Abstract
The human high-affinity copper transporter (hCtr1) plays an important role in the regulation of intracellular copper homeostasis. hCtr1 is involved in the transport of platinum-based antitumor agents such as cisplatin (CDDP); however, the mechanisms that regulate hCtr1-mediated transport of these agents have not been well elucidated. We compared the mechanisms of hCtr1-mediated transport of copper and CDDP. We found that replacements of several methionine residues that are essential for hCtr1-mediated copper transport conferred a dominant-negative effect on the endogenous hCtr1’s function, resulting in reduced rates of Cu(I) and CDDP transport and increased resistance to the toxicities of copper and CDDP treatments. Kinetic constant analyses revealed that although these mutations reduced maximal transport rates (Vmax) for Cu(I) and CDDP, reduction of Km only for Cu(I) but not for CDDP was observed. Mutation in Gly167, which is located in the third transmembrane domain and is involved in helix packing of hCtr1, also conferred dominant-negative property of Cu(I) transport but not of CDDP transport. Deleting the N-terminal 45 amino acids that contain two methionine-rich motifs resulted in cytoplasmic localization of the hCtr1 and abolished the dominant-negative function of these mutants. Nonetheless, these mutations did not affect the capacities of hCtr1 oligomerization induced by copper or CDDP, suggesting a distinct structural requirement between metal transport and oligomerization. Finally, we also observed that expressing the dominant-negative hCtr1 mutants up-regulates endogenous hCtr1 mRNA expression, consistent with our previous report that intracellular copper homeostasis and homeostatic levels of hCtr1 mRNA are mutually regulated.
Copper is an essential trace element required for a wide variety of enzymatic activities involved in many important physiological processes. Excess accumulation of copper is toxic because it generates elevated levels of reactive oxygen species that damage proteins, lipids, and nucleic acids. To balance the need for copper with its toxicity, all living organisms from yeast to humans have developed an evolutionarily conserved system to regulate copper homeostasis (Safaei and Howell, 2005; Kuo et al., 2007; Kim et al., 2008). Although copper ion entry can also be carried out by divalent metal transporter 1, the majority of copper acquisition is accomplished by Ctr1, which transports Cu(I). Extracellular copper exists in the oxidized [Cu(II)] form and is reduced to Cu(I) by membrane-associated cupric reductases, similar to the FRE1 and FRE2 reductases found in yeast (Georgatsou et al., 1997; Hassett et al., 2000).
Humans have two Ctr genes, hCtr1 and hCtr2. The encoded hCtr1 is cytoplasmic membrane located and mainly for Cu(I) acquisition (Safaei and Howell, 2005; Kuo et al., 2007; Kim et al., 2008); hCtr2 is primarily localized in the membrane of intracellular vacuoles (Rees and Thiele, 2007) and endosomes/lysosomes (van den Berghe et al., 2007) and primarily for copper storage. The hCtr1 has 190 amino acids spanning across three transmembrane domains (TM1–TM3). The N terminus is extracellularly located, and the C terminus is located inside the cytoplasm. Biochemical and genetic studies suggested that Ctr1 is present as an oligomer in the plasma membrane, most likely in a trimeric configuration (Dancis et al., 1994; Lee et al., 2002; Puig et al., 2002). A recent two-dimensional cryoelectron crystallographic study of hCtr1 supports the trimeric configuration with a central opening for the passage of Cu(I) ions (De Feo et al., 2009).
Recent studies have demonstrated that both human hCtr1 and its yeast counterparts, yCtr1 and yCtr3, are capable of transporting platinum-based antitumor agents (Ishida et al., 2002; Lin et al., 2002; Song et al., 2004) such as cisplatin (CDDP). Down-regulation of hCtr1 expression resulting in reduced drug uptake has been suggested as an important mechanism of CDDP resistance (Ishida et al., 2002; Lin et al., 2002; Song et al., 2004) .
The observations that hCtr1 can transport both Cu(I) ions and CDDP are intriguing, because the conventional inorganic physiological properties of CDDP and copper are quite different and raise a important question as to whether mechanisms underlying the hCtr1-mediated transport of these two different metal ions are different. Recent biochemical studies in yeast have demonstrated distinct mechanisms for the Cu(I) ions and CDDP transport mediated by yCtr1 (Sinani et al., 2007). In another study, CDDP induces intermolecular cross-linking of hCtr1 monomers that was not observed after copper treatment (Guo et al., 2004). Nonetheless, pharmacological studies showed that a reciprocal cross-resistance between cells selected from platinum-based drugs and those from copper salts (Lin et al., 2002; Safaei et al., 2004), suggesting that similar mechanisms are involved in the hCtr1-mediated CDDP and Cu transport.
Previous studies have identified several conserved amino acid sequences in hCtr1, including three highly conserved methionine-rich motifs (7MxMxxM, 40MMMMxM, and 150Mxxx154M). The first two methionine-rich motifs are located at the extracellular N terminus, whereas the last methionine-rich motif is located at the end of TM2 (Puig et al., 2002). Another conserved sequence is the 167GxxxG-motif (GG4-motif), which is embedded in TM3 (Aller et al., 2004; De Feo et al., 2007; Maryon et al., 2007). Mutagenic studies showed that substitutions of amino acid residues within these domains eliminate the ability of hCtr1 in complementation of genetic defects in yeast ΔyCtr/ΔyCtr3 mutants (Puig et al., 2002; Aller et al., 2004).
This study was initiated to investigate the mechanisms underlying hCtr1-mediated transport of CDDP and copper ions with specific reference to the roles of these conserved sequence motifs. We made several important new discoveries: 1) mutations at several methionine residues exhibited dominant-negative function by suppressing the rates of Cu(I) and CDDP transport with alterations of kinetic constants (Km values), as well as up-regulating the expression of endogenous hCtr1; 2) mutation of Gly167 also confers dominant-negative effects to copper ions but not to CDDP-transport; and 3) copper ions and CDDP facilitate oligomerization of hCtr1, regardless of these mutations, implying that single amino acid mutation in hCtr1 is not sufficient to destabilize its oligomerization induction by Cu(I) or by CDDP. Our present study revealed a conserved yet diverse mechanism of hCtr1-mediated transport in copper ions and CDDP.
Materials and Methods
Reagents.
