Abstract
Three Pavlovian fear conditioning experiments with rats as subjects explored the effect of extinction in the presence of a concurrent excitor. Our aim was to explore this particular treatment, documented in previous studies to deepen extinction, with novel control groups to shed light on the processes involved in extinction. Relative to subjects extinguished on the target CS alone, Experiments 1 and 2 found across a range of parameters that any appreciable effect of facilitated extinction due to the concurrent excitor was submerged by generalization decrement going from extinction to testing. In Experiment 3 we used different durations for the target and concurrent stimuli in order to discourage configuring and an ABC renewal design to increase sensitivity, and observed diminished renewal resulting from extinction in the presence of a second excitor. Our findings suggest that there are distinct limits to the observation of extinction in the presence of an excitor and identifies some of the sources of these limitations.
Keywords: Pavlovian fear conditioning, extinction, concurrent excitor, phobias, anxiety
In the last two decades, there has been renewed interest in the processes underlying experimental extinction after Pavlovian conditioning, in part because experimental extinction appears to model aspects of exposure-based therapies used to treat anxiety disorders (Hofmann, 2008). Experimental extinction after Pavlovian conditioning is a procedure in which subjects, who have previously learned an association between an initially neutral stimulus and aversive (or appetitive) consequences, learn, through repeated nonreinforced presentations of the stimulus, that the stimulus no longer is followed by the aversive consequences. This process is presumed to result in new [inhibitory-like] learning about this relationship that coexists with the original excitatory association but is highly context dependent (Bouton, 1993; 2002).
This interpretation of extinction is supported by demonstrations in which, without any further manipulations involving the target cue, recovery from extinction is observed (see Bouton, 2004, for a review). One such situation is renewal, which is the recovery from extinction observed when testing is conducted in a context different from that where extinction was conducted. Renewal can take three forms but all of them comply with the necessity of the test context being different from that of extinction. In ABA renewal, subjects experience reinforced training on one context (A), extinction training in a second context (B), and thereafter are tested in the reinforcement context (A; thus the nomenclature ABA refers to the context of training, extinction, and test, respectively). A second form of renewal is ABC renewal, in which subjects are tested in a neutral context (C). A third, albeit weaker, form of renewal is that in which reinforced training and extinction training are conducted in the same context (A), and testing is conducted in a different context (B; hence AAB renewal). In all three situations, recovery from extinction is observed.
Demonstrations of recovery from extinction have also been reported with humans in the laboratory (e.g., Effting & Kindt, 2007; Vasteenwegen et al. 2005), as well as in clinical settings (e.g., Mystowski, Craske, & Echiverri, 2002). In the clinical case, relapse from behavioral treatments (also called return of fear [ROF], Rachman, 1989) is often observed after the client leaves the therapist's office (analogous to renewal). Given these similarities between experimental extinction and behavior therapy, basic behavioral scientists have focused on finding ways to make the extinction memory more durable and more readily retrieved (Bouton, 2002). For example, renewal is attenuated when extinction is conducted in several different contexts (Chelonis, Calton, Hart, & Schachtman, 1999; Gunther, Denniston, & Miller, 1998; Neumann, 2006, with human participants, but see Bouton, García-Gutiérrez, Zilski, & Moody, 2006; Neumann, Lipp, & Cory, 2007). Renewal is similarly attenuated if large numbers of extinction trials (800) are presented relative to a fewer number of trials (160) which are sufficient to produce cessation of responding during the extinction session (Denniston, Chang, & Miller, 2003; also see Tamai & Nakajima, 2000). Widely spaced extinction trials (i.e., a long intertrial interval [ITI] between extinction trials) have also been shown to attenuate recovery from extinction relative to extinction trials separated by a short ITI as indexed by tests of renewal and spontaneous recovery (Urcelay, Wheeler, & Miller, 2009; but see Cain, Bouin & Barad, 2003).
The present series of experiments focused on another treatment that has shown to sometimes alleviate recovery from extinction, namely extinction in the presence of another excitor (Rescorla 2000; 2006; Thomas & Ayres, 2004; Wagner, 1969). In this treatment, during extinction trials the target cue is presented simultaneously with another cue that also received prior excitatory training. In addition to its implications for therapy, this effect is theoretically interesting because more durable extinction is anticipated by some current theories of associative learning (Rescorla & Wagner, 1972; Stout & Miller, 2007; Wagner, 1981) but not by others (Pearce, 1987, 1994; 2002).
Elemental theories of learning (e.g., Rescorla & Wagner, 1972; Wagner, 1981; Miller & Matzel, 1988) assume that a compound of two stimuli can be analyzed by looking at the contribution of each element to the compound. For example, the Rescorla-Wagner model computes changes in associative strength for each cue separately based on the predictiveness of all cues present on a given trial. Applied to an extinction situation in which a simultaneous compound of excitatory cues is presented, the model predicts that the additional excitor will increase the total error term on a given extinction trial and thus increase the rate of extinction to the target (but sometimes only during early extinction trials; see Rescorla, 2006, p 135, for a parametric qualification). The comparator hypothesis (Denniston, Savastano, & Miller, 2001; Miller & Matzel, 1988; also see, Stout & Miller, 2007, for a mathematical implementation), a response rule for the expression of associations, does not compute changes in associative strength based on all cues presented on a given trial, but rather computes the change in associative strength based on each cues' associative strength. According to this model conditioned responding is determined at the time of testing based on retrieval of the US representation by the target cue, but also on the retrieval of the US representation by other cues that were presented together with the target during training. At test, responding is directly related to the strength of the target cue's association with the US and inversely related to the product of the strength of the association of the target with other cues (i.e., comparator stimuli) that were presented together with the target during training, and of the association of these cues with the US. As a result, the model predicts enhancement of extinction after compound extinction trials of two excitatory cues. Specifically, according to this model extinction is driven by two processes, one of them being the loss of associative strength of the target CS→US association. The second process is the strengthening of CS→comparator stimulus (link 2 in the comparator framework) association, which should also diminish conditioned responding at test. If extinction is conducted with the target cue alone, the role of the comparator stimulus is taken by the context, which itself has a weak association with the US due to its relatively low salience. However, if the comparator stimulus is another excitatory punctate cue, then responding at test should be weaker (i.e., extinction should be enhanced), at least with few extinction trials, because the associative strength of the comparator stimulus to the US should further decrease responding to the target. Thus, both the Rescorla-Wagner model and the comparator hypothesis predict that with few extinction trials that extinction should be enhanced by the co-presentation of an additional excitor.
Contrary to elemental theories, Pearce's configural model (Pearce, 1987, 1994, 2002) assumes that organisms represent only the integrated features of the configured compound when several stimuli are presented together. At test, if only one of the stimuli is presented, the amount of responding to that stimulus is determined by the degree to which the inhibition acquired to the configuration generalizes to the element being tested. Applied to extinction in the presence of an added excitor, this model anticipates that compound extinction of two excitors should result in more extinction than compound extinction of an excitor and a novel stimulus because in the first situation both excitors will have excitation to generalize to the compound whereas in the second situation only one excitatory stimulus will. Thus, during extinction the inhibition acquired by the compound will be larger when two excitatory cues are combined than when one excitatory and a neutral cue are combined. Responding at test to one element is determined by the degree of generalization from the compound that underwent extinction to the element being tested, which should be the same for the two instances. However, this model predicts that, with respect to testing on a target cue, extinction of that cue alone should be roughly equivalent to extinction of a compound of that cue with an additional excitor because at test responding is determined by that cue's excitatory and inhibitory associative strengths; there is no generalization decrement because the stimulus tested is the same as the one extinguished. In other words, this model predicts that an additional excitatory stimulus should enhance extinction relative to group that received an additional neutral stimulus, but that responding to a cue after extinguishing it in compound with a second excitatory cue should not be appreciably different from extinction of a stimulus alone because in the compound case the loss in generalization is compensated for by the enhancement in extinction. Thus, Pearce's configural model makes a different prediction than elemental models.
One might turn to published data to resolve this theoretical disagreement, but the limited available data are not entirely consistent. Rescorla (2000; 2006) and Thomas and Ayres (2004) both observed enhanced extinction with the co-presentation of an additional excitor during extinction. Specifically, Rescorla (2000) separately paired two distinct conditioned stimuli (CSs) with an appetitive outcome (i.e., food). In the following phase he presented the target CS nonreinforced either alone or in compound with the second excitor. Using an immediate test and a single context for acquisition, extinction, and testing (AAA), he observed that extinction of the target CS in the presence of an additional excitor resulted in greater extinction of a conditioned response (CR) relative to when the target CS was extinguished alone. These observations are interesting, but the use of an appetitively motivated task raises questions concerning their generality to aversively motivated tasks which better model therapeutic exposure therapy. One might also question the generality of the effect to clinical settings because neither tests of spontaneous recovery nor renewal were employed to assess the durability of the enhanced extinction. Thomas and Ayres (2004) used fear conditioning and an ABA renewal paradigm to study the effects of this extinction treatment. In their experiment they assessed fear in Context A following extinction in Context B of three different excitors presented either in compound (all three excitors) or alone. However, they did not include a control for renewal; that is, they performed their study using only an ABA design without including an ABB control group. Their results were interpreted as a stronger renewal effect following elemental extinction (X alone) as compared to extinction in compound (VXY). Because they did not include the necessary control groups, it is not known whether facilitated extinction would be obtained without it's being embedded in a renewal procedure. Using an AAA design (i. e., no renewal) with appetitive reinforcement in an autoshaping preparation with pigeons, Pearce and Wilson (1991) observed the opposite pattern, that is, protection from extinction when extinction treatment was conducted in the presence of an excitor (also see Pineño, 2007, for parallel results in a conditioned taste aversion preparation). They interpreted this outcome as resulting from configural processes operating during extinction that, at the time of testing resulted in decreased generalization from extinction treatment to testing (i.e., generalization decrement of extinction). By generalization decrement, we mean that little compound extinction learning transfers to elemental testing. Consistent with this report, fear conditioning experiments with humans have not obtained the basic enhancement of extinction that is observed when extinction is conducted in the presence of another excitor (Lovibond, Davis, & O'Flaherty, 2000; Vervliet, Vansteenwegen, Hermans, & Eelen, 2007).
