Abstract
Applying jet propulsion-8 (JP-8) jet fuel to the skin of mice induces immune suppression. Applying JP-8 to the skin of mice suppresses T-cell–mediated immune reactions including, contact hypersensitivity (CHS) delayed-type hypersensitivity and T-cell proliferation. Because dermal mast cells play an important immune regulatory role in vivo, we tested the hypothesis that mast cells mediate jet fuel–induced immune suppression. When we applied JP-8 to the skin of mast cell deficient mice CHS was not suppressed. Reconstituting mast cell deficient mice with wild-type bone marrow derived mast cells (mast cell “knock-in mice”) restored JP-8–induced immune suppression. When, however, mast cells from prostaglandin E2 (PGE2)–deficient mice were used, the ability of JP-8 to suppress CHS was not restored, indicating that mast cell–derived PGE2 was activating immune suppression. Examining the density of mast cells in the skin and lymph nodes of JP-8-treated mice indicated that jet fuel treatment caused an initial increase in mast cell density in the skin, followed by increased numbers of mast cells in the subcutaneous space and then in draining lymph nodes. Applying JP-8 to the skin increased mast cell expression of CXCR4, and increased the expression of CXCL12 by draining lymph node cells. Because CXCL12 is a chemoattractant for CXCR4+ mast cells, we treated JP-8-treated mice with AMD3100, a CXCR4 antagonist. AMD3100 blocked the mobilization of mast cells to the draining lymph node and inhibited JP-8–induced immune suppression. Our findings demonstrate the importance of mast cells in mediating jet fuel–induced immune suppression.
Keywords: knockouts, exposure, environmental, percutaneous absorption, immunotoxicity
A byproduct of our modern industrialized society is exposure to chemicals released into our environment. It has been recognized for some time that chemical exposure can modulate immune function. Prominent examples include the induction of immune suppression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (Kerkvliet, 2002) arsenic (Patterson et al., 2004; Soto-Pena et al., 2006), and aromatic hydrocarbons (Veraldi et al., 2006). Common routes of exposure include oral ingestion, inhalation, and direct chemical contact with the skin. The immune modulation induced by the aromatic hydrocarbons found in jet fuel is an excellent example of dermal chemical exposure inducing immune suppression. Using a mouse model, we observed that direct application of military jet fuel (jet propulsion-8 [JP-8]) to the skin suppressed cell-mediated immune reactions. Both the induction and elicitation of delayed-in time hypersensitivity reactions (contact hypersensitivity to contact allergens, and classic delayed-type hypersensitivity to microbial antigens) as well as T-cell proliferation was suppressed following dermal application of jet fuel. On the other hand, antibody formation in vivo was not affected (Ramos et al., 2002; Ullrich, 1999; Ullrich and Lyons, 2000). One of the first steps in the cascade of events leading to immune suppression is the binding of the lipid mediator of inflammation, platelet-activating factor (PAF) to its receptor which induces the production of immune regulatory factors such as prostaglandin E2 (PGE2) and interleukin (IL)-10 (Ramos et al., 2004; Walterscheid et al., 2002). Blocking PAF receptor binding with a series of PAF receptor antagonists, or blocking PGE2 production with a selective cyclooxygenase-2 inhibitor blocked JP-8-induced immune suppression (Ramos et al., 2004). Similarly, neutralizing IL-10 function in vivo abrogated JP-8-induced immune suppression (Ullrich and Lyons, 2000). Although JP-8 is a complex mixture of over 260 different organic chemicals, we recently provided evidence indicating that the aromatic hydrocarbons found in JP-8 induce immune suppression (Ramos et al., 2007).
The skin is the largest organ of the body and its main purpose is to provide barrier function for the preservation of body homeostasis. However, contained within the skin are specialized elements that provide immunological function. Langerhans cells and dermal dendritic cells serve as antigen presenting cells that can initiate and/or regulate immune reactions (Kaplan et al., 2005; Kissenpfennig et al., 2005; Fukunaga et al., 2008). Epidermal keratinocytes secrete a wide variety of immune regulatory cytokines that have diverse effects on immune reactivity (Ullrich, 1995). Dermal mast cells also contribute to the immunological function of the skin. Due to the abundant expression of high-affinity Fcϵ receptors on their surface, and their ability to secrete histamine following IgE cross-linking, mast cells have been traditionally associated with allergic-type immune reactions (Turner and Kinet, 1999). However, newer findings indicate that mast cells influence a wide variety of nonallergic immune responses (Bischoff, 2007) and participate in inducing immune regulation and tolerance (Lu et al., 2006). In the skin, IL-10–secreting mast cells have been shown to limit pathology during a contact dermatitis reaction (Grimbaldeston et al., 2007) and play an essential role in the induction of immune suppression following exposure to the environmental carcinogen ultraviolet (UV) radiation (Byrne et al., 2008; Hart et al., 1998). In addition, mast cell density in human skin correlates with susceptibility to both nonmelanoma (Grimbaldeston et al., 2003) and melanoma skin cancers (Grimbaldeston et al., 2004) suggesting that the immunomodulatory function of mast cells is likely to be important for the development of skin cancer.
Because of their location in the dermis, and in view of the emerging appreciation that mast cells regulate immune function, we tested the hypothesis that mast cells are involved in the immune suppression activated by dermal chemical exposure. We used military jet fuel as our model because much is known about its mechanism of action (Ramos et al., 2002, 2004, 2007, 2009; Ullrich, 1999; Ullrich and Lyons, 2000). We were particularly interested in studying skin and lymph node mast cell density, because we recently provided data indicating that another well-known environmental immunosuppressive agent, UV radiation triggers dermal mast cell migration from the skin to draining lymph nodes, a process that is essential for the induction of immune suppression (Byrne et al., 2008). Here we report that we could not induce immune suppression by applying JP-8 to the skin of mast cell deficient mice. Immune suppression was restored when JP-8 was applied to the skin of mast cell deficient mice that were reconstituted with wild-type bone marrow derived mast cells (BMMC). When BMMC were isolated from PGE2 deficient mice, no immune suppression was noted, indicating that mast cells are the source of the soluble regulatory factors that drive JP-8–induced immune suppression. Within hours of chemical exposure we noted an increase in dermal mast cell numbers, followed by an increase in lymph node mast cell density. JP-8 treatment activated CXCR4 expression on mast cells, and administering a CXCR4 antagonist to jet fuel–treated mice blocked the increase in lymph node mast cell density and abrogated immune suppression. These data indicate that mast cells play an essential role in modulating immune suppression following the application of JP-8 to the skin.
