Abstract
Drug-induced valvular heart disease (VHD) is a serious side effect of a few medications, including some that are on the market. Pharmacological studies of VHD-associated medications (e.g., fenfluramine, pergolide, methysergide, and cabergoline) have revealed that they and/or their metabolites are potent 5-hydroxytryptamine2B (5-HT2B) receptor agonists. We have shown that activation of 5-HT2B receptors on human heart valve interstitial cells in vitro induces a proliferative response reminiscent of the fibrosis that typifies VHD. To identify current or future drugs that might induce VHD, we screened approximately 2200 U.S. Food and Drug Administration (FDA)-approved or investigational medications to identify 5-HT2B receptor agonists, using calcium-based high-throughput screening. Of these 2200 compounds, 27 were 5-HT2B receptor agonists (hits); 14 of these had previously been identified as 5-HT2B receptor agonists, including seven bona fide valvulopathogens. Six of the hits (guanfacine, quinidine, xylometazoline, oxymetazoline, fenoldopam, and ropinirole) are approved medications. Twenty-three of the hits were then “functionally profiled” (i.e., assayed in parallel for 5-HT2B receptor agonism using multiple readouts to test for functional selectivity). In these assays, the known valvulopathogens were efficacious at concentrations as low as 30 nM, whereas the other compounds were less so. Hierarchical clustering analysis of the pEC50 data revealed that ropinirole (which is not associated with valvulopathy) was clearly segregated from known valvulopathogens. Taken together, our data demonstrate that patterns of 5-HT2B receptor functional selectivity might be useful for identifying compounds likely to induce valvular heart disease.
In 1997, the anorexigen fenfluramine was voluntarily withdrawn from the U.S. market because of its association with valvular heart disease (VHD) and pulmonary hypertension (Connolly et al., 1997). Valve tissue obtained from affected persons revealed plaques of proliferating myofibroblasts beneath the elastic surface of the valves (Steffee et al., 1999). As lesions develop, valve function becomes impaired and valvular insufficiency ensues. Indistinguishable histopathologic results occur in patients with malignant carcinoid syndrome and in persons undergoing therapy with certain ergots and ergolines for migraines (e.g., methysergide, ergotamine) or Parkinson's disease [e.g., pergolide, cabergoline (Connolly et al., 1997; Pritchett et al., 2002; Horvath et al., 2004; Roth, 2007)].
We and others have identified the 5-HT2B receptor as a likely molecular target for drug-induced VHD based on the preferentially potent agonism of norfenfluramine (fenfluramine's active metabolite), methysergide, and methylergonovine (the active metabolite of ergotamine) at human cloned 5-HT2B receptors (Fitzgerald et al., 2000; Rothman et al., 2000; Setola et al., 2003). Additional evidence implicating 5-HT2B receptors in drug-induced VHD came from the observations that 1) 5-HT2B receptors are enriched in heart valve tissue from various species (Fitzgerald et al., 2000; Setola et al., 2003; Elangbam et al., 2005; Regard et al., 2008); 2) activation of human valvular interstitial 5-HT2B receptors is mitogenic, resulting in ERK1/2 phosphorylation and [3H]deoxythymidine incorporation (Setola et al., 2003); 3) 5-HT2B receptor activation has been implicated in 5-HT-induced valvulopathy in experimental animals (Elangbam et al., 2008); and 4) other drugs with potent 5-HT2B agonist activity [pergolide, cabergoline, MDMA, 3,4-dimethoxyamphetamine (Setola et al., 2003)] induce valvular heart disease in humans (Droogmans et al., 2007; Schade et al., 2007; Zanettini et al., 2007), whereas chemically similar drugs lacking 5-HT2B agonism [e.g., lisuride and bromocriptine (Roth, 2007; Berger et al., 2009)] are not associated with an increased risk of VHD (Schade et al., 2007; Zanettini et al., 2007).
Given the strong associations between 5-HT2B agonism and drug-induced VHD, we sought first to identify medications (either approved or investigational) that might induce VHD. Second, we attempted to determine which signal transduction pathway might be correlated with a drug's propensity to induce valvulopathy. To achieve the first goal, we screened a composite library of approximately 2200 approved or investigational medications and drug-like scaffolds for 5-HT2B receptor agonism using a high-throughput, calcium flux-based assay done on cells stably expressing exogenous human 5-HT2B receptors. Subsequently, we measured the activity of the bona fide agonists using five additional readouts of 5-HT2B receptor activation: nuclear factor of activated T cells (NFAT)-mediated transcription of a β-lactamase reporter gene, ERK2 phosphorylation, β-arrestin recruitment to agonist-occupied 5-HT2B receptors, accumulation of inositol phosphates (InsP), and cell proliferation. We reasoned that the valvulopathogens might share a pattern of functional selectivity (Urban et al., 2007) distinct from the non–VHD-associated medication ropinirole and/or other 5-HT2B receptor agonists.
Less than 1% of the compounds screened were 5-HT2B receptor agonists. Because the identification of 5-HT2B receptor agonists is relatively straightforward, we suggest that current and candidate drugs be screened for 5-HT2B agonism before clinical trials and that the clinical use of 5-HT2B agonist medications be avoided when possible.
Materials and Methods
Plasmid Construct and Cell Lines.
Cells stably expressing human 5-HT2B receptors were generated using the FlpIn system (Invitrogen Corp., Carlsbad, CA). In brief, a cDNA for the human 5-HT2B receptor was amplified by polymerase chain reaction from pUSF-5-HT2B (Setola et al., 2005) using 5′ and 3′ BamHI linkers, and then subcloned into the BamHI site of pcDNA5.0/Frt to produce pFlpIn-5-HT2B. Next, subconfluent (60%) FlpIn HEK293 cells in 10-cm dishes were cotransfected with 3 μg of pFlpIn-5-HT2B and 3 μg of pOG5 (bearing the Frt recombinase; Invitrogen Corp.) using 30 μl of FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN). Twenty-four hours after transfection, cells were split 1:5 into growth/selection medium (DMEM, 10% FBS, 500 mg/liter G418, and 50 mg/liter hygromycin B; all from Invitrogen) and allowed to expand. After selection, multiple aliquots of cells at passage 0 were collected, frozen in Cell Recovery Medium (Invitrogen) overnight at −80 degrees C, then stored in liquid nitrogen. Receptor expression was verified by 1) radioligand competition binding assays using [3H]d-lysergic acid diethylamide (∼1 nM final; PerkinElmer Life and Analytical Sciences, Waltham, MA) to label 5-HT2B receptors and varying concentrations of unlabeled ligand (spanning at least 5 orders of magnitude) to compete for radioligand binding (Setola et al., 2005) and 2) calcium flux assays (see Calcium Flux Assay) using varying concentrations of 5-HT and BW 723C86 to measure 5-HT2B receptor activation. Cells were passaged fewer than 10 times beyond passage 0 to ensure stable 5-HT2B receptor expression. The GeneBlazer cell line (CHO-NFAT-bla) stably expressing human 5-HT2B receptors was from Invitrogen (Madison, WI) and was maintained and prepared for assay exactly as described by the manufacturer.
Calcium Flux Assay.
FlpIn HEK239 5-HT2B cells were seeded in 384-well plates at a density of 10,000 cells/well in DMEM containing 1% dialyzed FBS 24 h before the calcium flux assay. The next day, the cells were incubated (20 μl/well) for 1 h at 37°C with Calcium Plus dye (Molecular Dynamics, Sunnyvale, CA) reconstituted in FLIPR buffer (Hanks' balanced salt solution, 2.5 mM probenecid, and 20 mM HEPES, pH 7.4) (15 ml buffer/bottle of lyophilized dye yielded a 30× dye stock). After the dye load, cells were placed in a FLIPRTETRA fluorescence imaging plate reader (Molecular Dynamics); drug dilutions, prepared at 2× final concentration in FLIPR buffer and aliquotted into 384-well plates, were also added to the FLIPRTETRA. The fluidics module and plate reader of the FLIPRTETRA were programmed to read baseline fluorescence for 10 s (1 read/s), then to add 20 μl of drug/well and to read for 6 min (1 read/s). Fluorescence in each well was normalized to the average of the first 10 reads (i.e., baseline fluorescence). Then, the maximum -fold increase, which occurred within 2 to 3 s after drug addition, over baseline fluorescence elicited by vehicle or drug was determined and plotted as a function of drug concentration. The data were analyzed by regression against a three-parameter logistic equation (Prism ver. 4.0; GraphPad Software, San Diego, CA), with the “bottom” shared for all samples in a plate. Finally, the data were normalized such that the baseline fluorescence was set to 0% and the Emax for 5-HT (measured on each plate) was set to 100%.
