotoxicity to develop. In particular, intravenous dosages of MDMA that engender high, but short-lived, peak concentrations of MDMA (Banks et al., 2007; M. Mueller and G. Ricaurte, unpublished observation) do not appear to be associated with neurotoxicity (Fantegrossi et al., 2004), presumably because of an insufficiently long duration of drug action. Likewise, repeated low doses of MDMA that fail to achieve a certain threshold concentration would not be expected to produce neurotoxic effects, even though, when considered in aggregate, they would lead to high AUC values. With respect to duration of action, coadministration of a selective 5-HT reuptake inhibitor (fluoxetine) up to 6 h after MDMA administration can protect from 5-HT neurotoxicity, suggesting that key events for the development of neurotoxicity take place within 6 h of drug administration (Schmidt, 1987). When these previously published data are considered along with the present findings, the most parsimonious explanation is that peak plasma drug concentrations must reach a threshold for a certain period of time (3–6 h) for 5-HT neurotoxicity to develop. Stated differently, it is likely that both Cmax and AUC are important determinants of MDMA-induced 5-HT neurotoxicity.
Although precise threshold neurotoxic Cmax and AUC MDMA values have yet to be determined, a working model of a potential mechanism underlying MDMA neurotoxicity can be proposed. This model, which emerges from data discussed above, relates the two principal outcome measures of the present study: pharmacokinetic parameters of MDMA and its metabolites during the period of drug (metabolite) exposure and 5-HT axonal markers (5-HT and 5-HIAA) measured 1 week later. The model assumes that, for neurotoxicity to occur, drug (or metabolite) must interact with the 5-HT transporter for 3 to 6 h. Furthermore, it assumes that a certain threshold drug level must be achieved and maintained during the 3 to 6 h that critical toxic drug/transporter interactions appear to take place. It is noteworthy that the model makes no assumption about serotonin or other monoamine or metabolite levels during the period of drug exposure. However, it does allow for a role of core temperature, with high temperatures facilitating and low core temperatures retarding toxic drug/transporter interactions (Malberg and Seiden, 1998).
It should be emphasized that correlation does not imply causation, that the relationship between MDMA (and MDA) and 5-HT deficits could be coincidental, and that other drug effects may be the most important mediators of neurotoxicity [e.g., transporter-based ion dysregulation, as postulated for methamphetamine (Callahan et al., 2001)]. As noted earlier (see Introduction), there are data indicating that when MDMA is injected directly into the brain, neurotoxicity does not develop. Although this may be viewed as incontrovertible evidence that MDMA is not the major mediator of MDMA-induced 5-HT injury, it is possible that peripheral pharmacological effects not reproduced by central administration (e.g., increased temperature) are required for neurotoxicity to occur. In addition, it is likely that centrally administered MDMA is only toxic when its concentration and duration of action are similar to those after peripheral administration.
The thioether metabolite of HHMA, 5-NAC-HHMA, has been directly implicated in MDMA neurotoxicity (Jones et al., 2005; Erives et al., 2008). In the present study, 5-NAC-HHMA, when administered repeatedly and in large doses into the striatum did not lead to statistically significant 5-HT depletions. Moreover, the modest effect of 5-NAC-HHMA on striatal 5-HT was neither dose related nor blocked by the 5-HT uptake inhibitor, fluoxetine, which is known to protect against MDMA neurotoxicity (Schmidt, 1987). These observations argue against a pivotal role for 5-NAC-HHMA in MDMA-induced 5-HT neurotoxicity but leave open the possibility that it may work in conjunction with MDMA or MDA in the neurotoxic process. Alternatively, 5-NAC-HHMA may require the presence of MDMA and/or elevated body temperature to be toxic, although an earlier study (McCann and Ricaurte, 1991) also suggested that the thioether adducts of HHA are not likely to be responsible for serotonergic neurotoxicity.
