Abstract
Persistent inflammation and the generation of reactive oxygen and nitrogen species play pivotal roles in tissue injury during disease pathogenesis and as a reaction to toxicant exposures. The associated oxidative and nitrative stress promote diverse pathologic reactions including neurodegenerative disorders, atherosclerosis, chronic inflammation, cancer, and premature labor and stillbirth. These effects occur via sustained inflammation, cellular proliferation and cytotoxicity and via induction of a proangiogenic environment. For example, exposure to the ubiquitous air pollutant ozone leads to generation of reactive oxygen and nitrogen species in lung macrophages that play a key role in subsequent tissue damage. Similarly, studies indicate that genes involved in regulating oxidative stress are altered by anesthetic treatment resulting in brain injury, most notable during development. In addition to a role in tissue injury in the brain, inflammation, and oxidative stress are implicated in Parkinson's disease, a neurodegenerative disease characterized by the loss of dopamine neurons. Recent data suggest a mechanistic link between oxidative stress and elevated levels of 3,4-dihydroxyphenylacetaldehyde, a neurotoxin endogenous to dopamine neurons. These findings have significant implications for development of therapeutics and identification of novel biomarkers for Parkinson's disease pathogenesis. Oxidative and nitrative stress is also thought to play a role in creating the proinflammatory microenvironment associated with the aggressive phenotype of inflammatory breast cancer. An understanding of fundamental concepts of oxidative and nitrative stress can underpin a rational plan of treatment for diseases and toxicities associated with excessive production of reactive oxygen and nitrogen species.
Keywords: cytokine signaling, carcinogenesis, developmental toxicity, prenatal, inflammation
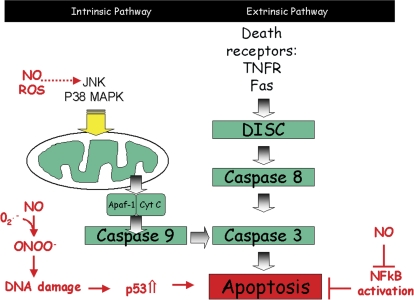
Toxicants cause tissue damage via diverse mechanisms, many of which involve activation of cell survival and apoptosis pathways. One of the key areas of recent interest is the role that oxidative and nitrative stress play in mediating the response to toxicants via this cytotoxic pathway. Two broad categories of apoptosis networks, referred to as the intrinsic and extrinsic pathways have been identified (reviewed in Aslan et al., 2008). The extrinsic pathway is driven by signaling from death receptors such as the tumor necrosis factor-α (TNF-α) receptor and Fas (Fig. 1) through the death-inducing signaling complex (DISC) and caspase 8. The intrinsic pathway is triggered by cellular stress and signals through survival kinases to the mitochondria which then engage caspase 9 through cytochrome c release. Both pathways converge on caspase 8 leading to cellular execution (reviewed in Aslan et al., 2008). Oxidative and nitrative species can impact on these pathways at several key points; reactive nitrogen species (RNS) and reactive oxygen species (ROS) can modulate survival signaling molecules such as c-Jun N-terminal kinase and p38 mitogen activated protein (MAP) kinase (reviewed in Aslan et al., 2008). Similarly RNS such as nitric oxide (NO) are able to cause DNA damage, leading to p53 stabilization and engagement of apoptosis pathways. Conversely, NO downregulates nuclear factor κB (NF-κB) which protects from apoptosis. This review analyzes the role for oxidative and nitrosative species in the response to diverse toxicants and presents evidence from different tissues to illustrate areas of consensus and those where more knowledge is needed. [The concepts and ideas were presented and debated at the Symposium ‘Oxidative and Nitrative Stress in Toxicology and Disease’ held at the annual Society of Toxicology Meeting, Baltimore, March 16, 2009.]. Although the examples chosen are deliberately diverse, there is much in common: “Replication ‘with difference’ builds the best case for a generality – for how can we prove a coordinating hypothesis unless we can apply it to multiple cases” (Gould, 2002). In addition, we propose new targets and therapeutic interventions for treatment of various disease pathologies.
FIG. 1.
A schematic depicting the impact of NO and ROS on the intrinsic and extrinsic pathways that regulate apoptosis.
WHAT IS OXIDATIVE STRESS?
Aerobic biologic systems have the capability of oxidizing some substances and reducing others, the ultimate oxidant being O2 and reducing species attributed to “food” (Fig. 2). The term “oxidative stress” is not defined intrinsically but balance models of oxidant stress based on chemical equilibrium concepts are most influenced by inhale/exhale and feast/fast effects (Smith, 1991). Lower glutathione (GSH) levels may reflect increased rates of oxidation of GSH, by reduction of H2O2 or other oxidants (Fig. 2). This would be associated with increased concentrations of glutathione disulfide (GSSG) and increased rates of production or pool turnover as additional indicators of increased oxidant activities. Conversely, when lower GSH levels compromise antioxidant defence functions, increases in oxidation of the substrate molecules proposed to be at greater exposure would be expected. Lower GSH concentrations may act through increased GSSG/GSH ratios, resulting in increased S-thiolations of critical protein thiols.
FIG. 2.
Cellular compartmentation of GSH and GSSG and metabolism of oxidants. Extracellular, intracellular, mitochondrial, and nuclear compartments are indicated, but other subcompartmental distinctions are not precluded. Although simplified, the schemes shown illustrate the fractal properties of branching in H2O2 disposition, as between GPx and other thiol/disulfide-dependent pathways, and Fe-chelate–directed reactions that are not reflected by thiol/disulfide reactions. Also illustrated is that cellular oxidation capacities are based on O2 and reduction capacities ultimately rely upon food, with NADPH as a key intermediary for any estimates of a global or ‘the’ redox status of a cell. Other concepts to consider are that H2O2 could react with Fe2+ to form hydroxyl radicals which may lead to lipid peroxides, but perhaps not directly to LOH. DNA (both nuclear and mitochondrial) may also be a target for such radicals. Abbreviations: CoASH, coenzyme A; CoASSG, mixed disulfide of CoASH with GSH; PSH, protein thiol, PSSG, mixed disulfide with GSSG; GPx, glutathione peroxidase; GR, GSSG reductase; ALF, alveolar lining fluid; LOH and LOOH, lipid hydroxy acids and hydroperoxides, respectively.
In experimental animals, massive increases in production of GSSG can be affected without initiation of tissue injury or even of appreciable decreases in tissue levels of GSH (Gupta et al., 1994; Smith, 1987). Conversely, much greater decreases in GSH levels can be produced without increasing production of GSSG or initiating tissue injury (Smith, 1991). Increases in GSSG formation can be observed in models in which injury also is produced, but the association with damage is more robust with other biomarkers. At the same time, the absence of measurable changes in cell or tissue levels of GSSG do not disprove hypotheses regarding oxidant contributions to a mechanism of damage or other effect, as compartmentalization of oxidant generation or molecular specificity of effect could obscure the relevant mechanisms from detection by less specific methods of analysis. Changes in concentrations or ratios of a given thiol/disulfide pair, such as GSH/GSSG, or other redox couple, such as nicotinamide adenine dinucleotide phosphate (reduced) (NADPH)/nicotinamide adenine dinucleotide phosphate (NADP+) are not necessarily accompanied by changes in other couples that would be predicted from chemical principles applicable to homogeneous solutions and should not be accepted uncritically as evidence of a parallel effect. Nevertheless, changes in biomarker pairs do offer evidence that something is different and thereby can provide useful clues for avenues for further investigation (Smith, 2005).
