Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a potent suppressor of humoral immunity but the specific molecular mechanisms responsible for immunosuppression by TCDD are poorly understood. In vivo and in vitro studies of the primary humoral IgM response demonstrated that the B cell is a sensitive cell type to modulation by TCDD. We hypothesized that in vivo administration of TCDD disrupts expression of transcription factors controlling B cell to plasma cell differentiation. Female C57BL6 mice were treated with a single dose of TCDD (3, 10, or 30 μg/kg) and/or vehicle (sesame oil). On day 4 post-TCDD administration mice were sensitized with 25 μg lipopolysacchride (LPS) by intraperitioneal injection to stimulate an immune response. Splenocytes were isolated on subsequent days following LPS, up to 3 days post-LPS, and the expression of IgM, XBP-1, PAX5, BCL-6, and Blimp-1 was assessed. TCDD treatment dose-dependently suppressed LPS-induced IgM antibody-forming cell number, which was correlated with decreased frequency of CD19+ CD138+ cells. Gene expression analysis revealed that TCDD caused a dose-dependent suppression of Igμ chain, Igκ chain, IgJ chain, XBP-1, and Blimp-1. TCDD also dose-dependently suppressed LPS-stimulated increases in Blimp-1 protein expression in CD19+ B cells. The deregulation of Blimp-1 expression by TCDD provides a partial explanation for the concomitant suppression of the IgM response and confirms previous observations established in vitro.
Keywords: LPS, TCDD, differentiation, B cell, Blimp-1, in vivo
Decades of research have established 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the prototypical aryl hydrocarbon receptor (AHR) ligand, as a health hazard. The immune system is highly sensitive to TCDD, as demonstrated by significant disruption of a range of immune responses in animal models at doses as low as 4 ng/kg (Burleson et al., 1996; Clark et al., 1983; House and Lauer, 1990). Humoral immune responses have been found to be especially sensitive to TCDD, as evidenced by suppression of both in vivo and in vitro primary IgM responses to T-cell dependent, T-cell independent, and polyclonal B-cell activators (reviewed in Holsapple et al., 1991; Kerkvliet, 2002), with maximal suppression of the primary anti-sheep erythrocytes (sRBC) IgM response observed at approximately 30 μg/kg TCDD in C57BL6 mice (Vecchi et al., 1980). In vitro studies have demonstrated that B cells, primary as well as certain cell lines, are highly sensitive to direct impairment by TCDD (Dooley and Holsapple, 1988; Sulentic et al., 1998) and that the ensuing suppression of the primary IgM response is dependent on activation of the AHR (Sulentic et al., 1998; Vorderstrasse et al., 2001).
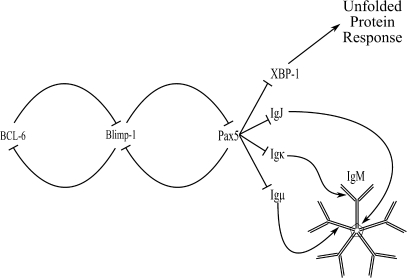
Robust humoral immunity requires large amounts of immunoglobulin (Ig) secretion, a function chiefly attributed to the antibody-forming cell (AFC). Differentiation into an AFC results in significant changes in morphology, phenotype, and gene expression profile. At the molecular level, cellular control of plasmacytic differentiation is accomplished by multiple transcription factors, including BCL-6, Blimp-1, Pax5, and XBP-1 (reviewed in Calame and Lin, 2003; Igarashi and Ochiai, 2007). Regulatory interaction of transcription factors is conceptualized in Figure 1, illustrating the interconnected and reciprocal regulation controlling plasmacytic differentiation. In the case of the primary IgM antibody response, the end product of plasmacytic differentiation is an AFC synthesizing and secreting large amounts of pentameric IgM, formed through Ig J chain (IgJ) coupling five individual IgM antibodies together, with each single IgM antibody made of two Ig light chains and two Ig heavy chains.
FIG. 1.
Schematic representation of proteins involved in plasmacytic differentiation.
Numerous methods of immune system activation initiate differentiation of B cells into AFCs, with LPS one of the most broadly applied polyclonal B cell stimulators resulting in IgM secretion. LPS stimulates B cells through Toll-like Receptor 4 (Hoshino et al., 1999) and RP105/CD180 (Ogata et al., 2000), initiating signaling cascades that converge on the transcription factors depicted in Figure 1, triggering the differentiation process culminating in the plasma cell phenotype.
We hypothesized that changes in gene and protein expression associated with in vitro TCDD-mediated suppression of IgM secretion would extend to the in vivo humoral IgM response. To test this hypothesis an extensive simultaneous analysis of dose response and kinetics during the humoral response for multiple outcomes, in the absence and presence of TCDD, was undertaken. The experimental design allows systematic assessment of TCDD effects on multiple regulatory proteins controlling B-cell differentiation, in vivo under physiologically relevant conditions. Our results verify and extend previous in vitro observations regarding the mechanism for TCDD suppression of the primary IgM response. Specifically, TCDD suppressed all phenotypic outcomes associated with the AFC response examined, implying TCDD-mediated disruption of one or more critical events prior to antibody production. In examining steps that precede antibody secretion, we observed TCDD disruption of LPS-induced changes in Pax5 mRNA abundance. TCDD decreased induction of both Blimp-1 mRNA and frequency of CD19+ Blimp-1elevated cells following LPS treatment. In addition, TCDD reduced expression of surface major histocompatibility complex (MHC) Class II induced by LPS. Cumulatively, these observations point to TCDD-mediated deregulation of either Blimp-1 expression or some proximal event responsible for controlling Blimp-1 expression.