MATERIALS AND METHODS
Mice.
C57BL/6 wild-type mice, mast cell deficient mice (KitW−sh/W−sh), IL-10 deficient mice (B6.129P2-IL10tmiCgn/J), and PGE2-deficient mice (B6.129 (FVB)-Ptgs2 tm2.1 (ptgs1)Fn/J), all rederived onto the C57BL/6 background were acquired from the Jackson Laboratory (Bar Harbor, ME). The animals were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health. All animal procedures were reviewed and approved by the MD Anderson Cancer Center Animal Care and Use Committee.
Application of jet fuel to the dorsal skin of mice.
JP-8 (lot # 3509) was acquired from the Operational Toxicology Branch, Air Force Research Laboratory, Wright Patterson Air Force Base (Dayton, OH). Synthetic jet fuel (S-8), produced from natural gas using the Fischer-Tropsch reaction was generated by the Syntroleum Corporation (Tulsa, OK) and supplied to us by the Operational Toxicology Branch. The S-8 fuel is very similar to JP-8 in composition (www.syntroleum.com/tech_specifications.aspx) but is devoid of aromatic compounds. Because we previously demonstrated that S-8 is not immunosuppressive (Ramos et al., 2007), we used S-8 in these experiments as a control. The fuels were stored and used in a chemical fume hood. Previous studies demonstrated maximal immune suppression, with no overt skin toxicity, when 300 μl (240 mg) of the undiluted fuel was applied directly to the shaved dorsal skin (Ullrich, 1999). Similarly, we noted no immune suppression when S-8 (100–300 μl) was applied to the skin (Ramos et al., 2007), so these doses were used here. The mice were held individually, using standard Plexiglas mouse cages, in the hood, for 3 h after exposure to prevent cage mates from grooming and ingesting the fuel. The animals had free range of motion during this time and were not restrained in any way. We placed the JP-8 high up on the back skin to prevent the mouse from ingesting any jet fuel during grooming, and applied the jet fuel in multiple small increments to prevent the fuel from running across the shaved back skin onto the flanks, but we cannot totally rule out any ingestion of jet fuel. After 3 h all the residual fuel was either absorbed or evaporated and the animals were returned to standard housing (five per cage) in a specific pathogen-free barrier facility.
Contact hypersensitivity reaction.
The contact hypersensitivity (CHS) reaction was used to measure the effect of in vivo chemical exposure on the immune response (Ullrich, 1999). The mice were first treated with JP-8 as described above. Four days later, 50 μl of 0.5% 2,4-dinitro-1-fluorobenzene (DNFB, Sigma-Aldrich, St Louis, MO) in 4:1 acetone:olive oil was applied to the shaved abdominal skin. Six days later, the ear thickness of each mouse was measured with a micrometer (Mitutoyo, Tokyo, Japan) and the mice were challenged by applying 10 μl of 0.2% DNFB to the ventral and dorsal surface of each ear. Twenty-four hours later, the thickness of each ear was remeasured, and the mean ear swelling for each mouse was calculated (left ear + right ear ÷ 2). The background response was measured in a group of mice that were not sensitized but were challenged. The data are expressed as mean change in ear swelling ± SD. There were at least five mice in each group.
Generation, activation, and reconstitution of BMMC.
Bone marrow hematopoietic stem cells were isolated from the femurs and tibias of 6-week-old C57BL/6 mice and cultured at a concentration of 106 cells/ml in complete RPMI 1640 (GIBCO-Invitrogen, Grand Island, NY) supplemented with murine recombinant IL-3 (10 ng/ml; Peprotech, Rocky Hill, NJ) and stem cell factor (10 ng/ml; Peprotech). Nonadherent cells were transferred to fresh culture medium twice a week for 4–5 weeks at which point more than 98% of viable cells were mast cells as verified by flow cytometry (CD45+ CD117+ FcϵR1α+ CD3− B220−) and positive staining with toluidine blue. The cells were washed, resuspended in sterile PBS and a total of 1 × 106 BMMC were injected into multiple sites underlying the dorsal skin of mast cell–deficient mice (Hart et al., 1998). Four to 6 weeks later, the mice were exposed to JP-8 or S-8, as described above.
To determine if jet fuel treatment could activate BMMC in vitro, the cells were treated for 2 h with 100 μg/ml of JP-8 or 100 μg/ml S-8 jet fuel. The doses were chosen based on a previous study that demonstrated no cellular toxicity at this dose of jet fuel (Ramos et al., 2009). The JP-8 and S-8 (≈800 mg/ml) were first diluted in ethanol (1:20 mixture), and then extensively diluted (1:400) in tissue culture medium, as described by Stoica et al. (2001). As a vehicle control for this experiment, mast cells were also treated with ethanol-diluted in tissue culture media (1:8000 dilution). As a positive control BMMC were activated by cross-linking surface IgE receptors using a modification of the procedure described by Swindle et al. (2007). The mast cells (106/ml) cells were incubated for 6 h with 5 μg/ml of mouse IgE specific for DNP-KLH (Sigma-Aldrich). The BMMC were then washed and incubated in RPMI containing 100 ng/ml DNP-KLH (Sigma-Aldrich) for 18 h. Twenty-four h post activation, the cells were washed and CXCR4 expression (BD Pharmingen, San Diego, CA) was determined by immunohistochemistry and flow cytometry.