OXIDATIVE STRESS, MITOCHONDRIAL DYSFUNCTION, AND BRAIN INJURY
Increasing evidence suggests a role for oxidative and nitrative stress in potential adverse reactions to anesthetics. Recent studies using in vitro approaches or in vivo animal models have indicated that blocking oxidative or nitrative stress will ameliorate anesthetic-induced brain cell death. The brain has been highlighted by advances in pediatric and obstetric surgery, which have necessitated an increase in the duration and complexity of anesthetic procedures. Specifically, it has been reported that anesthetic drugs cause widespread and dose-dependent apoptosis in the developing rat brain (Ikonomidou et al., 1999; Jevtovic-Todorovic et al., 2003; Scallet et al., 2004). In order to understand this phenomenon and its relevance to humans, studies have been undertaken in the monkey, a species with marked similarities to human physiology, pharmacology, metabolism, and reproductive systems. The window of vulnerability to the neuronal effects of anesthetics is restricted to the period of rapid synaptogenesis, also known as the brain growth spurt. Although the synaptogenesis is largely a early postnatal event in the rodent (first 2 weeks of life), in the human and nonhuman primate it extends from mid pregnancy through the first 2 years or first few months of life in the human and monkey, respectively.
In comparing the rodent and the nonhuman primate data, it is clear that the topography, characteristics and neuronal susceptibility to neurotoxic insult that leads to neuronal cell death depends on several factors including the dose and duration of exposure to anesthetic, the receptor subtype activated and the cell type, as well as the animal's stage of development (Ankarkona et al., 1995, Slikker et al., 2007; Wang et al., 2000). For one agent, the noncompetitive NMDA (N-methyl-d-aspartate) receptor antagonist and dissociative anesthetic, ketamine, the duration of exposure required to induce cell death under minimal exposure requirements is similar (4–6 h) for nonhuman primate and rodent brain cells in culture. In vivo, the susceptible stage or period of development has not been completely described but begins somewhere before the last quarter of pregnancy and continues to shortly after birth in the nonhuman primate. Shorter duration ketamine exposures (ketamine infusion 3 h vs. 9 or 24 h) do not produce neuronal cell death in this model (Slikker et al., 2007).
Reduction of Oxidative Stress Protects from Cell Death in the Brain
Several recent studies have indicated that reduction of oxidative stress may protect the developing animal from anesthetic-induced brain cell death. Thus, continuous exposure of developing brains to ketamine causes selective cell death by a mechanism that involves a compensatory upregulation of NMDA receptor subunits (Wang et al., 2005, 2006). This upregulation makes neurons bearing these receptors more vulnerable, after ketamine washout, to the excitotoxic effects of endogenous glutamate. In fact, this hypothesis was supported by the observation that the NR1 subunit mRNA was upregulated in ketamine-treated monkey fetuses (gestation day 122) and infants (post natal day 5) compared with their controls, and increased expression of NMDA receptor NR1 protein in ketamine-treated neurons (in vitro monkey). In addition, coadministration of NR1 antisense oligonucleotide (targeted to NR1 NMDA receptor subunit mRNA) is able to block the neuronal cell death induced by ketamine in rat and monkey cortical culture (Wang and Slikker, 2008). Associated with Ca2+ influx is an increase in ROS that appears to originate in the mitochondria (Johnson et al., 1998; Slikker et al., 2005). This Ca2+ loading by the mitochondria beyond its buffering capacity reduces the membrane potential and disrupts electron transport, which results in the increased production of the reactive free radical superoxide anion [O·−2] (Slikker et al., 2005, 2007; Wang et al., 2000). Meanwhile, the increased intracellular free calcium [Ca2+] is a potent activator of neuronal nitric oxide synthase (nNOS). Active nNOS generates NO, a multifaceted second messenger, which permeates neuronal cell membranes to alter specific tissue regions. Therefore, a significant issue to be resolved is whether increased neuronal NOS activity and increased generation of peroxynitrite are key regulatory steps in the apoptotic process induced by the administration of NMDA antagonists such as ketamine.
In experiments with cultured newborn rat forebrain cells, ketamine caused an increase in DNA fragmentation, elevated immunoreactivity to nitrotyrosine, a marked reduction in the expression of neuronal marker polysialic acid neural cell adhesion molecule and a reduction in mitochondrial metabolism. Among the potential downstream regulators, the ratio of Bax and BCL-XL was determined. Bax, a proapoptotic protein, is a pore-forming cytoplasmic protein which translocates to the outer mitochondrial membrane, influencing its permeability and inducing cytochrome c release from the intermembrane space of the mitochondria into the cytosol, subsequently leading to cell death (Cropton, 2000). Thus a decrease in the BCL-XL/Bax ratio is associated with apoptosis as was seen with ketamine. Interestingly, all these ketamine-induced neurotoxic effects were blocked by 7-Nitroindazole, an NOS inhibitor. Overall, these data suggest a role for NRS as mediated by NO in enhanced degeneration induced by ketamine in vitro and that blockage of nNOS may be beneficial for reducing the risk of pediatric use of ketamine (Wang et al., 2008). Further evidence for the role of oxidative stress in anesthetic-induced neurotoxicity has been generated in studies that apply oxidative stress blockers including L-carnitine (mitochondrial protector) and melatonin in vivo (Yon et al., 2006) and specific antioxidants in vitro including the superoxide dismutase mimetic as described above, M40403 and the NOS inhibitor, 7-nitroindazole.
The hypothesis that oxidative stress is associated with anesthetic-induced brain cell death is also supported by data generated by studies on the catalase pathway. Although the precise mechanisms by which NMDA regulates neuroapoptosis is unknown, blockade of this activity in vitro by the addition of catalase and superoxide dismutase (Wang et al., 2000), or in vivo by M40403 (superoxide dismutase mimetic) supports the involvement of ROS. Further evidence for the role of oxidative stress in anesthetic-induced neurotoxicity has been generated in studies that apply oxidative stress blockers including L-carnitine (mitochondrial protector) and melatonin in vivo and specific antioxidants in vitro including the superoxide dismutase mimetic as described above, M40403 and the NOS inhibitor, 7-nitroindazole. Recent gene expression assessments indicate that genes along the oxidative stress pathway are altered by a single, high dose ketamine treatment of the developing rat (Slikker et al., 2007). Together, the application of omics approaches along with traditional toxicological endpoints suggests that the susceptibility of the developing brain to anesthetics is mediated by oxidative stress.
Antioxidants Attenuate the Induction of Anesthetic-Induced Cell Death
The conclusion that the susceptibility of the developing brain to anesthetics is mediated by oxidative stress is further supported by studies on the anesthetic gas nitrous oxide (N2O) and the volatile anesthetic isoflurane (ISO). Both are commonly utilized in surgical procedures for human infants and in veterinary and laboratory animal practice to produce the salubrious properties such as loss of consciousness and analgesia. Recent reports indicate that exposure of the developing brain to general anesthetics that block NMDA glutamate receptors or potentiate gamma aminobutyric acid(A) receptors can trigger widespread apoptotic neurodegeneration (Jevtovic-Todorovic et al., 2003). A subsequent publication indicated that melatonin, an antioxidant, attenuated the effects produced by these anesthetic agents in the developing rat pup (Yon et al., 2006). Two main questions remain: what are the effects of equi-anesthetic levels of N2O and ISO alone or in combination and can a mitochondrial protector such as L-carnitine attenuate these effects?
In postnatal day 7 pups, no significant neurotoxic effects were observed in animals exposed to N2O or ISO alone. However, enhanced apoptotic cell death was apparent when N2O was combined with ISO, at exposure durations of 6 hours or more. Coadministration of L-carnitine protected neurons from inhaled anesthetic-induced damage. These data indicate that 6 h or more of inhaled anesthetic exposure results in enhanced neuronal apoptosis in developing rat brain and that L-carnitine effectively blocks this neuronal apoptosis. These data also indicate that the in vivo application of the mitochondrial protector L-carnitine may provide a strategy for clinical neuroprotection (Zou et al., 2009).