MATERIAL AND METHODS
Animals.
Female 6- to 8-week-old C57BL6 mice were purchased from the National Cancer Institute and housed in accordance with Michigan State University Institutional Animal Care & Use Committee policy. Mice were administered TCDD (3, 10, or 30 μg/kg) and/or vehicle (sesame oil) by a single oral gavage according to individual body weights 4 days prior to induction of an immune response. On day 0, mice received 25 μg Salmonella typhosa LPS or vehicle (phosphate buffered saline [PBS]) by intraperitoneal injection to initiate a primary humoral immune response. Each time point and treatment group consisted of six mice. Tissue samples were collected from mice that received only TCDD or vehicle treatment alone on day 0 of the study to establish baseline effects of TCDD on all outcomes measured, then on subsequent days tissues were collected from all treatment groups. Mice were euthanized by carbon dioxide asphyxiation and spleens removed for processing into single cell suspensions by mechanical disruption. Splenocytes from individual mice were divided into separate aliquots for flow cytometry, RNA and DNA isolation, protein isolation, and IgM AFC response enumeration.
Chemicals.
TCDD was purchased from Accustandard (New Haven, CT) and prepared in sesame oil (Sigma-Aldrich, St Loius, MO). S. typhosa LPS (Sigma-Aldrich) was prepared immediately prior to administration in PBS.
Flow cytometric analysis.
Spleen cell preparations were depleted of erythrocytes by ammonium chloride lysis. FcγIII/II (CD16/CD32) receptors were blocked with BD Biosciences Fc Block (2.4G2, San Jose, CA) followed by incubation with phycoerythrin-labeled anti-CD19 antibodies (2D4, BD Biosciences) to identify B cells. Cells were washed two times to remove excess antibody and fixed with 1× BD Cytofix. For detection of intracellular proteins cells were permeabilized with BD Biosciences 1× Perm/Wash according to manufacturer's instructions. Sources of primary and secondary antibodies used are listed in Supplemental Table S1. Excess antibody was removed with two washes before cells were resuspended in 1× FCM (flow cytometry) buffer (1× Hank's Balanced Salt Solution with 1% bovine serum albumin and 0.09% sodium azide). Cell analysis was performed on 20,000 CD19+ events collected using a BD FACSCalibur flow cytometer (BD Biosciences) with CellQuest Pro for acquisition. Data were analyzed using FlowJo 7.2 (Treestar Software, Ashland, OR).
Gene expression analysis.
RNA was isolated from splenocytes using Trizol (Sigma-Aldrich) according to manufacturer's protocol. RNA pellets obtained following isopropanol precipitation and ethanol wash were resuspended in Promega SV RNA Lysis Solution and then processed according to the manufacturer's protocol to further purify RNA (Promega, Madison, WI). cDNA was generated using Applied Biosystems High Capacity Archive kit according to manufacturer's instructions. TaqMan primer/probe sets were used for all gene expression analysis (listed in Supplemental Table S2), and quantified by ΔΔCt method using 18S ribosomal RNA for normalization (Livak and Schmittgen, 2001). Real-time PCR was performed using ABI 7900HT or Prism 7000 real-time PCR machines.
IgM AFC response.
Detailed methods used for enumeration of AFC can be found in Holsapple and Tucker (1984). Trinitophenol (TNP)-haptenated sRBC were prepared as described in (Rittenberg and Pratt, 1969). In brief, single cell suspensions of splenocytes were combined with a solution of warm 0.5% agar and TNP-haptenated sRBC. Guinea pig complement (Cedar Lane Labs, Burlington, NC) was added, then vortex mixed briefly and aliquots placed onto a Petri dish. Samples were overlaid with a glass coverslip and incubated at 37°C in 5% carbon dioxide overnight. Hemolytic plaques were counted under magnification and normalized to cell number as determined using Z1 series Coulter Counter (Beckman Coulter, Fullerton, CA) to determine IgM AFC per 106 cells.
Statistical analysis.
Results were analyzed by one-way ANOVA using Neuman-Keuls post hoc analysis to determine statistically significant differences among treatment groups within individual days using Graphpad Prism 4.05 (Graphpad Software, San Diego, CA). p Values less than or equal to 0.05 were considered significant. For legibility of figures, only treatment groups which differ significantly from sesame oil + PBS or sesame oil + LPS are indicated on graphs where space considerations do not allow for depiction of all statistically significant differences.
RESULTS
Suppression of the In Vivo LPS-Activated Primary IgM Response by TCDD
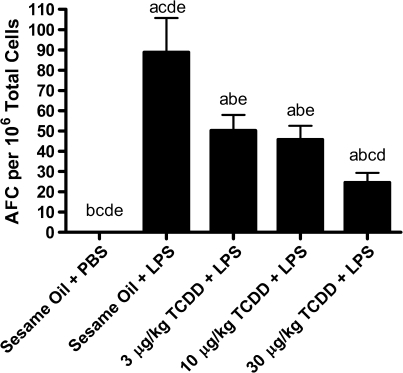
The rationale for using LPS in this study is that a strong polyclonal B-cell activator may drive a proportionally larger fraction of B cells in vivo than more specific antigens such as sRBC, resulting in a potentially greater sensitivity at early time points for detection of TCDD effects. To examine the in vivo effects of TCDD on the LPS-activated primary IgM response we selected a dose of 30 μg/kg TCDD, which is established to cause near-maximal suppression of the sRBC-activated primary IgM response in vivo (Vecchi et al., 1980), then selected half-log decreasing doses to profile the TCDD-mediated effects. In vivo LPS treatment induced a significant increase in the IgM AFC response, which was significantly suppressed by all concentrations of TCDD in a dose-dependent manner (Fig. 2). This may be the first study to demonstrate that in vivo exposure to the polyclonal B cell activator, LPS, results in an increase in IgM AFC response that is inhibited by TCDD.