Reagents were purchased from the following commercial sources: CDDP [Pt(NH3)2Cl2], CuSO4, bathocuproine disulfonic acid (C26H18N2Na2O6S2), G418 (Geneticin sulfate) [C20H40N4O10 · 2(H2SO4)], antibodies against hemagglutinin (HA), and Na+/K+-ATPase were from Sigma (St. Louis, MO); anti-tubulin and anti-lamin B antibodies were from Calbiochem (Darmstadt, Germany); LipofectAMINE and TRIzol were from Invitrogen (Carlsbad, CA); Western blotting detection system was from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK); 64CuCl2 was from Washington University Medical School (St. Louis, MO); and Bis(sulfosuccinimidyl)suberate (BS3) (C16H18N2O14S2Na2) was from Pierce (Rockford, IL).
Plasmid DNA and Transfection.
Recombinant plasmids hCtr1-wt (formerly pcDNA3.1-HA-hCtr1), δN1-hCtr1 (formerly pcDNA3.1-HA-δN1-hCtr1), and δN2-hCtr1 (formerly pcDNA3.1-HA-δN2-hCtr1) were prepared as described previously (Song et al., 2004). Site-directed mutations of hCtr1 recombinants were constructed using the QuikChange site-directed mutagenesis kit according to manufacturer’s instructions (Stratagene, La Jolla, CA) using primer pairs with sequences as indicated (Table 1). All plasmids were confirmed by DNA sequencing. Recombinant DNA was transfected into small-cell lung cancer cells (SCLCs), and stably transfected cell variants were established according to the procedures described previously (Song et al., 2004).
TABLE 1.
Primer sequences for hCtr1 site-direction mutagenesis
| Name | Sequence (5′→3′) | Location | Mutant |
|---|---|---|---|
| 15N-QL | GAGCTATATGGACTCCCGAAGTACCATGCAAC | 153–185 | AAC-CGA |
| 15N-QR | GTTGCATGGTACTTCGGGAGTCCATATAGCTC | ||
| M43-QL | CAGCAGCATGATGATGCAACCTATGACCTTCTAC | 237–270 | ATG-CAA |
| M43-QR | GTAGAAGGTCATAGGTTGCATCATCATGCTGCTG | ||
| M45-QL | CAGCAGCATGATGATGATGCCTCAAACCTTCTAC | 237–270 | ATG-CAA |
| M45-QR | GTAGAAGGTTTGAGGCATCATCATCATGCTGCTG | ||
| N112-QL | GCCTGTCCCAGGACCACAAGGAACCATCC | 444–472 | AAT-CAA |
| N112-QR | GGATGGTTCCTTGTGGTCCTGGGACAGGC | ||
| M127-QL | CCAAATGGAACCATCCTTCAAGAGACACACAAAAC | 457–490 | ATG-CAA |
| M127-QR | GTTTTGTGTGTCTCTTGAAGGATGGTTCCATTTGG | ||
| M150-QL | CATAAGCTACTTCCTCCAACTCATCTTCATGACCTACAAC | 558–497 | ATG-CAA |
| M150-QR | GTTGTAGGTCATGAAGATGAGTTGGAGGAAGTAGCTTATG | ||
| M154-QL | CTTCCTCATGCTCATCTTCCAAACCTACAACGGGTAC | 567–603 | ATG-CAA |
| M154-QR | GTACCCGTTGTAGGTTTGGAAGATGAGCATGAGGAAG | ||
| 167G-SL | GCATTGCAGTAGCAGCAAGCGCCGGTACAGGATACTTC | 608–645 | GGG-AGC |
| 167G-SR | GAAGTATCCTGTACCGGCGCTTGCTGCTACTGCAATGC |
Cellular Fractionation and Protein Cross-Linking.
Fractionation of cellular components into cytoplasmic, plasma membrane, and nuclear fractions followed the procedure described previously (Song et al., 2004). For protein cross-linking, cells were treated with 30 μM CuSO4 or 30 μM CDDP for various lengths of time. Cells were harvested, washed three times with phosphate-buffered saline, and pelleted by centrifugation followed by the addition of 10× the volumes of the cross-linking solution (5 mM BS3 and 20 mM HEPES, pH 7.0). The cross-linking reaction was incubated at 22°C for 30 min and stopped by the addition of 1 M Tris-HCl, pH 7.5, at a final concentration of 20 to 50 mM. After incubating for an additional 15 min, the protein was extracted for Western blotting analyses using anti-HA antibody or anti-hCtr1 antibody (Song et al., 2008).
Measurements of Km and Vmax.
For copper transport analyses, 2 × 105 cells/well of SCLC cells were plated in six-well plates. After 12 h, fresh medium containing various concentrations of 64CuCl2 was added and further cultured for various time intervals. Cells were washed four times with phosphate-buffered saline and then lysed in 400 μl of lysis buffer. The radioactivity was measured. For CDDP transport measurement, 5 × 106 cells/dish were treated with various concentrations of CDDP. Cells were harvested and lysed in 10 μl of benzethonium hydroxide at 50°C overnight. The lysates were acidified with 200 μl of 0.3 N HCl, and platinum contents were determined by atomic spectroscopy. The Vmax and Km values were calculated according Michaelis-Menten equation 1/V = 1/Vmax + Km/Vmax · [S], where [S] is copper or CDDP concentration, and V is copper or platinum concentration inside the cells/time.
Other Procedures.
Procedures for measuring the uptakes of 64Cu and CDDP, IC50, and RNase protection assay using RNase RPA III Ribonuclease Protection Assay Kit (Applied Biosystems/Ambion, Austin, TX) have been described previously (Song et al., 2004, 2008; Chen et al., 2008).
Results
Structural-Functional Analyses of hCtr1-Mediated Transport of Copper and CDDP.
