Abstract
Objective To describe the outcomes of clinical evaluation, skin testing, and vaccine challenge in adolescent schoolgirls with suspected hypersensitivity to the quadrivalent human papillomavirus vaccine introduced in Australian schools in 2007.
Design Retrospective cohort study.
Setting Two tertiary paediatric allergy centres in Victoria and South Australia, Australia.
Participants 35 schoolgirls aged 12 to 18.9 years with suspected hypersensitivity reactions to the quadrivalent human papillomavirus vaccine.
Main outcome measures Clinical review and skin prick and intradermal testing with the quadrivalent vaccine and subsequent challenge with the vaccine.
Results 35 schoolgirls with suspected hypersensitivity to the quadrivalent human papillomavirus vaccine were notified to the specialised immunisation services in 2007, after more than 380 000 doses had been administered in schools. Of these 35 schoolgirls, 25 agreed to further evaluation. Twenty three (92%) experienced reactions after the first dose. Thirteen (52%) experienced urticaria or angio-oedema, and of these, two experienced anaphylaxis. Thirteen had generalised rash, one with angio-oedema. The median time to reaction was 90 minutes. Nineteen (76%) underwent skin testing with the quadrivalent vaccine: all were skin prick test negative and one was intradermal test positive. Eighteen (72%) were subsequently challenged with the quadrivalent vaccine and three (12%) elected to receive the bivalent vaccine. Seventeen tolerated the challenge and one reported limited urticaria four hours after the vaccine had been administered. Only three of the 25 schoolgirls were found to have probable hypersensitivity to the quadrivalent vaccine.
Conclusion True hypersensitivity to the quadrivalent human papillomavirus vaccine in Australian schoolgirls was uncommon and most tolerated subsequent doses.
Introduction
A quadrivalent human papillomavirus vaccine (Gardasil; Merck, NJ, USA) was included in the Australian national immunisation programme in April 2007 for females aged 12-26 years. Adolescent schoolgirls received the vaccine in a secondary school vaccination programme and reports of vaccine related adverse events soon followed.1 Constituents of the quadrivalent vaccine, such as aluminium salts,2 3 polysorbate 80,4 and yeast,5 have been associated with hypersensitivity reactions. The vaccine also shares constituents with other vaccines, such as hepatitis B (H-B-Vax II; Merck, NJ, USA) and diphtheria, tetanus, and pertussis (Boostrix; GlaxoSmithKline, Rixensart, Belgium), which are given to Australian adolescents at age 13 and 15 years, respectively. A bivalent human papillomavirus vaccine (Cervarix; GlaxoSmithKline, Rixensart, Belgium) lacks these constituents and may be an alternative for patients with hypersensitivity to the quadrivalent vaccine (table 1).
Table 1.
Examples of constituents of vaccines
| Variables | Vaccine (manufacturer) | |||
|---|---|---|---|---|
| H-B-Vax II (Merck) | Boostrix (GlaxoSmithKline) | Gardasil (Merck) | Cervarix (GlaxoSmithKline) | |
| Microorganism | Double stranded DNA hepatitis virus family Hepadnaviridae | Bordetella pertussis, Corynebacterium diphtheriae, Clostridium tetani | Recombinant human papillomavirus proteins, virus-like particles 6, 11, 16, and 18 | Recombinant human papillomavirus proteins, virus-like particles 16 and 18 |
| Medium | Saccharomyces cerevisiae | Stainer-Scholte liquid; Fenton medium; Lantham medium | Saccharomyces cerevisiae | Baculovirus or Trichoplusnia |
| Preservative | None | Polysorbate 80 ≤100 μg; formaldehyde | Polysorbate 80 50 μg; L-histidine | None |
| Adjuvant | Aluminium hydroxyphosphate sulphate; potassium salt | Aluminium hydroxide; sodium chloride | Aluminium hydroxyphosphate sulphate; sodium chloride; sodium borate | Aluminium hydroxide and monophosphoryl lipid A; sodium chloride; sodium phosphate monobasic |
| Current immunisation schedule in Australia | Infant schedule; catch-up schedule 11-15 years | 15-17 years | School years 7, 10, 11, and 12 until 26 years (registered for 9-26 year olds) | (registered for 10-45 years) |
We describe the outcomes of clinical evaluation, skin testing, and vaccine challenge in Australian adolescent schoolgirls with suspected hypersensitivity to the quadrivalent human papillomavirus vaccine.