FIG. 2.
Suppression by TCDD of the LPS-activated IgM AFC response. Four days prior to stimulation of the immune response six mice per treatment group were administered a single dose of TCDD (3, 10, or 30 μg/kg) and/or vehicle (sesame oil) by oral gavage. On day 0 mice received single intraperitoneal injections of either PBS or 25 μg LPS. IgM AFCs were enumerated on day 3 following LPS. Results are depicted as mean AFC per 106 splenocytes ± SE of the mean. ap < 0.05 compared with sesame oil + PBS, bp < 0.05 compared with sesame oil + LPS, cp < 0.05 compared with 3 μg/kg TCDD + LPS, dp < 0.05 compared with 10 μg/kg TCDD + LPS, and ep < 0.05 compared with 30 μg/kg TCDD + LPS.
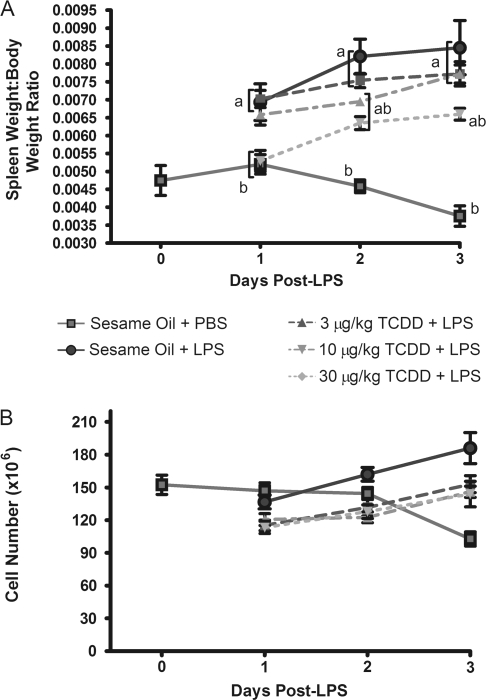
LPS treatment induced a significant increase in spleen size, as reflected in the spleen weight/body weight ratio, which was significantly attenuated by TCDD treatment (Fig. 3A). Treatment with TCDD alone did not significantly alter spleen weight/body weight ratio, and no treatments caused significant changes in body weight (data not shown). TCDD attenuation of LPS-induced increases in spleen weight/body weight ratio was associated with a commensurate decrease in total cellularity, suggesting a modest suppression in spleen cell proliferation.
FIG. 3.
Suppression by TCDD of LPS-induced spleen cell proliferation. Spleen weight, body weight, and total cell recovery per spleen were determined after mice were euthanized. (A) Ratio of spleen weight to total body weight. (B) Total splenocytes recovered per spleen following initial tissue processing into single cell suspensions. Results from six mice per group are depicted as mean ± SE of the mean. ap < 0.05 compared with sesame oil + PBS and bp < 0.05 compared with sesame oil + LPS.
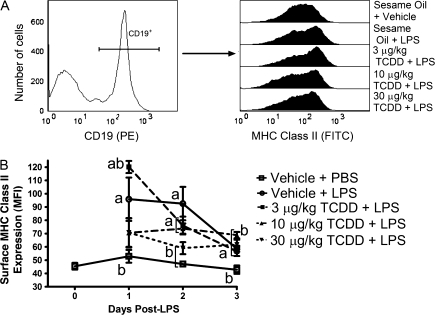
An extensive phenotyping of splenic B cells from all treatment groups was performed in which individual mice were evaluated for all outcomes detailed in this manuscript to maximize the statistical strength of this study. FCM was used to profile cell surface and intracellular protein abundance. In the normal course of an immune response LPS-activated B cells upregulate expression of several cell surface proteins, including MHC Class II to aid in antigen presentation. Surface MHC Class II expression on CD19+ B cells increased significantly in response to LPS, peaking on day 1 and modestly declined by day 3. As B cells proceed toward the AFC phenotype cell surface expression of MHC Class II declined, which is consistent with our observation of peak MHC Class II expression 1 day after LPS treatment and which then declined on days 2 and 3 (Fig. 4B). Administration of 30 μg/kg TCDD significantly attenuated the LPS-induced MHC Class II on the surface of CD19+ B cells at all time points evaluated, whereas lower doses of TCDD marginally attenuated LPS-induced cell surface MHC Class II upregulation.
FIG. 4.
Suppression by TCDD of LPS-induced cell surface MHC Class II expression. Isolated splenocytes were incubated with FcBlock, labeled with antibodies directed against CD19 and MHC Class II, then fixed with BD Cytofix. Cells were subsequently analyzed by FCM. Populations were gated by first identifying (A) CD19+ events, then (B) the individual median fluorescence (MFI) for MHC Class II. Results from six mice per group are depicted as mean MFI ± SE of the mean. ap < 0.05 compared with sesame oil + PBS and bp < 0.05 compared with sesame oil + LPS.