In summary, the data presented suggest that anesthetic exposure can cause apoptosis in the developing brain (Fig. 3). Although several mechanisms are proposed, upregulation of the NR1 subunit of the NMDA receptor appears to be an important first step in the pathway to anesthetic-induced neurotoxicity. Subsequently, excitotoxic effects of glutamate are largely mediated by increased Ca2+ influx through activated NMDA receptors. Associated with Ca2+ influx is an increase in the generation of ROS that appears to originate in mitochondria. This leads to dissociation and nuclear translocation of transcription factors such as NF-κB ultimately resulting in downregulation of Bcl-XL combined with an increase in Bax and apoptosis via mitochondrial-mediated mechanisms. Understanding these toxicity pathways allows postulation of protective approaches and their systematic assessment. Several recent studies using blockers of oxidative stress such as L-carnitine, melatonin, the superoxide dismutase mimetic, M40403, and the NOS inhibitor, 7-nitroindazole have indicated that reduction of oxidative stress may protect the developing animal from anesthetic-induced brain cell death.
FIG. 3.
A working model of NMDA antagonist-induced neuronal cell death. Excessive activation of upregulated NMDA receptors results in a calcium overload that exceeds the buffering capacity of the mitochondria and interferes with electron transport yielding ROS. This in turn causes dissociation and nuclear translocation of transcription proteins such as NF-κB that bind to genes such as P53 and Bcl-XL. The downregulation of Bcl-XL combined with an increase in Bax, diminishes the formation of antiapoptotic Bax/Bcl-XL heterodimers in favor of proapoptotic Bax/Bax homodimers.
MACROPHAGES, OXIDATIVE STRESS, AND LUNG INJURY
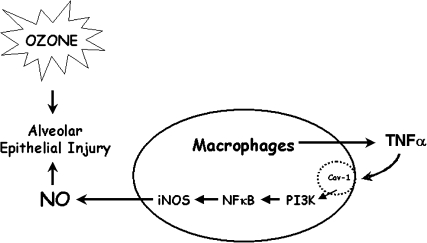
In addition to being implicated in brain injury, considerable evidence supports a role for reactive oxygen and RNS in lung injury induced by pulmonary irritants. These and other inflammatory mediators are released by phagocytic leukocytes and infiltrating macrophages in the lung (Benoit et al., 2008; Laskin and Laskin, 2001; Laskin et al., 2001; Libby, 2007). Of particular interest is the role of ROS and RNS in the toxicity of ozone, a ubiquitous urban air pollutant known to cause oxidative damage to the lower lung (Mudway and Kelly, 2000). Following ozone inhalation by rodents, alveolar macrophages are activated to generate excessive quantities of NO and peroxynitrite (Fakhrzadeh et al., 2002, 2004a, b, 2008; Pendino, et al., 1993, 1995, 1996) (Fig. 4). Moreover, inhibiting the activity of macrophages or production of these RNS abrogates ozone-induced tissue injury (Fakhrzadeh et al., 2002, 2004a, b; Pendino et al., 1995). These data provide direct support for the idea that macrophages and inflammatory mediators such as RNS can contribute to tissue damage.
FIG. 4.
Exposure to toxic levels of ozone results in alveolar epithelial damage. Macrophages responding to tissue injury release TNF-α which downregulates caveolin-1. This leads to release of signaling molecules such as PI3K, activation of NF-κB and upregulation of iNOS. RNS generated as a consequence contribute to ozone-induced lung injury.
Production of RNS in the Lung after Ozone Inhalation
There are several mechanisms proposed to explain the regulation of excessive production of RNS by macrophages in the lung after ozone inhalation. NO is produced in macrophages via an inducible form of the enzyme, NO synthase (iNOS or NOS2). This enzyme is upregulated by inflammatory mediators such as bacterially derived lipopolysaccharide (LPS), and cytokines like tumor necrosis factor alpha (TNF-α). TNF-α exerts its biological activity by binding to a receptor on macrophages; 2 major receptors have been identified: TNFR1 (p55) and TNFR2 (p75). Evidence suggests that the proinflammatory actions of TNF-α are mediated predominantly via activation of TNFR1. This receptor is localized in both lipid raft and non-raft regions of the plasma membrane. In response to TNF-α, TNFR1 rapidly associates with lipid rafts where it complexes with TNF-α (Doan et al., 2004). This leads to the recruitment of adaptor proteins and activation of a cascade of signaling molecules including phosphatidylinositol 3 (PI 3)-kinase, p44/42 MAP kinase, and consequent activation of NF-κB, a transcription factor important in the regulation of many inflammatory genes including iNOS (Aggarwal et al., 2002).
Following ozone inhalation, there is a rapid and persistent activation of NF-κB in alveolar macrophages. This is associated with a rapid induction of PI 3-kinase and its downstream target, protein kinase B (PKB). These data, together with the observation that inhibitors of PI 3-kinase block excessive NO production by alveolar macrophages from ozone treated mice suggest that NF-κB and upstream regulators PI 3-kinase and PKB are important in controlling expression of iNOS. This is supported by findings that ozone-induced increases in NF-κB nuclear binding activity are significantly attenuated in NF-κB p50−/− (knockout) transgenic mice (Fakhrzadeh et al., 2004b). Additionally, the loss of NF-κB p50 is associated with a marked reduction in ozone-induced upregulation of iNOS and an abrogation of NO or peroxynitrite generation by macrophages. Moreover, mice lacking NF-κB p50 were protected against ozone-induced toxicity. Taken together, these data demonstrate that ozone-induced expression of iNOS and NO production in the lung are dependent on NF-κB.
Ozone and Lung Toxicity: Role of TNF-α
As described above, TNF-α is known to be a major activator of NF-κB signaling. Treatment of mice with ozone resulted in increased expression of TNF-α in the lung (Fakhrzadeh et al., 2004b; Pendino et al., 1994). Using knockout mice with a targeted deletion of the gene for TNF-α, the role of this cytokine in ozone-induced NF-κB activation and iNOS induction was investigated. Ozone had no effect on NF-κB activity or on iNOS induction in TNF-α knockout mice. The fact that this was associated with protection from ozone toxicity suggests that TNF-α produced early after ozone inhalation contributes to tissue injury, in part, by stimulating the production of cytotoxic inflammatory mediators such as NO and peroxynitrite via activation of NF-κB.
Caveolae and TNF-α–Mediated Lung Responses
Caveolae are small (50–100 nm) vesicular invaginations of the plasma membrane. Because of their unique lipid composition, caveolae are classified as plasma membrane lipid rafts. The chief structural proteins of caveolae are caveolins. To date, 3 caveolins (Cav-1, Cav-2, and Cav-3) with unique tissue distribution have been characterized. Cav-1 is a 21- to 24-kDa protein that has been identified as the main structural and functional protein of caveolae, required for their formation and stabilization. Cav-1 functions as a membrane-organizing center, concentrating signaling molecules within a scaffolding domain and negatively regulating their activation state (Hnasko and Lisanti, 2003). Functional caveolin-binding motifs have been identified in a number of signaling molecules including TNFR1, in several serine/threonine kinases (p44/42 MAP kinases, PI 3-kinase) and tyrosine kinases (src kinase), as well as in endothelial NO synthase, and heme oxygenase-1 (Williams and Lisanti, 2004). Downregulation of Cav-1 leads to activation of these signaling pathways.