Transcription Factor Activation Assay.
The activation of the transcription factor NFAT was measured using the GeneBLAzer HTR2B-NFAT-bla CHO-K1 cell-based assay (Invitrogen Corp.), as specified by the manufacturer. In brief, cells were seeded in poly-lysine–coated 96-well plates at approximately 3 × 104 cells/well 1 day before assay in NFAT-bla assay medium (DMEM, 1% dialyzed FBS, 0.1 mM nonessential amino acids, and 25 mM HEPES, pH 7.3). On the day of the assay, dilutions of test compounds and reference compounds (prepared at 5× final concentration in PBS) were added to the cells, and plates were incubated for 4 h at 37°C in an atmosphere of 5% CO2. Next, the NFAT-β-lactamase (bla) FRET substrate was added to the cells and followed by 2-h incubation at room temperature in the dark. Cleavage of the substrate by bla results in the loss of FRET activity. Samples were excited at 409 nm and fluorescence was read from the bottom of the plates on a FlexStation II plate reader (Molecular Dynamics) at 460 nm (donor fluorescence) and 530 nm (FRET fluorescence). The ratio of the two fluorescence values (460 nm/530 nm) was calculated for each sample. These FRET ratios were analyzed as above for Ca2+ flux assays. The data were then normalized so that the shared baseline was set to 0%, and the Emax for 5-HT (measured on each plate) was set to 100%.
Arrestin Translocation Assay.
Arrestin recruitment to ligand activated 5-HT2B receptors was carried out using Tango HTR2B-bla U2OS cell line (Invitrogen). These cells stably express C-terminally modified 5-HT2B receptor, protease-tagged β-arrestin, and β-lactamase reporter gene. Arrestin recruited to the activated receptors was measured by the amount of β-lactamase activity. The cells were plated in 384-well plates at a density of 104 cells per well in 32 μl of assay medium (DMEM supplemented with 1% dialyzed FBS, 25 mM HEPES buffer, pH 7.3, 0.1 mM nonessential amino acids, and 1 mM sodium pyruvate). The cells were stimulated with indicated concentration of the test compounds or reference compound. After overnight incubation in a humidified incubator at 37°C with 5% CO2, the cells were loaded with cell-permeable LiveBLAzer FRET B/G substrate (Invitrogen) for 2 h at room temperature. FRET emission ratios were obtained on a Tecan Infinite 200 fluorescence plate reader (excitation at 409 nm, emission 450 nm and 525 nm). The ratio of two fluorescence values (450 nm/525 nm) was calculated for each sample. The data were analyzed as described above for transcription factor activation assay.
ERK2 Phosphorylation Assay.
The GFP-ERK2 expression vector was generated by Gateway cloning technology. Entry clone IOH12327 (Invitrogen) encoding ERK2 was recombined with a pLENTI-bsd destination vector modified with an N-terminal EmGFP tag. Using the resulting pLentiEmGFP-ERK2 construct, lentivirus was generated using the manufacturer's recommended protocol (Invitrogen). GeneBLAzer HTR2B-NFAT-bla CHO-K1 cells were then transduced with lentivirus and placed under blasticidin selection. Blasicidin-resistant clonal populations were isolated by fluorescence-activated cell sorting using GFP fluorescence as a sorting marker. GeneBLAzer HTR2B-NFAT-bla CHO-K1 GFP-ERK2 cells were seeded in white 384-well flat-bottomed cell culture-treated plates (Corning Life Sciences, Acton, MA) at densities of approximately 2 × 104 cells/well in 32 μl of assay medium (consisting of 99% Opti-MEM, supplemented with 0.1% charcoal/dextran-treated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 25 mM HEPES pH 7.3, and lacking phenol red). All media reagents were obtained from Invitrogen. After an overnight serum-starvation, the cells were exposed to 4 μl of antagonist or 1% DMSO (vehicle) for 10 min. Then, 4 μl of serial-diluted test compound was added to each well simultaneously using a Hamamatsu functional drug screening system instrument (Hamamatsu City, Japan). Immediately after a 6-min stimulation at room temperature, cell medium was removed by inverting the plate onto a dry paper towel. Cells were then lysed by addition of 20 μl of lysis buffer/well (consisting of 2 nM terbium-anti-ERK2 [pThr/pTyr 185/187] antibody (Invitrogen), 20 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 5 mM EDTA, 5 mM NaF, 150 mM NaCl, and 1:100 of protease and phosphatase inhibitor cocktails; Sigma, St. Louis, MO). After allowing the assay to equilibrate for 2 h at room temperature, time-resolved FRET emission ratios were determined on a BMG Pherastar fluorescence plate reader (BMG Labtech, Durham, NC) using the following settings: excitation at 340 nm, emission at 520 nm and 490 nm; 100-μs lag time, 200-μs integration time. Emission ratios were then calculated by dividing the 520 nm emission value by the 490 nm emission value. Data were then normalized as described above.
Immunoblot Analysis.
FlpIn HEK293 5-HT2B cells were plated in DMEM containing 5% dialyzed FBS. Twenty-four hours later, cells were washed and incubated in serum-free medium overnight. Drugs (except 5-HT) were initially dissolved at 10 mM in DMSO and then diluted in serum-free medium; 5-HT was dissolved directly in serum-free medium. When necessary, cells were pretreated for 15 min with 1 μM SB 206553 before addition of agonist or vehicle. Agonists were applied at 1 μM for 5 min, and then cells were immediately placed on ice, and lysed in lysis buffer [1.5% CHAPS, 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 10 mM Na4P2O7, 2 mM sodium orthovanadate, protease inhibitors (Complete EDTA-free Protease Cocktail; Roche Applied Science), pH 7.5] for 15 min at 4°C. Cells were scraped off plates, and the supernatants were collected after centrifugation. A Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) was performed to determine the protein concentration of each lysate (Bradford, 1976). Samples were diluted to equal concentrations in sample buffer containing SDS, heated, and separated by SDS-polyacrylamide gel electrophoresis, followed by transfer to polyvinylidene difluoride membranes. Blots were blocked in 3% BSA and probed for pERK with a rabbit anti-pERK antibody (Cell Signaling Technology, Danvers, MA) at a 1:1000 dilution, followed by horseradish peroxidase secondary antibody (1:1000; Vector Laboratories, Burlingame, CA), and detection as detailed previously (Sheffler et al., 2006). Blots were then stripped in stripping buffer containing 100 mM β-mercaptoethanol and 2% SDS and reprobed for total ERK using a rabbit anti-ERK antibody (Cell Signaling Technology) at 1:1000, followed by secondary antibody and detection as above. Blots were imaged on a Kodak Gel Logic 2200 (Carestream Health, Rochester, NY) imager and the bands quantified by densitometry using ImageJ software (http://rsbweb.nih.gov/ij/).
InsP Accumulation Assay.