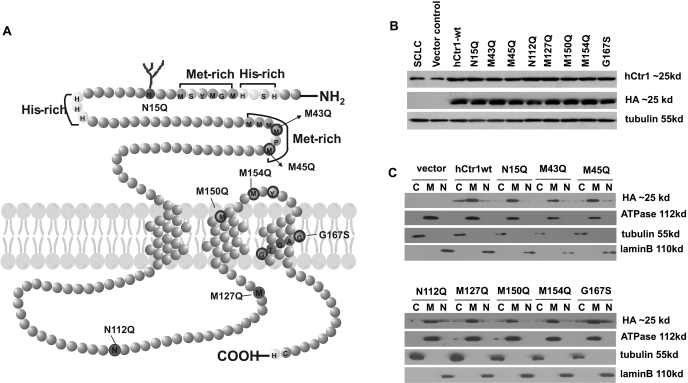
We established a series of stably transfected SCLC cell lines harboring site-specific hCtr1 mutants, including N15Q, M43Q, M45Q, N112Q, M127Q, M150Q, M154Q, and G167S (Fig. 1 A). These mutant recombinants were created in a eukaryotic expression vector and carried an HA-tag on their N termini. Multiple clones were picked from each transfection, and those with comparable hCtr1 expression levels were selected for the present study. Expression levels of hCtr1 from transfected hCtr1 recombinant DNA (hereafter referred to as exo-hCtr1) were determined by Western blotting using the anti-HA antibody, whereas total hCtr1 levels [i.e., endogenous hCtr1 (endo-hCtr1) and exo-hCtr1 combined] was determined using anti-hCtr1 antibody. Under strong detergent treatment, hCtr1 was presented as a monomeric form in Western blotting analysis (Chen et al., 2008; Song et al., 2008). Figure 1B shows that levels of exo-hCtr1 in these transfected cell lines were comparable (determined by anti-HA antibody). Because the current anti-hCtr1 antibody cannot differentiate between endo-hCtr1 and exo-hCtr1, the ratios between the two cannot be precisely determined; however, this can be determined at the hCtr1 mRNA levels using the RNase protection assay using specific probe that gives rise to distinguishable signals (below). Subcellular localization demonstrated that exo-hCtr1 proteins were mainly located in the cytoplasmic membrane fraction, as was the α-subunit of the Na+/K+-ATPase, a plasma membrane marker. Only minimal amounts of recombinant proteins were found in the cytoplasmic and nuclear fractions, as probed by antibodies against their respective marker proteins, tubulin and lamin B (Fig. 1C).
Fig. 1.
Expression and subcellular distributions of mutant hCtr1 in the stably transfected cell lines. A, diagram showing the topology of hCtr1. Each amino acid residue is represented by a circle. The hCtr1 has 190 amino acids, spanning into three transmembrane domains. The N terminus contains two histidine- and two methionine-rich motifs as indicated. Mutant hCtr1 with amino acid substitutions at the specific sites are indicated. B, Western blots showing levels of total hCtr1 monomers as probed by anti-hCtr1 antibody (Chen et al., 2008; Song et al., 2008) and exo-hCtr1 by anti-HA antibody and β-tubulin as a loading control. C, subcellular distribution of exo-hCtr1 in the transfected cells. Cells were fractionated into cytoplasmic (C), membrane (M), and nuclear (N) fractions and were probed by anti-β-tubulin, anti-ATPase, and anti-lamin B antibodies, respectively.
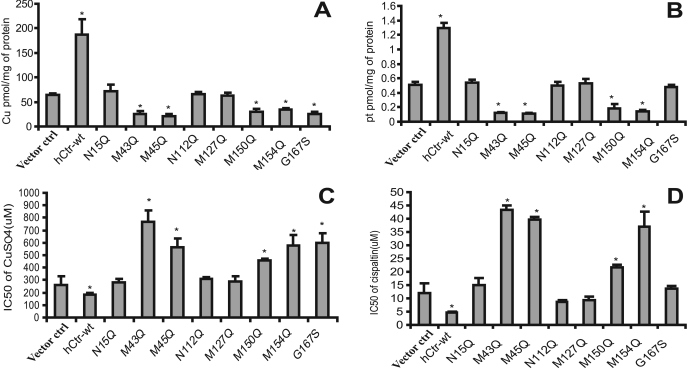
To investigate the effects of exo-hCtr1 expression on Cu(I) transport in the transfected cells, we carried out 64Cu transport analyses. The results can be categorized into three groups (Fig. 2 A). First, expression of hCtr1-wt was associated with an enhanced overall rate of Cu(I) uptake (approximately 3.5-fold increase). These results are consistent with those published previously showing that overexpressing hCtr1-wt stimulated copper uptake in mammalian cells (Puig et al., 2002; Song et al., 2004). Second, expression of N15Q, N112Q, and M127Q and empty vector had no effect on copper transport. Asn15 is an extracellular glycosylation site, and previous studies have demonstrated that mutations at this site had no effect on Cu(I) transport (Puig et al., 2002; Eisses and Kaplan, 2005). Our present results are consistent with these findings and show that additional mutations at N112Q and M127Q located in the intracellular loop linking TM1 and TM3 (Fig. 1A) have no effect on the transport function of hCtr1. Therefore, these mutations can be considered as dead mutants. Third, expression of mutants M43Q, M45Q, M150Q, M154Q, and G167S suppressed the Cu(I) transport function of hCtr1. It was demonstrated previously that mutated Met43 or Met45 into alanine (M43A and M45A) and that Met150 and Met154 into leucine (M150L and M154L) did not support the transport function of hCtr1 in human embryonic kidney 293 (Puig et al., 2002). Our results demonstrated that these mutations in fact exhibited a gain-of-function by suppressing the transport function of endo-hCtr1. Thus, these mutants can be considered as dominant-negative mutants.
Fig. 2.
Analyses of the effects of mutations of hCtr1 on the uptake and sensitivity to the cytotoxicities of copper and CDDP in the stably transfected cell lines. A, 64Cu uptake. B, CDDP uptake. Data represent the average ± S.D. of four independent measurements. ∗, P < 0.05 as determined by the Student’s t test. C and D, cellular sensitivity to CuSO4 and CDDP treatments, respectively. Cell viabilities to the treatments by these agents were analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium method, and the IC50 values were determined using the Prism software (GraphPad Software Inc., San Diego, CA). Arrow bars represent the mean ± S.D. of four independent measurements.
To investigate the effect of exo-hCtr1 expression on cellular sensitivity to copper toxicity, the IC50 (concentrations that inhibit 50% of cell proliferation) values of CuSO4 in these transfected cells were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium method. The result shows that the IC50 values were generally in a reverse correlation with the rates of 64Cu uptake among the mutants (i.e., cells transfected with hCtr1-wt exhibited elevated rates of 64Cu uptake but reduced IC50 values), whereas those with dominant-negative mutants (M43Q, M45Q, M150Q, M154Q, and G167S) had elevated IC50 values, indicating that these dominant-negative mutants display copper resistance (Fig. 2, A and C). Moreover, the dead mutants (N15Q, N112Q, and M127Q) did not alter the rate of 64Cu uptake, and no effect on sensitivity to copper toxicity was observed.