The IgM AFC response has been widely used to enumerate antibody-secreting plasma cells. Our results show that intraperitoneal administration of LPS induced a significant increase in splenic AFCs, which was suppressed in a dose-dependent manner by TCDD treatment. Using the IgM AFC response as a phenotypic anchor, splenocytes were further characterized for CD138 expression, also termed syndecan-1, by FCM as a complementary marker of plasma cells. Figure 5A shows LPS induced an increase in frequency of CD19+ CD138+ cells, which was attenuated by TCDD treatment, an indication that suppression of the IgM AFC response was not simply due to suppression of antibody secretion, but also involved a blockade in LPS-activated B cell differentiation into AFCs. Extending the observation that appearance of CD19+ CD138+ cells was impaired by TCDD, LPS-induced generation of CD19+ Igκhigh cells (Fig. 5D) and CD19+ IgJhigh cells (Fig. 5E) in TCDD-treated mice was also impaired, suggesting that TCDD blocks B-cell differentiation prior to the large increase in intracellular IgJ and Igκ associated with the plasma cell phenotype. Collectively, these results demonstrate TCDD-mediated suppression of the LPS-induced primary IgM response involves failure of individual cells to not only secrete IgM, but to express cell surface markers indicative of the AFC phenotype.
FIG. 5.
TCDD treatment impaired expression of phenotypic indicators for plasmacytic differentiation. To assess B cells for CD138 expression, isolated splenocytes were incubated with FcBlock, labeled with antibodies directed against CD19 and CD138, then fixed with BD Cytofix. For detection of total Igκ or IgJ, cells were blocked, labeled with CD19 antibodies, and fixed with BD Cytofix. At the completion of the time course, cells were permeabilized with BD Perm/Wash, incubated with antibodies directed against either IgJ or Igκ and analyzed by FCM. Populations were gated by first identifying CD19+ events as depicted for (A) CD138 and (B) Igκ, and then gated on populations that expressed elevated levels of (C) CD138, (D) Igκ, or (E) IgJ. Results from 6 mice per group are depicted as mean frequency of CD19+ cells ± SE of the mean for respective measurements. ap < 0.05 compared with sesame oil + PBS and bp < 0.05 compared with sesame oil + LPS.
TCDD-Mediated Suppression of LPS-Induced Changes in Gene and Protein Expression Involved in B Cell to AFC Transition
Differentiation of a resting B cell into an AFC is regulated by a network of reciprocally acting transcription factors, conceptualized in Figure 1. Previous in vitro studies established that TCDD impaired expression of several latter components of the plasma cell phenotype, including expression of IgJ, Igκ, Igμ, Pax5, and Blimp-1 (Schneider et al., 2008, (2009). High levels of IgJ, Igκ, Igμ, and XBP-1 are characteristic of antibody-secreting plasma cells, and splenocytes isolated from LPS-treated mice showed increasing levels of IgJ, Igκ, and Igμ mRNA peaking 2–3 days post-LPS (Figs. 6A, 6C, and 6D, respectively), with the largest fold increase in the expression of IgJ. TCDD impaired LPS-induced increases of IgJ, Igκ, and Igμ mRNA levels with IgJ significantly impaired relative to LPS treatment at all time points evaluated. Igκ and Igμ have a relatively high level of constitutive expression in B cells because they form the B cell antigen receptor, whereas IgJ is required only for the production of secreted pentameric IgM and dimeric IgA. In this study IgJ is likely to be the best marker of Ig destined for secretion, and thus an indicator of antibody-secreting plasma cells. The expression profile for total and spliced XBP-1 followed a similar pattern to IgJ, Igκ, and Igμ, peaking in response to LPS on days 2 and 3. Although not statistically significant, TCDD treatment did appear to partially impair induction of XBP-1, similar again to the patterns observed with Igκ and Igμ mRNA levels.
FIG. 6.
Gene expression profiles for LPS-induced plasmacytic differentiation in the presence and absence of TCDD treatment. Total RNA from splenocytes was isolated and cDNA synthesized for gene expression analysis. Target gene mRNA levels were normalized to 18S and the fold change was calculated by the ΔΔCt method. (A) IgJ, (B) CD138, (C) Igκ, (D) Igμ, (E) total XBP-1, or (F) spliced XBP-1. Results from six mice per group are depicted as mean fold change ± SE of the mean. ap < 0.05 compared with sesame oil + PBS and bp < 0.05 compared with sesame oil + LPS.
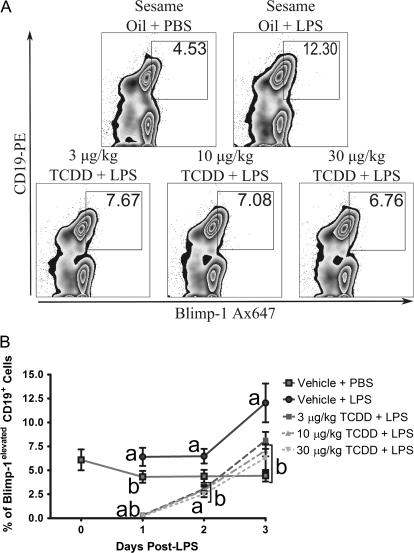
FCM analysis of Blimp-1 protein in CD19+ B cells showed LPS treatment induced an increase in the number of B cells expressing Blimp-1, consistent with activation that precedes the AFC stage. Expression of Blimp-1 protein is quantitatively associated with the AFC phenotype, increasing as B cells differentiate into plasma cells (Kallies and Hasbold, 2004), a finding supported by our own observation. The peak in vivo AFC response occurs 3–4 days post-LPS (data not shown), correlating with peak frequency for CD19+ Blimp-1elevated cells. TCDD treatment impaired generation of CD19+ Blimp-1elevated B cells in LPS-treated mice (Fig. 7). In agreement with protein expression, measurements of Blimp-1 mRNA levels in isolated splenocytes showed that LPS-treated mice possessed significantly increased Blimp-1 mRNA levels on days 2 and 3. For example, 10 and 30 μg/kg TCDD treatment caused significant dose-dependent suppression of LPS-induced Blimp-1 mRNA levels over the time course (Fig. 8B).
