Abstract
Objective To compare the effectiveness of ultrasound guided corticosteroid injection in the subacromial bursa with systemic corticosteroid injection in patients with rotator cuff disease.
Design Double blind randomised clinical trial.
Setting Outpatient clinic of a physical medicine and rehabilitation department in Oslo, Norway.
Patients 106 patients with rotator cuff disease lasting at least three months.
Interventions Ultrasound guided corticosteroid and lidocaine injection in the subacromial bursa and lidocaine injection in the gluteal region (local group); corticosteroid and lidocaine injection in the gluteal region and ultrasound guided lidocaine injection in the subacromial bursa (systemic group).
Main outcome measures Difference in improvement in the overall shoulder pain and disability index score after six weeks.
Results Six weeks after the intervention, the mean difference in improvement in overall shoulder pain and disability index score between the local group and the systemic group was −5.2 (95% confidence interval −13.9 to 3.5); it was −4.1 (−12.3 to 4.1, P=0.32) after adjustment for baseline score. A small but statistically significant difference in improvement between groups occurred in favour of the local group for two secondary outcome measures: the Western Ontario rotator cuff index (8.1, 0.7 to 15.6) and change in main complaint (2.0, 0 to 4).
Conclusions No important differences in short term outcomes were found between local ultrasound guided corticosteroid injection and systemic corticosteroid injection in rotator cuff disease.
Trial registration Clinical trials NCT00640575.
Introduction
Shoulder pain is a common medical problem; impingement syndrome or rotator cuff disease is the most frequent diagnosis.1 2 The exact source and mechanisms of pain in rotator cuff disease are not known.3 Histopathology studies reveal mainly degenerative changes of the rotator cuff tendons.4 Inflammatory mediators, free nerve endings, and nociceptive agents have been found in the subacromial bursa,5 6 but other factors may contribute to pain and dysfunction.
Non-operative treatment for rotator cuff disease primarily consists of active physiotherapy, which may be supplemented with non-steroidal anti-inflammatory drugs, steroid injections, and electrotherapy.3 Active physiotherapy has been reported to be superior to placebo and equivalent to surgery at long term follow-up.7 8 Despite extensive research, evidence for the effectiveness of steroid injections for rotator cuff disease is unconvincing. Conclusions of systematic reviews and meta-analyses are inconsistent and hampered by small sample sizes, variable methodological quality, and heterogeneity of the included studies.9 10 11
Corticosteroids are potent anti-inflammatory and pain modulating drugs with both systemic and local effects. The precise mechanism of local corticosteroid injections is not well understood. Possible therapeutic mechanisms include anti-inflammatory effects, relaxation of reflex muscle spasm, influence of local tissue metabolism, pain relief, mechanical improvement, and placebo effect.12
Thirty per cent to 80% of subacromial injections are reported to reach the subacromial bursa or the subacromial space when a blind injection technique is used.13 High frequency ultrasonography is a safe, accurate, readily available technique for guiding musculoskeletal aspiration and infiltration that ensures correct placement of the needle and delivery of the drug. Some studies have reported better short term improvement in patients when the injection has been placed accurately into an anatomical site or in the subacromial bursa.14 15 Valtonen reported that gluteal and subacromial corticosteroid injections significantly, and equally, improved supraspinatus tendonitis compared with placebo.16 Recently, two small randomised trials reported that ultrasonographically guided injections were significantly more effective than blind injections for short term pain relief and improved function.17 18 The participants were not blinded for treatment group in these two studies, which raises the possibility of a bias favouring ultrasound guided injections.
To investigate the importance of placement of corticosteroid injections in patients with rotator cuff disease, we did a randomised controlled study comparing the effectiveness of a systemic corticosteroid injection in the gluteal region with an ultrasound guided injection in the subacromial bursa in patients with rotator cuff disease. We used a double blind design.
Methods
This study was a prospective, double blind, randomised controlled trial. We recruited patients between April 2005 and October 2006. We invited general practitioners in Oslo, serving a population of half a million, to refer patients with rotator cuff disease to the outpatient clinic of the Physical Medicine and Rehabilitation Department at Ullevål University Hospital in Norway. We included patients who were at least 18 years old and had all of the following: shoulder pain for more than three months; pain on abduction; less than a 50% reduced glenohumeral range of motion in no more than one direction of external rotation, internal rotation, or abduction; pain on two of three isometric tests for abduction, external rotation, and internal rotation; and a positive Hawkins-Kennedy impingement sign.19
We excluded patients who had symptomatic acromioclavicular arthritis, clinical and radiological findings indicating glenohumeral joint pathology, referred pain from the neck or internal organs, generalised muscular pain syndrome with bilateral muscular pain in the neck and shoulders, a history of inflammatory arthritis, diabetes mellitus type 1, previous fractures or surgery to the shoulder, or contraindications to local steroid injections or if they had received any corticosteroids the last month before inclusion. We also excluded patients with a shoulder pain and disability index score below 30 points, representing minor complaints that did not indicate corticosteroid injections.