Alveolar macrophages from control animals constitutively express Cav-1 protein (Fakhrzadeh et al., 2008). Treatment of mice with ozone caused an immediate decrease in Cav-1 expression that began to return to control at 12 h. The suppressive effects of ozone on Cav-1 expression were not observed in TNFR1−/− mice demonstrating that ozone-induced alterations in Cav-1 are dependent on TNF-α signaling via this receptor. This conclusion is supported by the observation that TNF-α was effective in suppressing Cav-1 expression in macrophages in culture.
In summary, the data support a model where exposure to toxic levels of ozone results in injury to Type I alveolar epithelial cells in the lower lung and the release TNF-α by alveolar macrophages. Consequently, TNFR1 signaling via TNFR1 downregulates Cav-1 in macrophages leading to activation of signaling pathways like PI-3 kinase/PKB, subsequent activation NF-kB, upregulation of iNOS, and excessive production of reactive oxygen and nitrogen species. Understanding processes of ozone-induced tissue injury is critical for the development of clinical approaches for treating or abrogating toxicity induced not only by air pollutants, but also potentially, by chronic and episodic inflammatory lung diseases.
THE ROLE OF NITRATIVE AND OXIDATIVE STRESS IN SEPSIS
So far, we have considered basic mechanisms of oxidative and nitrative stress as illustrated by exposure of the developing brain and the lungs to anesthetics and ozone, respectively. Now, we consider the role of ROS and RNS in sepsis and preterm labor. Sepsis is a major challenge to health care in the United States, affecting about 750,000 people, causing about 215,000 deaths, and costing nearly $17 billion annually (Zhao et al., 2006). Oxidants, radicals, ROS, and oxidant stress mechanisms have been implicated in septic shock, but the mechanisms underlying their effects have not been defined with the specificity that will be essential in developing approaches to the prevention and treatment of this complex medical condition.
Inflammation and Premature Labor
In the United States, 12.7% of infants born in 2005 were delivered at less than 37 weeks gestational age, which represents a 34% increase from the rate of 9.5% reported in 1981. The annual economic cost of preterm labor and birth to the United States was estimated to be at least $26 billion (Behrman and Butler, 2006; Goldenberg et al., 2008; Shepherd et al., 2004; Zhao et al., 2006), and preterm delivery is associated with about half of long-term morbidities arising in newborns. Worldwide, an estimated 4 million deaths occur each year in neonates (less than 4 weeks of age), and 28% of these deaths are attributed to preterm birth as the primary cause (Lawn et al., 2005). In addition, an estimated 4 million infants are stillborn each year. The cause and effect mechanisms that contribute to inflammatory responses in preterm birth, stillbirth, and sepsis thus merit more research interest than has been directed at these problems to date.
Mechanisms of Preterm Birth
Chorioamnionitis, gingivitis, bacterial vaginosis, and other maternal infections have been implicated as major causal factors in premature births (Goldenberg et al., 2000), usually defined as births at less than 37 weeks gestational age. Despite the associations between maternal infections and preterm birth, prophylactic administration of antibiotics to pregnant women, even those in spontaneous preterm labor with intact membranes, has shown no efficacy for preventing preterm births (Kenyon et al., 2008). Further, adverse effects of antibiotic treatments have been observed, including increased incidence of cerebral palsy. Animal models of premature birth have shown that preterm delivery and intrauterine fetal death can be produced by administration of live pathogens or by purified bacterial products, such as LPS or lipoteichoic acid (Fidel et al., 1998; Kajikawa et al., 1998). The induction of preterm labor and of fetal demise by administration of killed bacteria or even purified bacterial components suggests an explanation as to why antibiotic treatments are not effective in preventing preterm birth. In addition, the observations of increased rates of fetal death in these experimental models suggest close mechanistic links between stillbirth and preterm birth in humans.
In both pregnant and nonpregnant experimental animals, administration of bacteria, or bacterial products initiates marked increases in cytokine levels, but the broad and overlapping responses in individual cytokines serves to obscure any critical mechanistic pathways that might exist. So, do oxidative mechanisms contribute to preterm delivery and fetal death? When LPS was administered to pregnant mice, decreases in hepatic GSH levels were observed in both the dams and the pups (Buhimschi et al., 2003). It was also reported that the effects of LPS on acceleration of delivery, fetal death, and hepatic GSH levels were attenuated by administration of N-acetylcysteine (NAC). The authors interpreted their data as indicating that free radicals generated in large quantities in response to LPS administration were shifting the maternal–fetal redox balance to an oxidative state, resulting in the observed fetal death and preterm delivery. These studies provide a useful first step in investigation of possible contributions of biochemical mechanisms to infection and preterm birth.
Oxidative Stress, Preterm Birth, and Fetal Death
In the report by Buhimschi, decreases in hepatic GSH were observed only at 16 h post LPS treatment (Buhimschi et al., 2003), whereas no differences in GSH were observed in fetuses or dams at 3 or 6 h. None of the fetuses examined 3 or 6 h after administration of LPS were dead, but over half of all the fetuses were dead by 16 h. The median time to onset of delivery in LPS-treated mice was 16.8 h, and all fetuses delivered were dead. Causes must precede effects, and breakdown of the compartmentalization of oxidants and reductants on which cell functions rely would be expected to arise during damage and/or death of aerobic cells and result in increased oxidations of biological molecules. Hence, the distinctions between cause and effect for hepatic GSH depletion, fetal demise, and preterm delivery are speculative at the moment, however logical and appealing the working hypothesis.
ROS Involved in Sepsis
Sepsis occurs as a result of complex host-pathogen interactions, leading to release of inflammatory mediators, as well as reactive oxygen intermediates (ROS) and reactive nitrogen intermediates (RNS). Neutrophils and monocyte/macrophages are the sentinel phagocytic cell primarily responsible for engulfment and destruction of pathogenic organisms during infection of the host. ROS and RNS are antimicrobial agents produced by these leukocytes that can directly destroy microbial pathogens. During sepsis, excess production of ROS and RNS can be a detriment, inducing significant cytotoxicity to organs and contributing to the sequelae of unresolved sepsis, multiorgan system failure (Fialkow et al., 2007).
The inflammatory mediators best characterized as having a role in sepsis are Interleukin-1 (IL-1), tumor necrosis factor (TNF), and IL-6. Very recent studies have identified a new sepsis-related cytokine, Interleukin-27, as a member of the IL-6/IL-12 cytokine family that is a negative regulator of innate immunity during sepsis (Wirtz et al., 2006).
These inflammatory mediators, as well as ROS and RNS induce cytotoxicity leading to damage to multiple organs, and without resolution of these activities may ultimately result in multisystem organ failure. ROS and RNS are known to directly induce cytotoxicity to organs and can also alter cell signaling pathways.
Interestingly the source of ROS may be a key to the extent of cellular cytotoxicity that occurs during sepsis and may also be involved in the ultimate ability of the host to limit sepsis. A very recent study reported that ROS derived from NADPH oxidase limited acute inflammatory responses in vivo induced by LPS administration, suggesting that the ROS derived from this enzyme source may limit the extent of cytotoxicity and alterations in oxidation reduction reactions such as changes in GSH during sepsis (Zhang et al., 2009). During LPS-induced endotoxemia, ROS was found to be significantly increased and was shown to limit the availability of NO, resulting in LPS-induced endothelial dysfunction, with the primary source of ROS identified as cyclooxygenase-2 (Cox-2) (Virdis et al., 2005).