Methods
In the Australian states of Victoria and South Australia, specialised immunisation services are notified of reported vaccine related adverse events. Adolescent schoolgirls with suspected hypersensitivity reactions to the quadrivalent human papillomavirus vaccine, including urticaria, generalised rash, angio-oedema, or anaphylaxis, were referred to tertiary paediatric allergy centres for further evaluation and are included in this retrospective cohort study. We include only girls who received the vaccine in school and not those who may have received the vaccine elsewhere. A detailed history of the reaction was obtained, including previous doses of the quadrivalent vaccine, concomitant vaccines, and time and severity of reaction. We also recorded any history of atopic disease, recurrent urticaria, or drug or vaccine related adverse reactions.
Skin prick and intradermal tests were carried out with 1:10 dilutions of both the quadrivalent and the bivalent human papillomavirus vaccines and 100 mg/ml polysorbate 80 (Tween 80; Merck, Darmstadt, Germany).6 We used histamine and normal saline as positive and negative controls. Additional skin prick tests to other potential allergens were done as guided by clinical history. We measured skin wheals 15 and 20 minutes after skin prick and intradermal testing, respectively, and considered diameters of 3 mm or more above the saline control as a positive result.
Vaccine challenges were administered intramuscularly under medical supervision. All the girls were offered challenge with the quadrivalent vaccine unless there was previous anaphylaxis or a positive skin test result to the vaccine. A 0.1 ml dose was followed 30 minutes later by a 0.4 ml dose. The bivalent vaccine (0.5 ml) was given if requested by the recipient. We followed up the schoolgirls by telephone one week after vaccination and recorded any adverse events. Further vaccinations were planned for those who tolerated the challenge, to complete the three dose schedule.
Results
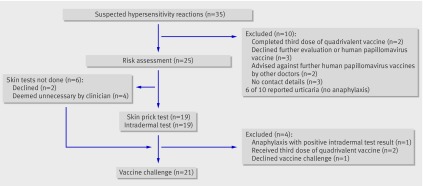
Thirty five schoolgirls with suspected hypersensitivity to the quadrivalent vaccine were reported in 2007, after more than 380 000 vaccine doses had been administered in schools in Victoria and South Australia. Twenty five of these schoolgirls (71%) agreed to undergo further evaluation and were reviewed between August 2007 and February 2008, at a median of 5.7 months (range 1.6-9.9 months) after the reaction (figure). The age of the schoolgirls, proportion with reactions to the first dose, and proportion with urticaria reactions were similar in those excluded and those evaluated. No cases of angio-oedema or anaphylaxis occurred in the excluded group (six in the evaluated group) and time to reaction was significantly longer (median 24 hours) and positively skewed than in the evaluated group.
Flow chart of clinical evaluation through trial
The median time to reaction after vaccination in the evaluated group was 90 minutes. Thirteen of the 25 evaluated schoolgirls experienced urticaria or angio-oedema, and of these, two experienced anaphylaxis (table 2). Thirteen experienced generalised rash, one with angio-oedema.
Table 2.
Details of 25 girls reporting adverse reactions to the quadrivalent human papillomavirus vaccine
| Vaccine category, dose, and concomitant vaccines | Suspected hypersensitivity reaction | Onset of reaction (min) | Skin prick test result | Intradermal test result | Vaccine challenge | Challenge reaction | Notes |
|---|---|---|---|---|---|---|---|
| Probable hypersensitivity (median 17.5 minutes): | |||||||
| Third dose | Urticaria, angio-oedema, laryngeal oedema, tachypnoea, palpitations | 390 | Negative | Negative | NA | NA | Anaphylaxis after third dose |
| First (and second) dose | Urticaria (urticaria, angio-oedema, hoarse voice, laryngeal oedema) | 20 (15) | Negative | Positive | NA | NA | Anaphylaxis after second dose |
| First dose | Urticaria | 15 | Negative | Negative | Quadrivalent HPV vaccine | Reported limited urticaria four hours later | |
| Possible hypersensitivity (median 16 hours): | |||||||
| Second dose | Urticaria | 960 | Negative | Negative | Elected not to proceed with challenge before evaluation | Elected not to proceed with challenge before evaluation | Hyperventilating after intradermal test. Reported non-specific limited rash several hours after intradermal test |
| Unlikely hypersensitivity (median 19 hours): | |||||||
| First dose plus H-B-Vax II | Generalised rash, angio-oedema | 2 | Negative | Negative | Bivalent HPV vaccine | None | Hypersensitivity unlikely as did not receive quadrivalent vaccine |