The same medical doctor (OME) examined all patients referred to the clinic for eligibility. They were thoroughly informed about the study and signed an informed consent if they were willing to participate.
Study protocol
We scheduled patients who were eligible and willing to participate in the study for an appointment at the clinic for baseline registrations and treatment according to the protocol. We recorded magnetic resonance imaging results. Patients not able or unwilling to have magnetic resonance imaging had diagnostic ultrasonography. Patients had an equal probability of assignment to the two treatment groups. We used the computer program Clinstat to generate a predetermined randomisation sequence with a randomised variable block size of three, four, and five. A member of the study group who was not participating in patients’ eligibility, allocation to treatment, or outcome assessment developed the randomisation sequence. The consultant physician responsible for the injections held the only copy of the randomisation sequence. After the baseline registrations, we gave each participant a study number. To ensure concealed allocation, each patient was referred to the consultant physician responsible for injections, who then allocated the patient to one of the treatment groups according to the randomisation sequence. Patients and the outcome assessor were blinded for treatment assignment. The consultant physician preparing and administering the injections was not blinded.
Treatment
We allocated patients randomly to either local or systemic steroid injection. Both groups received injections of local anaesthetic in the shoulder and the gluteal region. We provided local anaesthetic to improve blinding of participants by inducing a temporary pain relief (impingement test) and mask possible post-injection pain. The “local” group received a sonographically guided injection of 2 ml (10 mg/ml) triamcinolone (Kenacort-T, Bristol-Myers Squibb) and 5 ml (10 mg/ml) lidocaine hydrochloride (Xylocaine, AstraZeneca) to the subacromial bursa and an intramuscular injection of 4 ml (10 mg/ml) lidocaine hydrochloride to the upper gluteal region. The “systemic” group received a sonographically guided injection of 5 ml (10 mg/ml) lidocaine hydrochloride to the subacromial bursa and an intramuscular injection of 2 ml (10 mg/ml) triamcinolone and 2 ml (10 mg/ml) lidocaine hydrochloride to the upper gluteal region.
The injection procedure was standardised. The same independent consultant physician responsible for the injections prepared syringes immediately before use. She gave the sonographically guided injection first, using commercial ultrasound equipment (Medison 128 BW prime, Medison Co, Seoul, Korea) with a 5-9 MHz linear transducer for guidance. Patients sat facing the ultrasound screen with the affected arm rotated internally behind the back, elbow bent, and the back of the hand resting against the lower back. The physician sterilised patients’ skin and the ultrasound transducer with alcohol and applied sterile gel to the transducer. She used the ultrasound probe to visualise the insertion of the supraspinatus tendon and the subacromial bursa on the longitudinal axis, taking care that the contents of the syringes were never shown to the participants. She used the anterior approach with a 0.8×50 mm intramuscular needle for the subacromial injections, perforating the skin and tracking the needle in real time until it reached the subacromial bursa. She emptied the content of the syringe into the subacromial bursa, taking care to avoid direct injection into the rotator cuff tendons. The physician then gave patients the intramuscular injection in the upper gluteal region. We allowed patients to use analgesics in the trial period, and to continue any physiotherapy programme that they were attending at baseline. We did not allow other additional treatment in the research period.
