Abstract
We analyzed the outcomes of autologous stem cell transplantation (ASCT) following high-dose therapy with respect to remission status at the time of transplantation and induction regimen used in 56 consecutive patients with mantle cell lymphoma (MCL). Twenty-one patients received induction chemotherapy with HyperCVAD with or without rituximab (±R) followed by ASCT in first complete or partial remission (CR1/PR1), 15 received CHOP (±R) followed by ASCT in CR1/PR1, and 20 received ASCT following disease progression. Estimates of overall and progression-free survival (PFS) at three years among patients transplanted in CR1/PR1 were 93% and 63% compared with 46% and 36% for patients transplanted with relapsed/refractory disease, respectively. The hazard of mortality among patients transplanted with relapsed/refractory disease was 6.09 times that of patients transplanted in CR1/PR1 (P=.006). Patients in the CHOP (±R) group had a higher risk of failure for PFS compared to patients in the HyperCVAD (±R) group, though the difference did not reach statistical significance (hazard ratio 3.67, P=.11). These results suggest that ASCT in CR1/PR1 leads to improved survival outcomes for patients with MCL compared to ASCT with relapsed/refractory disease, and a HyperCVAD (±R) induction regimen may be associated with an improved PFS among patients transplanted in CR1/PR1.
Keywords: Mantle cell lymphoma, Autologous stem cell transplantation, HyperCVAD, CHOP, Non-Hodgkin lymphoma
INTRODUCTION
Mantle cell lymphoma (MCL) has the worst prognosis of all non-Hodgkin lymphoma (NHL) subtypes, with the possible exception of peripheral T cell lymphoma [1]. MCL is incurable with conventional therapies, with a median overall survival (OS) of 3–4 years from diagnosis in most series [1–3]. During the last 10 years, high-dose therapy followed by autologous stem cell transplantation (ASCT) has become an increasingly common treatment for MCL. Initally, ASCT was reserved as salvage therapy for relapsed or refractory disease, but outcomes were poor with this strategy [4,5]. In these studies, less heavily pre-treated patients had a longer duration of progression-free survival (PFS), suggesting that ASCT may lead to better outcomes if used earlier in the course of therapy. Subsequent trials assessed ASCT in first complete (CR1) or partial (PR1) remission, and although follow-up was generally short, compared to the earlier trials the median OS improved to approximately 5–6 years [6–12], and some studies demonstrated plateaus on the PFS curves, suggesting possible curability [8,9,13,14].
The first prospective evidence showing a benefit for ASCT in first remission compared with conventional chemotherapy alone came from a trial by Dreyling et al. [15] They reported that patients randomized to induction therapy with a CHOP-like regimen followed by ASCT in CR1/PR1 had an improved median PFS (39 mo. vs. 17 mo., P = .01) compared with patients who received chemotherapy followed by interferon maintenance.
Anthracycline-containing induction regimens appear to lead to the best response rates and longest response durations for MCL [16–21], with the most commonly employed regimens being HyperCVAD (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with cycles of high-dose methotrexate and cytarabine) with or without rituximab (±R), or CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) (±R) [20,21]. No randomized trials have compared these regimens, but limited retrospective data suggest that HyperCVAD may be superior to a CHOP-like regimen before ASCT [22,23]. We reviewed outcomes after ASCT at our institution with respect to remission status at the time of ASCT, as well as induction regimen, with particular attention to HyperCVAD (±R) vs. CHOP (±R), to assess differences in PFS and OS and to examine prognostic factors.
PATIENTS, MATERIALS, AND METHODS
Selection of Patients
Consecutive patients with a confirmed diagnosis of MCL treated with ASCT between August 1996 and July 2006 were included. Forty-eight of 56 patients had evidence of the (11;14) translocation either by polymerase chain reaction (PCR), fluorescence in situ hybridization (FISH), or cyclin D1 overexpression by immunohistochemistry, whereas 8 patients were diagnosed strictly based on histological appearance and immunophenotypic profile (CD5+, CD20+, CD23−) as outlined by the World Health Organization [24]. Patients were required to have acceptable organ function, performance status, and otherwise be deemed transplantation candidates by their primary oncologist. ASCT was performed at the Fred Hutchinson Cancer Research Center, the University of Washington Medical Center, or the Puget Sound Veterans Affairs Medical Center. Patients who received tandem ASCT followed by allogeneic stem cell transplantation were excluded from this analysis.