Histological analysis of mast cells.
Skin samples from mice treated with JP-8 or S-8, or shaved untreated controls, were embedded in paraffin and 7 μm serial sections cut, fixed and then stained for mast cells using toluidine blue. Inguinal draining lymph nodes were isolated from JP-8 or S-8–treated and control mice, placed in Tissue-Tek Optimal Cutting Temperature solution (Sakura Finetek-USA, Torrance, CA), and snap frozen in liquid nitrogen. Seven-micrometer frozen sections were cut, fixed and then stained for mast cells using toluidine blue. Care was taken to ensure that sectioning occurred in the same area of each individual lymph node. The lymph node mast cells density was determined by counting the total numbers of mast cells per each section. The area of each section was then determined by using NIH Image J software (http://rsb.info.nih.gov/nih-image/), and the number of cells per mm2 was calculated. All measurements were done in a blinded fashion.
Blocking the CXCR4-CXCL12 interaction in vivo.
AMD3100, a CXCR4 antagonist (Hatse et al., 2002) (Sigma-Aldrich) was added to the drinking water (80 μg/ml, diluted in the standard autoclaved water that is supplied to all mice in our animal facility) beginning 1 day prior to jet fuel treatment. For the analysis of mast cell density, AMD3100-supplemented drinking water was provided for the entire experiment (i.e., 1 day prior to JP-8 or S-8 treatment and throughout the experiment). To determine the effect of blocking CXCR4-CXCL12 interaction on immune suppression, AMD3100-supplemented drinking water was provided 1 day prior to and for 4 days following JP-8 exposure. AMD3100 treatment was stopped when the mice were sensitized with hapten, and normal drinking water was provided for the remainder of the experiment.
Real-time PCR for CXCL12 expression.
At various times after JP-8 or S-8 treatment, the mice were killed, the inguinal lymph nodes were removed, snap frozen in liquid nitrogen and pulverized with a mortal and pestle. Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) and further purified by treating with RNeasy RNA cleanup protocol (Qiagen, Valencia, CA). The concentration of isolated RNA was measured and 0.5 μg converted to cDNA using the Retroscript RT kit (Ambion, Austin, TX). Twenty-five ng of cDNA was subjected to real-time reverse trancriptase-PCR using a sequence detector (Model ABI Prism 7500, Applied Biosystems, Foster City, CA) and target mixes for CXCL12 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Taqman Gene Expression Assay, Applied Biosystems). Cycle threshold (CT) values for CXCL12 were normalized to GAPDH using the following equation: (1.8 (GAPDH−CxCL12) × 1000), where GAPDH is the CT of each GAPDH control, CXCL12 is the CT of CXCL12, and 1,000 is an arbitrary factor to bring all values above one. There were three mice in each group; RNA was isolated from each individual mouse.
Statistics.
The mean change in ear thickness ± the SD was calculated for each group (N = 5). Statistical differences between the control and experimental groups were then determined using a one-way ANOVA followed by the Bonferroni's multiple comparison test (GraphPad, Prism Software V4, San Diego, CA). In experiments where changes in lymph node mast cell numbers were measured, there were at least three mice per group. The number of mast cells per mm2 for each individual animal was calculated. The mean ± the SEM was then calculated for the group. Statistical differences between the experimental groups were determined using a one-way ANOVA followed by Bonferroni's multiple comparison test. Representative experiments are shown; each experiment was repeated at least three times.
RESULTS
Mast Cells are Required for Jet Fuel–Induced Immune Suppression
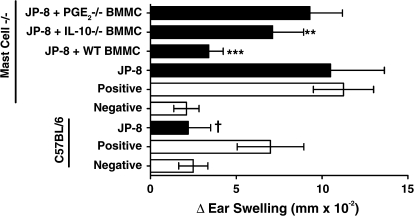
Because of the growing appreciation for the role of dermal mast cells in modulating the immune response, we first tested the hypothesis that mast cells are involved in the immune suppression induced by dermal application of JP-8 (Fig. 1). An immunosuppressive dose (240 mg) of JP-8 was applied to the skin of wild-type (C57BL/6) and syngeneic mast cell deficient (KitW−sh/W−sh) mice. Four days later, a contact allergen was applied to the skin, and the generation of CHS was measured as described previously (Ullrich, 1999). As expected, applying 240 mg of JP-8 to the skin of wild-type mice significantly suppressed CHS (p = 0.001 vs. positive control). However, when 240 mg of JP-8 was applied to the skin of mast cell deficient mice (mast cell−/−) no immune suppression was noted. To confirm that mast cells were essential for the induction of immune suppression, one group of mast cell deficient mice was reconstituted by injecting wild-type BMMC into multiple sites underlying the dorsal skin (mast cell “knock-in” mouse). Significant immune suppression was observed when JP-8 was applied to the skin of the mast cell reconstituted mice (mast cell−/− + BMMC; p = 0.001 vs. positive control). Previous studies have indicated that neutralizing the activity of IL-10, or inhibiting cyclooxygenase-2 activity with a selective cyclooxygenase-2 inhibitor abrogates JP-8–induced immune suppression (Ullrich and Lyons, 2000). Because mast cells secrete both IL-10 and PGE2 (Galli et al., 2005) we wanted to determine if mast cell–derived PGE2 or IL-10 is mediating immune suppression. To do this we reconstituted mast cell−/− mice with BMMC isolated from IL-10– or PGE2-deficient animals. When PGE2-deficient mast cells were used, we saw no immune suppression, in that the CHS reaction observed was not statistically different from that found in the positive control (p > 0.05) (Fig. 1). When IL-10 deficient mast cells were used, the CHS reaction was still suppressed (40% suppression vs. positive control), albeit not to the same degree as the suppression found in mice reconstituted with wild-type BMMC. However, because there was a significant difference (p = 0.01) between the CHS reaction found in the positive control mice and the reaction found in mice reconstituted with IL-10−/− mast cells, we conclude that mast cell derived IL-10 is not activating immune suppression in vivo. These data indicate that mast cells are essential for activating immune suppression following dermal application of jet fuel. Further they indicate that mast cell derived PGE2 is essential for inducing immune suppression in JP-8-treated mice.