FIG. 7.
TCDD treatment suppressed LPS-stimulated generation of CD19+ Blimp-1elevated cells. Isolated splenocytes were incubated with FcBlock, labeled with antibodies directed against CD19, then fixed with BD Cytofix. At the completion of the time course cells were permeabilized with BD Perm/Wash and incubated with antibodies directed against Blimp-1. Cells were subsequently analyzed by FCM. (A) Representative plots from individual mice 3 days post-LPS showing the CD19+ Blimp-1elevated cell population. (B) Time course and dose response for generation of CD19+ Blimp-1elevated cell population. Results from six mice per group are depicted as mean frequency of CD19+ Blimp-1elevated cells ± SE of the mean. ap < 0.05 compared with sesame oil + PBS and bp < 0.05 compared with sesame oil + LPS.
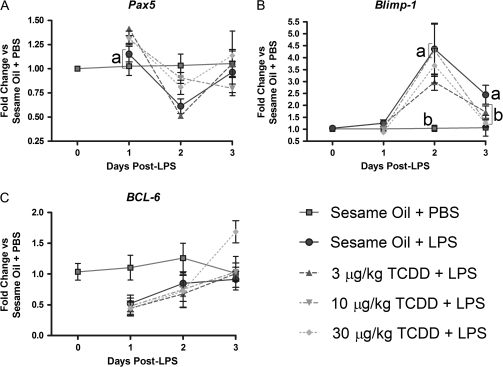
FIG. 8.
Gene expression profiles for transcription factors controlling plasma cell fate are influenced by LPS and TCDD treatments. Total RNA from splenocytes was isolated and cDNA synthesized for gene expression analysis. Target gene mRNA levels were normalized to 18S and the fold change was calculated by the ΔΔCt method. (A) Pax5, (B) Blimp-1, or (C) BCL-6. Results from six mice per group are depicted as mean fold change ± SE of the mean. ap < 0.05 compared with sesame oil + PBS and bp < 0.05 compared with sesame oil + LPS.
Previous in vitro studies with LPS-activated CH12.LX, a murine B cell line, and mouse splenocytes suggest that TCDD treatment impaired downregulation of Pax5 (Schneider et al., 2008; Yoo et al., 2004), a necessary regulatory event for plasmacytic differentiation (Lin and Angelin-Duclos, 2002; Nera et al., 2006). In agreement with prior in vitro observations, splenocytes from mice treated with LPS showed the greatest decrease in mRNA abundance of Pax5 on day 2 of the time course. Mice treated with TCDD prior to LPS had elevated Pax5 mRNA levels on day 1 of the time course, with 10 and 30 μg/kg TCDD doses preventing the Pax5 downregulation associated with LPS treatment observed most clearly on day 2 of the time course (Fig. 8A).
BCL-6, acting in concert with Pax5, is a negative regulator of Blimp-1 gene expression (Shaffer et al., 2000; Tunyaplin et al., 2004). B cells constitutively express BCL-6 under resting conditions, with initiation of the immune response causing a decrease in BCL-6 expression (Ohkubo and Arima, 2005), in turn allowing Blimp-1 expression to rise. In vivo LPS treatment decreased BCL-6 mRNA levels on days 1 and 2, but concomitant treatment with TCDD did not appear to alter BCL-6 gene expression (Fig. 8C).
DISCUSSION
This study demonstrates that in vitro events previously implicated in TCDD-mediated suppression of the primary IgM response occur in the context of an in vivo T-independent humoral immune response. TCDD modestly attenuated LPS-induced increases in splenocyte number, accompanied by profound suppression of the IgM response at all concentrations of TCDD evaluated. Assessment of multiple phenotypic endpoints associated with AFCs confirms that TCDD prevents LPS-induced increases in XBP-1 and all components of pentameric IgM. The observations that CD138 expression, IgJ, and Igκ production are suppressed advance our understanding of TCDD's mechanism of action, as previous studies have not directly shown suppression of intracellular antibody production on a single cell basis, and as such could never conclusively establish whether TCDD suppression of the primary IgM response resulted from suppression of antibody secretion and production or the differentiation of B cells into AFC. TCDD-impaired LPS-stimulated upregulation of Blimp-1, a transcription factor established to be a central regulator of B-cell differentiation. Collectively, these results strongly support our hypothesis that TCDD impairs B cell to plasma cell differentiation.
