Abstract
Pyridinium ketones have been found to exist as hydrates and hemiacetals in considerable amount in aqueous and alcoholic solutions, respectively. The relative position of the pyridinium positive charge has a large effect on the equilibrium constants. The polar substituent constants, σ*, of the pyridinium group substituted at different positions can be estimated from the hydration constants.
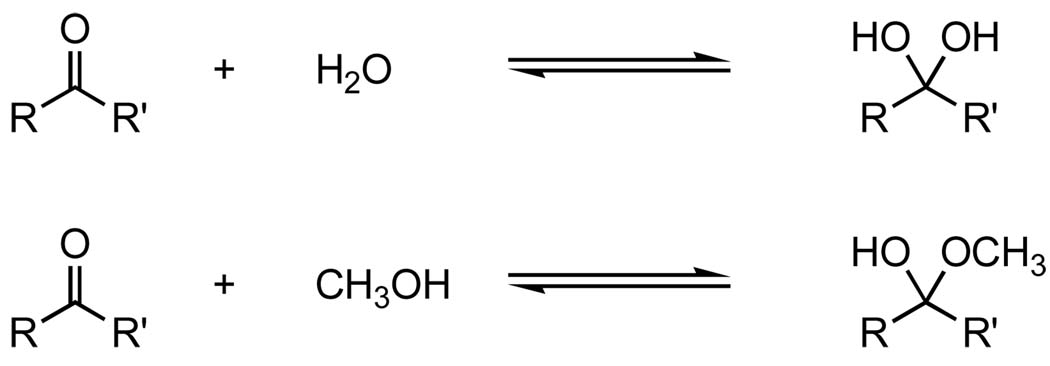
The reversible hydration of aldehydes and ketones in aqueous solution is one of the simplest addition reactions to the carbonyl group (Figure 1). The extent to which hydrates of aldehydes and ketones form gives a measure of the relative stability of the aldehydes and ketones. The greater extent to which the hydrate forms, the less stable the starting aldehyde or ketone. The reaction is of great importance in understanding reactions of the carbonyl group and has been the subject of many studies.1–4
Figure 1.
Equilibrium hydration and hemiacetal formation of aldehydes and ketones
The hydration constants of some aldehydes and ketones have been reported.1–3 Formaldehyde exists mostly as its hydrate in aqueous solution. Simple aliphatic aldehydes exist in equilibrium with their hydrates. Ketones usually exist predominately in the keto form, except for the α-halogenated ones, which exist in equilibrium with their hydrates. Typically α-substitution by electron-withdrawing groups favors the formation of hydrates.
Hemiacetal formation in alcohol follows the same mechanism as hydrate formation. The equilibrium constants for hemiacetal formation of some aldehydes and ketones have been reported as well.2
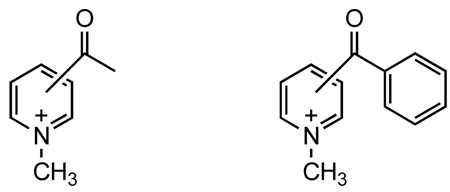
Hydration equilibria for most carbonyl compounds are too lopsided to permit their equilibrium constants to be measured accurately. In our study of pyridinium compounds5, however, we have found that 4-acetyl-N-methylpyridinium iodide exists in aqueous solution to a considerable extent as its hydrate. The pyridinium ketones have thus provided a unique opportunity to measure the thermodynamic parameters of the formation of the corresponding hydrates and hemiacetals. In this Letter, we report the ready formation of hydrates and hemiacetal from N-methylpyridinium ketones in aqueous and alcoholic solutions, respectively. The effect of the relative position of the pyridinium positive charge on the equilibrium is also studied.
During the course of our study of the NMR spectra of N-methylpyridinium compounds, we have observed that the 1H NMR spectrum of 4-acetyl-N-methylpyridinium iodide contains two sets of signals.5 Further study has revealed the existence of the compound as the ketone and hydrate structures. The electron-withdrawing nature of the pyridinium moiety increases the electron deficiency of the carbonyl group and thus makes it more favorable to form a hydrate.
We decided to study a series of methyl pyridinium ketones (acetylpyridinium) and phenyl pyridinium ketones (benzoylpyridinium) to measure their equilibrium constants for hydration and hemiacetal formation in aqueous and alcoholic solutions, respectively, and to investigate the effect of different positions of the positively charged nitrogen on the equilibrium constants.4,6 The compounds were prepared by reacting the corresponding pyridine ketones with methyl iodide as reported.4,5 The molar ratios of ketone to hydrate or hemiacetal in respective D2O or CD3OD solutions were measured by integration of 1H NMR peaks. The pyridinium hydrogens and the N-methyl groups of the hydrates and hemiacetals showed small upfield shifts relative to those of the ketones. Furthermore, in acetylpyridinium compounds, the acetyl methyl group has chemical shifts of about 2.8 ppm while the chemical shifts in the hydrate or hemiacetal form are around 1.8. The hydration constants (Kh, [hydrate]/[ketone]) and hemiacetal formation constants (K, [hemiacetal]/[ketone] in methanol) thus measured are shown in Table 1. The hydration constant for 2-acetylpyridinium iodide is in good agreement with the reported value.4
Table 1.