Treatment and Monitoring
Newly diagnosed patients were treated with induction chemotherapy with a variety of regimens, according to the preference of their primary oncologist. Most patients received either CHOP (±R) or HyperCVAD (±R) alternating with high-dose methotrexate (MTX) and cytarabine (Ara-C). The induction regimens used in the group of patients who subsequently underwent ASCT with relapsed or refractory disease (Group 3 under the Statistical Analysis section) were: CHOP (±R) (11 patients), CVP (±R) (3 patients), fludarabine (±R) (2 patients), R-HyperCVAD (1 patient), R-EPOCH (1 patient), antisense Bcl-2 inhibitor (1 patient), and local neck radiotherapy (1 patient). Some patients were referred to the transplant service for ASCT in CR1 or PR1, while others were referred after salvage therapy for relapsed/refractory disease. All patients underwent stem cell collection by apheresis after mobilization with filgrastim with or without chemotherapy. Patients were then treated with high-dose therapy followed by infusion of cryopreserved autologous peripheral blood stem cells. The conditioning regimens used were total body irradiation (TBI) with cyclophosphamide and etoposide (23 patients); iodine-131-labelled tositumomab alone (7 patients), or in combination with cyclophosphamide and etoposide (12 patients) or fludarabine (1 patient); and BEAM (BCNU, etoposide, cytarabine, and melphalan) or bulsulfan, thiotepa, and melphalan (13 patients). Stem cell products of selected patients with evidence of peripheral blood involvement by MCL prior to stem cell collection underwent ex vivo purging by immunoaffinity selection of CD34+ cells. The stem cell product of 1 patient underwent B-cell depletion in addition to ex vivo purging. Patients were observed for transplant-related toxicities in the immediate post-transplant period and then referred back to their primary oncologists for monitoring. Twenty-five patients were treated with post-ASCT rituximab maintenance therapy of varying schedules.
Statistical Analysis
Estimates of OS and PFS were obtained with the method of Kaplan and Meier and calculated from the time of transplant. The probability of relapse was summarized using cumulative incidence estimates, where death without relapse was regarded as a competing risk. Comparisons of the hazard of failure for OS and PFS were made using Cox regression. Comparisons of primary interest involved remission status at time of ASCT, where patients were categorized as being transplanted either in CR1/PR1 or with relapsed or refractory disease, and induction regimen, where patients received either HyperCVAD (±R), CHOP (±R). Because only one patient of 21 who received HyperCVAD was transplanted after relapse or progression compared to 11 of 26 patients who received CHOP for induction, a comparison of CHOP and HyperCVAD was restricted to patients transplanted in first remission. Patients were categorized based on remission status and induction regimen as follows: Group 1: HyperCVAD (±R) followed by ASCT in CR1/PR1; Group 2: CHOP (±R) followed by ASCT in CR1/PR1; and Group 3: ASCT with relapsed or refractory disease. Three patients in the CHOP group received an additional chemotherapy regimen in PR1 in an unsuccessful attempt to achieve a CR and were considered to be in PR1 at ASCT. A fourth patient in the CHOP group achieved a CR after 2 additional cycles of chemotherapy (R-HyperCVAD) in PR1 and was considered to be a CHOP patient in CR1 at ASCT. Groups 1 and 2 were then combined to analyze the differences between patients who underwent ASCT in CR1/PR1 and patients who underwent ASCT with relapsed or refractory disease (Group 3), and the impact of CHOP vs. HyperCVAD among patients transplanted in CR1/PR1 was assessed by comparing Groups 1 and 2.
Responses were determined according to the International Working Group criteria [25]. The pre-treatment serum lactate dehydrogenase (LDH) values of several patients who underwent induction therapy at other institutions were not available. For these patients, the International Prognostic Index (IPI) scores were calculated assuming a normal LDH value; thus, the IPI scores may be underestimated for those patients.
Factors other than remission status at ASCT and induction regimen were assessed for their association with outcome. These factors included age at diagnosis, sex, stage at diagnosis, serum LDH at diagnosis, performance status at diagnosis, IPI score, presence of bone marrow or gastrointestinal tract involvement, presence of splenomegaly, number of extranodal sites of disease, presence of B symptoms, serum hemoglobin (Hb) at diagnosis, number of cycles of induction chemotherapy, use of rituximab with induction, number of pre-ASCT chemotherapy regimens (not counting mobilization), response to induction therapy, preparatory regimen for ASCT, and post-ASCT rituximab therapy. The impact of using rituximab after ASCT was assessed by considering rituximab use as a time-dependent covariate, with the covariate assuming a value of 0 until the time of first administration of rituximab following ASCT, at which time the covariate assumes the value 1. The relatively small number of events limited the number of factors that could be included in a multivariate model to two. All two-sided p-values from regression models were calculated using the Wald test.