The present findings with 5-NAC-HHMA are at odds with findings of a previous study showing that this compound produced dose-related depletions of 5-HT in rats (Jones et al., 2005). The reasons for this discrepancy are not entirely clear. We established the identity of 5-NAC-HHMA by high-performance liquid chromatography and NMR spectra [methods available in the supporting information for Felim et al. (2007)]. Furthermore, the stability of 5-NAC-HHMA was confirmed after each injection by using LC-MS to monitor the abundance of its molecular mass ion [MH+] and one fragment ion (m/z = 343 and m/z = 181, respectively). Another potential reason for discrepant findings is inadequate drug delivery of an unstable compound to target tissues. However, 5,7-DHT (which is also unstable and has a tendency to oxidize) was injected using identical methods and was found to produce robust 5-HT deficits. Finally, it may be relevant that 5-NAC-HHMA used in the present studies was prepared using a biomimetic electrochemical synthetic method (Felim et al., 2007), whereas 5-NAC-HHMA used by Jones et al. (2005) was prepared with mushroom tyrosinase, which yields a different ratio of 5-NAC-HHMA diastereoisomers (Pizarro et al., 2008). Additional research will be required to determine the basis for discrepant findings between the present study and that of Jones et al. (2005).
In conclusion, the present results indicate that MDMA-induced 5-HT neurotoxicity is most closely related to plasma and brain concentrations of MDMA, with a weaker relationship to concentrations of MDA and no relationship to concentrations of HHMA or HMMA. The present results also indicate that the pharmacokinetic parameter of MDMA that best predicts subsequent 5-HT neurotoxicity is Cmax, although AUC is also a good predictor and both peak levels and duration of action are likely to be important. It is noteworthy that neither HHMA nor HMMA could be detected in brain, despite high concentrations of these MDMA metabolites in plasma, indicating that HHMA and HMMA do not readily penetrate the blood-brain barrier. Because brain concentrations of MDMA in the same animals were 5- to 10-fold higher than those in plasma, the absence of measurable amounts of HHMA and HMMA in their brains also suggests that biotransformation of MDMA to HHMA and HMMA does not occur to any appreciable degree in the brain. Finally, repeated intrastriatal administration of 5-NAC-HHMA produced a modest, nonsignificant decrease in striatal 5-HT content that was neither dose-related nor influenced by fluoxetine. Taken together, these results favor the view that MDMA and, possibly, MDA are the compounds that trigger brain 5-HT neurotoxicity in rats, and suggest that HHMA, HMMA, and the catechol thioether metabolite, 5-NAC-HHMA do not play a crucial role in MDMA-induced 5-HT neurotoxicity in vivo.
Acknowledgments.
We thank George Hadtzidimitriou and Gianluigi Tanda for help with these experiments.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- MDMA
- 3,4-methylenedioxymethamphetamine
- 5-HT
- serotonin
- HHMA
- 3,4-dihydroxymethamphetamine
- HHA
- 3,4-dihydroxyamphetamine
- THMA
- 2,4,5-trihydroxymethamphetamine
- SKF-525A
- 2-diethylaminoethyl 2:2-diphenylvalerate hydrochloride
- MDA
- 3,4-methylenedioxyamphetamine
- 5-NAC-HHMA
- 5-(N-acetylcystein-S-yl)-3,4-dihydroxymethamphetamine
- HMMA
- 4-hydroxy-3-methoxymethamphetamine
- 5-HIAA
- 5-hydroxyindol acetic acid
- 5,7-DHT
- 5,7-dihydroxytryptamine
- SMBS
- sodium metabisulfite
- LC-MS
- liquid chromatography-mass spectrometry
- AUC
- area under the curve
- aCSF
- artificial cerebrospinal fluid.