Studies within the last 5 years have identified a defect in neutrophil function of neonates that may be associated with the predisposition of preterm neonates to bacterial infection. In adults, neutrophils, and mast cells form extracellular traps (ETs) composed of extracellular DNA, chromatin, histones, and antibacterial peptides, proteolytic enzymes that destroy microbes. This phagocytic activity of neutrophils that have produced ETs results in induction of a specific death pathway defined as “Etosis” which these neutrophils. Etosis is mediated through ROS generated through the NADPH oxidase enzyme pathway (Wartha and Henriques-Normark, 2008). In preterm infants, neutrophils have an impaired formation of ETs which is associated with the lack of microbiocidal activity of neutrophils from preterm neonates and was not found to be linked to the presence of ROS. These defects in linked pathways in preterm infants are thought to be a common immunodeficiency in the lack of response of preterm neonates to respond to microbial challenge (Yost et al., 2009).
In summary, the lives lost and the social and economic costs of preterm birth and stillbirth are enormous in the United States and throughout the world. Maternal infections are associated with and likely responsible for a large fraction of these problems, but prevention of all exposures of pregnant women to bacteria or other potential pathogens is impractical and possibly even undesirable. Treatments of clinically significant infections in women clearly are essential, defining the more complex mechanisms relating maternal infections with stillbirths, preterm births, and other adverse effects on fetal health and development is essential for the rational design and investigation of approaches to amelioration of these underappreciated problems in human health. Much can be learned both from further study of this area and also from basic mechanistic work on oxidative and nitrative stress in other organ systems such as lung, brain and gut.
OXIDATIVE STRESS IN REGULATING THE AGGRESSIVE PHENOTYPE OF INFLAMMATORY BREAST CANCER
It is clear that oxidative and nitrative stress play a role in the response to toxicants and in proinflammatory conditions such as sepsis. The data presented above make a case for a role for the inflammatory response to bacteria in preterm birth and fetal death. Now we move on to consider the role oxidative stress plays in the development of diseases such as cancer, in particular, inflammatory breast cancer (IBC). IBC is the most aggressive and lethal form of breast cancer and has the worst prognosis of any variant of this disease (reviewed in Dawood et al., 2008). IBC tumors do not commonly present as a lump but are rather diffuse, requiring imaging modalities such as magnetic resonance imaging (MRI) and positron emission tomography (PET) for accurate diagnosis and tumor staging. IBC commonly presents as a skin rash due to infiltration of IBC tumor cells into dermal lymphatics, leading to common misdiagnosis of this type of breast cancer as mastitis and treatment of IBC patients with antibiotics, delaying correct diagnosis. IBC patients often have lymph node involvement, with late stage disease (stage IIIb or stage IV) at the time of first accurate diagnosis. Although the standard of care for IBC patients for the past 30 years has been multimodality treatment combining chemotherapy, surgery, and radiation, there has been no change in the very low survival rate of 2.9 years for patients diagnosed with IBC during this time (Ueno et al., 1997).
IBC and the Role of NF-κB and Inflammatory Mediators
Based on studies evaluating IBC tumors, an inflammatory signature has been defined suggesting that IBC is characterized by the involvement of genes regulated by the transcription factor NF-κB (Charafe-Jauffret et al., 2004). Thus, there is a common mechanism of signaling with the brain and lung injury described above but there are stark contrasts in the availability of model systems to study IBC because there are only 2 cell lines available, the SUM190 and SUM149 cell lines with which to study this disease. The SUM190 cell line was developed from the primary tumor of an IBC patient and the SUM149 cells were derived from metastatic tumor cells isolated from pleural effusion fluid of an IBC patient (Ethier, 1996).
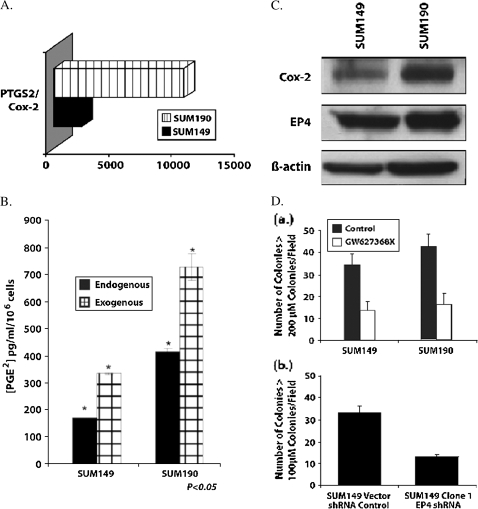
In order to understand basic mechanisms and to define potential therapeutic targets, real time PCR arrays were used in combination with Western blotting to elucidate IBC-related genes involved in oxidative and nitrative stress and associated inflammation. Both SUM149 and SUM190 IBC breast tumor cells express levels of the PTGS-2 gene that are 2500 and 11,000-fold increased, respectively, compared with the PTGS-2 null MCF-7 breast cancer cell line (Fig. 5A). PTGS-2 encodes for the inducible form of Cox-2, the enzyme responsible for production of the proinflammatory mediator, prostaglandin E2 (PGE2). Although previous studies have demonstrated that Cox-2 is highly elevated in IBC tumors (Charafe-Jauffret et al., 2004), the loss of selective Cox-2 inhibitors had lead the way in the search for alternative approaches to block Cox-2 driven inflammatory responses in this very aggressive form of breast cancer. EP4 appears to be one of the critical prostanoid receptors that may well serve as a target for Cox-2 associated therapeutics in breast cancer progression (Robertson et al., 2008).
FIG. 5.
Elucidation of IBC-related genes involved in oxidative and nitrative stress and associated inflammation. (A) PTGHS2/COX-2 mRNA is upregulated in SUM149 and SUM190 IBC cells compared with MCF-7 cells. (B) PGE2 production by SUM149 and SUM190 cells. (C) Western blot of COX-2 and EP4 proteins in SUM149 and SUM190 IBC tumor cells. (D) Inhibition of anchorage independent growth in soft agar by SUM149 IBC cells by EP4 antagonist and EP4 shRNA.
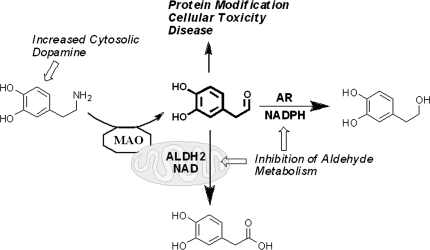
FIG. 6.
Schematic of DA catabolism. DA catabolism yields DOPAL, which undergoes oxidation to an acid (DOPAC) or reduction to an alcohol (DOPET) as a minor pathway. Increased cytosolic DA or impairment of aldehyde metabolism yields elevated levels of the reactive and toxic intermediate, DOPAL.
As a validation of the increased Cox-2 mRNA, we evaluated the levels of PGE2 production and Cox-2 protein produced by SUM149 and SUM190 (Figs. 5B and 5C). Both of these cell lines secrete elevated amounts of PGE2, which were increased with addition of the substrate for the Cox-2 enzyme, arachidonic acid (Fig. 5B). Both of the IBC cell lines also produced abundant Cox-2 protein (Fig. 5C). The importance of Cox-2 as a critical regulator of breast tumor proliferation, angiogenesis and invasion was demonstrated by previous studies showing that Cox-2 directly regulates these activities of breast tumor cells, in breast tumors and in breast tumor xenografts in immunocompromised mice (Parrett et al., 1997; Prosperi et al., 2004; Prosperi and Robertson, 2006).
To date, there have been no studies that have reported what ROS are generated in IBC tumors. There have been multiple studies documenting that IBC tumor cells produce abundant levels of inflammatory cytokines such as Interleukin-1 alpha and beta, Interleukin-8, Interleukin-8 and chemokines such as GRO and Rantes (Benoy et al., 2004; Pan et al., 2003; van Golen et al., 2000). Although some studies identified both Cox-2 and Nfκb transcription factor as being highly upregulated in IBC cell lines (Robertson et al., 2008; Van Laere et al., 2006) there been no studies that have evaluated these aggressive breast tumor types for the presence of oxidized products of arachidonic acid metabolism as a unique signature of ROS derived from oxidized products.