Thus, at both theoretical and empirical levels there is disagreement regarding the outcome of this treatment. Understanding the outcome of this treatment, and its underlying mechanisms, is important given the translational implications of experimental extinction, as there is increasing interest from clinicians to incorporate knowledge from basic research (Craske, Kircanskim, Zelikowsky, Mystkowski, Chowdhury, & Baker, 2008; Hoffman, 2008). The objective of the current experiments was to assess this effect with control groups better designed to illuminate the underlying processes involved in conducting extinction in the presence of a second excitatory stimulus. Moreover, we wanted to assess the generality of previous findings and perhaps shed light on the reasons why discrepant findings have been reported in the literature.
Experiment 1
In Experiment 1 we asked whether greater extinction effects would be observed when extinction occurred in the presence of an additional excitor. Extinction and testing occurred in the same context, distinct from that of conditioning (i.e., no renewal [ABB]). In other words, we trained subjects in Context A and extinguished and tested in a different Context B. We made use of a factorial mixed design in which the potential effect was assessed both, within and between subjects. The design of the experiment is shown in Table 1. In the critical group, denoted Excitor, subjects were given interspersed excitatory conditioning of three different stimuli (CSs V, X, and Y), each paired separately with an unconditioned stimulus (US; footshock). Following this training, two of the excitatory stimuli were extinguished in compound (VX) and the third stimulus was extinguished alone (Y). Then subjects were tested on both X and Y. Group Elemental was a redundant control for compound vs. elemental extinction; however, unlike Group Excitor it offered a between-subjects comparison rather than within subjects. This group was trained to the same three excitatory stimuli. Following training all three stimuli were extinguished elementally. If extinguishing in compound with an additional excitor does facilitate extinction, then we would expect that responding to X in this group would be stronger than responding to X in Group Excitor for which the subjects experienced extinction to X in compound with another excitor (V). A third group, which we denoted GenDec, was included to control for the effects of generalization decrement. Specifically, this group assessed whether the subjects processed the stimuli presented in compound during extinction as two separate stimuli or whether they partially treated the compound as a novel configured stimulus. This group was exposed to the same training as Group Excitor, except that during the extinction phase, instead of extinguishing X in compound with an excitatory stimulus V, it was extinguished in compound with a neutral stimulus Z. The occurrence of generalization decrement will be revealed in this group if X is extinguished to a lesser degree than Y. Additionally, if generalization decrement operates against enhanced extinction due to the presence of a second excitor, as is anticipated by some theories of learning (e. g., Pearce, 1987; 1994; 2002), the response to X in Group GenDec would be greater than that of Group Elemental (see above) because extinction to X would be reduced by stimulus configuration during extinction treatment. Rescorla (2000) suggested that generalization decrement could be reduced by repeated presentations of the neutral stimulus alone prior to extinction treatment. He reasoned that prior exposure of the stimulus would make it familiar and thus less subject to any conditional control. Thus, we included a second generalization decrement group (Group GenDec2) which, besides being trained with the three excitatory stimuli, also received exposure to a neutral stimulus Z, which was presented alone and nonreinforced during this phase. Following reinforced training, the target cue X was extinguished in compound with Z, and Y was extinguished alone. During the test, if prior exposures to Z alone decreased generalization decrement, then weaker responding to X in Group GenDec2 relative to responding to X in Group GenDec would be expected. Group Y-only was a control group in which we presented the same training as in the first two groups, but extinguished the subjects on stimulus Y only. We expected to see no differences in responding to Y in this group as compared to the rest of the groups, and also a strong responding to X, as it was not extinguished at all. This group was included to permit assessment of simple extinction without any possibility of extinction generalizing between X and Y. Finally, we included Group Control which received the same training, yet none of the stimuli received extinction treatment. Thus, we expected equally strong responding to X and Y.
Table 1.
Design of Experiment 1
| Group | Pre-Exposure (A) | Phase 1 Acquisition (A) | Phase 2 Extinction (B) | Test (B) | Test (B) |
|---|---|---|---|---|---|
| Excitor | 4 V / 4 Z | 6 V+ / 6 X+ / 6 Y+ | 8 VX- / 8 Y- | X | Y |
| Elemental | 4 V / 4 Z | 6 V+ / 6 X+ / 6 Y+ | 8 V- / 8 X- / 8 Y- | X | Y |
| GenDec | 4 V / 4 Z | 6 V+ / 6 X+ / 6 Y+ | 8 ZX- / 8 Y- | X | Y |
| GenDec2 | 4 V / 4 Z | 6 V+ / 6 X+ / 6 Y+ / 6 Z- | 8 ZX- / 8 Y- | X | Y |
| Y-only | 4 V / 4 Z | 6 V+ / 6 X+ / 6 Y+ | -- / 8 Y- | X | Y |
| Control | 4 V / 4 Z | 6 V+ / 6 X+ / 6 Y+ | -- / -- | X | Y |
Note: + = footshock. Numbers next to the stimuli indicate total number of trials in that phase. Slashes indicate interspersed trials. Stimuli X and Y were 60-s complex tone and 60-s click train, counterbalanced. Stimuli V and Z were 60-s white noise and 60-s Sonalert, also counterbalanced. Letters inside parentheses indicate the context.
Method
Subjects
Subjects were 36 male and 36 female, experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. Subjects were randomly assigned to one of six groups (ns = 12), counterbalanced within groups for sex. Subjects were individually housed and maintained on a 16-hr light/8-hr dark cycle. Experimental sessions occurred roughly midway through the light portion. Between weaning and the initiation of the experiment, all animals were handled for 30 s three times a week. Subjects had free access to food in the home cages. Prior to initiation of the experiment, water availability was progressively reduced to 10 min per day, provided approximately two hours after any scheduled treatment.
Apparatus and stimuli
The apparatus consisted of twelve operant chambers, each measuring 30 × 30 × 27 cm (l × w × h). The sidewalls of the chamber were made of stainless steel sheet metal, and the front wall, back wall, and ceiling of the chamber were made of clear Plexiglas. On the left side of one metal wall of each chamber there was a 3.5-cm wide operant lever, 4-cm above the floor, and on the right side of this wall there was a niche (2.5 × 4.5 × 4 cm), the bottom of which was 2 cm above the floor, where a drop (0.04 cc) of tap water could be presented by a solenoid valve into a small cup. The floor was constructed of 0.3 cm diameter rods, spaced 1.3 cm center-to-center, and connected by NE-2 neon bulbs that allowed a 0.7-mA, 0.5-s constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each chamber was housed in an environmental isolation chest, which was dimly illuminated by a houselight (1.12-W, #1820 incandescent bulb) mounted on a wall of the experimental chamber. A ventilation fan in each enclosure provided a constant 76-dB (C-scale) background noise. Three 45-Ω speakers mounted on the interior right, left and back side of each environmental chest were used to deliver a low frequency complex tone (300 & 320 Hz presented simultaneously), a click train (6 Hz), and a white noise, respectively, each at 6 dB (C-scale) above the background. Additionally, a Sonalert mounted on each environmental chest was able to deliver a high frequency (1900 Hz) tone at 8 dB above background. Tone and click served as CSs X and Y, counterbalanced within groups, while white noise and Sonalert served as Stimuli V and Z, also counterbalanced within groups. During training, all stimuli were 60 s in duration with the exception of the 0.5-s footshock US. Lastly, a visual stimulus that consisted of a 0.5-s light served as a discriminative stimulus, presented simultaneously with each presentation of water. The light was provided by a 100-W bulb, nominal at 120 VAC, but driven at 40 VAC. The bulbs were mounted on an inside wall of the environmental chest, approximately 30 cm from the center of the experimental chamber. Chamber assignments were fully counterbalanced within each group.
Various types of materials were used to create two distinct contexts. The physical identity of Contexts A and B was defined by different instances of the chambers. Moreover, Context A had an odor cue (methyl salycilate), no levers, the houselight off, and cylindrical grid floors for footshock administration. Context B had no odor cue, levers for lever-pressing, Plexiglas sheet floors, and the presence of the houselight.
Procedure
Shaping
On Days 1-5, prior to pre-exposure, acclimation to the apparatus and shaping of lever press behavior were conducted in 60-min sessions in Context B. Subjects were shaped to lever press for water on a variable-interval 20-s schedule in the following manner. On Days 1 and 2, a fixed-time 120-s schedule of noncontingent water delivery was in force with a continuous reinforcement schedule. On Day 3, noncontingent reinforcers were discontinued, and subjects were trained on the continuous reinforcement schedule alone. Subjects that made less than 50 responses were hand shaped to reach this criterion later on the same day. On Days 4 and 5, a variable-interval 20-s schedule was imposed. This schedule of reinforcement prevailed throughout the remainder of the experiment including testing (except for Phase 1 and the pre-exposure treatment). No nominal stimuli were presented during this phase. Water (H2O) presentation was always accompanied by 0.5-s presentation of the light.
Pre-Exposure
On Days 6 and 7, all subjects were exposed to two nonreinforced presentations of Stimulus V interspersed with two nonreinforced presentations of Stimulus Z on each day in Context A. This was done in order to mitigate against these cues being configured when they were later presented in compound. Each stimulus presentation occurred at 10-min intervals in a 60-min session, and the order of stimuli presentations was reversed on Day 7 relative to Day 6.