Measurements of InsP accumulation in agonist-stimulated FlpIn HEK293 5-HT2B cells were made using the scintillation proximity assay method (Bourdon et al., 2006; Jensen et al., 2008). In brief, 3 × 104 cells/well were plated into 96-well tissue culture plates in dialyzed culture medium. The cells were inositol-starved for 1.5 h and then incubated for 18 h at 37°C with labeling medium [inositol-free basal media Eagle's solution] (Lonza Walkersville, Inc., Walkersville, MD) with 5% dialyzed FBS and 0.01 μCi/ml [myo-3H]inositol (PerkinElmer Life and Analytical Sciences)]. Labeling medium was removed and agonists [dissolved in DMSO and diluted in assay buffer (1× Hanks' balanced salt solution, 24 mM NaHCO3, 11 mM glucose, and 35 mM LiCl, pH 7.4)] were added to the cells for 1 h at 37°C. The assay was terminated by replacement of the incubation medium with 40 μl of 50 mM formic acid. After a 20-min incubation in formic acid to extract the cytosolic fraction from the cells, the formic acid was incubated with 0.2 mg of yttrium silicate beads (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). Radioactivity was measured by scintillation counting using a Wallac MicroBeta TriLux plate reader (PerkinElmer Life and Analytical Sciences). InsP levels (in disintegrations per minute) were analyzed as above for Ca2+ flux assays. Finally, the data were normalized such that the shared baseline was set to 0% and the Emax for 5-HT (measured on each plate) was set to 100%.
Tetrazolium Salt-Based Cell Proliferation Assay.
Cell proliferation was assessed using the XTT Cell Proliferation assay (Roche Applied Science) according to the manufacturer's recommendations. Stable FlpIn HEK293-5-HT2B cells or the parental FlpIn HEK293 cells were seeded into poly-l-lysine–coated 96-well plates at density of 3 × 104 cells/well and grown for 16 h in DMEM containing 1% serum (37°C, 5% CO2). Cells were then exposed to various concentrations of test or reference compounds for 48 h. During the final 4 h of treatment, 50 μl of XTT tetrazolium salt reagent was added to each well, and incubation was continued at 37°C. Metabolic mitochondrial dehydrogenase activity of the cells converts the tetrazolium salt to a water-soluble formazan dye product, providing a colorimetric index proportional to cell number. The formazan dye absorbance peak at 490 nm was measured using a SpectraMax microplate reader (Molecular Dynamics). The absorbances obtained were normalized to the values of untreated cells and expressed as a percentage.
Statistics.
Data were analyzed for statistical significance by two-way analysis of variance followed by a Bonferroni post test using Prism 4.0. A p value less than 0.05 was considered significant.
Cluster Analysis.
For hierarchical clustering analysis, pEC50 data (expressed as pEC50 − 5) were clustered using GeneCluster (http://www.broadinstitute.org/cancer/software/genecluster2/gc2.html) with “Assays” and “Drugs” representing the two axes. The similarity matrix used was produced via the correlation (uncentered) method, and a complete linkage clustering was performed. The cluster was visualized with Java TreeView ver. 1.13 (http://jtreeview.sourceforge.net) with a contrast value set at 4.5.
Results
To identify potential valvulopathogenic 5-HT2B receptor agonists, we compiled a small molecule library of FDA-approved and investigational drugs and drug-like scaffolds. This composite library contained the Prestwick Chemical Library, the National Institutes of Health Clinical Collection, the National Institute of Mental Health-RTI International Screening set, and our own internal library (approximately 2200 compounds; see Supplementary Table 1, which contains all the drugs and the initial screening results). For the initial screen, we assayed compounds at 3 to 10 μM final concentration for agonist activity at recombinant human 5-HT2B receptors stably expressed in HEK293 FlpIn cells using a calcium flux-based FLIPR assay. In parallel, we also assessed compound activity in the parental HEK293 FlpIn cells to identify false positives. From the list of confirmed agonists (see Supplementary Table 2), several compounds were chosen for further study. The selected compounds were 1) known 5-HT2B agonists, 2) VHD-associated medications and/or metabolites thereof, and/or 3) investigational compounds and medications not previously known to activate 5-HT2B receptors (Table 1).
TABLE 1.
Agonist relative efficacies and potencies at 5-HT2B receptors measured using five assays of receptor activation
Known valvulopathic compounds are in bold type. Emax represents percentage of 5-HT.
| Drugs | Calcium Flux |
NFAT-bla Activity |
ERK2 Phosphorylation |
Arrestin Translocation |
InsP Accumulation |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | |
| % | % | % | % | % | ||||||
| 5-HT | 100.0 ± 0.7 | 8.75 ± 0.03 | 100 ± 2 | 9.14 ± 0.05 | 100 | 9.43 ± 0.02 | 100 ± 2 | 7.78 ± 0.06 | 100 ± 1 | 7.97 ± 0.03 |
| BW 723C86 | 92.9 ± 0.9 | 8.85 ± 0.03 | 96 ± 2 | 8.23 ± 0.05 | 65.3 ± 0.8 | 9.04 ± 0.04 | 100 ± 2 | 6.18 ± 0.05 | 89.5 ± 0.6 | 7.58 ± 0.02 |
| DOI | 88.2 ± 6.3 | 8.84 ± 0.13 | 106.6 ± 0.9 | 7.86 ± 0.02 | 54 ± 1 | 8.26 ± 0.06 | 96 ± 1 | 6.99 ± 0.03 | 103 ± 1 | 7.19 ± 0.03 |