References
- Bai F, Lau SS, Monks TJ . (1999) Glutathione and N-acetylcysteine conjugates of α-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol 12:1150–1157 [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA . (2007) Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 35:1840–1845 [DOI] [PubMed] [Google Scholar]
- Callahan BT, Cord BJ, Yuan J, McCann UD, Ricaurte GA. (2001) Inhibitors of Na+/H+ and Na+/Ca2+ exchange potentiate methamphetamine-induced dopamine neurotoxicity: possible role of ionic dysregulation in methamphetamine neurotoxicity. J Neurochem 77:1348–1362 [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remião F, Bastos ML, Meisel A, Carvalho F. (2009) Molecular and cellular mechanisms of Ecstasy-induced neurotoxicity: an overview. Mol Neurobiol 39:210–271 [DOI] [PubMed] [Google Scholar]
- Capela JP, Macedo C, Branco PS, Ferreira LM, Lobo AM, Fernandes E, Remião F, Bastos ML, Dirnagl U, Meisel A, et al. (2007) Neurotoxicity mechanisms of thioether ecstasy metabolites. Neuroscience 146:1743–1757 [DOI] [PubMed] [Google Scholar]
- Chu T, Kumagai Y, DiStefano EW, Cho AK . (1996) Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51:789–796 [DOI] [PubMed] [Google Scholar]
- Elayan I, Gibb JW, Hanson GR, Foltz RL, Lim HK, Johnson M . (1992) Long-term alteration in the central monoaminergic systems of the rat by 2,4,5-trihydroxyamphetamine but not by 2-hydroxy-4,5-methylenedioxymethamphetamine or 2-hydroxy-4,5-methylenedioxyamphetamine. Eur J Pharmacol 221:281–288 [DOI] [PubMed] [Google Scholar]
- Erives GV, Lau SS, Monks TJ . (2008) Accumulation of neurotoxic thioether metabolites of 3,4-(±)-methylenedioxymethamphetamine in rat brain. J Pharmacol Exp Ther 324:284–291 [DOI] [PubMed] [Google Scholar]
- Escobedo I, O'Shea E, Orio L, Sanchez V, Segura M, de la Torre R, Farre M, Green AR, Colado MI . (2005) A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. Br J Pharmacol 144:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban B, O'Shea E, Camarero J, Sanchez V, Green AR, Colado MI . (2001) 3,4-Methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacology (Berl) 154:251–260 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G, Ricaurte GA, Woods JH, Winger G . (2004) Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology 29:1270–1281 [DOI] [PubMed] [Google Scholar]
- Felim A, Urios A, Neudörffer A, Herrera G, Blanco M, Largeron M. (2007) Bacterial plate assays and electrochemical methods: an efficient tandem for evaluating the ability of catechol-thioether metabolites of MDMA (“ecstasy”) to induce toxic effects through redox-cycling. Chem Res Toxicol 20:685–693 [DOI] [PubMed] [Google Scholar]
- Gollamudi R, Ali SF, Lipe G, Newport G, Webb P, Lopez M, Leakey JE, Kolta M, Slikker W., Jr (1989) Influence of inducers and inhibitors on the metabolism in vitro and neurochemical effects in vivo of MDMA. Neurotoxicology 10:455–466 [PubMed] [Google Scholar]
- Goni-Allo B, O Mathúna B, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N . (2008) The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology (Berl) 197:263–278 [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Kumagai Y, Unger SE, Cho AK. (1990) Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther 254:521–527 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed.Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC: [Google Scholar]
- Johnson M, Elayan I, Hanson GR, Foltz RL, Gibb JW, Lim HK . (1992) Effects of 3,4-dihydroxymethamphetamine and 2,4,5-trihydroxymethamphetamine, two metabolites of 3,4-methylenedioxymethamphetamine, on central serotonergic and dopaminergic systems. J Pharmacol Exp Ther 261:447–453 [PubMed] [Google Scholar]
- Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, Monks TJ . (2005) Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther 313:422–431 [DOI] [PubMed] [Google Scholar]
- Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wickham DJ, Sherwin A, Tong J . (2009) Brain 5-HT transporter in human methamphetamine users. Psychopharmacology (Berl) 202:649–661 [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008) Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit 30:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Foltz RL . (1991a) In vivo formation of aromatic hydroxylated metabolites of 3,4-(methylenedioxy)methamphetamine in the rat: identification by ion trap tandem mass spectrometric (MS/MS and MS/MS/MS) techniques. Biol Mass Spectrom 20:677–686 [DOI] [PubMed] [Google Scholar]