IBC, Inflammation, and Therapeutic Opportunities
Although numerous studies have now reported that selective Cox-2 inhibitors have antitumor and antiangiogenesis activities and effectively block breast cancer progression, use of these agents has cardiovascular risks stimulating the search for other approaches to block Cox-2/PGE2 associated activities. One alternative is to inhibit the binding of PGE2 to its G protein coupled receptors, defined as the prostanoid receptors (EPs). There are 4 members of this receptor family, designated as EP1, EP2, EP3, and EP4. Although EP1 and EP2 appear to have roles in regulating the hyperplasia at early stages of tumor development, the EP4 receptor is more likely involved in regulation of breast tumor cell invasion and metastasis through its ability to block the effects of PGE2 following receptor-ligand binding (Robertson et al., 2008). IBC tumor cells with high Cox-2 expression also express high levels of EP4 receptor (Fig. 5C). Neither antagonists of the EP1 or EP2 prostanoid receptors nor agonists of the EP3 receptor, which is recognized as a downregulatory receptor, had any significant effect on biological activities of IBC tumor cells. In contrast, both the EP4 chemical antagonist GW 627368X and SUM149 stable clones containing EP4 specific short hairpin RNAi inhibited anchorage independent growth in soft agar (Fig. 5D). Because anchorage independent growth in soft agar is used as a gold standard to determine the effect of agents on in vivo tumorigenesis, these studies suggested it would be important to evaluate the effect of EP4 blockade in vivo. Key next steps are to evaluate the effects of targeting EP4 using pharmacological and molecular strategies that block Cox-2 driven invasion, angiogenesis and metastasis in IBC xenografts.
In summary, it is clear that oxidative stress is key in the development of diseases such as IBC mediated via activation of transcription factors such as NF-κB and induction of a proinflammatory environment. There are key mediators such as Cox-2 and PGEs and the present observations demonstrate that targeting molecules associated with the Cox-2/PGE2/EP4 receptor pathways may be useful as probes to define the specific role of Cox-2 and PGE2 in IBC and potentially may serve as targets for development of effective IBC-specific therapeutics to inhibit the aggressive phenotype of this lethal variant of breast cancer.
GENERATION OF REACTIVE INTERMEDIATES DURING DOPAMINE CATABOLISM: IMPLICATIONS FOR PARKINSON'S DISEASE
As described for IBC, knowledge of the inflammatory mediators provides opportunities to target these pathways in a rational plan of treatment. This is also well illustrated when we consider Parkinson's disease (PD). PD is one of the most common neurodegenerative diseases in the United States and is characterized by the loss of dopamine (DA) neurons. This neuronal loss translates into symptoms of motor dysfunction observable as asymmetric rigidity, tremors, bradykinesia, and postural instability. Several factors have been implicated in the pathogenesis of PD, including environmental toxicants, inflammation, and oxidative stress (Jenner, 2003).
In regards to environmental toxicants, a number of pesticides have been correlated to PD etiology, particularly the organochlorine, dieldrin (Kanthasamy et al., 2005). Elevated levels of this compound have been found in the post-mortem brains of PD patients, implicating a link between pesticide exposure and PD (Corrigan et al., 2000).
PD, Pesticides, and Oxidative Stress
Previous work has demonstrated the ability of dieldrin to generate oxidative stress in cellular models of PD (Kanthasamy et al., 2005). This was observed as an increase in the ROS superoxide anion, and the production of this ROS was preventable with the pretreatment of superoxide dismutase, the enzyme responsible for the conversion of superoxide anion to hydrogen peroxide. Dieldrin also disrupts a number of other cellular processes related to PD such as mitochondrial function, lipid peroxidation, and induces apoptosis (Kanthasamy et al., 2005). Current research efforts in this area include elucidating the structure–activity relationship of dieldrin in reference to the numerous adverse cellular effects it initiates, as well as determining specific cellular targets that may explain the disruptions in cellular function and generation of oxidative stress.
The mechanism by which oxidative stress translates into selective degeneration of DA neurons is unknown. Two biomarkers of oxidative stress are the lipid peroxidation products 4-hydroxynonenal (4HNE) and malondialdehyde (MDA) (Esterbauer et al., 1991). Lipid peroxidation products (i.e., 4HNE) were found to be elevated in PD patients (Yoritaka et al., 1996), and recently, were linked to impairments in DA catabolism (Rees et al., 2007).
DA is Both a Neurotransmitter and a Neurotoxicant
DA is an important neurotransmitter; however, it is also neurotoxic given its catechol group, which has the propensity to redox cycle and generate ROS (superoxide anion) in the presence of molecular oxygen (Fig. 6). Also, of note, the auto-oxidation of this compound yields a highly Cys-reactive ortho-quinone that readily modifies proteins. DA undergoes metabolism initially by monoamine oxidase (MAO) yielding the toxic and protein-reactive intermediate 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is further oxidized to 3,4-dihydroxyphenylacetic acid (DOPAC) via aldehyde dehydrogenase (ALDH) or reduced to 3,4-dihydroxyphenylethanol (DOPET) via reductases. An outline of this catabolism (Fig. 5) illustrates that there may be multiple ALDH (e.g., mitochondrial ALDH known as ALDH2) and cytosolic AR enzymes participating in metabolism of the DA-derived aldehyde.
Oxidative Stress and DA Catabolism
Previous work has demonstrated the enzyme ALDH to be highly sensitive to products of oxidative stress (Florang et al., 2007; Jinsmaa et al., 2009; Rees et al., 2007). Using models for DA metabolism such as rat brain mitochondria, rat striatal synaptosomes and PC6-3 cells, both 4HNE and MDA were found to disrupt DA catabolism via potent inhibition of ALDH but not MAO at physiologic concentrations, yielding a several-fold increase in the level of cellular DOPAL. Reductases can compensate for impaired ALDH activity, and indeed, treatment of dopaminergic PC6-3 cells with 4HNE yielded both an increase in (DOPAL) but also (DOPET), compared with control. Surprisingly, however, MDA but not 4HNE was shown to inhibit reductase-mediated metabolism of DOPAL at low μM concentrations. The impaired DA catabolism via lipid peroxidation products was found to yield elevated levels of DOPAL–protein adducts.
Of concern for the elevated concentration of DOPAL is that this DA-derived aldehyde was found to be highly toxic to dopaminergic cells and hypothesized to be a “chemical trigger” relevant to neurodegeneration such as PD (Burke, 2003; Burke et al., 2004). Previous studies reported DOPAL to be much more toxic to dopaminergic cells compared with DA, that is, 100-fold more in vitro and 1000-fold greater in vivo. The mechanism for DOPAL-mediated cytotoxicity may involve the following: protein modification, generation of the hydroxyl radical, or mitochondrial pore transition. With respect to protein modification, elevation of [DOPAL] was correlated with increased catechol–protein adducts (Rees et al., 2007). Because DOPAL contains both a catechol and aldehyde, protein reactivity is predicted to result from interaction of amines (e.g., Lys) with the aldehyde or thiols (i.e., Cys) with the oxidized catechol (i.e., ortho-quinone); however, the DA-derived aldehyde may also be a bifunctional electrophile capable of cross-linking proteins (Rees et al., in press). In support of such a hypothesis, a recent paper reported DOPAL-mediated cross-linking of α-synuclein, a protein found to aggregate in the PD brain (Burke et al., 2008).