Hydration constants and methanolic hemiacetal formation constants of some N-methylpyridinium ketones
| Substituent | Hydration, Kh | Hemiacetal, K |
|---|---|---|
| 2-Acetyl | 0.06 | 0.30 |
| 3-Acetyl | 0.03 | 0.29 |
| 4-Acetyl | 0.26 | 1.38 |
| 2-Benzoyl | <0.01a | 0.11 |
| 3-Benzoyl | <0.01a | 0.20 |
| 4-Benzoyl | 0.07 | 0.70 |
The hydration constant was measured to be about 0.008, but was deemed to be beyond the detection limit of 1H NMR.
It is expected that the benzoyl derivatives are less likely to form hydrates or hemiacetals due to their stronger hydrophobic nature and/or their less reactive ketone moieties. The effect of the relative position of the positive charge on the equilibrium is quite interesting. It appears that the carbonyl groups attached to the 4-position of the pyridinium group are substantially more reactive towards water or alcohol than those substituted at 2- or 3- position. The diminished reactivity of the 3-substituted pyridinium ketones is not unexpected because there are no resonance structures putting a positive charge adjacent to the carbonyl group. However, the absence of enhanced reactivity in the 2-substituted pyridinium ketones is mostly due to a steric effect. The proximity of the N-methyl group and the carbonyl group makes it possible that steric interaction may be more profound in the tetrahedral structure of the hydrate or hemiacetal than in the keto form.
The formation of hemiacetals appears to be more favorable as compared to that of hydrates, as in the case of aryl alkyl ketones such as α,α,α-trifluoroacetophenone.2 In most ketones and aldehydes, however, the formation of hydrate is usually more favorable.2
The hydration constants measured for acetylpyridinium compounds can be used to estimate the polar substituent constants, σ*, of the pyridinium group substituted at 2-, 3-, and 4- positions. In methyl ketones, the hydration constants are related to σ* by eq 1 developed empirically by Greenzaid et al.1,4 The substituent constants, σ*, of the pyridinium group substituted at 2-, 3-, and 4- positions were thus calculated to be 0.93, 0.76, and 1.31, respectively. These values of σ* can be used to estimate the polar effects of the pyridinium group substituted at different positions.
| (1) |
In summary, hydrates and hemiacetals form in substantial amount in aqueous and alcoholic solutions of pyridinium ketones. Unlike most ketones and aldehydes, hemiacetal formation appears to be more favorable than hydrate formation. The hydration constants of acetylpyridinium compounds provide an estimate of the polar substituent constants and thus the polar effect of the pyridinium group substituted at various positions of the heterocyclic ring.
Acknowledgements
This investigation was supported by the National Institutes of Health, MBRS SCORE Program – Grant #5 S06 GM52588. We thank Wee Tam for obtaining the NMR spectra. The NMR facility was funded by the National Science Foundation (DUE-9451624 and DBI 0521342). We also thank Professors James Keeffe and Ihsan Erden at SFSU for helpful discussions.
References and notes
- 1.Greenzaid P, Luz Z, Samuel D. J. Am. Chem. Soc. 1967;89:749–756. [Google Scholar]
- 2.Guthrie JP. Can. J. Chem. 1975;53:898–906. [Google Scholar]
- 3.Keeffe JR, Kresge AJ. Ch. 7. In: Rappoport Z, editor. The Chemistry of Enols. New York: Wiley; 1990. [Google Scholar]
- 4.Tobin JB, Frey PA. J. Am. Chem. Soc. 1996;118:12253–12260. [Google Scholar]
- 5.Huang S, Wong JCS, Leung AKC, Chan YM, Wong L, Fernendez MR, Miller AK, Wu W. Tetrahedron Lett. 2009;50:5018–5020. doi: 10.1016/j.tetlet.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A few ketones or aldehydes with positively charged heterocycles have been discussed. Benzimidazolium and thiazolium ketones were reported to form considerable amount of hydrate in water. Lienhard GE. J. Am. Chem. Soc. 1966;88:5642–5649. doi: 10.1021/ja00969a017. Owen TC. J. Heterocyclic Chem. 1990;27:987–990. Bunting JW, Stefanidis D. J. Am. Chem. Soc. 1988;110:4008–4017.