FIG. 1.
Mast cell deficient mice are resistant to immune suppression by JP-8. JP-8 was applied to the dorsal skin of wild-type C57Bl/6 mice or mast cell deficient mice (Mast Cell−/−) as described in “Material and Methods.” One group of mast cell deficient mice was reconstituted with C57BL/6 BMMC (wild-type BMMC), another was reconstituted with IL-10−/− deficient BMMC, and another was reconstituted with PGE2−/− BMMC. Four days after reconstitution, the mice were sensitized with DNFB. Negative refers to negative control mice that were not sensitized but were challenged with the happen. Positive refers to positive control mice that were sensitized with hapten and then challenged 6 days later. The positive and negative control mice received no further treatment (open bars). Closed bars represent the CHS response found in JP-8-treated mice. †p = 0.001 versus C57BL/6 positive control. **p = 0.01 versus mast cell−/− positive control. ***p = 0.001 versus mast cell−/− positive control.
Mast Cells Accumulate in Skin Draining Lymph Nodes following Jet Fuel Exposure
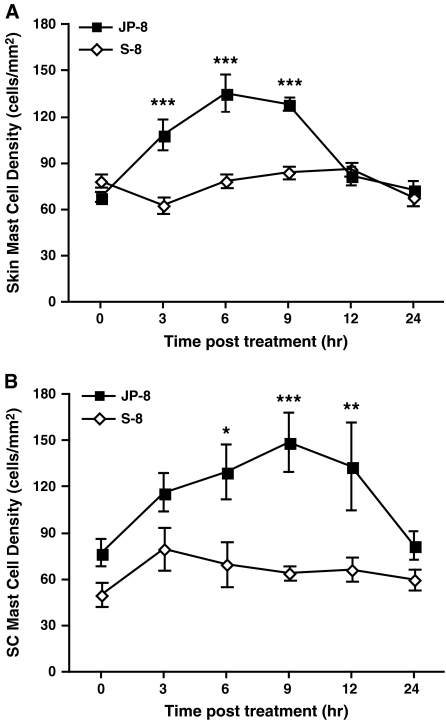
In many ways, the immune suppression induced by jet fuel is similar to that observed following UV exposure (i.e., selective suppression of cell-mediated immune reactions, a critical role for PAF and PGE2, a critical role for mast cells). In light of the fact that we recently reported that mast cell migration from the skin to the draining lymph nodes represents a key step in UV-induced immune suppression (Byrne et al., 2008), we decided to examine dermal and lymph node mast cell density following jet fuel exposure. An immunosuppressive dose (240 mg) of JP-8, or an equivalent amount of S-8 was applied to the skin of wild-type mice, and at various times afterwards the numbers of mast cells found in the skin was examined. At 3-, 6-, and 9-h post-JP-8 treatment, we noted a significant increase in skin mast cell numbers compared with the S-8–treated control (Fig. 2A; p = 0.0001). At 12-h postexposure, mast cell numbers in the skin returned to normal. We also measured mast cell numbers in the subcutaneous tissue underlying the skin (Fig. 2B). At 6-, 9-, and 12-h post JP-8-treatment, we noted a significant increase in the number of mast cells found in the subcutaneous tissue compared with the mast cell numbers found in the S-8–treated control mice.
FIG. 2.
JP-8 induces alteration in skin mast cell densities. JP-8 or the control (S-8) was applied to wild-type mice and mast cell density in the skin was measured at various times post-treatment: (A) Total skin mast cells per mm2 of skin. B, Mast cell per mm2 in subcutaneous (SC) tissue. *p = 0.05, **p = 0.01, ***p = 0.001 versus comparable time point in S-8–treated control. N = 3 mice per time point in each experiment.
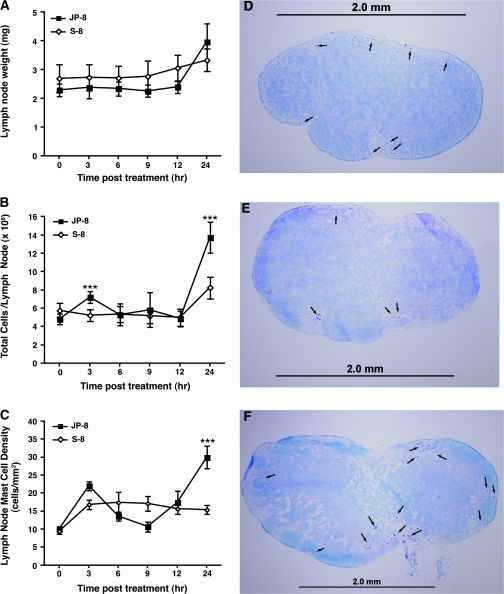
We next measured mast cell numbers in lymph nodes draining the skin (Fig. 3). Although lymph node weight did not increase significantly (Fig. 3A), lymph node cellularity (Fig. 3B) and the number of mast cells in the draining lymph nodes (Fig. 3C) were both significantly increased 24 h after jet fuel treatment. Representative lymph node sections, stained with toluidine blue to identify mast cells (arrows) are shown. Figure 3D shows a normal lymph node. A lymph node removed from a mouse 24 h after being treated with the S-8 is presented in Figure 3E. Figure 3F shows a lymph node removed from a mouse 24 h after JP-8 treatment. These data indicate that JP-8 treatment causes a transient increase in skin mast cell numbers, which peaks at 6 h, followed by an increase in the numbers of mast cells found in the subcutaneous tissue, peaking at 9 h, followed by an increase in the number of mast cells found in draining lymph node.