| Ergotamine | 87.2 ± 5.7 | 5.46 ± 0.18 | 88 ± 1 | 8.42 ± 0.04 | 74.0 ± 0.9 | 7.70 ± 0.03 | 98.8 ± 0.8 | 8.08 ± 0.03 | 82 ± 2 | 6.41 ± 0.04 |
| TFMPP | 40.3 ± 8.0 | 7.42 ± 0.11 | 24 ± 2 | 7.5 ± 0.2 | 49 ± 1 | 7.64 ± 0.06 | 126 ± 1 | 6.52 ± 0.02 | 18.3 ± 0.4 | 6.97 ± 0.05 |
| Quipazine | 8.4 ± 0.2 | 8.05 ± 0.09 | 7.0 ± 0.5 | 7.3 ± 0.2 | 21 ± 1 | 7.9 ± 0.1 | 60 ± 1 | 6.44 ± 0.04 | 7.1 ± 0.3 | 7.71 ± 0.09 |
| RU 24969 | 60.1 ± 13.3 | 7.89 ± 0.08 | 43 ± 1 | 7.04 ± 0.06 | 87 ± 2 | 8.00 ± 0.05 | 91 ± 1 | 6.26 ± 0.02 | 26.7 ± 0.6 | 6.47 ± 0.05 |
| SCH 23390 | 47.1 ± 6.4 | 7.18 ± 0.10 | 46 ± 1 | 7.06 ± 0.06 | 75 ± 2 | 7.05 ± 0.06 | 142 ± 2 | 5.76 ± 0.02 | 21.7 ± 0.6 | 5.95 ± 0.05 |
| WAY 161503 | 72 ± 1 | 8.44 ± 0.05 | 98 ± 3 | 7.64 ± 0.08 | 68 ± 1 | 7.71 ± 0.04 | 232 ± 3 | 6.50 ± 0.02 | 91 ± 1 | 6.93 ± 0.03 |
| Cabergoline | 98.5 ± 1.9 | 6.40 ± 0.10 | 108 ± 4 | 9.1 ± 0.1 | 60.1 ± 0.9 | 8.44 ± 0.04 | 90.6 ± 0.6 | 8.23 ± 0.03 | 77 ± 2 | 8.18 ± 0.07 |
| Dihydroergotamine | 81.6 ± 7.9 | 5.30 ± 0.22 | 92 ± 1 | 8.32 ± 0.04 | 70.6 ± 0.8 | 8.27 ± 0.03 | 175 ± 4 | 7.65 ± 0.06 | 76 ± 2 | 7.48 ± 0.06 |
| Ergonovine | 39.7 ± 4.0 | 7.15 ± 0.37 | 54.3 ± 0.6 | 9.03 ± 0.03 | 57 ± 1 | 8.48 ± 0.05 | 74.7 ± 0.9 | 7.82 ± 0.03 | 22.2 ± 0.2 | 8.64 ± 0.03 |
| Methylergonovine | 49.5 ± 6.7 | 7.67 ± 0.07 | 56 ± 2 | 9.3 ± 0.1 | 52.5 ± 0.8 | 8.78 ± 0.05 | 102.4 ± 0.7 | 8.96 ± 0.02 | 18.7 ± 0.1 | 8.52 ± 0.02 |
| Norfenfluramine | 107.4 ± 0.6 | 8.61 ± 0.02 | 101 ± 2 | 7.81 ± 0.04 | 75 ± 1 | 8.85 ± 0.04 | 52 ± 1 | 6.89 ± 0.05 | 84 ± 2 | 6.73 ± 0.05 |
| Pergolide | 88.5 ± 6.4 | 7.13 ± 0.08 | 108 ± 3 | 7.72 ± 0.05 | 79 ± 1 | 8.99 ± 0.05 | 92 ± 1 | 7.73 ± 0.04 | 95 ± 1 | 6.94 ± 0.02 |
| Ropinirole | 73 ± 1 | 5.59 ± 0.02 | 89 ± 6 | 5.0 ± 0.1 | 67 ± 3 | 5.60 ± 0.05 | N.D. | <5 | 163 ± 4 | 4.46 ± 0.02 |
| Guanfacine | 93 ± 1 | 6.91 ± 0.03 | 102 ± 2 | 6.26 ± 0.05 | 59 ± 1 | 7.04 ± 0.04 | 87 ± 4 | 5.32 ± 0.05 | 119 ± 3 | 5.16 ± 0.03 |
| Oxymetazoline | 70.9 ± 0.4 | 7.34 ± 0.01 | 80 ± 1 | 6.44 ± 0.03 | 60.0 ± 0.9 | 8.00 ± 0.04 | 130 ± 5 | 5.41 ± 0.04 | 51.8 ± 0.6 | 5.38 ± 0.02 |
| Quinidine | 55.9 ± 0.3 | 6.73 ± 0.01 | 63 ± 3 | 6.17 ± 0.09 | 51 ± 1 | 7.19 ± 0.05 | 119 ± 4 | 5.36 ± 0.03 | 32.2 ± 0.9 | 5.31 ± 0.04 |
| Xylometazoline | 55.7 ± 0.3 | 6.62 ± 0.01 | 56 ± 1 | 6.00 ± 0.04 | 53 ± 1 | 7.16 ± 0.06 | 140 ± 10 | 5.18 ± 0.07 | 47 ± 3 | 4.86 ± 0.06 |
| Fenoldopam | 92.5 ± 0.6 | 7.11 ± 0.01 | 87 ± 2 | 6.91 ± 0.06 | 91 ± 2 | 6.23 ± 0.03 | 207 ± 7 | 5.51 ± 0.04 | 71 ± 1 | 5.79 ± 0.03 |
| SR 57227A | 37.4 ± 0.4 | 6.97 ± 0.02 | 28 ± 2 | 7.2 ± 0.2 | 91 ± 2 | 6.72 ± 0.04 | 74 ± 2 | 5.94 ± 0.04 | 21.9 ± 0.2 | 6.58 ± 0.01 |
| L 694247 | 102.2 ± 4.4 | 7.27 ± 0.06 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| DM 360 | 84.6 ± 7.5 | 8.38 ± 0.33 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| SKF 83566 | 48.6 ± 3.5 | 6.90 ± 0.03 | 56 ± 2 | 7.24 ± 0.09 | 142 ± 3 | 6.54 ± 0.05 | 134 ± 1 | 5.97 ± 0.02 | 36 ± 1 | 5.43 ± 0.05 |
| CP 132484-42 | 92.7 ± 4.0 | 7.70 ± 0.28 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| CP 118,952 | 71.8 ± 10.2 | 7.89 ± 0.28 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| CP 123,479-11 | 93.9 ± 4.8 | 8.71 ± 0.17 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
TFMPP, 1-(3-trifluoromethylphenyl) piperazine; ND, not determined.
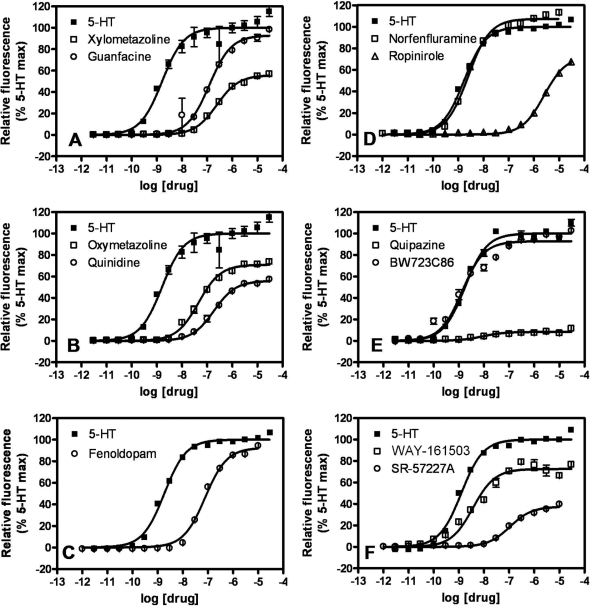
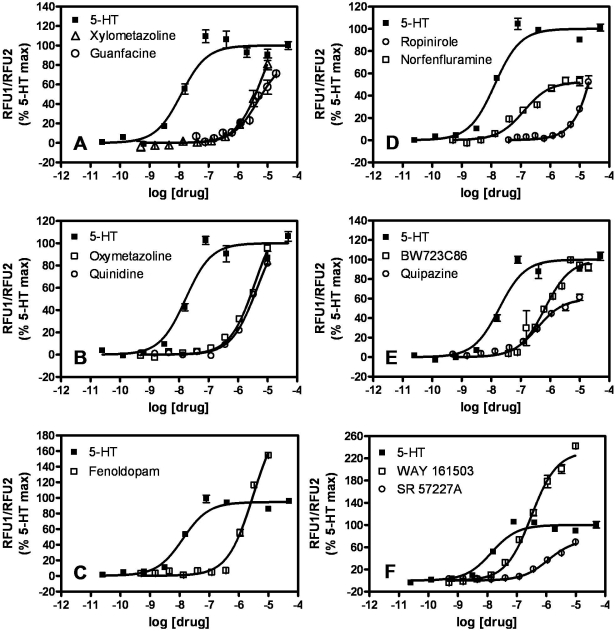
We next generated concentration-response isotherms for the selected, putative 5-HT2B agonists listed in Table 1 to obtain estimates of potency (pEC50) and efficacy relative to 5-HT (Emax) (Fig. 1, Table 1). It is noteworthy that the calcium response for each agonist was blocked by the 5-HT2B/2C receptor-selective antagonist SB 206553 (Supplementary Fig. 1). Fourteen of the 27 hits were previously identified 5-HT2B receptor agonists [quipazine, 1-(3-trifluoromethylphenyl) piperazine, RU 24969, SCH 23390, BW723C86, DOI, WAY 161503, pergolide, norfenfluramine, ergotamine, dihydroergotamine, cabergoline, ergonovine, methylergonovine]. Of the 14 known 5-HT2B receptor agonists, 7 were medications (or metabolites thereof) associated with VHD (pergolide, norfenfluramine, ergotamine, dihydroergotamine, cabergoline, ergonovine, methylergonovine). The remaining 13 hits were not previously known to be 5-HT2B receptor agonists, although some were known to be functionally active at other 5-HT receptors (L 694247, SR 57227A, CP 132484-42, and CP 123,479-11). SKF 83566 was a known D1-like receptor-selective antagonist. Six of the newly discovered 5-HT2B receptor agonists (guanfacine, quinidine, fenoldopam, oxymetazoline, xylometazoline, and ropinirole) are currently approved medications.
Fig. 1.
Agonist concentration-dependent stimulation of calcium flux in FlpIn HEK293 5-HT2B receptor-expressing cells. Maximal intracellular calcium response to each concentration of agonist, measured by Calcium Plus dye fluorescence, is expressed as a percentage of the 5-HT Emax. Each panel shows isotherms for two of the drugs listed in Table 1 and for 5-HT. Similar experiments were performed for all drugs listed in Table 1, and the data were analyzed as described under Materials and Methods to obtain potency (pEC50) and efficacy (Emax) estimates. Parental untransfected FlpIn HEK293 cells did not exhibit calcium flux responses to any of the tested drugs at any concentration (data not shown).