Institutional Approval
Institutional approval from the Fred Hutchinson Cancer Research Center was obtained to evaluate patients’ medical records retrospectively. Patients were either enrolled into institutionally approved studies or treated with standard transplantation protocols.
RESULTS
Patients
Fifty-six consecutive patients (median age at diagnosis 54.5, range 35–70) were treated with high-dose therapy followed by ASCT at our center between August 1996 and July 2006, with a median follow-up time among survivors of 24.6 months and maximum follow-up time of 10.1 years. Patient characteristics are listed in Table I. All patients had pathologically confirmed MCL, with 48 of 56 patients having either positive cyclin D1 by immunohistochemistry or evidence of t(11;14) by FISH or PCR. All patients but 1 (98%) had stage III–IV disease, and 77% of patients were male.
Table I.
Patient characteristics
| Parameter | All patients n (%) |
HyperCVAD (±R) + ASCT in CR1/PR1 n (%) |
CHOP (±R) + ASCT in CR1/PR1 n (%) |
ASCT with relapsed/refractory disease n (%) |
|---|---|---|---|---|
| Total no. | 56 | 21 | 15 | 20 |
| Sex | ||||
| Male | 43 (77) | 17 (81) | 11 (73) | 15 (75) |
| Female | 13 (23) | 4 (19) | 4 (27) | 5 (25) |
| Age, y, median | 54.5 | 53 | 56 | 57.5 |
| Range | 35–70 | 41–69 | 35–70 | 45–68 |
| Ann Arbor stage | ||||
| I–II | 1 (2) | 0 (0) | 0 (0) | 1 (5) |
| III–IV | 55 (98) | 21 (100) | 15 (100) | 19 (95) |
| Presence of B symptoms | 23 (41) | 8 (38) | 10 (67) | 5 (25) |
| LDH at diagnosis | ||||
| Normal | 26 (46) | 14 (67) | 6 (40) | 6 (30) |
| Abnormal | 16 (29) | 4 (19) | 5 (33) | 7 (35) |
| Unknown | 14 (25) | 3 (14) | 4 (27) | 7 (35) |
| IPI | ||||
| 0–1 | 23 (41) | 11 (52) | 7 (47) | 5 (25) |
| 2–3 | 30 (54) | 10 (48) | 5 (33) | 15 (75) |
| 4–5 | 3 (5) | 0 (0) | 3 (20) | 0 (0) |
| ECOG performance status | ||||
| 0–1 | 53 (95) | 21 (100) | 13 (87) | 19 (95) |
| 2–3 | 3 (5) | 0 (0) | 2 (13) | 1 (5) |
| 4–5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
|
Median no. of extranodal sites, exclusive of spleen |
1 | 1 | 1 | 1 |
| Serum hemoglobin (Hb) at diagnosis | ||||
| Hb ≥12 | 30 (54) | 13 (62) | 8 (54) | 9 (45) |
| Hb <12 | 17 (30) | 7 (33) | 5 (33) | 5 (25) |
| Unknown | 9 (16) | 1 (5) | 2 (13) | 6 (30) |
|
Rituximab used with induction therapy |
41 (73) | 20 (95) | 11 (73) | 10 (50) |
|
Treatment with 3 or more prior regimens |
0 (0) | 0 (0) | 0 (0) | 12 (60) |
|
Purged (CD34+ selected) stem cell product |
12 (21) | 0 (0) | 5 (33) | 7 (35) |
| ASCT conditioning | ||||
| TBI-containing regimen | 23 (41) | 16 (76) | 5 (33) | 2 (10) |
| I-131-labeled anti CD20 Ab- containing regimen |
20 (36) | 2 (10) | 5 (33) | 13 (65) |
| High-dose chemotherapy only | 13 (23) | 3 (14) | 5 (33) | 5 (25)† |
|
Use of post-ASCT rituximab maintenance therapy |
||||
| Yes | 25 (45) | 14 (67) | 7 (47) | 4 (20) |
| No | 27 (48) | 5 (24) | 7 (47) | 15 (75) |
| Unknown | 4 (7) | 2 (9) | 1 (6) | 1 (5) |
A bone marrow biopsy was not performed at diagnosis for 2 patients in this group.
One patient also received IL-2 in addition to high-dose chemotherapy.