FIG. 3.
Lymph node hypertrophy after skin exposure to JP-8. JP-8 or the control (S-8) was applied to wild-type mice at time zero. (A) Inguinal lymph node weights after chemical exposure. (B) Lymph node cell number after chemical exposure. (C) Lymph node mast cell density after chemical exposure. (D) Cross-section through the center of normal untreated lymph node, (E) 24 h after S-8 exposure. (F) 24 h after JP-8 exposure. Scale bar = 2 mm. **p = 0.01, ***p = 0.001 versus comparable time point in S-8–treated control. N = 5 mice/time point in each experiment.
Jet Fuel Exposure Upregulates the Receptors and Ligands Required for Mast Cell Migration
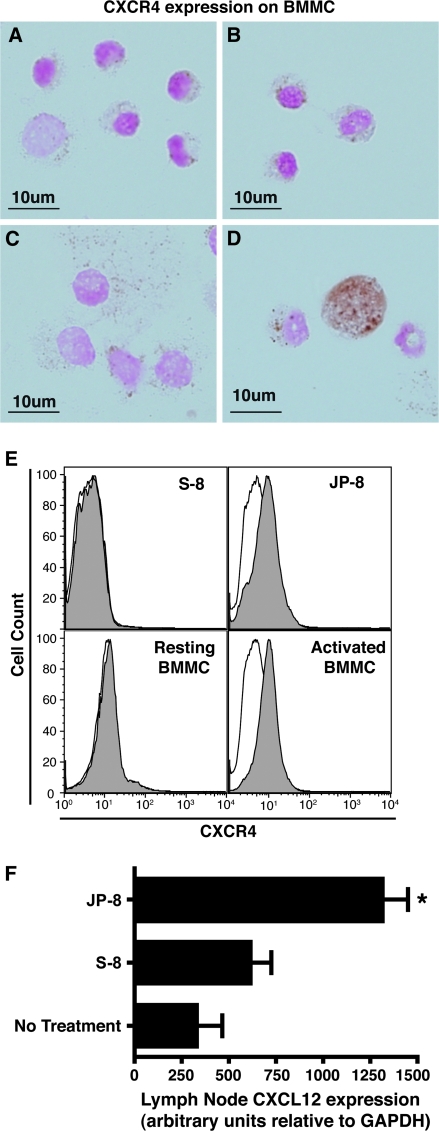
The chemokine receptor CXCR4 is up regulated on activated mast cells, and its ligand, CXCL12 acts as a mast cell chemotaxin (Juremalm et al., 2000). In light of the data presented in Figure 3, indicating increased numbers of mast cells in subcutaneous tissue and in the draining lymph nodes following JP-8, but not S-8 treatment, we decided to measure the effect of JP-8 on CXCR4 and CXCL12 expression. Cytospin preparations of resting (Fig. 4A), vehicle (ethanol diluted in medium) treated (Fig. 4B), S-8–treated (Fig. 4C), or JP-8–treated (Fig. 4D) BMMC were stained with anti-CXCR4. The chemokine receptor CXCR4 was only expressed on JP-8 activated mast cells. These data were confirmed by flow cytometry (Fig. 4E). In this experiment JP-8 treatment resulted in CXCR4 upregulation, treatment with the S-8 did not. For the sake of comparison, the CXCR4 expression on resting BMMC, and BMMC activated by cross-linking IgE receptors is also presented. We also measured the upregulation of CXCL12 in the draining lymph nodes (Fig. 4F). Mice were treated with S-8 or JP-8 as described. At various time post-treatment (6, 12, 24, and 48 h), the lymph nodes of the treated mice, and normal C57BL/6 mice were removed and CXCL12 expression was determined by quantitative real-time PCR. No upregulation of CXCL12 expression, compared with the background control, was observed at the 6-, 12-, and 24-h time points (data not shown). At 48-h post-treatment, we observed a significant increase in CXCL12 mRNA expression (p = 0.01 vs. no treatment control). The CXCL12 expression in the S-8–treated mice was not significantly different (p > 0.05) from the expression found in the no treatment control mice. These data indicate that JP-8 upregulates the expression of CXCR4 on mast cells, and also upregulates the expression of its ligand, CXCL12 on lymph node cells.
FIG. 4.
Mast cell CXCR4 and lymph node CXCL12 expression is up regulated by jet fuel treatment. BMMC were treated with: (A) tissue culture medium, (B) ethanol-diluted in tissue culture medium, C, S-8, D, JP-8, and then CXCR4 expression was measured by immunohistochemistry, Scale bar = 10 μm. (E) BMMC were activated with S-8, JP-8, and CXCR4 staining was measured by flow cytometry. For comparison the CXCR4 expression on resting BMMC and activated BMMC is also presented. CXCR4 staining illustrated by grey histograms; isotype controls in white. (F) Lymph node CXCL12 expression was measured by real-time PCR. *p = 0.05.
Blocking CXCR4/CXCL12 Interaction Blocks JP-8–Induced Immune Suppression and Inhibits Mast Cell Accumulation in the Draining Lymph Node
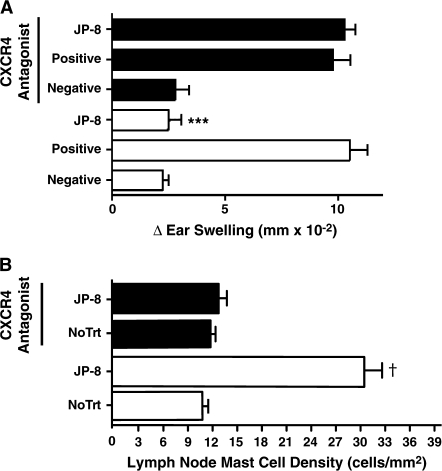
Jet fuel–induced upregulation of CXCR4 on mast cells and CXCL12 in the draining lymph nodes suggests that this receptor ligand pair may be involved in JP-8–induced immune suppression and mast cell mobilization to the draining lymph nodes. To determine if this was the case, we treated mice with AMD3100, a CXCR4 receptor antagonist (Hatse et al., 2002). Mice maintained on AMD3100-supplemented drinking water generated a CHS reaction that was indistinguishable from that found in mice maintained on normal drinking water (Fig. 5A; positive control open bars vs. positive control closed bars; p = 0.38). As expected, JP-8 treatment suppressed CHS in mice maintained on normal drinking water (p = 0.001 vs. positive control). When, however, mice maintained on AMD3100-supplemented drinking water were treated with JP-8, no immune suppression was noted.