On the basis of potency and efficacy estimates in the agonist-induced calcium flux assay, the VHD-associated hits (pergolide, norfenfluramine, ergotamine, dihydroergotamine, cabergoline, ergonovine, and methylergonovine) could not be clearly distinguished from ropinirole, which is thought to be “safe” in terms of VHD. The VHD-associated drugs cabergoline and dihydroergotamine had potencies similar to ropinirole (pEC50 values of 6.40 ± 0.10, 5.30 ± 0.22, and 5.59 ± 0.02, respectively) and were as efficacious (Emax values of 98.5 ± 1.9, 81.6 ± 7.9, and 73 ± 1%, respectively) (Table 1). Other VHD-associated compounds, such as pergolide and methylergonovine, were more potent (pEC50 values of 7.13 ± 0.08 and 7.67 ± 0.07, respectively) but not more efficacious (Emax values of 88.5 ± 6.4 and 49.5 ± 6.7%, respectively) than ropinirole (Table 1). Thus, we postulated that other readouts of 5-HT2B receptor activation might better distinguish the VHD-associated drugs from ropinirole, and thus permit us to assess the valvulopathic risk of the other FDA-approved hits (guanfacine, quinidine, fenoldopam, oxymetazoline, and xylometazoline).
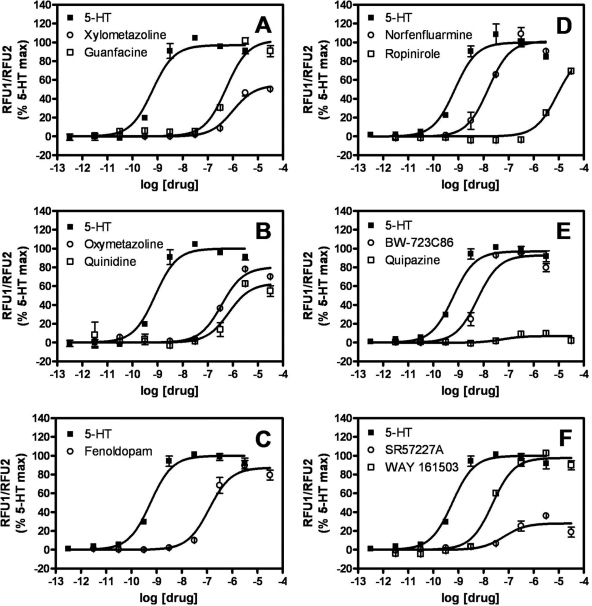
We generated concentration-response isotherms using four additional assays of 5-HT2B receptor activity as follows: 1) calcium-sensitive NFAT-mediated transcription of a β-lactamase reporter gene, 2) accumulation of InsPs in LiCl-treated cells, 3) recruitment of β-arrestin to agonist-occupied receptors, and 4) phosphorylation of the extracellular signal-regulated kinase ERK2. In control assays, we established that these agonist-induced responses were blocked by SB 206553, and/or absent in parental cell lines not expressing recombinant human 5-HT2B receptors (data not shown). It is noteworthy that the time scale of the four additional assays—but not of the calcium flux assay—permits measurement or response under equilibrium conditions of agonist-receptor occupancy.
The β-lactamase (bla) assays revealed all VHD-associated drugs to have pEC50 values greater than 7.5; i.e., they had EC50 values less than 30 nM (Table 1; representative isotherms appear in Fig. 2). In contrast, ropinirole was markedly less potent than known valvulopathogens in bla assays, having a pEC50 value of 5.0 ± 0.1 (i.e., an EC50 value of 10 μM) (Table 1, Fig. 2). The medications guanfacine, oxymetazoline, quinidine, xylometazoline, and fenoldopam had intermediate bla pEC50 values ranging from 6.91 ± 0.06 to 6.00 ± 0.04 (i.e., EC50 values between 123 and 1000 nM) (Table 1, Fig. 2). Relative efficacy measurements in bla assays did not distinguish ropinirole (Emax value of 89 ± 6%) from the VHD-associated compounds dihydroergotamine and methylergonovine (Emax values of 92 ± 1 and 56 ± 2%, respectively) (Table 1).
Fig. 2.
Agonist concentration-dependent stimulation of a 5-HT2B-mediated NFAT-β-lactamase reporter. The FRET ratio observed for each concentration of agonist is expressed as a percentage of the 5-HT Emax. Each panel shows isotherms for two of the drugs listed in Table 1 and for 5-HT. Similar experiments were performed for all drugs listed in Table 1, and the data were analyzed as described under Materials and Methods to obtain potency (pEC50) and efficacy (Emax) estimates.
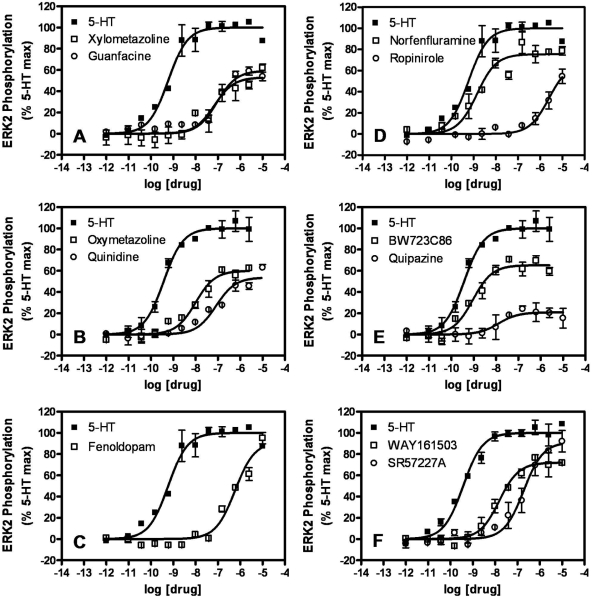
A similar distinction between the VHD-associated drugs and ropinirole was apparent in the phospho-ERK2 assays. The valvulopathogens all stimulated ERK2 phosphorylation with pEC50 values greater than 7.5 (i.e., EC50 values less than 30 nM) (Table 1; representative isotherms appear in Fig. 3), whereas ropinirole induced ERK2 phosphorylation with a pEC50 value of 5.60 ± 0.05 (Table 1, Fig. 3). The FDA-approved drugs guanfacine, oxymetazoline, quinidine, xylometazoline, and fenoldopam had pEC50 values for ERK2 phosphorylation ranging from 8.00 ± 0.04 to 6.23 ± 0.03 (i.e., EC50 values between 10 and 589 nM) (Table 1, Fig. 3). The relative efficacy of ropinirole at stimulating ERK2 phosphorylation (Emax value of 67 ± 3%) was not markedly different from the values for the VHD-associated drugs dihydroergotamine and cabergoline (Emax values of 70.6 ± 0.8 and 60.1 ± 0.9%, respectively) (Table 1).
Fig. 3.
Agonist concentration-dependent stimulation of ERK2 activation (phosphorylation) in U2OS 5-HT2B ERK2-GFP cells. Time-resolved FRET between ERK2-GFP and a terbium-labeled anti-phospho-ERK2 antibody is measured after 1) a 5-min agonist challenge and 2) a 2-h lysis/antibody incubation step. The time-resolved FRET observed for each concentration of agonist is expressed as a percentage of the 5-HT Emax. Each panel shows isotherms for two of the drugs listed in Table 1 and for 5-HT. Similar experiments were performed for all drugs listed in Table 1, and the data were analyzed as described under Materials and Methods to obtain potency (pEC50) and efficacy (Emax) estimates.