Different methods of B-cell activation result in distinctly different cell signaling programs and cumulative responses (Donahue and David, 2007). In order to maximize the validity of in vivo and in vitro comparisons it was deemed important to utilize similar modes of B-cell activation as in previous studies. TCDD suppression of the T-cell–dependent anti-sRBC IgM antibody response has been reported by many laboratories (Dooley and Holsapple, 1988; Luster and Germolec., 1988; Smialowicz et al., 1994; Tucker et al., 1986; Vecchi et al., 1980; Vorderstrasse et al., 2001), but to our knowledge no studies have evaluated TCDD-mediated suppression of the in vivo primary IgM response in the context of the commonly used polyclonal B-cell activating stimulus, LPS. Moreover, no published study to date has attempted to correlate alteration in the expression of regulatory transcription factors with in vivo TCDD suppression of the IgM response to any stimulus.
In prior studies by Smialowicz and coworkers only 30 μg/kg (single dose) TCDD, not 10 or 3 μg/kg TCDD, suppressed the T-cell–independent IgM AFC response. Unlike the present study, TCDD was delivered intraperitoneally followed by intravenous sensitization 7 days later with TNP-LPS (Smialowicz et al., 1996). Significant suppression of the IgM response at 3 μg/kg TCDD and higher in the present study is likely due to modest differences in the experimental design, including the strain of mouse used, time interval between TCDD administration and B cell activation, the B-cell activator (LPS vs. TNP-LPS) employed, route of TCDD administration, and methods for detection of humoral immune response.
In agreement with moderate attenuation of LPS-stimulated increases in cell number observed in this in vivo study, TCDD treatment modestly decreased proliferation, as measured by [3H] thymidine incorporation, in isolated splenocytes activated in vitro with LPS (Morris et al., 1993). A minor decrease in proliferation is insufficient to explain the magnitude of suppression in the IgM response observed in the present study, especially because the IgM AFC response was normalized to cell number. However, the observed effect on decreased spleen cellularity was consistent with the possibility that an event upstream of antibody production is disrupted by TCDD.
Disruption in the production of any single Ig component, or protein important in antibody secretion, impairs the primary IgM response. With that knowledge, we assayed expression of all immunoglobulin components. Expression of Igμ and Igκ was more modestly inhibited than the IgJ chain, and may provide insight on why the IgM response is highly sensitive to suppression by TCDD. B cells must express antigen receptors on their surface to receive survival signals (Rajewsky, 1996), hence the high constitutive levels of both Igμ and Igκ. TCDD inhibition of B-cell antigen receptor components may result in deletion of some B cells, preventing detection of some affected B cells using current techniques. Additionally, suppression of Igμ and Ig light chain expression can be expected to impair expression of all forms of immunoglobulin, whereas the IgJ chain is essential only for IgM and IgA classes of immunoglobulin. TCDD strongly attenuates IgJ chain expression, and can therefore be expected to markedly impair the assembly of pentameric IgM, an outcome verified by TCDD-mediated decrease in the AFC response. It is also plausible that less striking effects of TCDD on LPS-induced Igκ and Igμ expression are due to the high constitutive level of both Igκ and Igμ.
Impaired expression of Ig components disrupts antibody synthesis, but B cells must also resolve endoplasmic reticulum stress resulting from increased Ig protein synthesis necessary for robust secretion of antibody. Following activation B cells upregulate expression of XBP-1 mRNA, which can be spliced to an alternative mRNA translating to full length XBP-1, a transcription factor essential for resolution of the unfolded protein response (Reimold et al., 2001). Consistent with an interpretation that TCDD blocks plasmacytic differentiation upstream of antibody production and secretion, TCDD treatment reduced both total and spliced XBP-1 expression in LPS-treated mice (Figs. 6E and 6F).
Separate from their function in antibody synthesis and secretion, B cells change surface protein expression during transition into AFCs. Abundance of surface CD19, MHC Class II, and B-cell antigen receptor all decrease as B cells become plasma cells, whereas surface expression of CD138 is upregulated (reviewed in Fairfax et al., 2008). CD138 has been commonly used as a marker to enumerate plasma cells, and is a complementary phenotypic marker not directly involved in antibody secretion. LPS and TCDD cotreated mice were impaired in their ability to generate CD19+ CD138+ cells compared with mice treated with LPS in the absence of TCDD. Viewed cumulatively, TCDD affects expression of several proteins required for the synthesis and secretion of antibody, as well as CD138, and thus likely disrupts a step in B-cell differentiation preceding antibody production and establishment of the plasma cell phenotype in vivo.
Observations in this study indicate that TCDD interferes with LPS-induced Blimp-1 upregulation, and taken collectively, point toward TCDD-mediated disruption of a fundamental control switch during the primary IgM response. Blimp-1 expression is required for the generation of plasma cells (Shapiro-Shelef et al., 2003), thus failure to upregulate Blimp-1 expression leads to inhibition of plasma cell appearance, consistent with TCDD-mediated suppression of both IgM AFC response and frequency of CD19+ CD138+ B-cell frequency. Direct evidence for deregulation of Blimp-1 expression in vivo comes from the observations that TCDD treatment dose-dependently decreased the frequency of CD19+ Blimp-1elevated B cells and Blimp-1 mRNA abundance in LPS-treated mice. Previous results from this laboratory demonstrated TCDD-activated AHR could directly regulate the Igμ 3′ α enhancer (Sulentic et al., 2004a, b), an event in the IgM response distal to Blimp-1 upregulation during plasmacytic differentiation, implying the cumulative mechanism for TCDD-mediated suppression of the IgM is multifaceted. Suppression of both initiation of plasmacytic differentiation and Ig expression provides a partial explanation for the high sensitivity of B cells to TCDD.