FIG. 5.
Blocking CXCR4/CXCL12 interaction abrogates JP-8-induced immunosuppression and mast cell migration. (A) Immunosuppression: Mice were maintained on normal drinking water (open bars), or a CXCR4 antagonist (AMD-3100) was supplied in the drinking water (closed bars). Four days later all the mice were sensitized with DNFB and CHS was measured. Negative refers to negative control mice that were not sensitized but were challenged with the happen. Positive refers to positive control mice that were sensitized with hapten and then challenged 6 days later. The positive and negative control mice received no further treatment. ***p = 0.001 versus positive control. (B) Mast cell migration: mice were maintained on AMD3100-supplemented water (closed bars) or normal drinking water (open bars) for the entire experiment. Some mice received no further treatment (no TrT), others were exposed to JP-8. The number of mast cells in the draining lymph node was measured 24 h later. †p = 0.001 versus no treatment control maintained on normal drinking water. N = 5 for each group in each experiment.
The effect of the CXCR4 antagonist on mast cell accumulation in the draining lymph node is shown in Figure 5B. Treating mice with JP-8 caused a significant increase in lymph node mast cell number (p = 0.001 vs. no treatment control; open bars). When however, mice maintained on AMD3100-supplemented drinking water were treated with JP-8, no increase in lymph node mast cells numbers were noted (p = 0.22, vs. no treatment control, closed bars). Treating the mice with S-8 did not induce immune suppression nor did it activate mast cells accumulation in the draining lymph nodes, regardless of whether the mice were maintained on AMD3100-supplemented drinking water or not (data not shown). These data indicate that blocking the binding of CXCR4 to its ligand, CXCL12, blocks jet fuel–induced immune suppression and the accumulation of mast cells in the draining lymph nodes.
DISCUSSION
Exposure to environmental chemicals can modulate immune function. An example of this phenomenon is the induction of immune suppression following dermal exposure to the aromatic hydrocarbons found in jet fuel (Ramos et al., 2007). Estimates suggest that over 2 million people a year are exposed to 60 billion gallons of jet fuel, mostly during aviation-associated occupations, making jet fuel exposure a major source of human chemical exposure (Ritchie et al., 2003). A long-term focus of our research has been to understand the mechanisms underlying jet fuel–induced immune modulation. Here we tested the hypothesis that mast cells are critical for the induction of immune suppression following jet fuel exposure. Two observations lead to this hypothesis. First, there is a growing appreciation for the role of mast cells in regulating adaptive immune reactions (Galli et al., 2005). Second, we noted that the mechanisms of immune suppression induced following dermal application to jet fuel, and those observed following exposure to UV radiation, another common dermal immunotoxicant, are similar. Mast cells, and in particular, mast cell migration, plays a critical role in the immune suppression induced by UV radiation (Byrne et al., 2008; Hart et al., 1998; Ullrich et al., 2007). Here we report that mast cells are essential in the process leading to immune suppression following dermal jet fuel exposure. No immune suppression was observed in jet fuel–treated mast cell deficient mice, and immune suppression was restored after reconstituting the mast cell deficient with wild-type BMMC. Further, we observed that reconstituting mast cells deficient mice with PGE2−/− mast cells did not restore immune suppression, indicating that PGE2 is the critical mast cell product activating immune suppression. We did see a diminution in the amount of JP-8–induced immune suppression when IL-10−/− mast cells were used. However, because CHS was suppressed in these mice (40% immune suppression) and in light of the fact that there was a statistically significant difference between the CHS reaction observed in the IL-10−/− reconstituted mice and the positive control, we conclude that IL-10 is not the mast cell derived immune regulatory factor activating in JP-8–induced immune suppression.
We also noted increased numbers of mast cells in the draining lymph nodes of mice treated with jet fuel. We noted a JP-8–induced increase in mast cell CXCR4 expression, confirming data reported by others generated by microarray analysis (McDougal et al., 2007). Moreover, we found that treating the mice with an antileukemia drug, AMD3100, which is a CXCR4 antagonist and has been shown previously to block mast cell migration to skin draining lymph nodes (Byrne et al., 2008), abrogated the accumulation of mast cells in the lymph nodes and prevented the induction of immune suppression. We conclude that jet fuel–induced immune suppression is dependent on mast cell function, and suggest that mast cells migrate from the skin to draining lymph nodes thereby transmitting the immunosuppressive signal from the skin to the immune system.