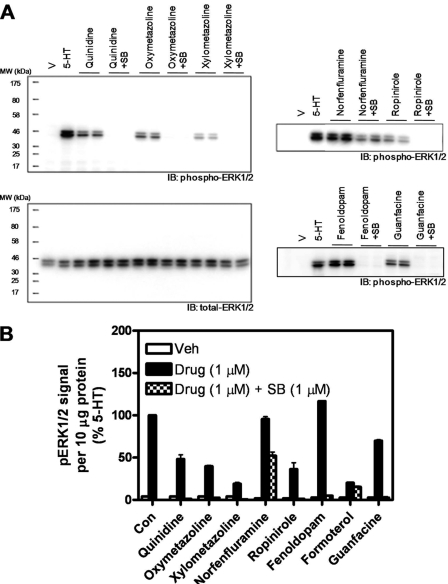
To validate the results of the ERK2 phosphorylation assays, we performed conventional immunoblot analyses. We focused our attention on the six currently prescribed medications (guanfacine, oxymetazoline, quinidine, xylometazoline, fenoldopam, and ropinirole) and included 5-HT and norfenfluramine as positive controls. At 1 μM after a 5-min challenge, all drugs stimulated significant ERK1/2 phosphorylation in serum-deprived HEK293 FlpIn cells stably expressing recombinant human 5-HT2B receptors, as assessed by immunoblot analysis using an anti-phospho-ERK1/2 antibody (Fig. 4). It is noteworthy that drug-induced ERK1/2 activation was blocked by the 5-HT2B/2C receptor-selective antagonist SB 206553 (Fig. 4) and was absent in parental HEK293 FlpIn cells (data not shown).
Fig. 4.
Immunoblot analysis of agonist-mediated ERK1/2 phosphorylation in FlpIn HEK293 5-HT2B receptor-expressing cells. A, representative immunoblots probed with anti-phospho-ERK1/2 or anti-total-ERK1/2 antibodies as indicated. B, image densitometry of immunoblot scans in A to quantify phospho-ERK1/2 content in cell lysates. Immunoreactivity for phospho-ERK1/2 (i.e., mean pixel intensity per region of interest that contained the p42 and p44 bands, minus the background) was measured for each sample. Blots were then stripped and reprobed for total-ERK1/2 and analyzed as before. The phospho-ERK1/2 immunoreactivity for each sample was normalized to its total-ERK1/2 immunoreactivity. The normalized phospho-ERK1/2 immunoractivity elicited by 5-HT (measured on each gel) was set to 100%. SB, SB-206,553 pretreatment.
In β-arrestin recruitment assays, the known valvulopathogens were again more potent [pEC50 values ranged from 8.96 ± 0.02 to 6.89 ± 0.05 (i.e., EC50 values between 1.1 and 129 nM)] than ropinirole [pEC50 value <5 (i.e., an EC50 value >10 μM)] (Table 1, Fig. 5). The currently prescribed drugs guanfacine, oxymetazoline, quinidine, xylometazoline, and fenoldopam stimulated β-arrestin translocation with pEC50 values ranging from 5.51 ± 0.04 to 5.18 ± 0.07 (i.e., EC50 values between 3100 and 6600 nM) (Table 1, Fig. 5). In terms of relative efficacy, the Emax value of ropinirole in β-arrestin recruitment assays was difficult to estimate because of its low affinity; however, ropinirole was at least as efficacious as the valvuopathogen norfenfluramine (Emax value of 52 ± 1%) (Table 1, Fig. 5). It is noteworthy that for the 23 drugs we characterized in all five functional assays, partial agonism (i.e., an Emax value less than 80%) was markedly rarer in β-arrestin translocation assays (4 of 23) than in the other assays (13 of 23 in calcium flux-based assays, 10 of 23 in bla assays, 18 of 23 in ERK2 phosphorylation assays, and 12 of 23 in InsP accumulation assays) (Table 1, Figs. 1, 2, 3, 5, and 6).
Fig. 5.
Agonist concentration-dependent stimulation of β-arrestin translocation. The FRET ratio observed for each concentration of agonist is expressed as a percentage of the 5-HT Emax. Each panel shows isotherms for two of the drugs listed in Table 1 and for 5-HT. Similar experiments were performed for all drugs listed in Table 1, and the data were analyzed as described under Materials and Methods to obtain potency (pEC50) and efficacy (Emax) estimates.
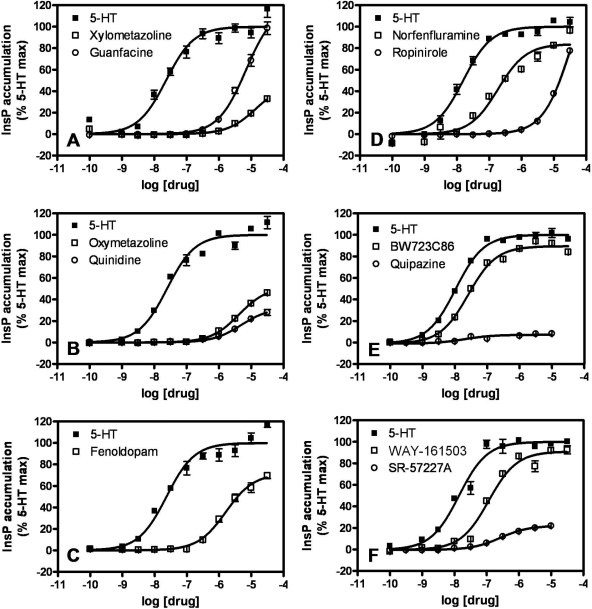
Fig. 6.
Agonist concentration-dependent stimulation of InsP accumulation in FlpIn HEK293 5-HT2B receptor-expressing cells. The InsP accumulation observed for each concentration of agonist is expressed as a percentage of the 5-HT Emax. Each panel shows isotherms for two drugs listed in Table 1 and for 5-HT. Similar experiments were performed for all drugs listed in Table 1, and the data were analyzed as described under Materials and Methods to obtain potency (pEC50) and efficacy (Emax) estimates. Note: InsP accumulation stimulated by all tested drugs (at 1 μM) was completely blocked by pretreatment with 10 μM SB-206593 (data not shown).
As was true in the previous assays, the bona fide VHD-associated drugs also exhibited greater potencies in InsP accumulation assays [pEC50 values ranged from 8.64 ± 0.03 to 6.73 ± 0.05 (i.e., EC50 values between 2.3 nM and 186 nM)] than did ropinirole [pEC50 value was 4.46 ± 0.02 (i.e., an EC50 value >10 μM)] (Table 1, Fig. 6). Ropinirole displayed an Emax of 163 ± 4% in InsP accumulation assays; as such, it is more efficacious by this measure than the valvulopathogen pergolide (Emax value of 95 ± 1%) (Table 1, Fig. 6).
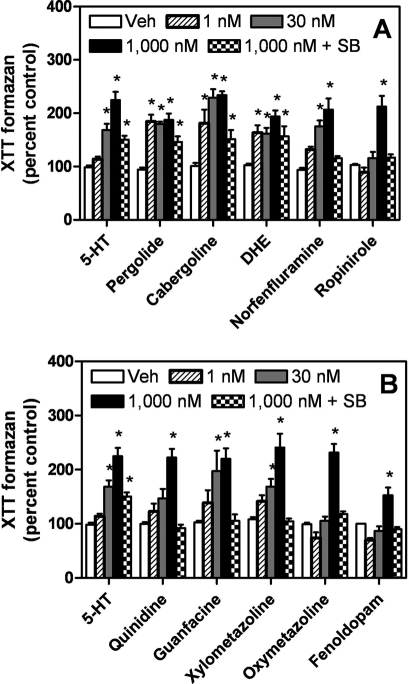
Valves resected from patients with VHD display proliferative interstitial foci, a hallmark feature of valvulopathy (Connolly et al., 1997; Steffee et al., 1999). The proliferative plaques precede, and probably contribute to, valve dysfunction (Roth, 2007). We showed that valvulopathogens elicit 5-HT2B receptor-dependent proliferative responses in primary cultures of human heart valve interstitial cells, consistent with the putative actions of VHD-associated drugs in vivo (Setola et al., 2003). Along these lines, we predicted that the six currently prescribed medications we had identified as 5-HT2B receptor agonists, as well as the known valvulopathogens, would stimulate the proliferation of HEK293 FlpIn cells stably expressing recombinant human 5-HT2B receptors. In XTT-based proliferation assays, all compounds seemed to stimulate proliferation at 1 μM, an effect that was blocked by the 5-HT2B/2C receptor selective antagonist SB 206553 (Fig. 7). In terms of apparent potency, 5-HT and the VHD-associated drugs tested (pergolide, cabergoline, dihydroergotamine, and norfenfluramine) were all active at 30 nM in the proliferation assays (Fig. 7A). Furthermore, the apparent proliferative activity at 30 nM distinguished ropinirole from the known valvulopathogens (Fig. 7A). Of the other FDA-approved medications tested, only guanfacine and xylometazoline were active at 30 nM, thus resembling the valvulopathogens (Fig. 7B). It is noteworthy that none of the drugs seemed to stimulate proliferation in HEK293 FlpIn cells not expressing recombinant human 5-HT2B receptors (Supplemental Fig. 2), ruling out a nonspecific effect (e.g., on metabolism of the XTT colorimetric substrate).