We then investigated the effects of exo-hCtr1 expression on CDDP uptake and toxicity in the transfected cells. The rates of CDDP uptake were correspondingly similar to those observed for copper, except for the G167S mutant (Fig. 2, A and C). CDDP uptake was increased in hCtr1-wt-transfected cells but was decreased in all but one of the dominant-negative mutants (i.e., G167S) (Fig. 2B). Moreover, a direct correlation was observed between increased uptake of CDDP and an enhanced sensitivity to its toxicity (Fig. 2, B and D) in these mutants. These results suggest that these methionine residues are important for hCtr1-mediated transport of both copper and CDDP, whereas Gly167 is only important for transport of Cu(I) but not of CDDP.
Effects of Exo-hCTR1 on the Expression of Endo-hCtr1.
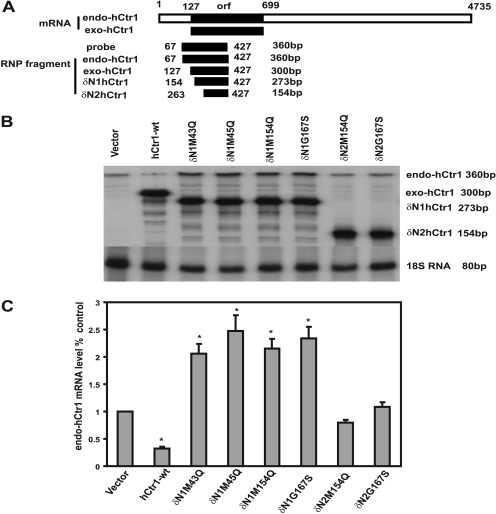
We recently demonstrated that maintenance of copper homeostasis is regulated at the hCtr1 mRNA levels. Levels of hCtr1 mRNA were increased in SCLC cells under copper-deplete conditions by treating cells with copper chelator bathocuproine disulfonic acid, whereas levels of hCtr1 mRNA were reduced under copper-replete conditions by treating cells with CuSO4 (Song et al., 2008). We further demonstrated that the steady-state levels of endo-hCtr1 mRNA were reduced in cells transfected with hCtr1-wt recombinants (Song et al., 2008). The establishment of dominant-negative mutant hCtr1-transfected cell lines provided an opportunity to further test the regulatory mechanism of hCtr1 mRNA in the context of copper homeostasis. Using a probe designed for simultaneously detecting both exo- and endo-hCtr1 mRNA in an RNase protection assay (Fig. 3 A), as consistent with the previous results (Song et al., 2008), we observed that overexpression of exo-hCtr1-wt down-regulates endo-hCtr1 mRNA expression (Fig. 3, B and C). Strikingly, expression of dominant-negative mutants (M43Q, M45Q, M150Q, M154Q, and G167S) resulted in increased endo-hCtr1 mRNA levels, whereas expression of the dead mutants (N15Q, N112Q, and M127Q) had no effect on the expression of endo-hCtr1 mRNA (Fig. 3, B and C). These results further support that the maintenance of copper homeostasis lies at the levels of hCtr1 mRNA (Song et al., 2008). Densitometric analyses revealed that in all of the transfected lines, levels of exo-hCtr1 mRNA ranged from 3- to 4-fold higher than that of endo-hCtr1 mRNA in the vector-transfected cells. Approximately 4-fold reduction and 3-fold increase in endo-hCtr1 mRNA were found in the hCtr1-wt and dominant-negative hCtr1-transfected cell lines, respectively (Fig. 3C).
Fig. 3.
Effects of stably expressed exogenous hCtr1 mRNA on endogenous hCtr1 mRNA expression as determined by the RNase protection assay. A, schematic diagram showing the design of hybridization probe that allows simultaneous detection of endo-hCtr1 mRNA (360 nucleotides) and transcripts from different transfected hCtr1 mutants (exo-hCtr1 mRNA) with the indicated protection fragment sizes. B, autoradiographs of the RNase protection assay. Note that levels of endo-hCtr1 RNA are down-regulated in the hCtr1-wt-transfected cells, up-regulated in dominant-negative mutant hCtr1-transfected cells and are unchanged in dead mutant hCtr1-transfected lines. 18S RNA was used as an internal loading control. C, densitometry of hybridization signals shown in B. Arrow bars represent average± S.D. of three independent experiments.
Effects of N-Terminal Extracellular Domain on the Dominant-Negative Function of Mutations.
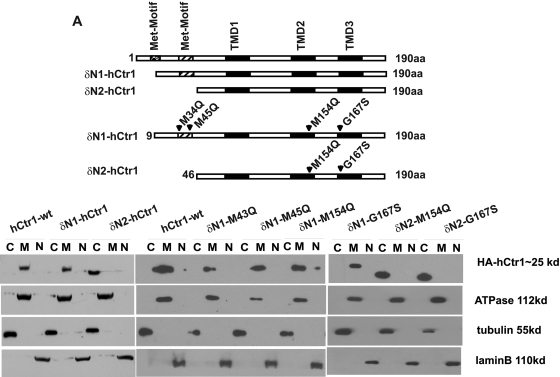
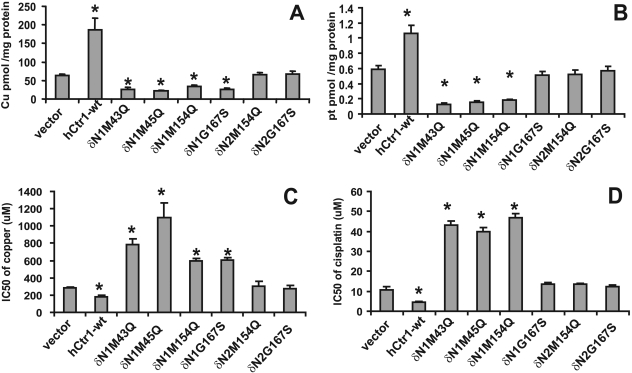
We hypothesized that to exert the dominant-negative function of a hCtr1 mutant, the mutant protein has to be targeted to the cytoplasmic membrane to interact with its endo-hCtr1 partner, and deleting the membrane-targeting signal peptide sequence in hCtr1 would retain the mutant hCtr1 in the cytosol and abolish its dominant-negative function. We further hypothesized that the membrane-targeting signal sequence of hCtr1 lies in the N-terminal, extracellular domain. This domain contains two methionine-rich motifs M7GMYSM12 and M40MMMPM45. To test these hypotheses, we generated two series of deletion mutants [i.e., the δN1 series (δN1-M43Q, δN1-M45Q, δN1-M154Q, and δN1-G167S) and the δN2 series (δN2-M154Q and δN2-G167S) (Fig. 4 A)]. Mutants in the δN1 series contain deletion of portion of the first methionine-rich motif (missing the first 8 amino acids), whereas in the δN2 series, the entire first and second methionine-rich motifs (missing the first 45 amino acids). These hCtr1 mutants were expressed in SCLC cells, and stable cell lines were established. Subcellular localization analyses revealed that mutant hCtr1 proteins in all of the δN1-hCtr1 mutants-transfected cells, just like their wild-type version (δN1-hCtr1-wt), were membrane-bound, whereas those in the δN2-hCtr2 mutant-transfected cells, also like their δN2-hCtr1-wt version, were localized in the cytosol (Fig. 4B). These results suggest that amino acids between 9 to 45 in the N-terminal extracellular domain of hCtr1 contain membrane-targeting signal. Figure 5, A and B, shows that the δN1-hCtr1 mutants (δN1-M43Q, δN1-M45Q, and δN1-M154Q) retained the dominant-negative functions of suppressing Cu(I) and CDDP uptakes and conferring resistance to the toxicities of copper (Fig. 5C) and CDDP (Fig. 5D), whereas δN1-G167S mutant only retained the dominant-negative function of Cu(I) uptake but not CDDP uptake. These results indicate that the first eight amino acids of hCtr1 are functionally dispensable in terms of membrane-targeting specificity and transport activity. Previous results also showed that replacing all the methionine residues in the first methionine motif into alanine (7AGASYA12) did not alter hCtr1-mediated Cu(I) transport function (Puig et al., 2002). In contrast, the δN2-hCtr1 mutants (δN2-M154Q and δN2-G167S), in which the first 45 amino acids were removed, abolished the dominant-negative functions on Cu(I) and CDDP uptakes (Fig. 5, A and B) and behaved like dead mutants. We also reported previously that δN2-hCtr1-wt possessed no copper transport function (Song et al., 2004). Figure 5, C and D, shows that mutants with altered uptakes of Cu(I) and CDDP, also showed altered sensitivity to copper and CDDP toxicities, whereas those with no alterations in Cu(I) and CDDP uptake also showed no changes in sensitivity to copper and CDDP toxicity.