In summary, there are much data demonstrating the sensitivity of DA catabolism to products of oxidative stress and such work implicates a mechanistic link between oxidative stress and elevated levels of DOPAL, a neurotoxin endogenous to DA neurons hypothesized to be a factor in neurodegeneration (Fig. 5). Current and future studies seek to define the pathway by which DOPAL interacts with proteins, elucidate other mechanisms for generation of the DA-derived aldehyde at aberrant levels and define the link between DOPAL and PD-relevant neurodegeneration. Such work has significant implications for development of therapeutics as DOPAL or other disease-relevant carbonyls may represent a therapeutic target such as carbonyl scavengers that prevent protein modification and/or cross-linking (Burcham and Pyke, 2006). In addition, this research may yield biomarkers for early disease diagnosis.
CONCLUSIONS
It is clear from the examples presented that oxidative and nitrative stress play a pivotal role in disease pathogenesis and in tissue damage in response to toxicant exposures. Inflammation and subsequent adaptation or cell death are common features and several signaling pathways are implicated in these processes. Clearly, TNF-α and subsequent abrogation of associated downstream signaling via survival networks such as that mediated by NF-κB are central in most if not all of the tissues considered. Additionally, there is evidence to support an interaction between oxidative and nitrative species, mitochondrial signaling and the caspase cascade. Conversely, certain routes of investigation such as the role of caveolae have only been explored in some model systems and pathological conditions such as the response of the lung to ozone; this suggests that this could be an interesting and fruitful route of investigation for other problems such as IBC, sepsis, and PD.
FUNDING
National Institutes of Health grants (ES004738, GM034310, CA132624, AR055073, and ES005022) to D.L.L.; and National Institutes of Health Grant (R01 ES15507) to J.A.D.
Acknowledgments
The views presented in this manuscript do not necessarily reflect those of the United States Food and Drug Administration.
References
- Aggarwal BB, Shishodia S, Ashikawa K, Bharti AC. The role of TNF and its family members in inflammation and cancer: Lessons from gene deletion. Curr. Drug Targets Inflamm. Allergy. 2002;1:327–341. doi: 10.2174/1568010023344571. [DOI] [PubMed] [Google Scholar]
- Ankarkona MM, Dybukt JM, Bonfoko E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic. Biol. Med. 2008;45:367–376. doi: 10.1016/j.freeradbiomed.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Behrman R, Butler A. Preterm Birth. Causes, Consequences, and Prevention. Washington, D.C: The National Academies Press; 2006. [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J. Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, Vermeulen PB, Dirix LY. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin. Cancer Res. 2004;10(21):7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am. J. Obstet. Gynecol. 2003;188:203–208. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: Role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol. Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- Burke WJ. 3,4-dihydroxyphenylacetaldehyde: A potential target for neuroprotective therapy in Parkinson's disease. Curr. Drug Targets CNS Neurol. Disord. 2003;2:143–148. doi: 10.2174/1568007033482913. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, O'Dell M, Li SW, Pan Y, Chung HD, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol. 2008;115:193–203. doi: 10.1007/s00401-007-0303-9. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, Zahm DS. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: Role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Tarpin C, Bardou VJ, Bertucci F, Ginestier C, Braud AC, Puig B, Geneix J, Hassoun J, Birnbaum D, et al. Immunophenotypic analysis of inflammatory breast cancers: Identification of an ‘inflammatory signature’. J. Pathol. 2004;202:265–273. doi: 10.1002/path.1515. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson's disease. J. Toxicol. Environ. Health A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Cropton M. Bax, bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr. Opin. Cell Biol. 2000;12:414–419. doi: 10.1016/s0955-0674(00)00110-1. [DOI] [PubMed] [Google Scholar]
- Dawood S, Ueno NT, Cristofanilli M. The medical treatment of inflammatory breast cancer. Semin. Oncol. 2008;35(1):64–71. doi: 10.1053/j.seminoncol.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Doan JE, Windmiller DA, Riches DW. Differential regulation of TNF-R1 signaling: Lipid raft dependency of p42mapk/erk2 activation, but not NF-kappaB activation. J. Immunol. 2004;172:7654–7660. doi: 10.4049/jimmunol.172.12.7654. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Ethier SP. Human breast cancer cell lines as models of growth regulation and disease progression. J. Mamm. Gland Biol. Neoplasia. 1996;1(1):111–121. doi: 10.1007/BF02096306. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Gardner CR, Laskin DL. Superoxide dismutase overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-a. Am. J. Respir. Cell Mol. Biol. 2004a;30:280–287. doi: 10.1165/rcmb.2003-0044OC. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNFα and tissue injury are dependent on NF-κB. Am. J. Physiol. Lung Cell Physiol. 2004b;287:L279–L285. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Laskin DL. Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am. J. Respir. Cell Mol. Biol. 2002;26:413–419. doi: 10.1165/ajrcmb.26.4.4516. [DOI] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Laskin DL. Regulation of caveolin-1 expression, nitric oxide production and tissue injury by tumor necrosis factor alpha following ozone inhalation. Toxicol. Appl. Pharmacol. 2008;227:380–389. doi: 10.1016/j.taap.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Fidel PI, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J. Matern. Fetal Med. 1998;7:222–226. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Florang VR, Rees JN, Brogden NK, Anderson DG, Hurley TD, Doorn JA. Inhibition of the oxidative metabolism of 3,4-dihydroxyphenylacetaldehyde, a reactive intermediate of dopamine metabolism, by 4-hydroxy-2-nonenal. Neurotoxicology. 2007;28:76–82. doi: 10.1016/j.neuro.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;18:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Gould SJ. I Have Landed. London: Jonathan Cape; 2002. [Google Scholar]
- Gupta S, Rogers LK, Smith CV. Biliary excretion of lysosomal enzymes, iron, and oxidized protein in Fischer-344 and Sprague-Dawley rats and the effects of diquat and acetaminophen. Toxicol. Appl. Pharmacol. 1994;125:42–50. doi: 10.1006/taap.1994.1047. [DOI] [PubMed] [Google Scholar]
- Hnasko R, Lisanti MP. The biology of caveolae: Lessons from caveolin knockout mice and implications for human disease. Mol. Interv. 2003;3:445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson's disease. Ann. Neurol. 2003;53(Suppl. 3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat rain and persistent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinsmaa Y, Florang VR, Rees JN, Anderson DG, Strack S, Doorn JA. Products of oxidative stress inhibit aldehyde oxidation and reduction pathways in dopamine catabolism yielding elevated levels of a reactive intermediate. Chem. Res. Toxicol. 2009;22:835–841. doi: 10.1021/tx800405v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Phillips M, Wang C, Kevetter GA. Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J. Neurosci. Res. 1998;52:709–722. doi: 10.1002/(SICI)1097-4547(19980615)52:6<709::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J. Pharmacol. Toxicol. Methods. 1998;39:147–154. doi: 10.1016/s1056-8719(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: Relevance to Parkinson's disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, Taylor DJ. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372:1319–1327. doi: 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Fakhrzadeh L, Laskin JD. Nitric oxide and peroxynitrite in ozone-induced lung injury. Adv. Exp. Med. Biol. 2001;500:183–190. doi: 10.1007/978-1-4615-0667-6_24. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Laskin JD. Role of macrophages and inflammatory mediators in chemically induced toxicity. Toxicology. 2001;160:111–118. doi: 10.1016/s0300-483x(00)00437-6. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007;65:S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Mudway IS, Kelly FJ. Ozone and the lung: A sensitive issue. Mol. Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- Parrett ML, Harris RE, Joarder FS, Ross MS, Clausen KP, Robertson FM. Cyclooxygenase-2 gene expression in human breast cancer. Int. J. Oncol. 1997;10:503–507. doi: 10.3892/ijo.10.3.503. [DOI] [PubMed] [Google Scholar]