- Lim HK, Foltz RL . (1991b) Ion trap tandem mass spectrometric evidence for the metabolism of 3,4-(methylenedioxy)methamphetamine to the potent neurotoxins 2,4,5-trihydroxymethamphetamine and 2,4,5-trihydroxyamphetamine. Chem Res Toxicol 4:626–632 [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS . (1998) Small changes in ambient temperature cause large changes in 3,4-methylenedioxy-methamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 18:5086–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Ricaurte GA. (1991) Major metabolites of (±)3,4-methylenedioxyamphetamine (MDA) do not mediate its toxic effects on brain serotonin neurons. Brain Res 545:279–282 [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. (1998) Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 352:1433–1437 [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. (2005) Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology 30:1741–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechan A, Yuan J, Hatzidimitriou G, Irvine RJ, McCann UD, Ricaurte GA. (2006) Pharmacokinetic profile of single and repeated oral doses of MDMA in squirrel monkeys: relationship to lasting effects on brain serotonin neurons. Neuropsychopharmacology 31:339–350 [DOI] [PubMed] [Google Scholar]
- Meyer MR, Peters FT, Maurer HH . (2008) The role of human hepatic cytochrome P450 isozymes in the metabolism of racemic 3,4-methylenedioxy-methamphetamine and its enantiomers. Drug Metab Dispos 36:2345–2354 [DOI] [PubMed] [Google Scholar]
- Miller RT, Lau SS, Monks TJ . (1997) 2,5-Bis-(glutathion-S-yl)-α-methyldopamine, a putative metabolite of (±)-3,4-methylenedioxyamphetamine, decreases brain serotonin concentrations. Eur J Pharmacol 323:173–180 [DOI] [PubMed] [Google Scholar]
- Molliver ME, Berger UV, Mamounas LA, Molliver DC, O'Hearn E, Wilson MA . (1990) Neurotoxicity of MDMA and related compounds: anatomic studies. Ann N Y Acad Sci 600:649–661 [DOI] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, Lau SS . (2004) The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit 26:132–136 [DOI] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Ricaurte GA, Maurer HH . (2007) Validated liquid chromatographic-electrospray ionization mass spectrometric assay for simultaneous determination of 3,4-methylenedioxymethamphetamine and its metabolites 3,4-methylenedioxyamphetamine, 3,4-dihydroxymethamphetamine, and 4-hydroxy-3-methoxymethamphetamine in squirrel monkey plasma. J Chromatogr B Analyt Technol Biomed Life Sci 855:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Ricaurte GA, Maurer HH . (2008) Liquid chromatographic-electrospray ionization mass spectrometric assay for simultaneous determination of 3,4-methylenedioxymethamphetamine and its metabolites 3,4-methylenedioxyamphetamine, 3,4-dihydroxymethamphetamine, and 4-hydroxy-3-methoxymethamphetamine in rat brain. J Chromatogr B Analyt Technol Biomed Life Sci 874:119–124 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1986) The Brain in Stereotaxic Coordinates 2nd ed, Academic Press, Inc., New York: [Google Scholar]
- Perfetti X, O'Mathúna B, Pizarro N, Cuyàs E, Khymenets O, Almeida B, Pellegrini M, Pichini S, Lau SS, Monks TJ, et al. (2009) Neurotoxic thioether adducts of 3,4-methylenedioxymethamphetamine identified in human urine after Ecstasy ingestion. Drug Metab Dispos 37:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro N, de la Torre R, Joglar J, Okumura N, Perfetti X, Lau SS, Monks TJ . (2008) Serotonergic neurotoxic thioether metabolites of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”): synthesis, isolation, and characterization of diastereoisomers. Chem Res Toxicol 21:2272–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte G, Bryan G, Strauss L, Seiden L, Schuster C. (1985) Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science 229:986–988 [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Martello AL, Katz JL, Martello MB. (1992) Lasting effects of (±)-3,4-methylenedioxymethamphetamine (MDMA) on central serotonergic neurons in nonhuman primates: neurochemical observations. J Pharmacol Exp Ther 261:616–622 [PubMed] [Google Scholar]
- Schmidt CJ. (1987) Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J Pharmacol Exp Ther 240:1–7 [PubMed] [Google Scholar]
- Schmidt CJ, Taylor VL . (1988) Direct central effects of acute methylenedioxymethamphetamine on serotonergic neurons. Eur J Pharmacol 156:121–131 [DOI] [PubMed] [Google Scholar]
- Steele TD, Brewster WK, Johnson MP, Nichols DE, Yim GK . (1991) Assessment of the role of α-methylepinine in the neurotoxicity of MDMA. Pharmacol Biochem Behav 38:345–351 [DOI] [PubMed] [Google Scholar]
- Zhao ZY, Castagnoli N, Jr, Ricaurte GA, Steele T, Martello M . (1992) Synthesis and neurotoxicological evaluation of putative metabolites of the serotonergic neurotoxin 2-(methylamino)-1-[3,4-(methylenedioxy)phenyl] propane [(methylenedioxy)methamphetamine]. Chem Res Toxicol 5:89–94 [DOI] [PubMed] [Google Scholar]