Fig. 4.
Effects of N-terminal deletions on the subcellular distributions of transfected mutants hCtr1. A, schematic diagram showing the wild-type and various N-terminal deletion mutants of hCtr1. B, subcellular distributions of exo-hCtr1 in the transfected cells.
Fig. 5.
Effects of N-terminal deletion mutants of hCtr1 on the uptake and cytotoxicity of copper and CDDP in the transfected cells. A, 64Cu uptake. B, CDDP uptake. C, sensitivity to copper assay. D, sensitivity to CDDP assay. Details are the same as described in the legend to Fig. 2.
The dominant-negative function in the δN1-hCtr1 mutants (δN1-M43Q, δN1-M45Q, δN1-M154Q, and δN1-G167S) was also evidenced by their ability to induce up-regulation of endo-hCtr1. In contrast, the abolishment of the dominant-negative function in the δN2-hCtr1 mutants (δN2-M154Q and δN2-G167S) was associated with their failure to up-regulate endo-hCtr1 expression (Fig. 6). These results, collectively, support our hypothesis that membrane-targeting of hCtr1 is required for the dominant-negative function of these hCtr1 mutants.
Fig. 6.
Expression levels of endogenous hCtr1 mRNA in the N-terminal deletion mutant hCtr1-transfected cells. A, schematic diagram showing the sizes of endo-hCtr1 mRNA, exo-hCtr1 mRNA, the probe used, and the anticipated protected fragment by the RNase protection assay. B, autoradiograph of RNase protection assays. C, densitometric analyses of the autoradiograph shown in B. Details are the same as described in the legend to Fig. 3.
Effects of Mutations on the Vmax and Km Values for hCtr1-Mediated Uptake of Copper and CDDP.
To more precisely determine whether mutations in hCtr1 change the kinetics of hCtr1-mediated Cu(I) and CDDP transport, we measured the kinetic constants (i.e., Km and Vmax values). The Km values for 64Cu uptake in the untransfected and vector-transfected SCLC cells were 4.86 ± 0.5 and 5.12 ± 0.4 μM, respectively (Table 2). These values are in agreement with those reported for Ctr1-mediated Cu(I) transporters in a variety of organisms (between 1 and 5 μM) (Lee et al., 2002; Puig et al., 2002; Eisses and Kaplan, 2005). Transfection with hCtr1-wt did not affect the Km values but greatly increased Vmax (>5- fold) for Cu(I) transport. In contrast, transfection with dominant-negative hCtr1 mutant (M43Q, M45Q, M154Q, and G167S) significantly reduced both Km (∼30% to the values from untransfected or vector-transfected cells) and Vmax (55–67%) values. Deleting the membrane-targeting motifs from these dominant-negative mutants (δN2-M154Q and δN2 G167S) returned the Km and Vmax values to those comparable with the untransfected or empty vector-transfected controls.
TABLE 2.
Kinetic constants for copper and CDDP uptakes by SCLC and various mutant hCtr1-transfected cells
Data from copper and CDDP uptake experiments at various concentrations were analyzed using the Michaelis-Menten equation, 1/V = 1/Vmax+ Km/Vmax · [S] (where [S] is the copper or cisplatin concentration). Km values and Vmax were derived from three experiments (experiments for each protein, each experiment consisting of triplicate determinations). S.E. for the Km and Vmax values are shown.