Baseline characteristics were similar among the primary comparison groups, with a few exceptions. A higher percentage of patients treated with CHOP (±R) followed by ASCT in CR1/PR1 had B symptoms (67%) than patients treated with HyperCVAD (±R) followed by ASCT in CR1/PR1 (38%) or patients with relapsed/refractory disease at the time of ASCT (25%). A slightly higher percentage of patients in the CHOP group had a performance status of 2 or greater compared with patients in the HyperCVAD group (13% and 0%, respectively). More patients with relapsed/refractory disease at ASCT had an IPI score at diagnosis of 2 or greater than patients transplanted in first remission (75% and 50%, respectively).
Response to Induction
With respect to the 3 comparison groups, 21 patients were treated with a HyperCVAD (±R) regimen followed by ASCT in CR1/PR1, 15 patients were treated with CHOP (±R) followed by ASCT in CR1/PR1, and 20 patients underwent ASCT with relapsed/refractory disease (Table I). Among the 21 patients in the HyperCVAD group, 17 (81%) had a complete response or complete response unconfirmed (CR/CRu) to induction, compared with only 4 of 15 patients (27%) in the CHOP group (P = .002). Among patients who underwent ASCT after relapse, 15 (75%) had a response to initial induction therapy (7 CR/CRu, 8 PR), 3 patients had stable disease, and 2 had progressive disease (Table II). Fifteen of the 20 patients had chemosensitive disease at ASCT, as defined by cumulative response (CR or PR) since the time of last relapse/progression. All 5 of the primary refractory patients subsequently responded to salvage chemotherapy regimens. Patients were more likely to receive a CHOP (±R) regimen in the early years of the study and more likely to receive HyperCVAD (±R) in more recent years. The median follow-up of survivors in the CHOP group was 31 months, and the median follow-up for survivors in the HyperCVAD group was 14 months. This difference was due primarily to the three long-term survivors in the CHOP group; the number of patients with follow-up less than 3 years was more similar between the two groups: 13/20 (65%) of the HyperCVAD patients and 7/13 (54%) of the CHOP patients. There is only one death beyond 3 years (at 3.6 years in CHOP group).
Table II.
Response to induction therapy and remission status at ASCT
| Parameter | All patients n (%) |
HyperCVAD (±R) + ASCT in CR1/PR1 n (%) |
CHOP (±R) + ASCT in CR1/PR1 n (%) |
ASCT with relapsed/refractory disease n (%) |
|---|---|---|---|---|
| Response to induction therapy | ||||
| CR/CRu | 28 (50) | 17 (81) | 4 (27) | 7 (35) |
| PR | 23 (41) | 4 (19) | 11 (73) | 8 (40) |
| Other | 5 (9) | 0 (0) | 0 (0) | 5 (25) |
| Remission status at ASCT | ||||
| CR1/CRu1 | 23 (41) | 18 (86)* | 5† (33) | 0 (0) |
| PR1 | 13 (23) | 3 (14) | 10 (67) | 0 (0) |
| Other | 20 (36) | 0 (0) | 0 (0) | 20 (100) |
One patient converted from a PR to a CR with chemomobilization prior to stem cell collection.
One patient converted from a PR to a CR after receiving 2 cycles of R-HyperCVAD after the initial R-CHOP×4 cycles
Outcomes After Stem Cell Transplantation
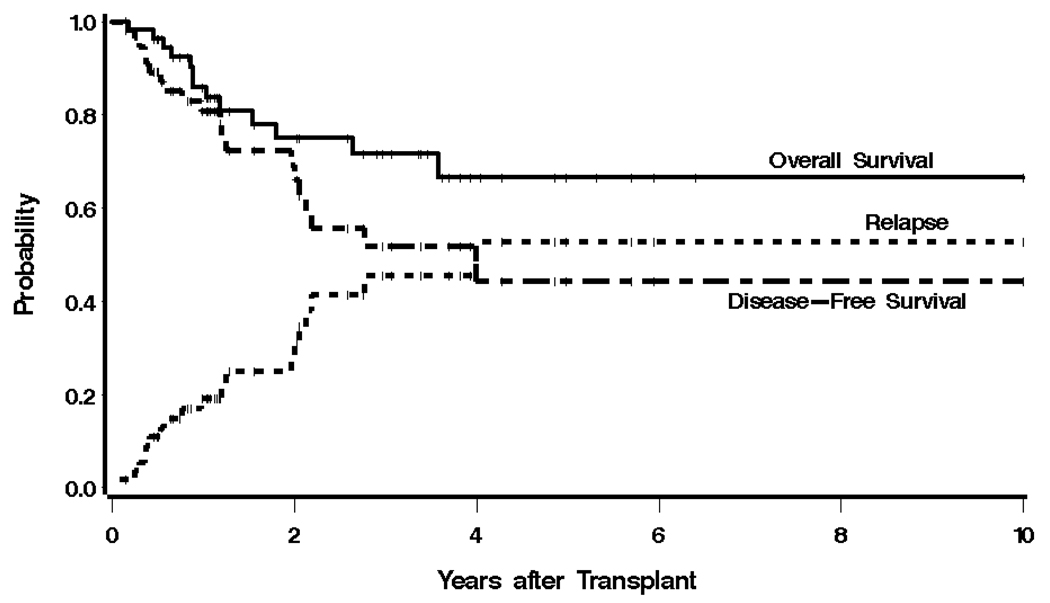
At most recent follow-up, 13 patients had died, 12 of progressive disease, and 1 patient, in the relapsed/refractory group, from refractory cytopenias leading to a fatal infection (1.8% transplant-related mortality). There were 19 relapses, 12 of which led to death. The median OS of the entire group was not yet reached, and the median PFS was 4.0 years, with estimated 3-year OS and PFS of 72% and 52%, respectively (Figure 1).