Fig. 7.
Agonist-mediated proliferation responses in FlpIn HEK293 5-HT2B receptor-expressing cells. XTT-based proliferation assay of agonist-treated FlpIn HEK293 5-HT2B cells. Cells were stimulated for 48 h with the indicated drug at 1 nM, 30 nM, or 1 μM. Four hours before the end of the drug treatment phase, XTT reagent was added. Then, the 490-nm absorbance (ODmax of the XTT formazan metabolite) was read. OD490 was proportional to cell number (data not shown). *, p < 0.05 compared with vehicle (two-way analysis of variance followed by Bonferroni post test). A, VHD-associated drugs were all potent at 30 nM; ropinirole was distinct from the valvulopathogens in that is was inactive at 30 nM. B, of the five FDA-approved medications we identified, only guanfacine and xylometazoline were active at 30 nM.
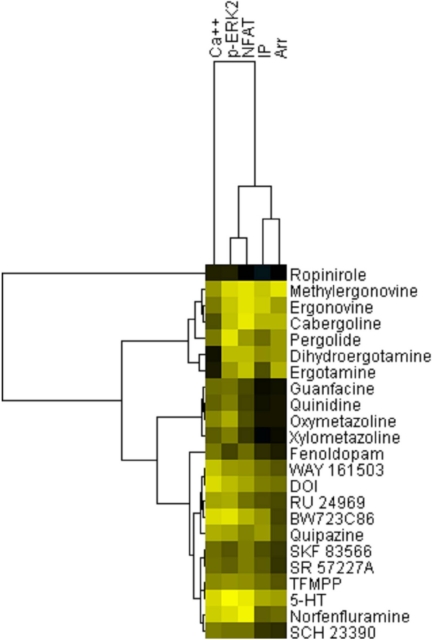
We finally conducted hierarchical clustering analysis to determine whether any patterns of functional activity at the various read-outs of 5-HT2B agonism distinguish bona fide from potential valvulopathogens. As shown in Fig. 8, ropinirole (a drug not associated with VHD) was clearly separated from known VHD-inducing drugs. In addition, ergoline and ergot-like drugs associated with VHD were all clustered on the same node. Finally, norfenfluramine was clustered on a node with 5-HT, indicating a similar pattern of functional selectivity for these two known valvulopathogens. These results indicate that ergot and ergoline medications induce a pattern of functional selectivity at 5-HT2B receptors that is distinct from that induced by indoleamines and other small molecules.
Fig. 8.
Hierarchical clustering analysis reveals a separation between ropinirole and valvulopathogens. Shown is an image generated by TreeView ver. 1.1.3 of pEC50 data derived from Table 1 (see Materials and Methods for details). The x-axis represents “Assays,” whereas the y-axis represents “Drugs tested.” Data are colored for activity: <5, black; >9, yellow.
Discussion
The major findings of this study are that known valvulopathic 5-HT2B agonists are distinguished by relatively high potencies across a variety of signaling measures and that potent 5-HT2B receptor agonism is a relatively rare occurrence among drugs and drug-like compounds. In addition, our results demonstrate that no single pattern of functional selectivity (Urban et al., 2007) distinguishes bona fide valvulopathic drugs from nonvalvulopathic drugs. On the other hand, a composite analysis of the signaling data indicates that ropinirole (a 5-HT2B agonist not associated with VHD) has a distinctly different pattern of functional selectivity compared with known VHD-inducing medications.
To arrive at these conclusions, we screened a composite library containing three publicly available collections of FDA-approved and investigational medications and one internally compiled library (approximately 2200 compounds in all). After removing nonspecific “agonists” (false positives) from the initial hit list, 27 bona fide 5-HT2B receptor agonists remained; thus, the validated hit rate was 1.2%. Among the hits identified in our blinded screen were 1) previously identified 5-HT2B receptor agonists used in preclinical biomedical research (e.g., BW 723C86, DOI, WAY 161503) and 2) all seven VHD-associated medications/metabolites in the composite library (pergolide, norfenfluramine, ergotamine, dihydroergotamine, cabergoline, ergonovine, and methylergonovine), all of which are reported 5-HT2B receptor agonists (Fitzgerald et al., 2000; Rothman et al., 2000; Setola et al., 2003). These findings validate our screening strategy.
Another major finding is the identification of six currently prescribed medications (guanfacine, oxymetazoline, quinidine, xylometazoline, fenoldopam, and ropinirole) as 5-HT2B receptor agonists. It is noteworthy that, in 2003, we discovered that MDMA, its metabolite 3,4-dimethoxyamphetamine, and pergolide were potent 5-HT2B receptor agonists, and that these drugs stimulated heart valve cell proliferation in vitro (Setola et al., 2003). Thus, we predicted that MDMA and pergolide use might be associated with VHD—predictions that were validated in 2007 (Droogmans et al., 2007; Roth, 2007; Schade et al., 2007; Zanettini et al., 2007). Therefore, there is precedent for predicting VHD liability based solely on agonist activity at recombinant 5-HT2B receptors. In this regard, it is noteworthy that ropinirole, which is approved for treating Parkinson's disease and restless legs syndrome, seems not to induce VHD. If one assumes that ropinirole is “safe” with respect to valvulopathy, then what additional factor(s) distinguish VHD-associated 5-HT2B receptor agonists from 5-HT2B receptor agonists?
The present results suggest that ropinirole is distinct from the seven known valvulopathic 5-HT2B receptor agonists we studied in that it is much less potent, albeit not less efficacious, than the VHD-associated drugs in all but one of the 5-HT2B receptor functional assays employed. In bla assays, ropinirole was 526-fold less potent than the least potent VHD-associated drug (pergolide); in ERK2 phosphorylation assays, ropinirole was 631-fold less potent than the least potent valvulopathogen (cabergoline); in β-arrestin recruitment assays, ropinirole was 77-fold less potent than the least potent VHD-inducing compound (norfenfluramine); finally, in InsP accumulation assays, ropinirole was 301-fold less potent than the least potent valvulopathic compound (pergolide) (Table 1). In cell proliferation assays, all VHD-associated drugs elicited a robust response (in terms of the maximum measured 5-HT response) at 30 nM; ropinirole was active only at 1 μM but not at 30 nM (Fig. 7A). In none of the assays was there a clear distinction between ropinirole's relative efficacy and the relative efficacies of the VHD-associated drugs. It is not surprising that hierarchical clustering analysis revealed a clear separation of ropinirole from known VHD-inducing medications.