Outcome measures
Blind follow-up measures were carried out at two and six weeks after treatment. Baseline demographics and clinical characteristics included sex, age, duration of symptoms, sickness leave, worker’s compensation claims, dominant side affected, previous episodes of shoulder pain, and drug use. We used the Hopkins symptom checklist to measure emotional distress.20 We asked patients to report additional treatment at follow-ups. The main outcome measure was the shoulder pain and disability index.21 This is a self administered, validated, shoulder specific questionnaire consisting of five pain and eight disability items. Patients recorded responses on each item on visual analogue scales on the basis of the previous week’s symptoms. We calculated the score by summing and then averaging the two subscales to give a score out of 100. Secondary outcome measures were the Western Ontario rotator cuff index22; active range of abduction and flexion; the participant’s assessment of change in the main complaint compared with baseline, measured on an 18 point ordinal scale; and two separate questions about pain at rest and pain during activity measured on a nine point ordinal scale.23 Patients completed all questionnaires before the consultation and were assessed by the same physician (OME) blinded for treatment group. He measured active abduction and flexion with an electronic digital inclinometer (EDI 320, Cybex Inc, Ronkonkoma, NY) according to a standardised protocol.24
Statistical analysis
After a pilot study, we estimated the standard deviation of change in shoulder pain and disability index to be approximately 20 points. The minimal clinically important difference has been variously reported to be 10 points and 13.2 points.25 26 We designed this study to detect a clinically relevant difference of 10 points with 95% probability and 80% power. We needed a sample size of 63 patients per group for a two sided t test and 48 patients per group for the more efficient analysis of covariance.27 To account for a 10% rate of loss at follow-up and still meet these requirements, we included 106 patients in the study. We did not plan or do any interim analyses.
We analysed data according to the principle of intention to treat. All hypothesis tests were two tailed with a significance level of 5%. We calculated point estimates with associated 95% confidence intervals for mean differences in improvement between groups in shoulder pain and disability index score and Western Ontario rotator cuff index score. For analysis of participants’ change in main complaint, pain at rest, and pain during activity, we calculated non-parametric estimates with median, interquartile range, and median difference in improvement between groups with 95% confidence intervals. As recommended, we planned and used an analysis of covariance model with adjustment for baseline differences.28 We used the Mann-Whitney U test for hypothesis testing of group differences in main complaint. We used Minitab 15.1.1.0 and Confidence Interval Analysis 2.1.2 to calculate non-parametric statistics and SPSS 16.0 for Mac for all other statistical analyses. We reported the study according to the CONSORT rules.
Results
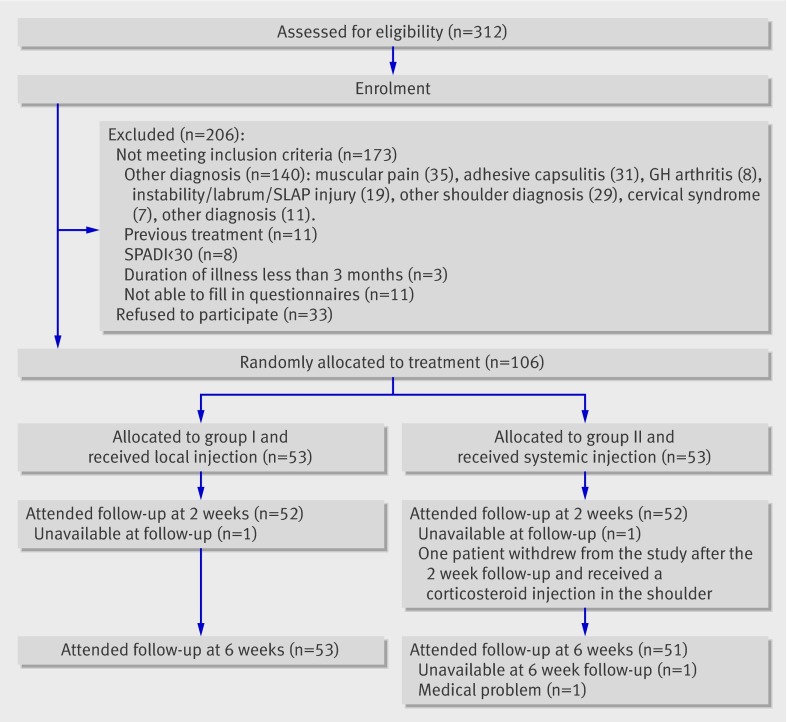
Of the 312 patients evaluated for inclusion, we randomised 106. The figure shows the reasons for exclusion and the follow-up of patients through the trial. One patient in each treatment group did not attend the two week follow-up. Two patients in the systemic group were not available at the six week follow-up (one had a medical condition and the other could not be reached). For these patients, we carried forward the last reported values in the intention to treat analysis. One patient in the systemic group withdrew from the study after the two week follow-up and received an additional corticosteroid injection in the shoulder. We included these results in the intention to treat analyses. The outcome for this patient was poor.