Importantly, recent studies of Blimp-1 conditional knockout mice and identification of XBP-1 as a regulator of hepatic lipid metabolism provide interesting, indirect links for two well-recognized human TCDD toxicities, chloracne and hepatosteatosis, to Blimp-1 expression. Magnúsdóttir and coworkers described a keratinocyte-specific knockout of Blimp-1 in mice resulting in a phenotype remarkably similar to the most consistently observed human health outcome produced by high dose TCDD exposure, chloracne (Zugerman, 1990). Blimp-1 knockout in keratinocytes resulted in a sequela termed by the investigators as “a cyst-like structure that proved to be filled with lipid” in the skin of adult mice (Magnusdottir et al., 2007). If TCDD inhibits Blimp-1 expression in keratinocytes similarly to B cells, then a shared mechanism for TCDD toxicity involving deregulation of Blimp-1 in different tissues becomes more plausible. Along these lines, XBP-1, a transcription factor that is indirectly controlled by Blimp-1 in B cells, is a regulator of lipid homeostasis in the mouse liver (Lee and Scapa, 2008). Human serum dioxin-like chemical levels are associated with hepatosteatosis (Lee and Yao, 2006), and furthermore, TCDD induces hepatosteatosis in mice (Boverhof et al., 2005). Coupling the knowledge TCDD reduced XBP-1 expression, and is downstream of Blimp-1, it is tempting to speculate that TCDD-induced deregulation of Blimp-1 is a key event in some of the toxicities produced by TCDD.
Our study did not detect a consistent TCDD-induced alteration in BCL-6 mRNA levels for LPS-treated mice. It is possible the mechanism of TCDD-induced suppression of the primary IgM response does not involve alteration in BCL-6 gene expression, or owing to high constitutive levels of BCL-6 and multiple cell types in the spleen expressing BCL-6 (Zhou and Ono, 2005), it may be difficult to detect TCDD effects on LPS-induced decreases in BCL-6 expression for B cells. In contrast, it is possible to observe effects of TCDD on Pax5 because B cells are the predominant source of Pax5 mRNA in splenocytes, thus provided greater sensitivity for detection than BCL-6.
Results of this study provide compelling evidence for TCDD impairment of the in vivo primary IgM response via a block in plasmacytic differentiation at, or upstream of, Blimp-1 expression, not simply at the level of antibody production. Upregulation of several phenotypic indicators for AFCs, including CD138 and intracellular levels of Igκ and IgJ, were impaired by TCDD exposure. Collectively, these results validate and enhance the value of mechanistic in vitro studies establishing TCDD-induced deregulation of the Igμ 3′ α enhancer (Sulentic et al., 2004a, b), Pax5 (Schneider et al., 2008), and Blimp-1 (Schneider et al., 2009). Future studies are planned to examine TCDD alteration of events preceding Blimp-1 upregulation in B cells.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (P42 ES04911, R01 ES02520, and T32 ES07255).
Acknowledgments
We thank Mrs Kimberly Hambleton for the excellent administrative assistance in preparation and submission of the manuscript.
References
- Boverhof DR, Burgoon LD, Tashiro C, Chittim B, Harkema JR, Jump DB, Zacharewski TR. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol. Sci. 2005;85:1048–1063. doi: 10.1093/toxsci/kfi162. [DOI] [PubMed] [Google Scholar]
- Burleson GR, Lebrec H, Yang YG, Ibanes JD, Pennington KN, Birnbaum LS. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on influenza virus host resistance in mice. Fundam. Appl. Toxicol. 1996;29:40–47. doi: 10.1006/faat.1996.0004. [DOI] [PubMed] [Google Scholar]
- Calame KL, Lin K-I, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu. Rev. Immunol. 2003;21:205–230. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- Clark DA, Sweeney G, Safe S, Hancock E, Kilburn DG, Gauldie J. Cellular and genetic basis for suppression of cytotoxic T cell generation by haloaromatic hydrocarbons. Immunopharmacology. 1983;6:143–153. doi: 10.1016/0162-3109(83)90007-3. [DOI] [PubMed] [Google Scholar]
- Donahue AC, Fruman DA. Distinct signaling mechanisms activate the target of rapamycin in response to different B-cell stimuli. Eur. J. Immunol. 2007;37:2923–2936. doi: 10.1002/eji.200737281. [DOI] [PubMed] [Google Scholar]
- Dooley RK, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988;16:167–180. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: From B-cell subsets to long-term survival niches. Semin. Immunol. 2008;20:49–58. doi: 10.1016/j.smim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Snyder NK, Wood SC, Morris DL. A review of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: 1991 update. Toxicology. 1991;69:219–255. doi: 10.1016/0300-483x(91)90184-3. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Tucker AN, McNerney PJ, White KL., Jr Effects of N-nitrosodimethylamine on humoral immunity. J. Pharmacol. Exp. Ther. 1984;229:493–500. [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda, Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- House RV, Lauer LD, Murray MJ, Thomas PT, Ehrlich JP, Burleson GR, Dean JH. Examination of immune parameters and host resistance mechanisms in B6C3F1 mice following adult exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Toxicol. Environ. Health. 1990;31:203–215. doi: 10.1080/15287399009531449. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Ochiai K, Muto A. Architecture and dynamics of the transcription factor network that regulates B-to-plasma cell differentiation. J. Biochem. 2007;141:783–789. doi: 10.1093/jb/mvm106. [DOI] [PubMed] [Google Scholar]
- Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL. Plasma cell ontogeny defined by quantitative changes in Blimp-1 expression. J. Exp. Med. 2004;200:967–977. doi: 10.1084/jem.20040973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int. Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Lee A-H, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Yao YJ, Chen HL, Guo YL, Su HJ. Fatty liver and hepatic function for residents with markedly high serum PCDD/Fs levels in Taiwan. J. Toxicol. Environ. Health A. 2006;69:367–380. doi: 10.1080/15287390500244972. [DOI] [PubMed] [Google Scholar]
- Lin K-I, Angelin-Duclos C, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol. Cell. Biol. 2002;22:4771–4780. doi: 10.1128/MCB.22.13.4771-4780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luster MI, Germolec DR, Clark G, Weigand GW, Rosenthal GJ. Selective effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and corticosteroid on in vitro lymphocyte maturation. J. Immunol. 1988;140:928–935. [PubMed] [Google Scholar]
- Magnusdottir E, Kalachikov S, Mizukoshi K, Savitsky D, Ishida-Yamamoto A, Panteleyev AA, Calame K. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14988–14993. doi: 10.1073/pnas.0707323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL, Karras JG, Holsapple MP. Direct effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on responses to lipopolysaccharide (LPS) by isolated murine B-cells. Immunopharmacology. 1993;26:105–112. doi: 10.1016/0162-3109(93)90002-8. [DOI] [PubMed] [Google Scholar]
- Nera K-P, Kohonen P, Narvi E, Peippo A, Mustonen L, Terho P, Koskela K, Buerstedde JM, Lassila O. Loss of Pax5 promotes plasma cell differentiation. Immunity. 2006;24:283–293. doi: 10.1016/j.immuni.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 2000;192:23–29. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo Y, Arima M, Arguni E, Okada S, Yamashita K, Asari S, Obata S, Sakamoto A, Hatano M, J OW, et al. A role for c-fos/activator protein 1 in B lymphocyte terminal differentiation. J. Immunol. 2005;174:7703–7710. doi: 10.4049/jimmunol.174.12.7703. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, Friend D, Grusby MJ, Alt F, Glimcher LH. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- Rittenberg MB, Pratt KL. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc. Soc. Exp. Biol. Med. 1969;132:575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Crawford RB, Chen W, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated impairment of B cell differentiation involves dysregulation of paired box 5 (Pax5) isoform, Pax5a. J. Pharmacol. Exp. Ther. 2008;326:463–474. doi: 10.1124/jpet.108.139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Manzan MA, Yoo BS, Crawford RB, Kaminski N. Involvement of Blimp-1 and AP-1 dysregulation in the 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated suppression of the IgM response by B cells. Toxicol. Sci. 2009;108(2):377–378. doi: 10.1093/toxsci/kfp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- Smialowicz RJ, Riddle MM, Williams WC, Diliberto JJ. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on humoral immunity and lymphocyte subpopulations: Differences between mice and rats. Toxicol. Appl. Pharmacol. 1994;124:248–256. doi: 10.1006/taap.1994.1029. [DOI] [PubMed] [Google Scholar]
- Smialowicz RJ, Williams WC, Riddle MM. Comparison of the T cell-independent antibody response of mice and rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1996;32:293–297. doi: 10.1006/faat.1996.0133. [DOI] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol. Pharmacol. 1998;53:623–629. [PubMed] [Google Scholar]
- Sulentic CEW, Kang JS, Na YJ, Kaminski NE. Interactions at a dioxin responsive element (DRE) and an overlapping kappaB site within the hs4 domain of the 3′alpha immunoglobulin heavy chain enhancer. Toxicology. 2004a;200:235–246. doi: 10.1016/j.tox.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Sulentic CEW, Zhang W, Na YJ, Kaminski NE. 2,3,7,8-tetrachlorodibenzo-p-dioxin, an exogenous modulator of the 3′alpha immunoglobulin heavy chain enhancer in the CH12.LX mouse cell line. J. Pharmacol. Exp. Ther. 2004b;309:71–78. doi: 10.1124/jpet.103.059493. [DOI] [PubMed] [Google Scholar]
- Tucker AN, Vore SJ, Luster MI. Suppression of B cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Pharmacol. 1986;29:372–377. [PubMed] [Google Scholar]
- Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by bcl-6 inhibits plasmacytic differentiation. J. Immunol. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- Vecchi A, Mantovani A, Sironi M, Luini W, Cairo M, Garattini S. Effect of acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin on humoral antibody production in mice. Chem. Biol. Interact. 1980;30:337–342. doi: 10.1016/0009-2797(80)90056-3. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol. Appl. Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- Yoo BS, Boverhof DR, Shnaider D, Crawford RB, Zacharewski TR, Kaminski NE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the regulation of pax5 in lipopolysaccharide-activated B cells. Toxicol. Sci. 2004;77:272–279. doi: 10.1093/toxsci/kfh013. [DOI] [PubMed] [Google Scholar]
- Zhou G, Ono SJ. Induction of BCL-6 gene expression by interferon-gamma and identification of an IRE in exon I. Exp. Mol. Pathol. 2005;78:25–35. doi: 10.1016/j.yexmp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Zugerman C. Chloracne. Clinical manifestations and etiology. Dermatol. Clin. 1990;8(1):209–213. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.