Safety and efficacy studies are under way examining the anorexigen lorcaserin, a 5-HT2C/2B receptor full agonist with a reported 100-fold selectivity for 5-HT2C receptors over 5-HT2B receptors in vitro (Thomsen et al., 2008). In terms of VHD risk, the results seem promising: after 12 weeks, daily lorcaserin use did not seem to have fenfluramine-like valvulopathic liability (Smith et al., 2009). One possible explanation for the apparent safety of lorcaserin is its biodistribution; in rodents, levels of lorcaserin in the brain exceed plasma levels by a factor of 13 (Thomsen et al., 2008). Assuming similar pharmacokinetics and biodistribution in humans, therapeutic doses of lorcaserin may not generate sufficiently high levels of plasma lorcaserin to activate heart valve interstitial cell 5-HT2B receptors or other 5-HT2B receptors relevant to VHD.
In light of our present results, and our previous work linking 5-HT2B receptor agonists to VHD, we believe it would be prudent for guanfacine, oxymetazoline, quinidine, xylometazoline, and fenoldopam to be studied further in terms of their pharmacodynamics to determine whether they are safe with respect to VHD. Of particular concern are guanfacine (an antihypertensive agent) and quinidine (an antiarrhythmic agent), each of which is administered over sustained periods. Furthermore, given the recent FDA approval of guanfacine for the treatment of ADHD, increasing numbers of patients (and children) might be exposed to a potentially valvulopathic agent. Because duration of therapy with VHD-associated drugs is an important determinant of valvulopathic risk (Connolly et al., 1997; Roth, 2007; Schade et al., 2007), the short-term use of xylometazoline and oxymetazoline (nasal decongestants) and the one-time use of fenoldopam (antihypertensive agent used postoperatively and in-hospital during hypertensive crisis) may not be as risky.
In conclusion, we report that 5-HT2B receptor agonism is rare among drugs and drug-like compounds. In addition, we provide evidence that 5-HT2B receptor agonist potency in several functional assays might afford a means for separating compounds likely to induce VHD in humans from those that are not. Based on our data, we suggest that calcium flux-based screening is well suited to the initial identification of 5-HT2B receptor agonists but not to the discrimination of ones that might induce VHD from ones that are unlikely to do so. Hierarchical clustering analysis revealed a clear separation between ropinirole (which is not known to induce VHD) and other known valvuopathogens when a multiplicity of functional readouts was considered. Finally, our results suggest that parallel studies of the in vitro pharmacology and the pharmacokinetics of guanfacine and quinidine and their metabolites are warranted.
Supplementary Material
Acknowledgments
We are grateful to Dr. Wesley K. Kroeze for critical reading of the manuscript.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported in part by the National Institutes of Health National Institute of Mental Health [Grants R01-MH61887, U19-MH82441, HHSN-271-2008-00025]; National Institutes of Health National Institute of Child Health & Human Development[Grant T32-HD040127]; the University of North Carolina Neurodevelopmental Disorders Research Center; and the University of North Carolina Medical Scientist Training Program.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- VHD
- valvular heart disease
- MDMA
- 3,4-methylenedioxymethaphetamine
- 5-HT
- 5-hydroxytryptamine (serotonin)
- ERK
- extracellular signal-regulated kinase
- FRET
- fluorescence resonance energy transfer
- SB 206553
- 3,5-diydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b′]dipyrrole-1(2H)-carboxamide hydrochloride
- NFAT
- nuclear factor activated in T cells
- bla
- β-lactamase
- BW 723C86
- α-methyl-5-(2-thienylmethoxy)-1H-indole-3-ethanamine hydrochloride
- YSi
- yttrium silicate
- InsP
- inositol phosphates
- XTT
- tetrazolium hydroxide
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- CHO
- Chinese hamster ovary
- GFP
- green fluorescent protein
- DMSO
- dimethyl sulfoxide
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate
- HEK
- human embryonic kidney
- FLIPR
- fluorometric imaging plate reader
- RU 24969
- 5-methoxy 3-(1,2,3,6-tetrahydro-4-pyridinyl)1H indole
- SCH 23390
- R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- DOI
- 4-iodo-2,5-dimethoxyphenylisopropylamine
- WAY 161503
- 8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazino(1,2-a)quinoxalin-5(6H)-one
- L 694247
- N-(4-((5-(3-(2-aminoethyl)-1H-indol-5-yl)-1,2,4-oxadiazol-3-yl)methyl)phenyl)-methanesulfonamide
- SR 57227A
- 4-amino-1-(6-chloro-2-pyridyl)piperidine hydrochloride
- SKF 83566
- 7-bromo-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- FDA
- Food and Drug Administration
- CP-132484-42
- 1-(2-aminoethyl)-3-methyl-8,9-dihydropyrano(3,2-e)indole
- CP-123479-11
- 1-(2-aminoethyl)-8,9-dihydropyrano(3,2-e)indole
- CP-118,952
- N,N-dimethyl-2-(3,7,8,9-tetrahydropyrano[3,2-e]indol-1-yl)ethanamine
- DM360
- 2,5-dimethoxy-4-bromoamphetamine.
References
- Berger M, Gray JA, Roth BL. (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon DM, Wing MR, Edwards EB, Sondek J, Harden TK. (2006) Quantification of isozyme-specific activation of phospholipase C-beta2 by Rac GTPases and phospholipase C-epsilon by Rho GTPases in an intact cell assay system. Methods Enzymol 406:489–499 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. (1997) Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337:581–588 [DOI] [PubMed] [Google Scholar]
- Droogmans S, Cosyns B, D'haenen H, Creeten E, Weytjens C, Franken PR, Scott B, Schoors D, Kemdem A, Close L, et al. (2007) Possible association between 3,4-methylenedioxymethamphetamine abuse and valvular heart disease. Am J Cardiol 100:1442–1445 [DOI] [PubMed] [Google Scholar]
- Elangbam CS, Job LE, Zadrozny LM, Barton JC, Yoon LW, Gates LD, Slocum N. (2008) 5-Hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2B receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp Toxicol Pathol 60:253–262 [DOI] [PubMed] [Google Scholar]
- Elangbam CS, Lightfoot RM, Yoon LW, Creech DR, Geske RS, Crumbley CW, Gates LD, Wall HG. (2005) 5-Hydroxytryptamine (5HT) receptors in the heart valves of cynomolgus monkeys and Sprague-Dawley rats. J Histochem Cytochem 53:671–677 [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, et al. (2000) Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 57:75–81 [PubMed] [Google Scholar]
- Horvath J, Fross RD, Kleiner-Fisman G, Lerch R, Stalder H, Liaudat S, Raskoff WJ, Flachsbart KD, Rakowski H, Pache JC, et al. (2004) Severe multivalvular heart disease: a new complication of the ergot derivative dopamine agonists. Mov Disord 19:656–662 [DOI] [PubMed] [Google Scholar]
- Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. (2008) N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsychopharmacology 33:2303–2312 [DOI] [PubMed] [Google Scholar]
- Pritchett AM, Morrison JF, Edwards WD, Schaff HV, Connolly HM, Espinosa RE. (2002) Valvular heart disease in patients taking pergolide. Mayo Clin Proc 77:1280–1286 [DOI] [PubMed] [Google Scholar]
- Regard JB, Sato IT, Coughlin SR. (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. (2007) Drugs and valvular heart disease. N Engl J Med 356:6–9 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. (2000) Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102:2836–2841 [DOI] [PubMed] [Google Scholar]
- Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. (2007) Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 356:29–38 [DOI] [PubMed] [Google Scholar]
- Setola V, Dukat M, Glennon RA, Roth BL. (2005) Molecular determinants for the interaction of the valvulopathic anorexigen norfenfluramine with the 5-HT2B receptor. Mol Pharmacol 68:20–33 [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. (2003) 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol 63:1223–1229 [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Kroeze WK, Garcia BG, Deutch AY, Hufeisen SJ, Leahy P, Brüning JC, Roth BL. (2006) p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci U S A 103:4717–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WRAPD356–004 Study Group (2009) Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 17:494–503 [DOI] [PubMed] [Google Scholar]
- Steffee CH, Singh HK, Chitwood WR. (1999) Histologic changes in three explanted native cardiac valves following use of fenfluramines. Cardiovasc Pathol 8:245–253 [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587 [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, et al. (2007) Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 320:1–13 [DOI] [PubMed] [Google Scholar]
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. (2007) Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med 356:39–46 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.