- Pan Q, Bao LW, Merajver SD. Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NF-kappaB signaling cascade. Mol. Cancer Res. 2003;1:701–706. [PubMed] [Google Scholar]
- Pendino KJ, Gardner CR, Shuler RL, Laskin JD, Durham SK, Baron DS, Tsuyoshi OS, Ohnishi T, Laskin DL. Inhibition of ozone-induced nitric oxide synthase expression in the lung by endotoxin. Am. J. Respir. Cell Mol. Biol. 1996;14:516–525. doi: 10.1165/ajrcmb.14.6.8652180. [DOI] [PubMed] [Google Scholar]
- Pendino KJ, Laskin JD, Shuler RL, Punjabi CJ, Laskin DL. Enhanced production of nitric oxide by rat alveolar macrophages after inhalation of a pulmonary irritant is associated with increased expression of nitric oxide synthase. J. Immunol. 1993;151:7196–7205. [PubMed] [Google Scholar]
- Pendino KJ, Meidhof TM, Heck DE, Laskin JD, Laskin DL. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am. J. Respir. Cell Mol. Biol. 1995;13:125–132. doi: 10.1165/ajrcmb.13.2.7542894. [DOI] [PubMed] [Google Scholar]
- Pendino KJ, Schuler R, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-α and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am. J. Respir. Cell Mol. Biol. 1994;11:279–286. doi: 10.1165/ajrcmb.11.3.8086166. [DOI] [PubMed] [Google Scholar]
- Prosperi JR, Mallery SR, Kigerl KA, Erfurt AA, Robertson FM. Invasive and angiogenic phenotype of MCF-7 human breast tumor cells expressing human cyclooxygenase-2. Prostaglandins Other Lipid Mediat. 2004;73:249–264. doi: 10.1016/j.prostaglandins.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Prosperi JR, Robertson FM. Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, pI.3 and pI.7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006;81:55–70. doi: 10.1016/j.prostaglandins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Anderson DG, Doorn JA. Lipid peroxidation products inhibit dopamine catabolism yielding aberrant levels of a reactive intermediate. Chem. Res. Toxicol. 2007;20:1536–1542. doi: 10.1021/tx700248y. [DOI] [PubMed] [Google Scholar]
- Rees JN, Florang VR, Eckert LL, Doorn JA. Protein reactivity of 3,4-dihydroxyphenylacetaldehyde, a toxic dopamine metabolite, is dependent on both the aldehyde and the catechol. Chem. Res. Toxicol. in press;22:1256–1263. doi: 10.1021/tx9000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson FM, Simeone AM, Mazumdar A, Shah AH, McMurray JS, Ghosh S, Cristofanilli M. Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. J. Exp. Ther. Oncol. 2008;7:299–312. [PubMed] [Google Scholar]
- Scallet A, Schmued LC, Slikker W, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: Morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol. Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- Shepherd EG, Zhao Q, Welty SE, Hansen TN, Smith CV, Liu Y. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J. Biol. Chem. 2004;279:54023–54031. doi: 10.1074/jbc.M408444200. [DOI] [PubMed] [Google Scholar]
- Slikker W, Paule M, Wright KM, Patterson TA, Wang C. Systems biology approaches for toxicology. J. Appl. Toxicol. 2007;27:201–217. doi: 10.1002/jat.1207. [DOI] [PubMed] [Google Scholar]
- Slikker W, Xu Z, Wang C. Application of a systems biology approach to developmental neurotoxicology. Reprod. Toxicol. 2005;19:305–319. doi: 10.1016/j.reprotox.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Smith CV. Evidence for participation of lipid peroxidation and iron in diquat-induced hepatic necrosis in vivo. Mol. Pharmacol. 1987;32:417–422. [PubMed] [Google Scholar]
- Smith CV. Correlations and apparent contradictions in assessment of oxidant stress status in vivo. Free Radic. Biol. Med. 1991;10:217–224. doi: 10.1016/0891-5849(91)90079-i. [DOI] [PubMed] [Google Scholar]
- Smith CV. Compartmentalization of redox regulation of cell response. Toxicol. Sci. 2005;83:1–3. doi: 10.1093/toxsci/kfi036. [DOI] [PubMed] [Google Scholar]
- Ueno NT, Buzdar AU, Singletary SE, Ames FC, McNeese MD, Holmes FA, Theriault RL, Strom EA, Wasaff BJ, Asmar L, et al. Combined-modality treatment of inflammatory breast carcinoma: Twenty years of experience at M.D. Anderson Cancer Center. Cancer Chemother. Pharmacol. 1997;40:321–329. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2:418–425. doi: 10.1038/sj.neo.7900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laere SJ, Van der Auwera I, Van den Eynden GG, Elst HJ, Weyler J, Harris AL, van Dam P, Van Marck EA, Vermeulen PB, Dirix LY. Nuclear factor-kappaB signature of inflammatory breast cancer by cDNA microarray validated by quantitative real-time reverse transcription-PCR, immunohistochemistry, and nuclear factor-kappaB DNA-binding. Clin. Cancer Res. 2006;12:3249–3256. doi: 10.1158/1078-0432.CCR-05-2800. [DOI] [PubMed] [Google Scholar]
- Virdis A, Colucci R, Fornai M, Blandizzi C, Duranti E, Pinto S, Bernardini N, Segnani C, Antonioli L, Taddei S, et al. Cyclooxygenase-2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: Role of inducible nitric-oxide synthase and oxidative stress. J. Pharmacol. Exp. Ther. 2005;312:945–953. doi: 10.1124/jpet.104.077644. [DOI] [PubMed] [Google Scholar]
- Wang C, Kaufmann JA, Sanchez-Ross MG, Johnson KM. Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J. Pharmacol. Exp. Ther. 2000;294:287–295. [PubMed] [Google Scholar]
- Wang C, Sadovova N, Fu X, Scallet A, Hanig J, Slikker W. The role of NMDA receptors in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–977. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- Wang C, Sadovova N, Patterson T, Zou X, Fu X, Hanig J, Paule M, Ali S, Zhang X, Slikker W. Protective effects of 7-nitroindazole on ketamine-induced neurotoxicity in rat forebrain culture. Neurotoxicology. 2008;29:613–620. doi: 10.1016/j.neuro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: Effects on the developing nervous system. Anesth. Analg. 2008;106:1643–1658. doi: 10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W., Jr Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol. Sci. 2006;91:192–201. doi: 10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- Wartha F, Henriques-Normark B. ETosis: A novel cell death pathway. Sci. Signal. 2008;1:p25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. The Caveolin genes: From cell biology to medicine. Ann. Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- Wirtz S, Tubbe I, Galle PR, Schild HJ, Birkenbach M, Blumberg RS, Neurath MF. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J. Exp. Med. 2006;203:1875–1810. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon JH, Carter LB, Reiter RJ, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol. Dis. 2006;21:522–530. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, Chandler NB, Rodesch CK, Albertine KH, Petti CA, et al. Impaired neutrophil extracellular trap (NET) formation: A novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Frei B. Genetic deficiency of NADPH oxidase does not diminish, but rather enhances, LPS-induced acute inflammatory responses in vivo. Free Radic. Biol. Med. 2009;46:791–798. doi: 10.1016/j.freeradbiomed.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J. Exp. Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Sadavova NV, Patterson TA, Divine BL, Hotchkiss CE, Ali SF, Hanig JP, Paule MG, Slikker W, Wang C. The effects of L-carnitine on the combination of inhalation anesthetic-induced developmental neuronal apoptosis in the rat frontal cortex. Neuroscience. 2009;151:1053–1065. doi: 10.1016/j.neuroscience.2007.12.013. [DOI] [PubMed] [Google Scholar]