| Copper |
Cisplatin |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Km | KmMutant/Vector | P | Vmax | Vmax Mutant/Vector | P | Km | KmMutant/Vector | P | Vmax | Vmax Mutant/Vector | P | |
| μM | pmol/mg protein/min | μM | pmol/mg protein/min | |||||||||
| SCLC | 4.86 ± 0.5 | 0.95 | 16.2 ± 1.4 | 1.05 | 13.1 ± 1.9 | 1.10 | 22.8 ± 1.9 | 1.02 | ||||
| vector | 5.12 ± 0.4 | 15.4 ± 1.4 | 11.9 ± 1.5 | 22.4 ± 1.9 | ||||||||
| WT | 5.42 ± 0.4 | 1.06 | 87.2 ± 9.5 | 5.66 | <0.01 | 17.2 ± 2.6 | 1.45 | 117 ± 10 | 5.22 | <0.01 | ||
| M43-Q | 3.54 ± 0.3 | 0.69 | <0.01 | 5.24 ± 0.4 | 0.34 | <0.01 | 11.1 ± 1.0 | 0.93 | 8.43 ± 1.3 | 0.38 | <0.05 | |
| M45-Q | 3.99 ± 0.3 | 0.78 | <0.05 | 6.99 ± 0.5 | 0.45 | <0.01 | 15.9 ± 1.0 | 1.34 | 8.47 ± 1.0 | 0.38 | <0.05 | |
| M154-Q | 3.55 ± 0.3 | 0.69 | <0.01 | 5.02 ± 0.4 | 0.33 | <0.01 | 12.2 ± 1.3 | 1.03 | 11.1 ± 0.5 | 0.50 | <0.05 | |
| G167-S | 3.78 ± 0.2 | 0.74 | <0.05 | 6.26 ± 0.5 | 0.41 | <0.01 | 6.82 ± 0.7 | 0.57 | <0.05 | 16.9 ± 0.7 | 0.75 | |
| δN2-M154-Q | 5.25 ± 0.4 | 1.03 | 15.5 ± 1.2 | 1.01 | 11.5 ± 1.1 | 0.97 | 21.8 ± 1.6 | 0.97 | ||||
| δN2-G167-S | 4.99 ± 0.4 | 0.97 | 14.9 ± 1.1 | 0.97 | 13.7 ± 1.2 | 1.15 | 22.8 ± 0.9 | 1.02 | ||||
Effects of mutations on the kinetic parameters of hCtr1-mediated CDDP transport were also investigated (Table 2). The Km values for the untransfected and empty vector-transfected SCLC cells were 13.1 ± 1.9 and 11.9 ± 1.5 μM, respectively (Table 2), approximately 2.5-fold higher than that for Cu(I) transport; yet, approximately 5-fold increase in Vmax value in hCtr1-wt transfected cells compared with those in the untransfected control was observed. This value is similar to that for 64Cu transport in the same comparison. Strikingly, in the dominant-negative mutants, significant reduction of Vmax with no significant effect on Km values was observed; again, deleting the N-terminal 45 amino acids (δN2-M154Q) abolished the effects on Vmax. Moreover, reduction of Km but not of Vmax was found in G167S, which exhibited a dominant-negative function for Cu(I) transport but not for CDDP transport. These results clearly show the variations in the kinetic parameters for hCtr1-mediated transport between Cu(I) and CDDP. Moreover, the significance of Gly167 in the hCtr1-mediated transport between these two substrates is also different.
Effects of Mutations on Induction of hCtr1 Oligomerization by Copper and CDDP.
As alluded to above, many studies have demonstrated that hCtr1 prepared from cultured cells is exhibited in multiple configurations including monomer, dimer, and trimer as viewed by SDS polyacrylamide gel electrophoresis. It is unclear how the fate of these multiple species of hCtr1 in the plasma membrane when cells are exposed to copper and CDDP. Here, we used BS3 to probe hCtr1 oligomers. BS3 is a water-soluble, membrane-impermeable, homobifunctional primary amine cross-linker. This allows identification of weak or transient protein interactions in cellular membrane before cell lysis. SCLC cells treated with increasing concentrations of CuSO4 were harvested after BS3 treatment before cells were lysed. Total cellular proteins were prepared and separated on SDS-polyacrylamide gel electrophoresis. Exo-hCtr1 proteins were detected by Western blotting analysis using the anti-HA antibody. Figure 7, A and B, shows that, under the conditions used, a time- and concentration-dependent formation of dimers/trimers in hCtr1-wt was observed, reaching to a plateau between 2 and 3 h after copper treatment. The intracellular copper concentration in SCLC cells grown in the regular medium is 7.14 ± 0.08 μM as measured by inductively coupled plasma mass spectrometry (Song et al., 2008). We observed formation of hCtr1 oligomers in CuSO4-treated hCtr1-wt-transfected cells at concentration as low as 10 μM copper (Fig. 7B). Similar time- and concentration-dependent formation of oligomers in hCtr1-wt-transfected cells treated with CDDP was observed (data not shown). These results suggest that the copper and CDDP treatments induce stabilization of hCtr1 oligomers.
Fig. 7.
Induction of oligomerization of hCtr1 in the transfected cells by Cu and CDDP. A, time-dependent induction. SCLC cells were treated with 30 μM CuSO4 for different lengths of time as indicated. Cells were harvested and treated with cross-linking agent BS3. hCtr1 oligomerization was detected by SDS-polyacrylamide gel electrophoresis followed by Western blot. B, concentration-dependent induction of oligomerization. SCLC cells were treated with various concentrations of CuSO4 for 2 h. Oligomerization of hCtr1 was analyzed. C, time-dependent induction of hCtr1 oligomerization by CDDP in various transfected cells. D, time-dependent induction of hCtr1 oligomerization by copper in different transfected cells. E, induction of hCtr1 oligomerization by copper (top) and by CDDP (bottom) in various mutant hCtr1-transfected cells. Monomeric to trimeric species are denoted by one to three black ovals, respectively.
The time-dependent formations of multimers were also observed in mutant hCtr1-transfected SCLC cells treated with 30 μM CuSO4 (Fig. 7C, right) or 30 μM CDDP (Fig. 7C, left). Strikingly, stabilization of hCtr1 oligomers in response to copper and CDDP treatments was found in all of the three classes of mutant hCtr1, including hCtr1-wt, dominant-negative mutants (M43Q and M45Q), and dead mutants (N15Q and N112Q) (Fig. 7C). However, deleting the first 45 amino acid residues (δN2-M154Q and δN2-G167S) abolished the ability of copper- (Fig. 7D, top) and CDDP-induced stabilization of hCtr1 oligomers (Fig. 7D, bottom). It seems that rates of Cu(I)-induced hCtr1 stabilization were slower in the dominant-negative mutants (M43Q, M45Q, δN1-M43Q, δN1-M45Q, and δN1M154Q) compared with those in the hCtr1-wt and dead mutants (N15Q, N112Q, and δN1-G167S) (Fig. 7C, left), as judged by the rates of disappearance of monomers in the Western blots. However, the effects of mutation on the kinetics of CDDP-induced hCtr1 oligomerization were not as clear (Fig. 7C, right). These results demonstrate that mutations in single amino acid residues are not sufficient to affect the oligomerization of hCtr1 induced by the treatments of copper and CDDP, although the transport function of hCtr1 is compromised by these mutations.