Acquisition
On Days 8 – 10, all subjects received two reinforced presentations of CSs V, X, and Y per day in Context A. Group GenDec2 also received two nonreinforced presentation of Stimulus Z per day. The mean intertrial interval (ITI) for the presentation of CSs was 8 min, with a range of 6 – 10 min, except for Group GenDec2, for which the mean ITI was 6 min, with a range of 4 – 8 min. Mean ITIs reported here were measured from CS termination to CS onset. During acquisition the subjects had no access to water.
Extinction
On Days 11 and 12, the extinction treatment was administered in Context B. On each day subjects in Group Excitor received 4 nonreinforced presentations of a VX compound, and 4 nonreinforced presentations of stimulus Y alone. Subjects in Groups GenDec and GenDec2 received 4 nonreinforced presentations of a ZX compound and 4 nonreinforced presentations of Y on each day. The mean ITI in these three groups was 6 min, with a range of 4 - 8 min. The subjects in Group Y-only received 4 nonreinforced presentations of CS Y alone, with a mean ITI of 12 min and a range of 10 – 14 min. Subjects in Group Elemental received 4 nonreinforced elemental presentations of stimuli V, X, and Y. The mean ITI for this group was 4 min, with the range of 2 – 6 min, from CS termination to CS onset. Finally, subjects in Group Control were exposed to the context (B) alone without any nominal stimulus presentation. The order of CS presentations for all groups was pseudo-randomized for each of the two days.
Reshaping
On Days 13 and 14, all subjects were reshaped in Context B to restabilize lever pressing that may have been disrupted by presentations of the US. Subjects experienced daily 60-min sessions to restabilize lever pressing on the variable-interval 20-s schedule.
Test
On Days 15 and 16, all subjects were tested on CS X on one day and CS Y on another day, with the order being counterbalanced between subjects within groups. All testing was conducted in Context B. During testing, suppression of baseline responding during presentation of the CS was assessed. Each subject received four nonreinforced 60-s presentations of the CS each day. CS onset times were 4, 8, 12, and 16 min into a 18-min session.
Data Analysis
In all experiments reported here (with the exception of Experiment 3 in which only X was tested), four suppression ratios (Annau & Kamin, 1961) were calculated for each subject: one for responding to the first two X test trials, one for the second two X trials, one for the first two Y trials, and one for the second two Y trials. The ratio was calculated by the formula A/(A+B), where A is the rate of lever-pressing during two 60-s CS (X or Y) and B is the mean rate of lever-pressing during the two 60-s pre-CS periods. This ratio was used as an index of the subject's fear to the presentations of the target CS. Two blocks of test trials for each cue (X and Y) were used because we wanted sensitivity to differences between groups that may have emerged (or faded) during the course of extinction at test. Ratios typically range from 0 to 0.5, with lower values indicating more fear and 0.5 indicating no fear. All experiments were analyzed with analyses of variance (ANOVAs) and subsequent planned comparisons. When appropriate, we report effect size calculated using the algorithm provided by Myers and Well (2003, p. 210). We adopted the conventional criteria for effect sizes (small = 0.10, medium = 0.25, large = 0.40).
Results and Discussion
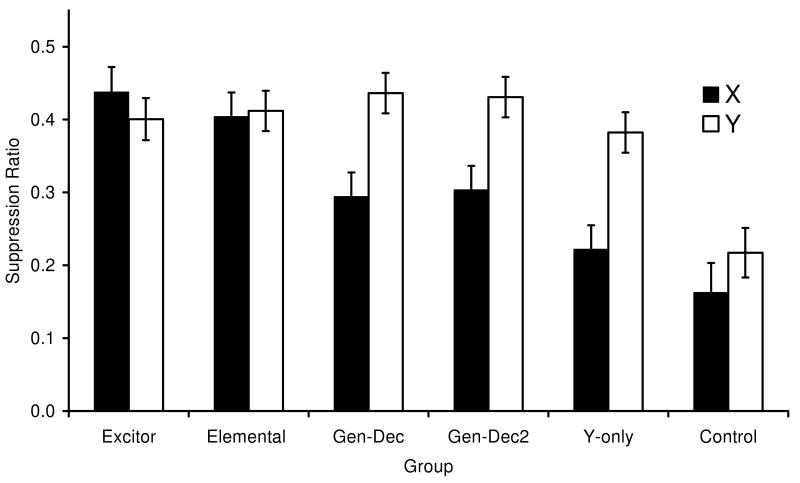
The group means of Experiment 1 are depicted in Figure 1. As it is apparent in the figure, there were no differences in suppression between CSs X and Y in Group Excitor, which experienced extinction of X in the presence of a second excitor and extinction of Y alone. Moreover, responding to CS X in this group was not appreciably different from responding to X in Group Elemental, suggesting that extinction of X was not enhanced when it was conducted in the presence of a second excitatory stimulus. Group GenDec's behavior suggests that mere extinction in the presence of a second neutral stimulus (Z) significantly decreases extinction (i.e., protects the target cue from extinction), as suppression to X was greater than to Y, perhaps due to generalization decrement from extinction with a compound (ZX) to testing with a single cue (X). These observations were verified with the following statistical analyses.
Figure 1.
Mean suppression ratios to X (black bars) and Y (white bars), averaged within subjects over four test trials with each cue in Experiment 1 (ABB design). See Table 1 for group treatments and procedural details. Note that lower values denote more suppression and larger values denote less suppression (i.e., more extinction). Error brackets depict the standard error of the mean for each group.
Due to a lack of responding during pre-CS periods on either the X or Y test trials, one subject from Group Excitor and four subjects from Group Control were eliminated from all the analyses. A 6 (Group: Excitor vs. GenDec vs. GenDec2 vs. Y-only vs. Elemental vs. Control) × 2 (Stimulus: X vs. Y) × 2 (Block: 1 vs. 2) mixed ANOVA conducted on the lever-pressing rates during blocks of two 60-s periods immediately before each CS presentation did not reveal any main effects or interactions (smallest p = .11), suggesting that there were no appreciable differences among groups in baseline responding during the pre-CS temporal windows used to calculate the suppression ratios. A similar ANOVA conducted on the suppression ratios revealed a main effect of group, F(5, 61) = 8.69, p < 0.01, MSE = 0.03, a main effect of stimulus, F(1, 61) = 26.32, p < 0.01, MSE = 0.01, and a main effect of block, F(1, 61) = 153.16, p < 0.01, MSE = 0.01. More important, the ANOVA also revealed an interaction between group and stimulus, F(5, 61) = 5.13, p < 0.01, MSE = 0.01, Cohen's f = 0.53, and an interaction between stimulus and block, F(1, 61) = 12.75, p < 0.01, MSE = 0.006, but no group × stimulus × block interaction, p = .28. The stimulus × block interaction reflects differential overall extinction of X and Y during testing. This may have occurred because there was overall more suppression to X than to Y, so that more extinction of X during the test session was observed. Nevertheless, the interaction is not central to the focus of this paper.
The lack of a three-way interaction suggests that the interaction between group and stimulus was relatively stable across blocks of test trials, and therefore we assessed the source of that interaction with planned comparisons collapsing the two blocks for each CS. A within-subjects comparison of suppression to X and Y in Group Excitor did not reveal any difference, F(1, 61) = 1.04, p = .31, MSE = 0.01. Moreover, a between-subjects comparison of Groups Excitor and Elemental in responding to X did not reveal any differences in suppression, F(1, 61) = 0.48, p = 0.48, MSE = 0.02. These two critical comparisons suggest, both within and between subjects, that extinguishing the target X in the presence of another excitor V did not appreciably enhance overt extinction. Additional comparisons suggested a large generalization decrement effect in that suppression to X was greater than to Y in Groups GenDec, F(1, 61)= 16.89, p < 0.01, MSE = 0.01, Cohen's f = 0.46, and GenDec2, F(1, 61) = 13.52, p < 0.01, MSE = 0.01, Cohen's f = 0.42, after experiencing extinction of X in the presence of a neutral cue (Z) compared with extinction of Y alone. Notably, these two groups did not differ in their differences between X and Y, suggesting that nonreinforced preexposure to Z in Group GenDec2 did not decrease generalization decrement going from extinction of ZX to testing with X. Moreover, a between-subjects comparison of Groups Excitor and GenDec on responding to X revealed a difference, F(1, 61) = 8.91, p < 0.01, MSE = 0.03, which suggests that facilitated extinction actually occurred in Group Excitor and compensated for the generalization decrement caused by the presentation of a second stimulus during extinction treatment. However, the absence of any difference in suppression to X in Groups Excitor and Elemental indicates that any facilitatory effect of V on Group Excitor is diminished by generalization decrement so that net behavioral control by X after extinction in the presence of V was no different than after extinction alone. A final comparison between X and Y in Group Y-only suggests that the cues were highly discriminated in that extinction of only one of them (Y) did not alter responding to the nonextinguished X, F(1, 61) = 21.52, p < 0.01, MSE = 0.01. Thus, little if any generalized extinction from one to the other cue was observed.