| First dose plus H-B-Vax II | Generalised rash | 120 | Negative | Negative | Bivalent HPV vaccine | None | Hypersensitivity unlikely as did not receive quadrivalent vaccine |
| First dose | Generalised rash | 2160 | Negative | Negative | Quadrivalent HPV vaccine | Reported nausea, vomiting, and lethargy two days later | Hypersensitivity unlikely as reaction was different to previous reaction |
| First dose plus H-B-Vax II | Urticaria, angio-oedema | 2880 | Negative | Negative | Bivalent HPV vaccine | None | Hypersensitivity unlikely as did not receive quadrivalent vaccine |
| Not hypersensitivity (median 90 minutes): | |||||||
| First dose plus Varilrix plus tetanus | Generalised rash | 1440 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose plus H-B-Vax II | Generalised rash (eczema) | 1440 | NA | NA | Quadrivalent HPV vaccine | None | Skin testing deemed unnecessary |
| First dose | Generalised rash | 1080 | NA | NA | Quadrivalent HPV vaccine | None | Skin testing deemed unnecessary |
| First dose | Generalised rash | 1080 | NA | NA | Quadrivalent HPV vaccine | None | Declined skin testing |
| First dose | Generalised rash | 180 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose plus Boostrix | Generalised rash | 720 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose | Urticaria | 2880 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose | Angio-oedema | 5 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose | Generalised rash | 90 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose | Generalised rash | 1440 | NA | NA | Quadrivalent HPV vaccine | None | Declined skin testing |
| First dose | Angio-oedema | 1440 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First dose | Urticaria | 15 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First (and second) dose | Urticaria (urticaria) | 30 (20) | Negative | Negative | Quadrivalent HPV vaccine | None | Twin of schoolgirl |
| Third dose | Urticaria | 10 | Negative | Negative | NA | NA | Twin of schoolgirl |
| First dose | Generalised rash, tachypnoea | 20 | Negative | Negative | Quadrivalent HPV vaccine | None | Thought to hyperventilate after first dose |
| First dose | Generalised rash | 30 | Negative | Negative | Quadrivalent HPV vaccine | None | |
| First (and second) dose | Urticaria (urticaria) | 10 (10) | Negative | Negative | Quadrivalent HPV vaccine | None |
HPV=human papillomavirus; NA=not applicable; H-B-Vax II=vaccine against hepatitis B (Merck); Varilix=vaccine against varicella (GlaxoSmithKline); Boostrix=vaccine against diphtheria, tetanus, and pertussis (GlaxoSmithKline).
Nineteen (76%) of the 25 evaluated schoolgirls received only the quadrivalent vaccine, whereas six had concomitant vaccines (table 2). Twenty three of the 25 (92%) reported reactions after the first dose of quadrivalent vaccine. Four of the 25 reported reactions after the second dose, and of these, three reported reactions after the first and the second doses. One patient reported a reaction after the third dose.
Fifteen (60%) of the 25 evaluated schoolgirls had a history of current atopic disease: allergic rhinitis in 12 (48%), asthma in eight (32%), atopic dermatitis in five (20%), allergic conjunctivitis in five (20%), and food allergy in three (12%). Two girls had recurrent urticaria and none had a history of hypersensitivity to yeast, drugs, or vaccines. Food, environmental allergens, and drug allergens that may have been associated with the vaccine related adverse event were excluded by a detailed history taking and, if clinically indicated, skin prick tests.
Nineteen of the 25 evaluated schoolgirls (76%) underwent skin prick testing to the quadrivalent vaccine, polysorbate 80, and bivalent vaccine. All the results were negative. The 19 schoolgirls underwent intradermal testing, of which one (table 2) had a positive result to the quadrivalent vaccine and negative results to polysorbate 80 and the bivalent vaccine. One schoolgirl experienced hyperventilation during intradermal testing (table 2) and reported a limited non-specific rash several hours later. She declined further vaccination against human papillomavirus.
Challenge with the quadrivalent vaccine was carried out in 18 (72%) of the 25 evaluated schoolgirls. Three of the seven schoolgirls who were not challenged with the quadrivalent vaccine elected to receive the bivalent vaccine as they had concerns about the quadrivalent vaccine despite a negative skin test result. Vaccine challenges were not done in the two schoolgirls who had completed the three doses of the schedule or the one girl who declined further vaccination, and challenge was contraindicated in one girl who had anaphylaxis and a positive skin test result to the quadrivalent vaccine.