As mentioned above, evidence exists demonstrating that the aromatics hydrocarbons in jet fuel are the active agents that induce immune suppression (Ramos et al., 2007). Others have shown that the aromatic hydrocarbons found in jet fuel penetrate through the skin (McDougal et al., 2000; Riviere et al., 1999), and breakdown products of naphthalene are recovered from the urine of military personnel after dermal jet fuel exposure (Chao et al., 2006), indicating that fuel components can be distributed system wide. On the other hand, McDougal and colleagues, after estimating the amount of jet fuel that could penetrate through human skin after holding one's hands in JP-8 for approximately 8h, suggest that the level of JP-8 that can be absorbed through the skin is too low to induce systemic toxicity (McDougal et al., 2000). One way to reconcile these opposing observations is to suggest that jet fuel–induced immune suppression occurs by an indirect mechanism. Our data supports this idea. We base this conclusion on the data generated with mast cell deficient mice. Mice deficient in mast cells are resistant to the immune suppressive effects of jet fuel, and reconstituting these mice, by injecting BMMC into the subcutaneous space underlying the dermis, reconstitutes immune suppression (Fig. 1). One would assume, based on the data reported by others (McDougal et al., 2000; Riviere et al., 1999), that the aromatic hydrocarbons found in jet fuel are penetrating through the skin of mast cell deficient mice. The absence of immune suppression in these mice suggests that the induction of immune suppression occurs via an indirect mechanism, and not through direct interaction of JP-8 with immune cells in the lymph node. We suggest the following scenario. After dermal exposure, jet fuel penetrates through the stratum corneum and interacts with the PAF receptor on epidermal keratinocytes (Ramos et al., 2004). This activates a cascade of biological effects including the production of more PAF, and the upregulation of immune modulatory factors such as PGE2. At the same time, JP-8 exposure activates CXCR4 expression on resident mast cells (Fig. 4). One of the factors produced by the jet fuel–treated keratinocytes, most likely PGE2, activates lymph node CXCL12 expression (Kim et al., 2006), which sets up the gradient needed for mast cell migration. Mast cells move to the draining lymph nodes and once there secrete PGE2, which induces the production of other immunoregulatory factors. Evidence to support this last idea is provided by a previous study showing that injecting PGE2 into mice upregulates serum IL-10 levels (Shreedhar et al., 1998). Studies are currently in progress to test these hypotheses.
Over the course of studying the immune modulation induced by JP-8, we were struck by the similarity between the mechanisms of immune suppression induced by the aromatic hydrocarbons found in jet fuel and the immunosuppression induced by UV radiation (reviewed by Ullrich, 2005). First, like UV, jet fuel application to the skin preferentially affects cell-mediated immune reactions. We noted that delayed-type hypersensitivity, CHS, and T-cell proliferation, but not antibody production was suppressed by JP-8 treatment (Ullrich, 1999; Ullrich and Lyons, 2000). Both primary and secondary immune reactions are suppressed by application of jet fuel (Ramos et al., 2002) and UV radiation. Also, similar to UV, repeated exposure to small doses of jet fuel will induce immune suppression (Ramos et al., 2002). Second, activation of cytokine production by JP-8-treatment appears to play a important role, in that suppressing PGE2 production with a selective COX-2 inhibitor, or neutralizing IL-10 activity with monoclonal antibody, blocked JP-8-induced immune suppression (Ullrich and Lyons, 2000). Third, like UV, PAF receptor binding triggers JP-8–induced immune suppression. PAF-treated keratinocytes secrete PGE2, and treating JP-8–treated mice with a series of selective PAF receptor antagonists blocked immune suppression (Ramos et al., 2004). Moreover, PAF induces the transcription of IL-10 (Walterscheid et al., 2002), a cytokine found in the serum of JP-8–treated mice (Ullrich, 1999). These findings suggest that jet fuel–induced PAF is initiating a cytokine cascade similar to that found after exposure to UV radiation. Based on the findings presented here we can add mast cell function, and mast cell migration, which for years has been recognized to be important in the immune suppression induced by UV radiation (Byrne et al., 2008; Hart et al., 1998; Ullrich et al., 2007), to the list of similarities. This is somewhat surprising based on the fact that these two immunosuppressive agents (UV and jet fuel) differ considerably; one being a chemical mixture and the other being a physical agent. Whether other agents that cause immune suppression after interacting with the skin, for example thermal trauma (Miller et al., 2007), behave similarly remains to be seen.
In summary, our findings add to the growing appreciation of the immunoregulatory function of mast cells by demonstrating that their function is critical for the induction of immune suppression following the dermal application of the aromatic hydrocarbons found in a complex chemical mixture, such as jet fuel. They add to our appreciation of how dermal immunosuppressive agents induce immune suppression by showing that jet fuel activates mast cell migration to the underlying lymph nodes. We suggest that this may represent a common mechanism by which dermal immunotoxicants induce immune suppression.
FUNDING
National Cancer Institute (CA112660 and CA131207); United States Air Force Office of Scientific Research (FA9550-05-1-402); USAF Institute of Technology scholarship supported G.R.; National School of Biological Sciences (ENCB-IPN) funding supported R.C.-S; and NCI Cancer Center Support Grant (CA16672) supported animal, histology and flow cytometry facilities at the MD Anderson Cancer Center.
Acknowledgments
We thank Nasser Kazimi for his help with the animal experiments. The views and opinions expressed here are those of the authors and do not reflect the official policy or position of the United States Air Force.