From observation of Figure 1, one might conjecture that we did not observe differences in extinction due to the presence of an additional excitor because we administered too many extinction trials so that floor effects precluded the observation of enhanced extinction. A Group × Stimulus mixed ANOVA conducted on the first block of test trials (with no subject excluded because of lack of baseline responding because no subject met the exclusion criterion on the first block of trials) revealed a main effect of Group, F(5, 66) = 8.67, p < 0.01, MSE = 0.02, a main effect of Stimulus, F(1, 61) = 34.49, p < 0.01, MSE = 0.01, and an interaction F(1, 66) = 4.61, p < 0.01, MSE = 0.01. This indicates a similar pattern to that observed with both blocks of trials. All of the pairwise comparisons reported above here achieve similar results. In Group Excitor, the mean suppression to stimulus X during the first trial was M = 0.25, SE = 0.04 and to stimulus Y was M = 0.26, SE = 0.05. Thus, early during the test, suppression was vigorous enough to allow the observation of differences due to the different extinction treatments, but these differences were not observed. This additional analysis confirms that our results are not due to floor effects.
In this experiment, we assessed the effects of conducting extinction in the presence of a second excitatory stimulus. Relative to a cue that was extinguished alone (Y), extinction in the presence of an excitor (VX in Group Excitor) did not facilitate net extinction of fear, as suggested by both within- and between-subjects comparisons. This is not to say that enhanced extinction did not occur. Rather an enhanced extinction effect seems to have occurred, but was countered by a strong generalization decrement resulting from extinguishing a compound and testing on only one element of that compound. This is supported by the pattern observed in GenDec Groups in which the addition of a neutral cue (Z) during extinction had a protective effect from extinction regardless of whether or not there had been preexposure to that cue itself. That is, both GenDec and GenDec2 groups showed more suppression to X (i.e., less extinction) at the time of test than to Y, which was extinguished alone. In other words, our preexposure to Z treatment failed to eliminate generalization decrement. Thus, these results are consistent with a configural account of learning (extinction here) such as that proposed by Pearce (1987, 1994, 2002). This model predicts similar effective extinction to X when it is extinguished alone (X) and when it is extinguished with another excitor (VX), but less extinction when the target is extinguished with a neutral cue (ZX). In the VX case, the VX configured stimulus undergoes greater decrease in associative strength than X given X-alone presentations, but generalization decrement reduces the effect of this on responding to X alone at test.
Although the results of Experiment 1 lend support for a configural account of learning, this view does not explain the observations of Rescorla (2000, 2006), in which he found enhanced extinction with the co-presentation of a second excitor during extinction in a number of different conditioning preparations. It is important to keep in mind that Rescorla did not observe a generalization decrement effect as large as the one we observed in Groups GenDec and GenDec2. Rescorla's analogous groups (that is, groups that experienced extinction in the presence of a neutral cue) all showed similar extinction to that observed in groups receiving elemental extinction. Perhaps the facilitative effects of extinction treatment in the presence of another excitor that Rescorla documented are most readily observed when generalization decrement resulting from a compound extinction procedure is minimized. In Rescorla's studies, generalization decrement may have been reduced by his choice of stimulus modalities and parameters (see General Discussion). Based on this logic, Experiment 2 was run with the same design as Experiment 1 but with some parametric changes that would decrease configural processing of compound stimuli and thus would presumably reduce generalization decrement from extinction to test.
Experiment 2
In Experiment 2, we used the same design to that of Experiment 1 but with a number of parametric variations that we expected would diminish configural processing and thus increase the possibility of observing enhanced extinction when it occurred in the presence of a second excitor. This would shed light on the reasons why some studies observe the effect whereas others do not. Specifically, in Experiment 2 we changed the modality of the two stimuli (V and Z) that were co-administered with the target (X) during extinction from auditory to visual, with the thought that a difference in stimulus modality between X and its companion in extinction would favor elemental processing of cues over configural processing and thus allow for the observation of increased extinction in the VX condition (Kehoe, Horne, Horne, & Macrae, 1994). Moreover, in order to further decrease configural processing during extinction, we decreased the duration of all cues from 60 s to 15 s. The reasoning was that short duration cues favor the observation of overshadowing (which results from elemental processing), whereas long duration cues favor the observation of potentiation resulting presumably from configural processing (Westbrook, Homewood, Horn, & Clarke, 1983). Thus, there was less time in which X and its companion was presented in compound. Because cues of short duration acquire more associative strength with USs than cues of long duration (a CS-duration effect; e.g., Urushihara, Stout & Miller, 2004), we decreased the number of reinforced trials during Phase 1 from 6 to 4, and we increased the number of extinction trials from 8 to 12 trials. These changes were done mainly to avoid ceiling or floor effects of the extinction treatment. Overall, the total duration the two cues were presented during extinction was reduced from 480 s (8 trials with 60-s CSs) to 180 s (12 trials with 15-s CSs). We expected that these changes would favor elemental processing of the cues, thus revealing enhanced extinction with the co-presentation of a second excitor. The design of Experiment 2 is shown in Table 2.
Table 2.
Design of Experiment 2
| Group | Pre-Exposure (A) | Phase 1 Acquisition (A) | Phase 2 Extinction (B) | Test (B) | Test (B) |
|---|---|---|---|---|---|
| Excitor | 4 V / 4 Z | 4 V+ / 4 X+ / 4 Y+ | 12 VX- / 12 Y- | X | Y |
| Elemental | 4 V / 4 Z | 4 V+ / 4 X+ / 4 Y+ | 12 V- / 12 X- / 12Y- | X | Y |
| GenDec | 4 V / 4 Z | 4 V+ / 4 X+ / 4 Y+ | 12 ZX- / 12 Y- | X | Y |
| GenDec2 | 4 V / 4 Z | 4 V+ / 4 X+ / 4 Y+ / 4 Z- | 12 ZX- / 12 Y- | X | Y |
| Y-only | 4 V / 4 Z | 4 V+ / 4 X+ / 4 Y+ | -- / 12 Y- | X | Y |
| Control | 4 V / 4 Z | 4 V+ / 4 X+ / 4 Y+ | -- / -- | X | Y |
Note: + = footshock. Numbers next to the stimuli indicate total number of trials in that phase. Slashes indicate interspersed trials. Stimuli X and Y were 15-s complex tone and 15-s click train, counterbalanced. Stimuli V and Z were 15-s house-light on and 15-s flashing light, also counterbalanced. Letters inside parentheses indicate the context.
Methods
Subjects
The subjects were 36 male and 36 female, experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. Subjects were randomly assigned to one of six groups (ns = 12), counterbalanced within groups for sex. Subjects were individually housed, maintained, and water deprived in a similar manner to Experiment 1.
Apparatus and stimuli
The apparatus was the same as that used in Experiment 1. Also similar to Experiment 1, the tone and click served as CSs X and Y, counterbalanced within groups. However, the houselight being turned on and the brighter light (now turned into a 0.5s on / 0.5s off flashing light) served as stimuli V and Z, also counterbalanced within groups. The flashing light was provided by a 100-W bulb, nominal at 120 VAC, but now driven at 60 VAC. During the entire experiment, all stimuli were 15-s in duration with the exception of the 0.5-s footshock US, 60-s test stimuli, and an auditory stimulus that consisted of a white noise (0.5 s) which served as a discriminative stimulus, presented contingent upon the presentation of water. All sessions were conducted with houselight off other than when it served as a cue.
Procedure
Shaping
On Days 1-5, prior to pre-exposure, acclimation to the apparatus and shaping of lever press behavior were conducted in 60-min sessions in a similar way as in Experiment 1. Water presentation was always accompanied by 0.5-s of the white noise.
Pre-Exposure
On Days 6 and 7, all subjects were exposed to two presentations of Stimulus V and two presentations of Stimulus Z on each day in Context A. Each stimulus presentation occurred at 10-min intervals in a 60-min session, and the order of stimuli presentations was reversed on Day 7 relative to Day 6.
Acquisition
On Days 8 – 11, all subjects received reinforced presentations of stimuli V, X, and Y, one presentation of each stimulus per day, except Group GenDec2, which also received one nonreinforced presentation of stimulus Z per day. Training occurred in Context A. The mean ITI for the presentation of CSs was 15.75 min, with a range of 10.75 – 20.75 min, from CS termination to CS onset, except for Group GenDec2, for which mean ITI was 12.75 min, and the range of 8.75 – 16.75 min, from CS offset to CS onset. The order of CS presentations was pseudo-randomized for each day. During acquisition the subjects had no access to water in the chambers.
Extinction
On Days 12 and 13, extinction occurred in Context B with a session length of 60 min. On each day subjects in Group Excitor received 6 nonreinforced presentations of a VX compound, and 6 nonreinforced presentations of CS Y alone. Subjects in Groups GenDec and GenDec2 received 6 nonreinforced presentations of a ZX compound and 6 nonreinforced presentations of Y alone on each day. The mean ITI for groups Excitor, GenDec, and GenDec2 was 4.42 min, with a range of 2.45 - 6.45 min. Group Elemental received 6 nonreinforced elemental presentations of stimuli V, X, and Y. The mean ITI for this group was 3 min, with the range of 1.75 – 4.25 min. The subjects in Group Y-only received 6 presentations of nonreinforced stimulus Y alone, with the mean ITI of 9.75 min and a range of 6.75 – 12.75 min. Subjects in Group Control were exposed to the context alone without any nominal stimulus being presented. The order of CS presentations for all groups was pseudo-randomized for each of the two days. During extinction the levers were presented and the subjects could lever-press for water.
Reshaping
On Days 14 and 15, all subjects were reshaped in Context B. On each day, subjects experienced one 60-min session to restabilize lever pressing on the VI 20-s schedule.
Test
On Days 16 and 17, all subjects were tested in Context B on CS X on one day and CS Y on another day, counterbalanced between subjects within groups. During testing, suppression of baseline responding during presentation of the CS was also assessed. Each subject received four nonreinforced 60-s presentations of the CS each day.