Seventeen of the 18 schoolgirls challenged with the quadrivalent vaccine and all three challenged with the bivalent vaccine remained well one week after vaccination. One schoolgirl reported limited urticaria over the limbs and trunk four hours after challenge with the quadrivalent vaccine (table 2). Supervised challenge with the bivalent vaccine for her third dose of human papillomavirus vaccine was well tolerated.
The 25 evaluated schoolgirls were classified into one of four categories (table 2): probable hypersensitivity—those with anaphylaxis, a positive skin test result for the quadrivalent vaccine, or reproducible reactions to challenge with the quadrivalent vaccine; possible hypersensitivity—those with reactions to skin testing who were not challenged with the quadrivalent vaccine; unlikely hypersensitivity—those with negative skin test results to the quadrivalent vaccine who were not challenged with the quadrivalent vaccine, or were challenged with the quadrivalent vaccine but did not experience a reproducible reaction; and not hypersensitivity—those with negative skin test results for the quadrivalent vaccine and no adverse reaction to subsequent challenge with the quadrivalent vaccine.
Schoolgirls in the probable hypersensitivity group were more likely to present with urticaria than those in the unlikely hypersensitivity group (likelihood ratio 9.0) and not hypersensitivity group (10.2), and had a median time to reaction of 17.5 minutes compared with 19 hours in the unlikely hypersensitivity group and 90 minutes in the not hypersensitivity group (table 2). Other clinical features, including number of doses of the quadrivalent vaccine, concomitant vaccines, recurrence of reactions to the quadrivalent vaccine, and current atopic disease or recurrent urticaria, did not predict hypersensitivity to the quadrivalent vaccine.
Discussion
We evaluated suspected hypersensitivity in adolescent females immunised with a human papillomavirus vaccine in Australian schools. Only three of the 25 evaluated schoolgirls had probable hypersensitivity to the quadrivalent human papillomavirus vaccine after 380 000 doses had been administered in schools. Seventeen of the 18 girls subsequently challenged with the quadrivalent vaccine tolerated revaccination. Our data suggest that true hypersensitivity to the quadrivalent vaccine is uncommon and that suspected hypersensitivity reactions such as urticaria are often idiosyncratic and not usually a contraindication to further vaccinations. Studies of other vaccines have found that most reactions after immunisation are not due to hypersensitivity and revaccination is usually well tolerated.7 8 9
Although we excluded 10 of 35 schoolgirls with suspected hypersensitivity to the quadrivalent vaccine from our evaluation, reactions in the excluded group were mostly mild and delayed in presentation, suggesting that we did not miss any important cases of suspected hypersensitivity to the quadrivalent vaccine. All reported cases of anaphylaxis were evaluated.
Time to anaphylaxis was 15 minutes in one girl and 6.5 hours in another. As anaphylaxis after childhood vaccinations usually occurs within one hour,10 11 6.5 hours is beyond any standard observation period after immunisation. Consistent with the delayed presentation, one of the girls had no evidence of IgE mediated hypersensitivity to the quadrivalent vaccine and we postulate her reaction was mediated by IgG or complement, or both. As she was not rechallenged with the quadrivalent vaccine, however, hypersensitivity was not confirmed.
One of the girls had a positive intradermal test result to the quadrivalent vaccine that was consistent with IgE mediated hypersensitivity. We were unable to determine whether her reaction was due to the recombinant viral-like particles or other constituents of the vaccine such as aluminium hydroxyphosphate sulphate. As she had no history of reactions to yeast, and skin testing for polysorbate 80 gave a negative result, IgE mediated hypersensitivity to these components was unlikely. For females with probable hypersensitivity to the quadrivalent vaccine, immunoblot analysis and measurement of specific IgG and IgE to the individual vaccine components would provide further information.
Our study describes two cases of anaphylaxis after 380 000 doses of the quadrivalent vaccine had been administered. Although we have a passive surveillance system for reporting vaccine related adverse events in Australia, the quadrivalent human papillomavirus vaccine is a new vaccine and there is a high level of awareness of the importance of reporting adverse events in the school immunisation programme. One study estimated that if 80% of eligible US adolescent females were to receive a saline injection according to the vaccination schedule for human papillomavirus, 3 per 100 000 adolescents would require emergency care for asthma or allergy within 24 hours of vaccination.12 As allergic symptoms are common, studies of adverse events to the quadrivalent vaccine should take these “baseline” rates into consideration. An Australian human papillomavirus vaccination programme register (www.hpvregister.org.au/), established in August 2008, will facilitate more accurate determination of rates of hypersensitivity reactions not possible from current data sources.