References
- Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Limon-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J. Immunol. 2008;180:4648–4655. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao YC, Kupper LL, Serdar B, Egeghy PP, Rappaport SM, Nylander-French LA. Dermal exposure to jet fuel JP-8 significantly contributes to the production of urinary naphthols in fuel-cell maintenance workers. Environ. Health Perspect. 2006;114:182–185. doi: 10.1289/ehp.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J. Immunol. 2008;180:3057–3064. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Green A, Darlington S, Robertson BO, Marshman G, Finlay-Jones JJ, Hart PH. Susceptibility to basal cell carcinoma is associated with high dermal mast cell prevalence in non-sun-exposed skin for Australian populations. Photochem. Photobiol. 2003;78:633–639. doi: 10.1562/0031-8655(2003)078<0633:stbcci>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Pearce AL, Robertson BO, Coventry BJ, Marshman G, Finlay-Jones JJ, Hart PH. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br. J. Dermatol. 2004;150:895–903. doi: 10.1111/j.1365-2133.2004.05966.x. [DOI] [PubMed] [Google Scholar]
- Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- Juremalm M, Hjertson M, Olsson N, Harvima I, Nilsson K, Nilsson G. The chemokine receptor CXCR4 is expressed within the mast cell lineage and its ligand stromal cell-derived factor-1alpha acts as a mast cell chemotaxin. Eur. J. Immunol. 2000;30:3614–3622. doi: 10.1002/1521-4141(200012)30:12<3614::AID-IMMU3614>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int. Immunopharmacol. 2002;2:277–291. doi: 10.1016/s1567-5769(01)00179-5. [DOI] [PubMed] [Google Scholar]
- Kim YS, Bigliani LU, Fujisawa M, Murakami K, Chang SS, Lee HJ, Lee FY, Blaine TA. Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J. Orthop. Res. 2006;24:1756–1764. doi: 10.1002/jor.20197. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: Dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Garrett CM, Amato CM, Berberich SJ. Effects of brief cutaneous JP-8 jet fuel exposures on time course of gene expression in the epidermis. Toxicol. Sci. 2007;95:495–510. doi: 10.1093/toxsci/kfl154. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Pollard DL, Weisman W, Garrett CM, Miller TE. Assessment of skin absorption and penetration of JP-8 jet fuel and its components. Toxicol. Sci. 2000;55:247–255. doi: 10.1093/toxsci/55.2.247. [DOI] [PubMed] [Google Scholar]
- Miller AC, Rashid RM, Elamin EM. The “T” in trauma: The helper T-cell response and the role of immunomodulation in trauma and burn patients. J. Trauma. 2007;63:1407–1417. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- Patterson R, Vega L, Trouba K, Bortner C, Germolec D. Arsenic-induced alterations in the contact hypersensitivity response in Balb/c mice. Toxicol. Appl. Pharmacol. 2004;198:434–443. doi: 10.1016/j.taap.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Ramos G, Kazimi N, Nghiem DX, Walterscheid JP, Ullrich SE. Platelet activating factor receptor binding plays a critical role in jet fuel-induced immune suppression. Toxicol. Appl. Pharmacol. 2004;195:331–338. doi: 10.1016/j.taap.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Ramos G, Limon-Flores AY, Ullrich SE. Dermal exposure to jet fuel suppresses delayed-type hypersensitivity: A critical role for aromatic hydrocarbons. Toxicol. Sci. 2007;100:415–422. doi: 10.1093/toxsci/kfm247. [DOI] [PubMed] [Google Scholar]
- Ramos G, Limon-Flores AY, Ullrich SE. JP-8 induces immune suppression via a reactive oxygen species NF-kappa beta-dependent mechanism. Toxicol. Sci. 2009;108:100–109. doi: 10.1093/toxsci/kfn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos G, Nghiem DX, Walterscheid JP, Ullrich SE. Dermal application of jet fuel suppresses secondary immune reactions. Toxicol. Appl. Pharmacol. 2002;180:136–144. doi: 10.1006/taap.2002.9380. [DOI] [PubMed] [Google Scholar]
- Ritchie G, Still K, Rossi J, 3rd, Bekkedal M, Bobb A, Arfsten D. Biological and health effects of exposure to kerosene-based jet fuels and performance additives. J. Toxicol. Environ. Health B Crit. Rev. 2003;6:357–451. doi: 10.1080/10937400306473. [DOI] [PubMed] [Google Scholar]
- Riviere JE, Brooks JD, Monteiro-Riviere NA, Budsaba K, Smith CE. Dermal absorption and distribution of topically dosed jet fuels jet-A, JP-8, and JP-8(100) Toxicol. Appl. Pharmacol. 1999;160:60–75. doi: 10.1006/taap.1999.8744. [DOI] [PubMed] [Google Scholar]
- Shreedhar V, Giese T, Sung VW, Ullrich SE. A cytokine cascade including prostaglandin E2, IL-4, and IL-10 is responsible for UV-induced systemic immune suppression. J. Immunol. 1998;160:3783–3789. [PubMed] [Google Scholar]
- Soto-Pena GA, Luna AL, Acosta-Saavedra L, Conde P, Lopez-Carrillo L, Cebrian ME, Bastida M, Calderon-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20:779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Boulares AH, Rosenthal DS, Iyer S, Hamilton ID, Smulson ME. Mechanisms of JP-8 jet fuel toxicity. I. Induction of apoptosis in rat lung epithelial cells. Toxicol. Appl. Pharmacol. 2001;171:94–106. doi: 10.1006/taap.2000.9108. [DOI] [PubMed] [Google Scholar]
- Swindle EJ, Coleman JW, DeLeo FR, Metcalfe DD. FcepsilonRI- and Fcgamma receptor-mediated production of reactive oxygen species by mast cells is lipoxygenase- and cyclooxygenase-dependent and NADPH oxidase-independent. J. Immunol. 2007;179:7059–7071. doi: 10.4049/jimmunol.179.10.7059. [DOI] [PubMed] [Google Scholar]
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. The role of epidermal cytokines in the generation of cutaneous immune reactions and ultraviolet radiation-induced immune suppression. Photochem. Photobiol. 1995;62:389–401. doi: 10.1111/j.1751-1097.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Dermal application of JP-8 jet fuel induces immune suppression. Toxicol. Sci. 1999;52:61–67. doi: 10.1093/toxsci/52.1.61. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat. Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Ullrich SE, Lyons HJ. Mechanisms involved in the immunotoxicity induced by dermal application of JP-8 jet fuel. Toxicol. Sci. 2000;58:290–298. doi: 10.1093/toxsci/58.2.290. [DOI] [PubMed] [Google Scholar]
- Ullrich SE, Nghiem DX, Khaskina P. Suppression of an established immune response by UVA—A critical role for mast cells. Photochem. Photobiol. 2007;83:1095–1100. doi: 10.1111/j.1751-1097.2007.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraldi A, Costantini AS, Bolejack V, Miligi L, Vineis P, van Loveren H. Immunotoxic effects of chemicals: A matrix for occupational and environmental epidemiological studies. Am. J. Ind. Med. 2006;49:1046–1055. doi: 10.1002/ajim.20364. [DOI] [PubMed] [Google Scholar]
- Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]