Flow chart of study. GH=glenohumeral; SLAP=superior labrum, anterior to posterior; SPADI=shoulder pain and disability index
Table 1 shows the baseline characteristics of the study sample. At baseline, the groups were similar with respect to sex, age, duration of symptoms, sickness leave, worker’s compensation claims, dominant side affected, concomitant neck pain, previous episodes of shoulder pain, emotional distress, and use of analgesics. Eight patients were not able or were unwilling to have magnetic resonance imaging and had diagnostic ultrasonography. Five patients in each group had a full thickness rotator cuff tear. Eight patients in the local group and five patients in the systemic group attended physiotherapy between baseline and the six week follow-up. The groups did not differ in drug use, and no patient reported attending for other treatments in the trial period. All procedures were implemented without difficulties. Seven (24%) of 29 patients in the local group and 19 (50%) of 38 patients in the systemic group correctly identified the treatment group they were assigned to (P=0.055). Thirty nine (37%) patients did not answer the question about which treatment they believed they had received.
Table 1.
Baseline characteristics according to group. Values are numbers (percentages) unless stated otherwise
| Characteristic | Local group (n=53) | Systemic group (n=53) |
|---|---|---|
| Sex (male/female) | 21/32 | 20/33 |
| Mean (SD) age (years) | 51 (11) | 50 (12) |
| Symptom duration: | ||
| <6 months | 15 (28) | 15 (28) |
| 6 months to 1 year | 17 (32) | 15 (28) |
| 1-2 years | 7 (13) | 11 (21) |
| >2 years | 14 (26) | 12 (23) |
| Work: | ||
| On sick leave | 17 (32) | 14 (26) |
| Median (interquartile range) duration of sick leave (months) | 8 (1-14) | 4 (3-17) |
| Worker’s compensation claim | 5 (9) | 3 (6) |
| Bilateral pain | 8 (15) | 9 (17) |
| Dominant side affected | 34 (64) | 35 (66) |
| Full thickness rotator cuff rupture | 5 (9) | 5 (9) |
| Hopkins symptom checklist—mean (SD) sum score* | 1.53 (0.42) | 1.53 (0.38) |
| Previous treatment: | ||
| Physiotherapy | 29 (55) | 23 (43) |
| Corticosteroid injection | 22 (42) | 20 (38) |
| Current additional treatment: | ||
| Physiotherapy | 9 (17) | 6 (11) |
| Analgesics | 19 (36) | 21 (40) |
*Local group n=48; systemic group n=50.
Table 2 shows the improvement in shoulder pain and disability index scores for both groups over the six week period. The mean difference from baseline to the six week follow-up was 24.4 (SD 22.5, P<0.001) for the local group and 19.2 (SD 22.7, P<0.001) for the systemic group. The results at the six week follow-up were slightly in favour of the group receiving local injections for all outcome measures. The difference in effectiveness of treatment between the two groups in the primary outcome measure was small and not statistically significant at any time point, even after adjustment for baseline difference in shoulder pain and disability index score (mean difference −4.1, 95% confidence interval −12.3 to 4.1, P=0.32) (table 2).
Table 2.
Outcome measures
| Measure | Local group (n=53) | Systemic group (n=53) | Difference in improvement (95% CI) | Adjusted difference (95% CI) | P value |
|---|---|---|---|---|---|
| Shoulder pain and disability index—mean (SD) | |||||
| Baseline | 53 (18) | 51 (17) | – | – | – |
| 2 weeks | 32 (25) | 28 (23) | 0.8 (−7.9 to 9.4) | – | – |
| 6 weeks | 29 (21) | 32 (23) | −5.2 (−13.9 to 3.5) | −4.1 (−12.3 to 4.1) | 0.32 |
| Western Ontario rotator cuff index*—mean (SD) | |||||
| Baseline | 45 (17) | 47 (16) | – | – | – |
| 2 weeks | 64 (23) | 63 (22) | 3.0 (−4.6 to 10.6) | – | – |
| 6 weeks | 67 (21) | 60 (22) | 9.0 (1.2 to 16.8) | 8.1 (0.7 to 15.6) | 0.032 |
| Abduction†—median (interquartile range) | |||||
| Baseline | 131 (98-144) | 126 (88-144) | – | – | – |
| 2 weeks | 140 (130-148) | 133 (108-146) | −2 (−11 to 7) | – | – |
| 6 weeks | 141 (122-150) | 121 (99-144) | −4 (−12 to 4) | −6 (−15.9 to 3.8) | 0.23 |
| Flexion†—median (interquartile range) | |||||
| Baseline | 151 (132-160) | 150 (129-158) | – | – | – |
| 2 weeks | 158 (148-164) | 150 (134-161) | −4 (−10 to 1) | – | – |
| 6 weeks | 156 (148-166) | 152 (132-160) | −2 (−8 to 5) | −4.4 (−14.7 to 5.9) | 0.40 |
| Pain at rest†—median | |||||
| Baseline | 6.0 | 7.0 | – | – | – |
| 2 weeks | 4.0 | 4.0 | 0 (−1.0 to 1.0) | – | – |
| 6 weeks | 3.0 | 5.0 | 1.0 (0 to 2.0) | −0.6 (−1.5 to 0.2) | 0.13 |
| Pain in activity†—median | |||||
| Baseline | 6.0 | 7.0 | – | – | – |
| 2 weeks | 3.0 | 2.0 | 0 (−1.0 to 1.0) | – | – |
| 6 weeks | 2.0 | 3.0 | 1.0 (0 to 2.0) | −0.5 (−1.1 to 0.2) | 0.19 |
| Change in main complaint†—median | |||||
| 2 weeks | 5.0 | 4.0 | 1.0 (0 to 2.0) | – | – |
| 6 weeks | 6.0 | 2.0 | 2.0 (0 to 4.0) | –‡ | 0.009§ |
*Local group n=52; systemic group n=52.