Results and Discussion
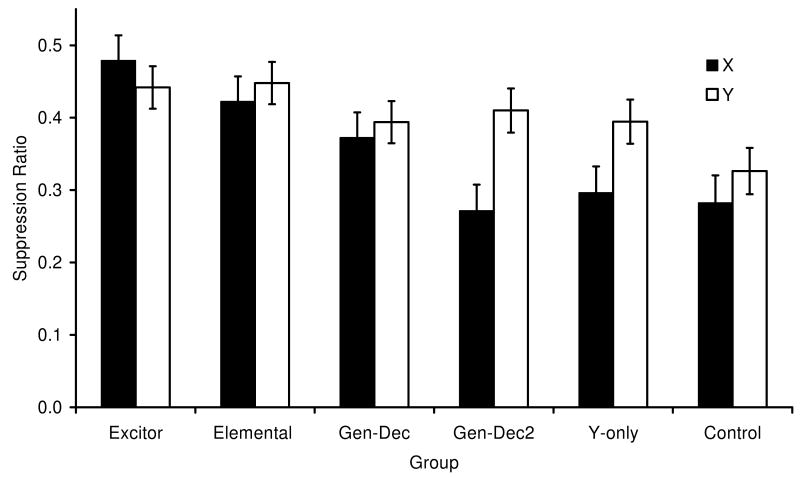
The main findings of Experiment 2 are depicted in Figure 2. The figure suggests that there were no appreciable differences in conditioned responding to CS X regardless of whether it was extinguished alone (Group Elemental) or in the presence of an additional excitor (Group Excitor). Thus in the present experiment, as in Experiment 1, we did not observe enhanced extinction by the co-presentation of an additional excitor. At the same time, we observed generalization decrement from extinction of the target with a neutral cue (Group GenDec) to testing the target cue alone (Group Elemental), probably because of configural processing during extinction. These results largely replicated what we had observed in Experiment 1.
Figure 2.
Mean suppression ratios to X (black bars) and Y (white bars), averaged within subjects over four test trials with each cue in Experiment 2 (ABB design). See Table 2 for group treatments and procedural details. Note that lower values denote more suppression and larger values denote less suppression (i.e., more extinction). Error brackets depict the standard error of the mean for each group.
Due to the lack of responding during pre-CS periods on certain test trials, one subject from Group GenDec2, one from Group Y-only, and two subjects from Group Control were eliminated from all the analyses. A 6 (Group: Excitor vs. GenDec vs. GenDec2 vs. Y-only vs. Elemental vs. Control) × 2 (Stimulus: X vs. Y) × 2 (Block: 1 and 2) mixed ANOVA conducted on the lever-pressing rates during blocks of two 60-s periods immediately before each CS presentation did not reveal any main effects of group nor an interaction of this factor with other factors (smallest p = .33), suggesting the absence of appreciable differences between groups in baseline responding. A similar ANOVA conducted on the suppression ratios of each block revealed a main effect of group, F(5, 62) = 5.25, p < 0.01, MSE = 0.03, a main effect of stimulus, F(1, 62) = 8.47, p < 0.01, MSE = 0.01, and a main effect of block, F(1, 62) = 194.69, p < 0.01, MSE = 0.01. More important, the ANOVA also revealed a group × stimulus interaction, F(5, 62) = 2.36, p = 0.050, MSE = 0.01, Cohen's f = 0.42, as well as an interaction between group and block, F(5, 62) = 3.54, p < 0.01, MSE = 0.007, and stimulus and block, F(1, 62) = 4.57, p < 0.05, MSE = 0.006, but no group × stimulus × block interaction, p = .31. Analogous to Experiment 1, the lack of a three-way interaction among group, stimulus, and block suggests that the interaction between group and stimulus was stable across blocks of test trials; therefore, we assessed the source of that interaction with planned comparisons pooling the two blocks of trials for each CS. Similar to Experiment 1, and thus providing a replication of that finding, a within-subjects comparison of suppression to X and Y in the Group Excitor revealed no appreciable difference, F(1, 62) = 0.89, p = .34, MSE = 0.02, nor did a between-subjects comparison of Groups Excitor and Elemental in responding to X, F(1, 62) = 1.33, p = 0.25, MSE = 0.03. These two critical comparisons again suggest that extinguishing the target X in the presence of another excitor V did not enhance extinction. But like Experiment 1, there was a nonsignificant tendency toward greater extinction of X when it was extinguished in compound with another excitor.
Additional comparisons indicated a moderate generalization decrement effect, in that suppression to X was greater than to Y in Group GenDec2, F(1, 62) = 11.33, p < 0.01, MSE = 0.02, after experiencing extinction of X in the presence of a preexposed neutral cue (Z) compared with extinction of Y alone, but this difference was not observed in Group GenDec, F(1, 62) = 0.28, p = 0.59, MSE = 0.02. In Group GenDec we did not see a large effect of generalization decrement, and surprisingly it seems as if the additional exposure to Z that GenDec2 received during Phase 1, if any, decreased the amount of extinction accrued to X. It is possible that nonreinforced exposure to a CS alone during reinforcement training of other alternative cues made this nonreinforced CS a differential inhibitor. Differential inhibition depends on the training trials being relatively massed (Urcelay & Miller, 2006), and this experiment training was conducted with widely spaced trials. However, it is possible that Z acquired sub-threshold inhibitory properties during Phase 1 that resulted in X being protected from extinction (Rescorla, 2003), which is opposite to the effect being investigated in the present experiment. Furthermore, a between-subjects comparison of Groups Excitor and GenDec on responding to X revealed a difference, F(1, 62) = 4.73, p < 0.05, MSE = 0.03, which again suggests that extinction in the presence of another excitor is enhanced but the enhancement is reduced by generalization decrement. A final comparison between X and Y in Group Y-only suggests that the cues were highly discriminated in that extinction of only one of them (Y) did not alter responding to the nonextinguished X, F(1, 62) = 5.67, p < 0.01, MSE = 0.01. Thus, little generalized extinction from one cue to the other was observed.
Finally, in order to evaluate the alternative explanation that floor effects precluded the observation of enhanced extinction, we conducted a Group × Stimulus mixed ANOVA on the first block of test trials. No subjects were excluded in this analysis. This analysis revealed a main effect of Group, F(5, 66) = 6.71, p < 0.01, MSE = 0.02, a main effect of Stimulus, F(1, 61) = 12.99, p < 0.01, MSE = 0.01, and a marginally significant interaction F(1, 66) = 2.01, p = 0.08, MSE = 0.01. All the comparisons reported above here resulted in similar results, indicating that a similar pattern was observed within the first two test trials, when responding was vigorous. In Group Excitor, the mean suppression to stimulus X during the first trial was M = 0.29, SE = 0.04 and to stimulus Y was M = 0.33, SE = 0.05.
The results of the present experiment agree with those of Experiment 1 in that both experiments failed to show appreciable facilitated extinction as a result of extinction treatment in the presence of an additional excitor. Regardless of the smaller generalization decrement as indicated by Group GenDec in Experiment 2 compared to Experiment 1, both experiments failed to detect an effect of facilitated extinction when presenting a second excitor during extinction relative to elemental extinction of the target cue. Two different types of generalization decrement may actually occur: one going from training (stimuli are elementally reinforced) to extinction (stimuli are presented in compound) and the other going from extinction to testing (stimuli are tested elementally). In order to minimize both sources of generalization decrement, in Experiment 3 we implemented two major changes from the previous studies to overcome enhanced extinction being constrained due to generalization decrement. One change involved decreasing the duration of the added stimulus during extinction (V or Z) relative to X in order to maximize discrimination among the stimuli and reduce configuring, and consequently generalization decrement going from acquisition to extinction and going from extinction to testing. The reasoning for this was based on the argument that cues are better configured when their onsets and termination are similar (Riley, 1984). The second change was based on the theoretical view that generalization decrement is context specific. For example, in Pearce's configural theory (Pearce, 1987, 1994, 2002) and Wagner's elemental theory (Brandon, Vogel, & Wagner, 2000), inputs from stimuli presented together (in compound) activate configural memory nodes (that is, nodes activated when multiple stimuli are presented) by excitatory and inhibitory connections. In both models, part or all of the configural nodes activated by a compound of cues are context dependent, so that activation of one node (by one of the elements of the compound, for example) in a different context will produce less activation of the configural node (i.e., less generalization decrement). In other words, generalization decrement from the compound to one of the elements is context dependent because the context dependent links are not activated by only one cue of the compound. Based on this theoretical assumption, we conducted testing either in the same context in which extinction was administered, as in the two previous experiments (ABB) or in a different context in which we expected recovery from extinction (ABC renewal; see Bouton & Bolles, 1979). Either because generalization decrement is context specific, or because renewal may constrain the expression of extinction, or both, we expected this manipulation to maximize sensitivity to any existing enhanced extinction as a result of co-presentation of another excitor during extinction treatment. Notably, an ABC renewal design more closely models clinical situations in which exposure therapy is administered in a therapist's office, after which the patient must bring the treatment to bear on behavior outside the therapeutic setting.
Experiment 3
In Experiment 3 we used a 2 × 3 factorial design in which 6 groups of subjects could receive one of three types of extinction treatment. They could experience extinction in the presence of an excitor (Condition Excitor), in the presence of a neutral cue (Condition GenDec), or elementally (Condition Elemental). Subjects in each of these three conditions were then tested either in the extinction context (Condition ABB) or in a third context (Condition ABC). The design of Experiment 3 is shown in Table 3. Groups Excitor-ABB and Excitor-ABC experienced extinction of X in the presence of an added excitor V. Groups GenDec-ABB and GenDec-ABC controlled for generalization decrement, in that for these groups X was extinguished in the presence of a neutral stimulus (Z). Finally, Groups Elemental-ABB and Elemental-ABC experienced extinction of the two excitatory stimuli (X and Y) elementally. Testing of X alone for Groups Excitor-ABB, GenDec-ABB, and Elemental-ABB occurred in Context B, the extinction context, whereas for Groups Excitor-ABC, GenDec-ABC, and Elemental-ABC it occurred in a third neutral Context (C). The expectation was that we would see a renewal effect when extinction of the target was conducted alone (X) and a weaker renewal effect when extinction of X occurred in the presence of a second excitor (XV).