Discussion
The discovery that hCtr1 functions as a transporter for CDDP and other platinum-based antitumor agents underscores the importance of hCtr1 in cancer chemotherapy. Whereas mechanisms by which hCtr1-mediated Cu(I) transport have been investigated to a great extent, the underlying mechanisms as to how hCtr1 transports CDDP remain largely unknown. As an initial step to elucidate the underlying mechanism by which hCtr1 transports CDDP, we investigated the roles of amino acids known to be important for hCtr1-mediated copper transport for CDDP transport. Several new findings are presented in this communication. First, there is a consistency among the amino acid residues involved in the transport of these two substrates: all of the methionine residues that are important for Cu(I) transport (Met43, Met45, Met150, and Met154) are also important for CDDP transport. However, Gly167, which is important for Cu(I) transport, is not essential for CDDP transport. Second, mutations of these important methionine residues resulted in dominant-negative gain-of-function by suppressing the transport of both Cu(I) and CDDP, but the effects of Km values are not the same. This dominant-negative function was not reported in previous publications expressing exo-hCtr1 in heterologous cellular background (Puig et al., 2002; Eisses and Kaplan, 2005). Third, treating SCLC cells with CuSO4 or CDDP induces the stabilization of the hCtr1 multimers. Guo et al. (2004) reported that treating cultured cells with high concentrations of CDDP, but not copper, resulted in a cross-linking of hCtr1 monomers and the formation of hCtr1 oligomers. These results, collectively, suggest that the gross mechanisms of hCtr1-mediated Cu(I) and CDDP transport are similar, but the importance of several key amino acid residues in the kinetics of hCtr1-mediated transport of these metal ions are different. We discuss the mechanisms of hCtr1-mediated transport of copper ions and CDDP within the context of these findings.
The current model of hCtr1-mediated copper transport suggests that hCtr1-mediated Cu(I) transport involves the binding of several important amino acid residues distributed along the entire hCtr1 monomer, which is in turn organized in trimeric configuration with a pore that stretches across the membrane bilayer (De Feo et al., 2009). The methionine residues described here are essential for hCtr1-mediated Cu(I) transport. These methionine residues, perhaps also contributed by the side chains of their neighboring residues, provide coordinate chemistry for direct interactions with Cu(I) through thiolation (Davis and O’Halloran, 2008; De Feo et al., 2009), much like the mechanism that permits the transfer of Cu(I) between its chaperones (Wernimont et al., 2000; van Dongen et al., 2004; Banci et al., 2006). These methionine residues may be statically arranged in an interface that allows Cu(I) to “slide through.” Alternatively, Cu(I) thiolation may induce conformational changes that bring the essential amino acid residues into close proximity, creating an intermolecular surface that allows Cu(I) to permeate into the cytoplasmic compartment. Previous in vitro assays demonstrated that bindings of Cu(I) (Hassett and Kosman, 1995) and CDDP (Arnesano et al., 2007) to methionine-rich motifs require completely naked forms of the metal ions. The interaction of CDDP with methionine, like Cu(I), was mostly by means of S-thiolation (Borch and Pleasants, 1979; Guo et al., 2004; Arnesano et al., 2007). We demonstrated that both methionine mutations and membrane localization of hCtr1 are required for the dominant negative function for Cu(I) and CDDP transport. These findings suggest that hCtr1 mutants are integrated into hCtr1 oligomeric configuration in such a way that they can be stabilized by copper and CDDP exposures. Mutations in these methionine residues disrupt the coordination chemistry and hinder the permeation of Cu(I) and CDDP through the processes, resulting in the observed dominant-negative effects on the transport of metal ions.
Recent results from inductive plasma-optical emission spectroscopy suggested that two Cu(I) molecules are bound on each trimeric hCtr1 complex, each binding site involves three copper-sulfur coordinate interactions (De Feo et al., 2009). It is plausible that two Pt(IV) may also occupy one trimeric hCtr1 complex but may involve four platinum-sulfur coordinate interactions (Kuo et al., 2007). Our present results demonstrating that mutation of only one methionine residue per hCtr1 monomer is not sufficient to effectively destabilize copper- and CDDP-induced stabilizations of hCtr1 oligomers are consistent with the interactions of multiple metal ions with one hCtr1 trimer in the transport process.
We observed that the apparent Km value for CDDP transport in SCLC cells is 13.1 ± 1.9, approximately 2.5-fold higher than that seen in copper. Because hCtr1 is the major transporter for the transport of both Cu(I) and CDDP, this implies that CDDP has a reduced apparent affinity to hCtr1 compared with Cu(I). Although thiolation of Cu(I) is almost spontaneous and requires no enzymatic catalysis (Freedman et al., 1989; Waud et al., 1990), thiolation of CDDP is a slow process and usually takes hours (Kuo et al., 2007). This difference in affinity may explain why mutations in these methionine residues result in a drastic reduction in Km values for Cu(I) transport but only a marginal effect for CDDP transport. The kinetic differences in hCtr1-mediated transport between CDDP and Cu(I) were also reported by Sinani et al. (2007). These investigators reported that coexpression of functional monomers fused with cyan or yellow fluorescent protein in ΔyCtr1/ΔyCtr3 strain resulted in copper-mediated fluorescence resonance energy transfer (FRET). However, CDDP did not induce yCtr1 FRET or compete against Cu(I) for FRET, despite yCtr1-dependent cellular accumulation of CDDP.
Another mechanistic difference between Cu(I) and CDDP transport mediated by hCtr1 is the role of Gly167. We found that mutation in this amino acid confers a dominant-negative property for copper transport with reduced Km and Vmax values compared with those in the hCtr1-wt control. However, a reduction of Km but not Vmax (therefore no dominant-negative function) for CDDP transport was observed. Gly167 is part of a highly conserved GXXXG (GG4) motif that is located in TM3 of Ctr1 family and plays an important role in helix packaging in the transmembrane domain. Previous study demonstrated that expressing mutant hCtr1 containing substituted Gly167 with leucine in yeast cells resulted in mislocation of mutant hCtr1, unable to oligomerize, and loss of hCtr1 transport function (Aller et al., 2004). However, we show that Gly167, like other methionine residues described here, is essential for hCtr1-mediated copper transport but not for CDDP transport. Moreover, G167S mutation did not mislocate hCtr1 and remained sensitive to metal-induced stabilization of hCtr1 oligomer. The discrepancy between these results may be due to the use of different amino acid substitutions and different expression systems in different cellular backgrounds. Nonetheless, the structural insights into why Gly167 is essential for hCtr1-mediated Cu(I) transport but not for CDDP transport need to be further elucidated.
Finally, another important finding described here is the demonstration that expression of dominant-negative exo-hCtr1 mRNA up-regulates the expression of endo-hCtr1 mRNA. These findings support our previous observations (Chen et al., 2008; Song et al., 2008) of a self-regulatory mechanism for the maintenance of intracellular functional levels of hCtr1 mRNA. By feedback and feed-forward mechanisms, mammalian cells intricately self-readjust the steady-state levels of hCtr1 mRNA. Together, these studies support the new concept that intracellular copper homeostasis is maintained by homeostatic regulation of hCtr1 mRNA.