†Non-parametric statistics.
‡No adjustment possible for baseline score.
§Mann-Whitney test of hypothesis of difference between medians v no difference.
After adjusting for baseline difference in Western Ontario rotator cuff index score, we found a significant difference between groups of 8.1 (95% confidence interval 0.7 to 15.6, P=0.032) points at the six week follow-up in favour of patients receiving local injections. The participants’ reported change in main complaint from baseline to six week follow-up was 6 (range 3-7) versus 2 (range 0-7), and the median difference between the groups was 2 (95% confidence interval 0 to 4, P=0.009) in favour of the local group. We found no significant difference between groups in range of abduction, range of flexion, or the two separate pain questions at two week and six week follow-ups (table 2).
Only five patients receiving local injections and three patients receiving systemic injections had a normal and pain-free range of abduction and negative clinical tests. Nine patients from both groups reported mild adverse effects such as facial redness, dizziness, and feeling of warmth. One patient in the local group and four patients in the systemic group reported post-injection pain in the shoulder. No serious side effects were reported.
Discussion
Our main objective was to compare the effectiveness of ultrasound guided subacromial injection and systemic gluteal injection of corticosteroids in patients with rotator cuff disease. We did not find significant between group differences in the primary outcome measure. Although 4.1 points is the best estimate of the between group differences in improvement of the shoulder pain and disability index score in this study, the 95% confidence interval includes the 10 point threshold we used as a minimal clinically important difference when designing the study. We therefore cannot exclude the possibility of a between group difference. A recent study estimated the minimal clinically important difference in shoulder pain and disability index score to be 13.2 points; accordingly, our observed results are not clinically important.26
Interpretation of results
We reported statistically significant, but clinically small, group differences for two secondary outcome measures. The observed inconsistency between outcome measures may be due to the effects of multiple testing, which increase the probability of positive results. With Bonferroni corrections, no results remained statistically significant. Conclusions of effectiveness of an intervention may be influenced by the choice of scoring system for rating patients.29 30 We cannot rule out the possibility that the Western Ontario rotator cuff index is a more sensitive outcome measure than the shoulder pain and disability index. Clinimetric studies investigating the responsiveness of the shoulder pain and disability index and the Western Ontario rotator cuff index with other shoulder questionnaires indicate that both outcome measures are responsive.31 32 The two scores may measure different constructs, however, as the shoulder pain and disability index is a pain and disability score and the Western Ontario rotator cuff index is a health related quality of life score. Consensus on a core set of outcome measures in shoulder interventions is warranted.
The most likely interpretation of our results is that local ultrasound guided corticosteroid injections do not improve patients’ outcomes compared with systemic corticosteroid injection in rotator cuff disease.
Comparison with existing literature
Previous randomised trials and systematic reviews have reported contradictory results on the effectiveness of corticosteroid injections for rotator cuff disease.9 11 Alvarez et al compared blind injection of betamethasone with Xylocaine in patients with chronic rotator cuff disease. They found a moderate improvement in symptoms in both groups but found no difference in improvement between groups.33 Considerable placebo effects have been seen with injection treatment, acupuncture, and extracorporeal shock wave therapy for shoulder disease.34 35 36 Thus, our results could be attributed to the systemic effect of corticosteroids, injections of lidocaine into the subacromial bursa, and placebo effects.