Table 3.
Design of Experiment 3
| Group | Pre-Exposure (A) | Phase 1 Acquisition (A) | Phase 2 Extinction (B) | Test |
|---|---|---|---|---|
| Excitor-ABB | 4 V / 4 Z | 4 V+ / 4 X+ | 12 XV- | (B) X |
| GenDec-ABB | 4 V / 4 Z | 4 V+ / 4 X+ | 12 XZ- | (B) X |
| Elemental-ABB | 4 V / 4 Z | 4 V+ / 4 X+ | 12 X- / 12 V- | (B) X |
| Excitor-ABC | 4 V / 4 Z | 4 V+ / 4 X+ | 12 XV- | (C) X |
| GenDec-ABC | 4 V / 4 Z | 4 V+ / 4 X+ | 12 XZ- | (C) X |
| Elemental-ABC | 4 V / 4 Z | 4 V+ / 4 X+ | 12 X- / 12 V- | (C) X |
Note: + = footshock. Numbers next to the stimuli indicate total number of trials in that phase. Slashes indicate interspersed trials. Letters in prentices indicate the contexts of test. Stimulus X was a 15-s click train. Stimuli Z and V were 10-s house-light on and flashing light, counterbalanced. Letters between parentheses represent the context. Acquisition occurred in Context A and extinction occurred in context B.
Methods
Subjects and apparatus
The subjects were 36 male and 36 female, experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. Subjects were randomly assigned to one of six groups (ns = 12), counterbalanced within groups for sex. The apparatus, stimuli, and Phase 1 of treatment were identical to those used in Experiments 1 and 2, except for the omission of the Z trials and V being reduced to 10s in duration. Stimuli V and Z were 10-s of house-light presentation and 10-s flashing light instead of 15-s as in Experiment 2. In addition we used three different physical contexts instead of two. Various types of materials were used to create three distinct contexts. Context A was a standard chamber with a grid floor for presentation of shocks and no odor cues present. The physical identity of Contexts B and C were defined by different instances of the chambers, odor cues (methyl vs. banana), and floor surfaces (Plexiglas vs. 0.64 × 0.64 cm wire mesh square grid). Contexts B and C were counterbalanced within groups. All sessions were conducted with the houselights normally off.
Procedure
Shaping
On Days 1-5, prior to pre-exposure, acclimation to the apparatus and shaping of lever press behavior were conducted in two daily 30-min sessions, one each in Contexts B and C, with the order of context counterbalanced within each group. Subjects were shaped to lever press for water on a variable-interval 20-s schedule in the same manner as in Experiments 1 and 2.
Pre-Exposure
On Days 6 and 7, Phase 1 was conducted in the same manner as in Experiments 1 and 2. Preexposure occurred in Context A for all subjects.
Acquisition
On Days 8 and 9, all subjects received two daily reinforced presentations of CSs X and V. Training occurred in Context A and subjects had no access to the levers. Mean ITI for the presentation of CSs was 15.75 min, with a range of 10.75 – 20.75 min, from CS termination to CS onset. The order of CS presentations was pseudo-randomized on each day. Session length was 60 minutes.
Extinction and exposure
On Days 10-13, subjects experienced extinction in Context B, and equal exposure to Context C on alternate days. The order of exposure to each context was counterbalanced within groups. That is, half of the subjects in each group experienced extinction in Context B on Days 10 and 12 and exposure to Context C on Days 11 and 13. The remaining half experienced the same treatments, but in the opposite order. This training was conducted in the presence of the levers, which was a feature of these two contexts, but subjects did not receive water for lever-pressing. Session length was 60 min. On each extinction day, subjects in Groups Excitor-ABB and Excitor-ABC received 6 nonreinforced presentations of the VX compound. Subjects in Groups GenDec-ABB and GenDec-ABC received 6 nonreinforced presentations of a compound ZX on each day. The mean ITI for Groups Excitor-ABB, Excitor-ABC, GenDec-ABB and GenDec-ABC was 4.42 min, with a range of 2.45-6.45 min, from CS termination to CS onset. Groups Elemental-ABB and Elemental-ABC received 6 nonreinforced elemental presentations of CSs X and V. The mean ITI for this group was 3 min, with a range of 1.75 – 4.25 min, from CS termination to CS onset. On each trial, the target cue, which was 15-s long, onset alone, and the additional cue, which was 10-s long onset five seconds later. Both cues terminated simultaneously. No nominal stimuli were presented during exposure to Context C.
Reshaping
On Days 14 and 15, all subjects were reshaped in Contexts B and C. Subjects experienced half-hour daily sessions in each context to restabilize lever pressing on the VI 20-s schedule. The order of context exposure was counterbalanced within each group each day.
Test
On Day 16, all subjects were tested on CS X in Context B or C, depending on group assignment. During testing, suppression of baseline responding during presentation of the CS was assessed. Each subject received four nonreinforced 60-s presentations of the CS.
Results and Discussion
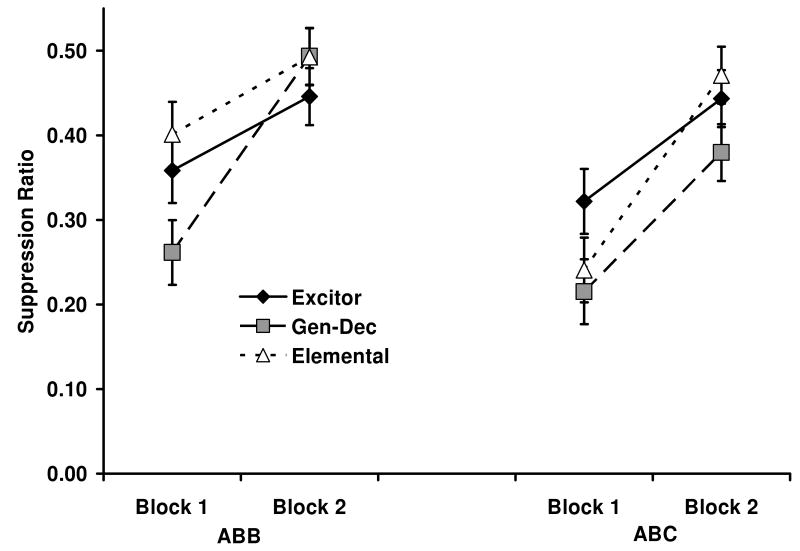
The mean suppression ratios for each block of test trials as a within-subjects variable are depicted in Figure 3. As the figure shows, differences between groups were evident in Block 1, but they waned with further testing (Block 2) presumably due to extinction during testing. In the first block, subjects in Condition ABB showed a pattern similar to that observed in Experiments 1 and 2. That is, when testing was conducted in the extinction context, extinction in the presence of an excitor (Excitor-ABB) did not result in significantly more extinction than extinction of the target alone (Elemental-ABB). This null result was likely due to generalization decrement that still constrained extinction as evidenced by the larger suppression in Group GenDec-ABB relative to the other two ABB groups. However, this pattern changed when subjects were tested in a third context (Condition ABC). In this instance, Group Excitor-ABC displayed less suppression (i.e., enhanced extinction) than the two groups that served as controls (GenDec-ABC and Elemental-ABC). In other words, extinction in the presence of an excitor alleviated the ABC renewal effect. Only subjects that experienced elemental extinction recovered their fear when tested outside of the extinction context. These impressions were supported by the following statistics.
Figure 3.
See Table 3 for group treatments and procedural details. Mean suppression ratios to the target CS X across two blocks of two test trials in Experiment 3. Subjects tested in the extinction context (ABB design) are depicted in the left panel. Subjects tested in a third context (ABC design) are depicted in the right panel. Note that lower values denote more suppression and larger values denote less suppression (i.e., more extinction). Error brackets depict the standard error of the mean for each block in each group.
No subjects had to be eliminated from the analyses in this experiment due to a failure to lever press during the pre-CS periods. A 3 (Type of Extinction: Excitor vs. GenDec vs. Elemental) × 2 (Test Context: B vs. C) × 2 (Block: 1 vs. 2) mixed ANOVA conducted on the lever-pressing rates during blocks of two 60-s periods immediately before each CS presentation did not reveal any main effects of type of extinction, test context, nor an interaction of these factors with block (smallest p = .46), suggesting that baseline responding across groups did not differ appreciably. A similar ANOVA conducted on the suppression ratios revealed a main effect of test context, F(1, 66) = 6.95, p < 0.05, MSE = 0.02, a main effect of block, F(1, 66) = 83.73, p < 0.01 MSE = 0.01, and a marginal three-way interaction between extinction, test, and block, F(2, 66) = 3.1, p = 0.051, MSE = 0.01, Cohen's f = 0.17. The triple interaction suggests that differences between groups were present in Block 1 but not in Block 2, as it is shown in Figure 3. In fact, we depicted the data broken by blocks to let the reader assess the differences between Blocks 1 and 2 that justify the present analyses. Therefore, we conducted planned comparisons focusing only on the first block of test trials. These comparisons suggested that there was no difference between Groups Excitor-ABB and Elemental-ABB, F < 1, thus replicating the absence of facilitated extinction in an ABB design following extinction with an excitor that we observed in Experiments 1 and 2. We also observed a generalization decrement effect in an ABB design, indicated by the difference between Groups GenDec-ABB and Elemental-ABB, F(1, 66) = 6.65, p < 0.05, MSE = 0.02. Group Elemental-ABC suppressed more than Group Elemental-ABB, F(1, 66) = 8.78, p < 0.01, MSE = 0.02, Cohen's f = 0.32, suggesting a renewal effect after extinction of the target stimulus alone. Centrally, such a renewal effect was not observed when extinction was conducted in the presence of an additional excitor; Groups Excitor-ABB and Excitor-ABC did not differ, F < 1. Moreover, a comparison between Groups GenDec-ABB and GenDec-ABC detected no differences, F < 1, which suggests that the generalization decrement observed when extinction is conducted in the presence of a neutral cue is not context dependent. Because these two control groups for extinction in the presence of an excitor did not differ from each other when testing was conducted in the new context (Groups GenDec-ABC and Elemental-ABC, F < 1), we conducted a final comparison between Group Excitor-ABC and Groups GenDec-ABC and Elemental-ABC pooled together, F(1, 66) = 4.01, p < 0.05, MSE = 0.02, Cohen's f = 0.20, which suggested that when extinction occurred in the presence of an excitor, the renewal effect was attenuated relative to its ABC control groups. We discuss the implications of this finding in the General Discussion.