Acknowledgments
We thank Ben Beltz for technical support, Dr. L. Klomp for anti-hCtr1 antibody, Dr. Zahid H. Siddik for allowing us to use the atomic spectroscope, and Maude Veech and Diane Hackett in the Department of Scientific Publications (M.D. Anderson Cancer Center) for editing the manuscript.
This study was supported in part by the National Institutes of Health National Cancer Institute [Grants R01-CA79085, R01-CA89541, R01-CA16672] and the Veterans Administration Merit Research Fund.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- TM
- transmembrane domain
- wt
- wild-type
- BS3
- bis(sulfosuccinimidyl)suberate
- CDDP
- cisplatin [
- cis-diamminedichloroplatinum(II)]; hCtr1
- human high-affinity copper transporter 1
- FRET
- fluorescence resonance energy transfer
- SCLC
- small-cell lung cancer
- HA
- hemagglutinin.
References
- Aller SG, Eng ET, De Feo CJ, Unger VM. (2004) Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem 279: 53435–53441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnesano F, Scintilla S, Natile G. (2007) Interaction between platinum complexes and a methionine motif found in copper transport proteins. Angew Chem Int Ed Engl 46: 9062–9064 [DOI] [PubMed] [Google Scholar]
- Banci L, Bertini I, Cantini F, Felli IC, Gonnelli L, Hadjiliadis N, Pierattelli R, Rosato A, Voulgaris P. (2006) The Atx1-Ccc2 complex is a metal-mediated protein-protein interaction. Nat Chem Biol 2: 367–368 [DOI] [PubMed] [Google Scholar]
- Borch RF, Pleasants ME. (1979) 76: Proc Natl Acad Sci U S A 6611–6614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, Lu J, Wu LY, Siddik ZH, Klomp LW, et al. (2008) Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol 74: 697– 704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A, Haile D, Yuan DS, Klausner RD. (1994) The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem 269: 25660–25667 [PubMed] [Google Scholar]
- Davis AV, O’Halloran TV. (2008) A place for thioether chemistry in cellular copper ion recognition and trafficking. Nat Chem Biol 4: 148–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. (2009) 106: Proc Natl Acad Sci U S A 4237–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Feo CJ, Aller SG, Unger VM. (2007) A structural perspective on copper uptake in eukaryotes. Biometals 20: 705–716 [DOI] [PubMed] [Google Scholar]
- Eisses JF, Kaplan JH. (2005) The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J Biol Chem 280: 37159–37168 [DOI] [PubMed] [Google Scholar]
- Freedman JH, Ciriolo MR, Peisach J. (1989) The role of glutathione in copper metabolism and toxicity. J Biol Chem 264: 5598–5605 [PubMed] [Google Scholar]
- Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D. (1997) The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem 272: 13786–13792 [DOI] [PubMed] [Google Scholar]
- Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. (2004) Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem 279: 17428–17433 [DOI] [PubMed] [Google Scholar]
- Hassett R, Dix DR, Eide DJ, Kosman DJ. (2000) The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem J 351: 477–484 [PMC free article] [PubMed] [Google Scholar]
- Hassett R, Kosman DJ. (1995) Evidence for Cu(II) reduction as a component of copper uptake by Saccharomyces cerevisiae. J Biol Chem 270: 128–134 [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. (2002) 99: Proc Natl Acad Sci U S A 14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, Thiele DJ. (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4: 176–185 [DOI] [PubMed] [Google Scholar]
- Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. (2007) The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev 26: 71–83 [DOI] [PubMed] [Google Scholar]
- Lee J, Peña MM, Nose Y, Thiele DJ. (2002) Biochemical characterization of the human copper transporter Ctr1. J Biol Chem 277: 4380–4387 [DOI] [PubMed] [Google Scholar]
- Lin X, Okuda T, Holzer A, Howell SB. (2002) The copper transporter CTR1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol 62: 1154–1159 [DOI] [PubMed] [Google Scholar]
- Maryon EB, Molloy SA, Zimnicka AM, Kaplan JH. (2007) Copper entry into human cells: progress and unanswered questions. Biometals 20: 355–364 [DOI] [PubMed] [Google Scholar]
- Puig S, Lee J, Lau M, Thiele DJ. (2002) Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem 277: 26021–26030 [DOI] [PubMed] [Google Scholar]
- Rees EM, Thiele DJ. (2007) Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J Biol Chem 282: 21629–21638 [DOI] [PubMed] [Google Scholar]
- Safaei R, Holzer AK, Katano K, Samimi G, Howell SB. (2004) The role of copper transporters in the development of resistance to Pt drugs. J Inorg Biochem 98: 1607–1613 [DOI] [PubMed] [Google Scholar]
- Safaei R, Howell SB. (2005) Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol 53: 13–23 [DOI] [PubMed] [Google Scholar]
- Sinani D, Adle DJ, Kim H, Lee J. (2007) Distinct mechanisms for Ctr1-mediated copper and cisplatin transport. J Biol Chem 282: 26775–26785 [DOI] [PubMed] [Google Scholar]
- Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, Kuo MT. (2008) Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol 74: 705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3: 1543–1549 [PubMed] [Google Scholar]
- van den Berghe PV, Folmer DE, Malingré HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. (2007) Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J 407: 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen EM, Klomp LW, Merkx M. (2004) Copper-dependent protein-protein interactions studied by yeast two-hybrid analysis. Biochem Biophys Res Commun 323: 789–795 [DOI] [PubMed] [Google Scholar]
- Waud WR, Leopold WR, Elliott WL, Dykes DJ, Laster WR, Jr, Temple CG, Jr, Harrison SD, Jr, Griswold DP., Jr (1990) Antitumor activity of ethyl 5-amino-1,2-dihydro-2-methyl-3-phenyl-pyrido [3,4-b]pyrazin-7-ylcarbamate, 2-hydroxyethanesulfonate, hydrate (NSC 370147) against selected tumor systems in culture and in mice. Cancer Res 50: 3239–3244 [PubMed] [Google Scholar]
- Wernimont AK, Huffman DL, Lamb AL, O’Halloran TV, Rosenzweig AC. (2000) Structural basis for copper transfer by the metallochaperone for the Menkes/Wilson disease proteins. Nat Struct Biol 7: 766–771 [DOI] [PubMed] [Google Scholar]