Naredo et al and Chen et al have reported better outcomes for pain, disability, and range of motion after ultrasound guided injections than blind subacromial injections.17 18 Thus, we cannot rule out the possibility that the use of ultrasound for better placement of lidocaine injections contributed to the results of our study. We used 20 mg of triamcinolone to parallel the dose used in the study by Naredo et al. Several previous studies have used higher doses of corticosteroid injections for rotator cuff disease. Limited evidence exists for better efficacy with higher corticosteroid dosage,10 but 20 mg of triamcinolone is generally regarded as a low dose for systemic treatment. A higher dosage would be likely to reduce the difference between groups and increase adverse effects.
Possible confounders and weaknesses
The effectiveness of corticosteroid injections might be influenced by the duration of rotator cuff disease. The favourable result of ultrasound guided corticosteroid injections in the study by Naredo et al occurred in a group of patients with a first flare of shoulder pain.18 Longstanding symptoms are a negative prognostic factor for clinical outcome.37 38 A large portion of patients in our study had had symptoms for more than six months, and many patients had recurring episodes of shoulder pain. No differences existed in duration of disease between groups in our study.
We based inclusion of patients on strict clinical criteria.23 This strategy is in accordance with Park et al, who found that the use of a combination of clinical tests increased the accuracy of clinical diagnosis.39 Our inclusion criteria are quite similar, but not identical, to the criteria used in the study by Park et al. By combining the Hawkins-Kennedy impingement sign, the painful arc sign, and the infraspinatus muscle strength test, they achieved a post-test probability of 95% for any degree of impingement syndrome. In our study, the clinical classification of soft tissue shoulder disorders and use of strict inclusion criteria lowered the external validity with respect to the 312 patients considered for inclusion.
Twenty patients in the local group and 18 patients in the systemic group had had previous shoulder injections. Eight patients in the local group and five in the systemic group had concurrent physiotherapy. Both previous and concurrent treatment might have influenced individual treatment response, but these possible confounders existed in the same number of patients in each intervention group. We did not register type of physiotherapy or use a structured reporting system for additional drug use such as the defined daily dose or the anatomical therapeutic chemical classification system that could have revealed a bias in compliance caused by these potential confounders.
A weakness in the design of the study was the lack of blinding of the physician who gave the injections. Even though we standardised the procedure and took care not to reveal group assignment, a bias may have been introduced. If present, we believe the bias was most likely to be in favour of the local group on the basis of the good results of recent studies using ultrasound guided corticosteroid injections and the fact that the physician responsible for giving injections had specialised in diagnostic ultrasonography and ultrasound guided injections.
We set the period of time between treatment and follow-up testing to optimise the anticipated pharmacological effect of the injected steroid. Evidence of the effectiveness of long term treatment is scant.40 Recent studies have reported better short term and inferior long term results from corticosteroid intervention than from physiotherapy and no intervention (controls).41 42
The modest improvements in self reported complaints and range of motion after steroid injection seen in this and previous studies suggest that steroid injection is not a sufficient treatment strategy for patients with rotator cuff disease. Better outcome in terms of range of motion is reported after attendance at an active physiotherapy programme.7 8 43
Conclusion
The results of this study do not indicate that local corticosteroid injection is more effective than systemic corticosteroid injection for short term improvement in rotator cuff disease. Ultrasound guided injection of lidocaine into the subacromial bursa might have contributed to the observed improvement in both groups.
What is already known on this topic
Insufficient evidence exists for the efficacy of corticosteroid injections in rotator cuff disease of the shoulder
Recent studies have shown favourable results with ultrasound guided injections in the subacromial bursa
Corticosteroids are potent anti-inflammatory and pain modulating drugs and may act through both local and systemic mechanisms
What this study adds
Local ultrasound guided corticosteroid injection is unlikely to be substantially more effective than systemic corticosteroid injection for short term improvement of pain and disability in rotator cuff disease
The study did not include a sham injection group, so whether either treatment is superior to placebo could not be determined
We thank the employees at the Physical Medicine and Rehabilitation Department at Ullevål University Hospital, Charlotte Begby for help with patients’ logistics, and all the patients who made this study possible.
Contributors: All authors participated in the planning of the study and contributed to the protocol. SK was responsible for the injection treatment. OME analysed the data and drafted the manuscript, which were revised by the other authors. All authors approved the final version. OME is the guarantor.