General Discussion
In this series of experiments, we investigated the consequences of conducting extinction in the presence of a second excitatory conditioned stimulus. Some theories of learning (Miller & Matzel, 1988; Rescorla & Wagner, 1972, Stout & Miller, 2007) predict that extinction of a target cue should be enhanced when an additional excitor is presented in compound with the target cue during extinction, whereas other theories predict that no differences between extinction in the presence of an excitor and elemental extinction (Pearce, 1987, 1994, 2002). In Experiment 1, we failed to observe appreciable enhanced extinction after co-presenting another excitatory stimulus during extinction relative to a cue that underwent the extinction treatment elementally. Moreover, control groups suggested that the extinction memory of the compound presented during extinction generalizes poorly to a test situation in which the target cue is tested alone (i.e., generalization decrement). That is, extinguishing the compound VX and then testing on X alone resulted in generalization decrement that counteracted any enhanced extinction effect. This resulted in a net excitatory status equal to an excitatory stimulus extinguished alone. In Experiment 2, we used a similar design to that of Experiment 1, but we made significant parametric changes that we hypothesized would diminish generalization decrement occurring from extinction to test thereby enhancing sensitivity to observing any benefit of conducting extinction in the presence of another excitor. Despite these parametric variations, Experiment 2 also failed to reveal enhanced extinction after co-presenting an additional excitor. Although a within-subjects comparison revealed less generalization decrement (X vs. Y in Group GenDec), a between-subject comparison still revealed a generalization decrement effect (X in Group GenDec vs. Group Elemental). Taken together, Experiments 1 and 2 support Pearce's configural theory (1987, 1994, 2002).
In Experiment 3, we varied the duration of the added stimuli from that of the target in order to facilitate elemental processing during extinction treatment, and we conducted testing under conditions known to produce recovery from extinction, specifically ABC renewal (Bouton & Bolles, 1979). Although we still observed a generalization decrement effect when subjects were tested in the extinction context (ABB), ABC renewal was alleviated when extinction was conducted in the presence of an additional excitor. That is, subjects that received elemental extinction showed a reliable ABC renewal effect, but subjects that experienced compound extinction in the presence of an excitor did not recover responding when tested in a context different from that of extinction. Even though comparisons with their respective ABB controls clearly suggest that ABC renewal occurred after elemental extinction but not after extinction in the presence of an additional excitor, we should note that we pooled the two control groups (Elemental and GenDec) to obtain a significant difference between extinction in the presence of an excitor and the control conditions, and the effect size was between small and intermediate. Thus, the advantage of conducting extinction in the presence of an excitor that was revealed in the ABC renewal design is not particularly large. The results of Experiment 3 are problematic for Pearce's configural model because, with a change in the context (assuming that the context is like any other stimulus, perhaps with less salience), the theory predicts more generalization decrement rather than less generalization decrement after extinction in the presence of an additional excitor. This later result suggests that, as we discuss below, additional factors may contribute to the phenomenon of extinction in the presence of an excitor.
There are a number of reports on the effects of extinguishing an excitor in the presence of a second excitatory stimulus. For example, Wagner (1969) using an eyeblink conditioning preparation observed enhanced extinction of a target cue when it was conducted in the presence of a second highly excitatory stimulus relative to a condition in which extinction occurred in the presence of a less excitatory stimulus. Our data from Experiments 1, 2, and 3 are consistent with his data. However, Wagner's experiment did not include a control condition that experienced extinction of a cue alone, so we cannot determine whether extinction in the presence of an additional excitor was better than extinction of the target cue alone. Rescorla (2000, 2006) reported two series of experiments in which he observed that extinction in the presence of an excitor was superior to extinction of a cue alone. However, Rescorla (2000) using magazine conditioning (Experiments 1, 3, and 5) and operant preparations (Experiments 2 and 4) administered hundreds of reinforced trials before he administered a few extinction trials of either a compound of cues or one cue alone. With these parameters, that is, many elemental presentations and few compound presentations, configural processes should have been minimal. Therefore, he was likely able to observe an effect of enhanced extinction of a cue when extinction was conducted in the presence of another excitatory cue relative to a condition that experienced extinction of a cue alone because of the lack of configuring.
Moreover, in the two experiments in which Rescorla (2000) included a control for generalization decrement (Experiments 1 and 2), he administered nonreinforced presentations of this cue during Phase 1, when he was also training three excitatory stimuli. This treatment could have easily converted the stimulus used to control for generalization decrement into a differential inhibitor, and Rescorla himself found conditioned inhibitors to protect extinction when co-administered with an excitor during extinction training (Rescorla, 2003). Also consistent with a generalization decrement view, Rescorla (2006) recently replicated those findings in an aversively motivated preparation that required parameters more akin to those employed here, but in those experiments he extinguished each cue separately before he conducted compound extinction. Thus, it is possible that extinction of both CSs alone diminished the possibility that subjects configured the two cues during the compound presentations. One may argue that we did not observe generalization decrement in the GenDec Groups but rather an instance of protection from extinction (Rescorla, 2003; McConnell & Miller, 2009). That is, according to the Rescorla-Wagner model, after a few extinction trials the neutral cue would acquire inhibitory properties which, in turn, protect further extinction by the target excitor. This explanation, although theoretically possible, fails to account for our data in light of studies from our laboratory that demonstrate that, with the number of trials that we used here, the added neutral stimulus would have become a second-order excitor, not a conditioned inhibitor (Yin, Barnet, & Miller, 1994). In those studies, conditioned inhibition was observed after 48 nonreinforced trials but only when these were interspersed with reinforced trials. When all reinforced trials were followed by nonreinforced trials (which resembles the procedures used here), conditioned inhibition was not observed even after 96 reinforced trials followed by 48 nonreinforced trials (Yin et al., 1994). Thus, it is very unlikely that our neutral stimulus in Groups GenDec became a conditioned inhibitor and protected extinction of the target stimulus.
Using an aversive preparation similar to the one used in the present experiments, Thomas and Ayers (2004) assessed fear in the training context (i.e., ABA renewal) following extinction in a different context (B) of three different excitors presented either as a compound triplet or individually. Although they found less renewal after extinction in the presence of other excitors, the effect was not reliable for the first-tested target CS, which was a tone. Notably, testing involved elemental extinction of the tone. On subsequent test trials, they observed less renewal to the other CSs. Thus, they extinguished one of the elements of the compound before finding a benefit of extinction in the presence of two excitors. Moreover, they did not include a control for renewal (ABB), so their results may be interpreted as a stronger renewal effect following elemental extinction (Y alone) as compared to extinction in compound (XV), but they do not inform us concerning the effect of extinction in an ABB design. However, our Experiments 1 and 2 suggest that they would have not observed enhanced extinction without their procedure being embedded in a renewal preparation. In summary, our results are consistent with theirs, but further illuminate the boundaries and underlying processes involved in the compound extinction procedure.
Our and others results can best be interpreted in terms of configural processes mitigating the size of this effect. When configural processes are reduced, either by the use of stimuli of different modalities and different duration or by extensive elemental presentation of the cues alone before or after compound extinction, a benefit of extinction in the presence of another excitor is observed (Experiment 3 of the present report; Rescorla, 2000, 2006; Thomas & Ayres, 2004). However, the benefit of extinction in the presence of an additional excitor is diminished when configural processes are operating, as it was the case in our Experiments 1 and 2 and in Pearce and Wilson's study (1991). Consistent with this view is a recent experiment conducted in taste aversion (Pineño, 2007). In this study, different groups experienced extinction of the target CS in the presence of a second excitatory CS that had previously received zero, five or ten elemental extinction trials. He observed that compound extinction of the target was similar to elemental extinction only when the added CS had previously been extinguished for five trials (and presumably was still somewhat excitatory), but diminished extinction (i.e., generalization decrement) when the added CS had been extinguished ten times (and presumably rendered neutral) or not extinguished at all (favoring maximal configuring). Therefore, it is perhaps best to view the effect of extinction in the presence of another excitor as the net result of two processes that operate in opposite directions. One such process is the added excitation due to the presence of another excitor, which enhances extinction. The second process is generalization decrement from extinction to test, which diminished the expression of the extinction memory when an element is tested. Applying this reasoning to Pineño's data suggests that extinction in the presence of an already extinguished CS provides no benefit of greater excitation during extinction. Extinction with a highly excitatory CS diminishes expression of the extinction memory at test through generalization decrement. But extinction with a stimulus that has intermediate excitatory value provides some benefit of greater excitation during extinction while allowing only limited configuring, which is similar to what we observed in Experiments 1 and 2 with extinction in the presence of an excitatory CS that did not undergo any prior extinction trials but which incorporated parameters designed to limit configuring.