In conclusion, suspected hypersensitivity reactions to the human papillomavirus quadrivalent vaccine require further evaluation to exclude IgE mediated reactions. Most females with suspected hypersensitivity to this vaccine tolerate revaccination. Our clinical recommendation is that females with suspected hypersensitivity to the quadrivalent vaccine should be evaluated before receiving more doses, and any challenges with the same vaccine should be carried out in a supervised setting. Further studies are required to investigate the mechanisms of hypersensitivity to this vaccine.
What is already known on this topic
Hypersensitivity reactions to vaccines are uncommon
What this study adds
True hypersensitivity to the quadrivalent human papillomavirus vaccine is uncommon and most females tolerate subsequent doses
We thank the allergy and immunology department and immunisation nurse consultants from the Royal Children’s Hospital and the South Australian Immunisation Coordination Unit for their assistance. NC acknowledges support from a National health and Medical Research Council PhD postgraduate public health research scholarship.
Contributors: WK, SC, MT, and MG developed the study protocol. WK, NC, CZ, SC, SE, and PQ evaluated the participants. WK collated and analysed the data, WK and SC wrote the draft manuscript. MG, NC, MT, JB, and JR contributed to revisions of the manuscript. All authors gave their approval of this version to be published. SC is the guarantor.
Funding: None.
Competing interests: MT is chairperson of an incorporated association Asia Pacific Immunoglobulins in Immunology Expert Group that is supported by an unrestricted grant from CSL. JB has served on an advisory board for GSK and serves on a data safety monitoring board for CSL. MCRI receives reimbursement from both GSK and CSL for JB’s attendance at advisory board and scientific meetings.
Ethical approval: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Cite this as: BMJ 2008;337:a2642
References
- 1.Tanne JH. Questions over human papillomavirus vaccine in US and Australia. BMJ 2007;334:1182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox NH, Moss C, Forsyth A. Cutaneous reactions to aluminium in vaccines: an avoidable problem. Lancet 1988;2:43. [DOI] [PubMed] [Google Scholar]
- 3.Baylor NW, Egan W, Richman P. Aluminium salts in vaccines—US perspective. Vaccine 2002;(Suppl 3):S18-23. [DOI] [PubMed]
- 4.Shelley WB, Talanin M, Shelley ED. Polysorbate 80 hypersensitivity. Lancet 1995;345:1312-3. [DOI] [PubMed] [Google Scholar]
- 5.Brightman CA, Scadding GK, Dumbreck LA, Latchman Y, Brostoff J. Yeast-derived hepatitis B vaccine and yeast hypersensitivity. Lancet 1989;22:903. [DOI] [PubMed] [Google Scholar]
- 6.Wood RA, Setse R, Halsey N. Irritant skin test reactions to common vaccines. J Allergy Clin Immunol 2007;120:478-81. [DOI] [PubMed] [Google Scholar]
- 7.Ponvert C, Scheinmann P. Vaccine allergy and pseudo-allergy. Eur J Dermatol 2003;13:10-5. [PubMed] [Google Scholar]
- 8.Andrews RM, Kempe AE, Herceg A. Vaccinating children with a history of serious reactions after vaccination or of egg allergy. Med J Austr 1998;168:491. [DOI] [PubMed] [Google Scholar]
- 9.Gold M, Goodwin H, Botham S, Burgess M, Nash M, Kempe A. Revaccination of 421 children with a past history of an adverse reaction in a special immunization service. Arch Dis Child 2000;83:128-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama T, Aizawa C, Kuno-Sakai H. A clinical analysis of gelatin allergy and determination of its causal relationship to the previous administration of gelatin-containing acellular pertussis vaccine combined with diphtheria and tetanus toxoid. J Allergy Clin Immunol 1993;103:321-5. [DOI] [PubMed] [Google Scholar]
- 11.Patja A, Davidkin I, Kurki T, Kallio MJ, Valle M, Peltola H. Serious adverse events after measles-mumps-rubella vaccination during a fourteen year prospective follow-up. Pediatr Infect Dis J 2000;19:1127-34. [DOI] [PubMed] [Google Scholar]
- 12.Siegrist CA, Lewis EM, Eskola J, Evans SJ, Black SB. Human papilloma virus immunisation in adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J 2007;26:979-84. [DOI] [PubMed] [Google Scholar]