Funding: The University of Oslo funded this study.
Competing interests: None declared.
Ethical approval: The regional ethics committee approved this study. Included patients gave written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Cite this as: BMJ 2009;338:a3112
References
- 1.Van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis 1995;54:959-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vecchio P, Kavanagh R, Hazleman BL, King RH. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol 1995;34:440-2. [DOI] [PubMed] [Google Scholar]
- 3.Brox JI. Regional musculoskeletal conditions: shoulder pain. Best Pract Res Clin Rheumatol 2003;17:33-56. [DOI] [PubMed] [Google Scholar]
- 4.Kahn KM. Histopathology of common tendinopathies: update and implication for clinical management. Sports Med 1999;27:393-408. [DOI] [PubMed] [Google Scholar]
- 5.Blaine TA, Kim YS, Voloshin I, Chen D, Murakami K, Chang SS, et al. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J Shoulder Elbow Surg 2005;14(suppl S):84-9S. [DOI] [PubMed] [Google Scholar]
- 6.Vangsness CT Jr, Ennis M, Taylor JG, Atkinson R. Neural anatomy of the glenohumeral ligaments, labrum, and subacromial bursa. Arthroscopy 1995;11:180-4. [DOI] [PubMed] [Google Scholar]
- 7.Brox JI, Staff PH, Ljunggren AE, Brevik JI. Arthroscopic surgery compared with supervised exercises in patients with rotator cuff disease (stage II impingement syndrome). BMJ 1993;307:899-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haahr JP, Ostergaard S, Dalsgaard J, Norup K, Frost P, Lausen S, et al. Exercises versus arthroscopic decompression in patients with subacromial impingement: a randomised, controlled study in 90 cases with a one year follow up. Ann Rheum Dis 2005;64:760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arroll B, Goodyear-Smith F. Corticosteroid injections for painful shoulder: a meta-analysis. Br J Gen Pract 2005;55:224-8. [PMC free article] [PubMed] [Google Scholar]
- 10.Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev 2003(1):CD004016. [DOI] [PMC free article] [PubMed]
- 11.Koester MC, Dunn WR, Kuhn JE, Spindler KP. The efficacy of subacromial corticosteroid injection in the treatment of rotator cuff disease: a systematic review. J Am Acad Orthop Surg 2007;15:3-11. [DOI] [PubMed] [Google Scholar]
- 12.Neustadt DH. Local corticosteroid injection therapy in soft tissue rheumatic conditions of the hand and wrist. Arthritis Rheum 1991;34:923-6. [DOI] [PubMed] [Google Scholar]
- 13.Gruson KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg 2008;17(suppl):118-30S. [DOI] [PubMed] [Google Scholar]
- 14.Hollingworth GR, Ellis RM, Hattersley TS. Comparison of injection techniques for shoulder pain: results of a double blind, randomised study. BMJ 1983;287:1339-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henkus HE, Cobben LP, Coerkamp EG, Nelissen RG, van Arkel ER. The accuracy of subacromial injections: a prospective randomized magnetic resonance imaging study. Arthroscopy 2006;22:277-82. [DOI] [PubMed] [Google Scholar]
- 16.Valtonen EJ. Double acting betamethasone (Celestone Chronodose) in the treatment of supraspinatus tendinitis: a comparison of subacromial and gluteal single injections with placebo. J Int Med Res 1978;6:463-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen MJ, Lew HL, Hsu TC, Tsai WC, Lin WC, Tang SF, et al. Ultrasound-guided shoulder injections in the treatment of subacromial bursitis. Am J Phys Med Rehabil 2006;85:31-5. [DOI] [PubMed] [Google Scholar]
- 18.Naredo E, Cabero F, Beneyto P, Cruz A, Mondejar B, Uson J, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol 2004;31:308-14. [PubMed] [Google Scholar]
- 19.Hawkins RH, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med 1980;8:151-8. [DOI] [PubMed] [Google Scholar]
- 20.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins symptom checklist (HSCL): a self-report symptom inventory. Behav Sci 1974;19:1-15. [DOI] [PubMed] [Google Scholar]
- 21.Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. Development of a shoulder pain and disability index. Arthritis Care Res 1991;4:143-9. [PubMed] [Google Scholar]