We should note that our conclusion tentatively requires two assumptions about the interactions between stimuli. One supposes the operation of elemental processing that favors extinction in the presence of an excitor, whereas the second assumption supposes the operation of configural processes decreases this effect. These two processes, however, should not be seen in contradiction with one another. According to recent theoretical proposals, in any cognitive task subjects are capable of both elemental and configural processing (Melchers, Shanks, & Lachnit, 2008). Whether subjects will process cues elementally or configurally seems to depend on several parameters, including task demands, verbal instructions (in the case of humans), prior experiences, and the properties of the stimuli. According to this view, elemental and configural processing are end points of a continuum, so it is possible that in any middle point both processes simultaneously operate and can cancel each other, as we are proposing here.
Generalization decrement from extinction treatment to test limiting the extent to which the presence of an additional excitor during extinction treatment increases extinction is also consistent with reports using fear conditioning with humans (Lovibond et al., 2000; Vervliet et al., 2007). Lovibond et al. observed in Experiment 1 diminished extinction at test when extinction training was conducted in the presence of a conditioned inhibitor, which is similar to Rescorla's (2003) observation of protection from extinction. However, they did not include a control for generalization decrement. In their Experiment 2, they observed protection from extinction regardless of the associative value of the co-administered stimulus during extinction. That is, both an added inhibitor and excitor protected extinction during a subsequent test, suggesting that generalization decrements going from extinction to test masked the interaction between the cues during extinction. In a related study, Vervliet et al. observed online behavior indicative of extinction (i.e., response cessation) with a compound of two excitors but no behavior indicative of extinction when they tested one of the elements alone. They concluded that in their preparation recovery from extinction occurs when subjects are tested with one of the elements alone. These observations in fear conditioning with humans were consistent with two different indices of conditioned fear: rated shock expectancy and electrodermal responding. Thus, in human fear conditioning, similar to our findings, generalization decrement due to configural processing during extinction strongly limited extinction in the presence of an additional excitor when the target CS was tested alone.
In summary, in this series of experiments we observed no appreciable benefit of conducting extinction treatment in the presence of an additional excitor relative to extinction of the target alone when configural processes during extinction were most strongly operating (Experiments 1 and 2). When we minimized these processes and conducted testing in a different context (Experiment 3), we observed a benefit of conducting extinction in the presence of an excitor. Although one could speculate that generalization decrement is context specific and therefore the benefits of this treatment are only observed when testing is conducted in a different context, our GenDec groups in Experiment 3 argue against this interpretation. In that experiment, groups that showed generalization decrement operating when testing was conducted in the extinction context (Group GenDec-ABB) also showed this effect in a third context (Group GenDec-ABC). Therefore, the present results seem better explained as the result of enhanced extinction due either to larger error correction (Rescorla & Wagner, 1972) or retrieval processes at the time of testing (Denniston et al., 2001; Miller & Matzel, 1988; Stout & Miller, 2007) which is/are constrained by generalization decrement from extinction to test, as configural theory would anticipate (Pearce, 1987, 1994, 2002). In addition, the use of an ABC renewal paradigm, which is known to constrain the observation of extinction, was presumably more sensitive in detecting differences in responding based on procedural differences during extinction.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 33881. The authors thank Jeremie Jozefowiez, Mario Laborda, Bridget McConnell, Mikael Molet, Heather Sissons, and James Witnauer for their comments on an earlier version of this manuscript. Inquiries concerning all aspects of this research should be addressed to Ralph R. Miller, Department of Psychology, SUNY- Binghamton, Binghamton, NY 13902-6000, USA; rmiller@binghamton.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. Journal of Comparative and Physiological Psychology. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Bouton ME, García-Gutiérrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behaviour Research and Therapy. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Brandon SE, Vogel EH, Wagner AR. A componential view of configural cues in generalization and discrimination in Pavlovian conditioning. Behavioral Brain Research. 2000;110:67–72. doi: 10.1016/s0166-4328(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Temporally massed CS presentations generate more fear extinction than spaced presentations. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:323–333. doi: 10.1037/0097-7403.29.4.323. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Calton JL, Hart JA, Schachtman TR. Attenuation of the renewal effect by extinction in multiple contexts. Learning and Motivation. 1999;30:1–14. [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Chang RC, Miller RR. Massive extinction treatment attenuates the renewal effect. Learning and Motivation. 2003;34:68–86. [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding y relative strength. In: Mower RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Effting M, Kindt M. Contextual control of human fear associations in a renewal paradigm. Behaviour Research and Therapy. 2007;45:2002–2018. doi: 10.1016/j.brat.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Gunther LM, Denniston JC, Miller RR. Conducting exposure treatment in multiple contexts can prevent relapse. Behaviour Research and Therapy. 1998;36:75–91. doi: 10.1016/s0005-7967(97)10019-5. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clinical Psychology Review. 2008;28:200–211. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe E, Horne A, Horne P, Macrae M. Summation and configuration between and within sensory modalities in classical conditioning of the rabbit. Animal Learning & Behavior. 1994;22:19–26. [Google Scholar]
- Lovibond PF, Davis NR, O'Flaherty AS. Protection from extinction in human fear conditioning. Behaviour research and therapy. 2000;38:967–983. doi: 10.1016/s0005-7967(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Melchers KG, Shanks DR, Lachnit H. Stimulus coding in human associative learning: Flexible representations of parts and wholes. Behavioural Processes. 2008;77:413–427. doi: 10.1016/j.beproc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Orlando, FL: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Myers JL, Well AD. Research design and statistical analysis. 2nd. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Mystkowski JL, Craske MG, Echiverri AM. Treatment context and return of fear in spider phobia. Behavior Therapy. 2002;33:399–416. doi: 10.1016/j.beth.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Neumann DL. The effects of physical context changes and multiple extinction contexts on two forms of renewal in a conditioned suppression task with humans. Learning and Motivation. 2006;37:149–175. [Google Scholar]
- Neumann DL, Lipp OV, Cory SE. Conducting extinction in multiple contexts does not necessarily attenuate the renewal of shock expectancy in a fear-conditioning procedure with humans. Behaviour Research and Therapy. 2007;45:385–394. doi: 10.1016/j.brat.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Pearce JM. Evaluation and development of a connectionist theory of configural learning. Animal Learning & Behavior. 2002;30:73–95. doi: 10.3758/bf03192911. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Wilson PN. Effects of extinction with a compound conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:151–162. doi: 10.1037//0097-7403.18.4.379. [DOI] [PubMed] [Google Scholar]
- Pineño O. Protection from extinction by concurrent presentation of an excitor or an extensively extinguished CS. Psicológica. 2007;28:151–166. [Google Scholar]
- Rachman SJ. The return of fear: Review and prospect. Clinical Psychology Review. 1989;9:147–168. [Google Scholar]
- Rescorla RA. Extinction can be enhanced by a concurrent excitor. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:251–260. doi: 10.1037//0097-7403.26.3.251. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2001. pp. 119–154. [Google Scholar]
- Rescorla RA. Protection from extinction. Learning and Behavior. 2003;31:124–32. doi: 10.3758/bf03195975. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Deepened extinction from compound stimulus presentation. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:135–144. doi: 10.1037/0097-7403.32.2.135. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Riley DA. Do pigeons decompose stimulus compounds? In: Roiblatt H, Bever T, Terrance H, editors. Animal Cognition. Hillsdale, NJ: Erlbaum; 1984. pp. 333–352. [Google Scholar]
- Stout SC, Miller RR. Sometimes competing retrieval (SOCR): A formalization of the comparator hypothesis. Psychological Review. 2007;114:759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Tamai N, Nakajima S. Renewal of formerly conditioned fear in rats after extensive extinction training. International Journal of Comparative Psychology. 2000;13:137–146. [Google Scholar]
- Thomas BL, Ayres JJB. Use of the ABA fear renewal paradigm to assess the effects of extinction with co-present fear inhibitors or excitors: Implications for theories of extinction and for treating human fears and phobias. Learning and Motivation. 2004;35:22–52. [Google Scholar]
- Urcelay GP, Miller RR. A comparator view of Pavlovian and differential inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:271–283. doi: 10.1037/0097-7403.32.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay GP, Wheeler DS, Miller RR. Spacing extinction trials alleviates renewal and spontaneous recovery. Learning & Behavior. 2009;37:60–73. doi: 10.3758/LB.37.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Stout SC, Miller RR. The basic laws of conditioning differ for elemental cues and cues trained in compound. Psychological Science. 2004;15:268–271. doi: 10.1111/j.0956-7976.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, et al. Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behaviour Research and Therapy. 2005;43:323–336. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wagner AR. Stimulus selection and a “modified continuity theory.”. In: Bower GH, Spence JT, editors. The psychology of learning and motivation. Vol. 3. New York: Academic Press; 1969. pp. 1–41. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Wagner AR. Context-sensitive elemental theory. Quarterly Journal of Experimental Psychology. 2003;56B:7–29. doi: 10.1080/02724990244000133. [DOI] [PubMed] [Google Scholar]
- Westbrook RF, Homewood J, Horn K, Clarke JC. Flavour-odour compound conditioning: Odour-potentiation and flavour-attenuation. Quarterly Journal of Experimental Psychology. 1983;35B:13–33. doi: 10.1080/14640748308400911. [DOI] [PubMed] [Google Scholar]
- Yin H, Barnet RC, Miller RR. Second-order conditioning and Pavlovian conditioned inhibition: Operational similarities and differences. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:419–428. [PubMed] [Google Scholar]