- 22.Kirkley A, Alvarez C, Griffin S. The development and evaluation of a disease-specific quality-of-life questionnaire for disorders of the rotator cuff: the Western Ontario rotator cuff index. Clin J Sport Med 2003;13:84-92. [DOI] [PubMed] [Google Scholar]
- 23.Brox JI, Gjengedal E, Uppheim G, Bohmer AS, Brevik JI, Ljunggren AE, et al. Arthroscopic surgery versus supervised exercises in patients with rotator cuff disease (stage II impingement syndrome): a prospective, randomized, controlled study in 125 patients with a 2 1/2-year follow-up. J Shoulder Elbow Surg 1999;8:102-11. [DOI] [PubMed] [Google Scholar]
- 24.Green S, Buchbinder R, Forbes A, Bellamy N. A standardized protocol for measurement of range of movement of the shoulder using the Plurimeter-V inclinometer and assessment of its intrarater and interrater reliability. Arthritis Care Res 1998;11:43-52. [DOI] [PubMed] [Google Scholar]
- 25.Williams JW Jr, Holleman DR Jr, Simel DL. Measuring shoulder function with the shoulder pain and disability index. J Rheumatol 1995;22:727-32. [PubMed] [Google Scholar]
- 26.Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. J Clin Epidemiol 2004;57:1008-18. [DOI] [PubMed] [Google Scholar]
- 27.Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 1992;11:1685-704. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AJ. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med Res Methodol 2005;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lirette R, Morin F, Kinnard P. The difficulties in assessment of results of anterior acromioplasty. Clin Orthop Relat Res 1992;(278):14-6. [PubMed]
- 30.Romeo AA, Bach BR Jr, O’Halloran KL. Scoring systems for shoulder conditions. Am J Sports Med 1996;24:472-6. [DOI] [PubMed] [Google Scholar]
- 31.Paul A, Lewis M, Shadforth MF, Croft PR, Van Der Windt DA, Hay EM. A comparison of four shoulder-specific questionnaires in primary care. Ann Rheum Dis 2004;63:1293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razmjou H, Bean A, van Osnabrugge V, MacDermid JC, Holtby R. Cross-sectional and longitudinal construct validity of two rotator cuff disease-specific outcome measures. BMC Musculoskelet Disord 2006;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez CM, Litchfield R, Jackowski D, Griffin S, Kirkley A. A prospective, double-blind, randomized clinical trial comparing subacromial injection of betamethasone and xylocaine to xylocaine alone in chronic rotator cuff tendinosis. Am J Sports Med 2005;33:255-62. [DOI] [PubMed] [Google Scholar]
- 34.Carette S, Moffet H, Tardif J, Bessette L, Morin F, Frémont P, et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: a placebo-controlled trial. Arthritis Rheum 2003;48:829-38. [DOI] [PubMed] [Google Scholar]
- 35.Guerra de Hoyos JA, Andrés Martín Mdel C, Bassas y Baena de Leon E, Vigára Lopez M, Molina López T, Verdugo Morilla FA, et al. Randomised trial of long term effect of acupuncture for shoulder pain. Pain 2004;112:289-98. [DOI] [PubMed] [Google Scholar]
- 36.Speed CA, Richards C, Nichols D, Burnet S, Wies JT, Humphreys H, et al. Extracorporeal shock-wave therapy for tendonitis of the rotator cuff: a double-blind, randomised, controlled trial. J Bone Joint Surg Br 2002;84:509-12. [DOI] [PubMed] [Google Scholar]
- 37.Bartolozzi A, Andreychik D, Ahmad S. Determinants of outcome in the treatment of rotator cuff disease. Clin Orthop Relat Res 1994;(308):90-7. [PubMed]
- 38.Croft P, Pope D, Silman A. The clinical course of shoulder pain: prospective cohort study in primary care. BMJ 1996;313:601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park HB, Yokota A, Gill HS, El Rassi G, McFarland EG. Diagnostic accuracy of clinical tests for the different degrees of subacromial impingement syndrome. J Bone Joint Surg 2005;87:1446-55. [DOI] [PubMed] [Google Scholar]
- 40.Speed CA. Injection therapies for soft-tissue lesions. Best Pract Res Clin Rheumatol 2007;21:333-47. [DOI] [PubMed] [Google Scholar]
- 41.Bisset L, Beller E, Jull G, Brooks P, Darnell R, Vicenzino B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ 2006;333:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Ann Rheum Dis 2003;62:394-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ginn KA, Herbert RD, Khouw W, Lee R. A randomized, controlled clinical trial of a treatment for shoulder pain. Physical Therapy 1997;77:802-11. [DOI] [PubMed] [Google Scholar]