Figure 1.
Kaplan-Meier estimates of overall survival, progression free-survival, and relapse for the entire cohort. The probability of survival, progression-free survival, and relapse at 3 years were 72%, 52%, and 45%, respectively.
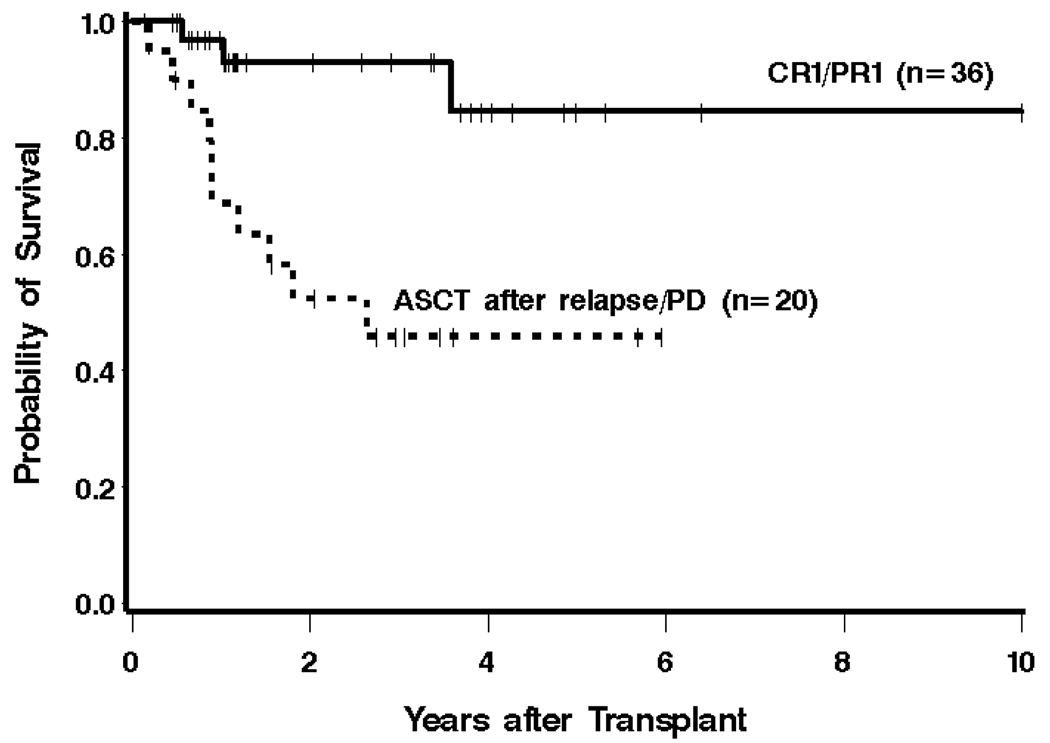
Patients who underwent ASCT in CR1/PR1 had an estimated 3-year OS of 93% compared to 46% for patients who were transplanted with relapsed/refractory disease (Figure 2). PFS at 3 years was estimated to be 63% and 35%, respectively, in these 2 groups of patients. Risk of mortality and risk of PFS failure were increased among patients undergoing ASCT with relapsed/refractory disease compared to patients transplanted in CR1/PR1 (HR 6.09 [95% CI 1.66–22.30], P = .006, and HR 3.15 [1.28–7.71], P = .01, respectively).
Figure 2.
Kaplan-Meier estimates of overall survival from the time of ASCT, with respect to remission status at ASCT. The estimated 3-year survival for patients treated with ASCT in CR1/PR1 was 93%, compared with 46% in patients who underwent ASCT with relapsed or refractory disease.
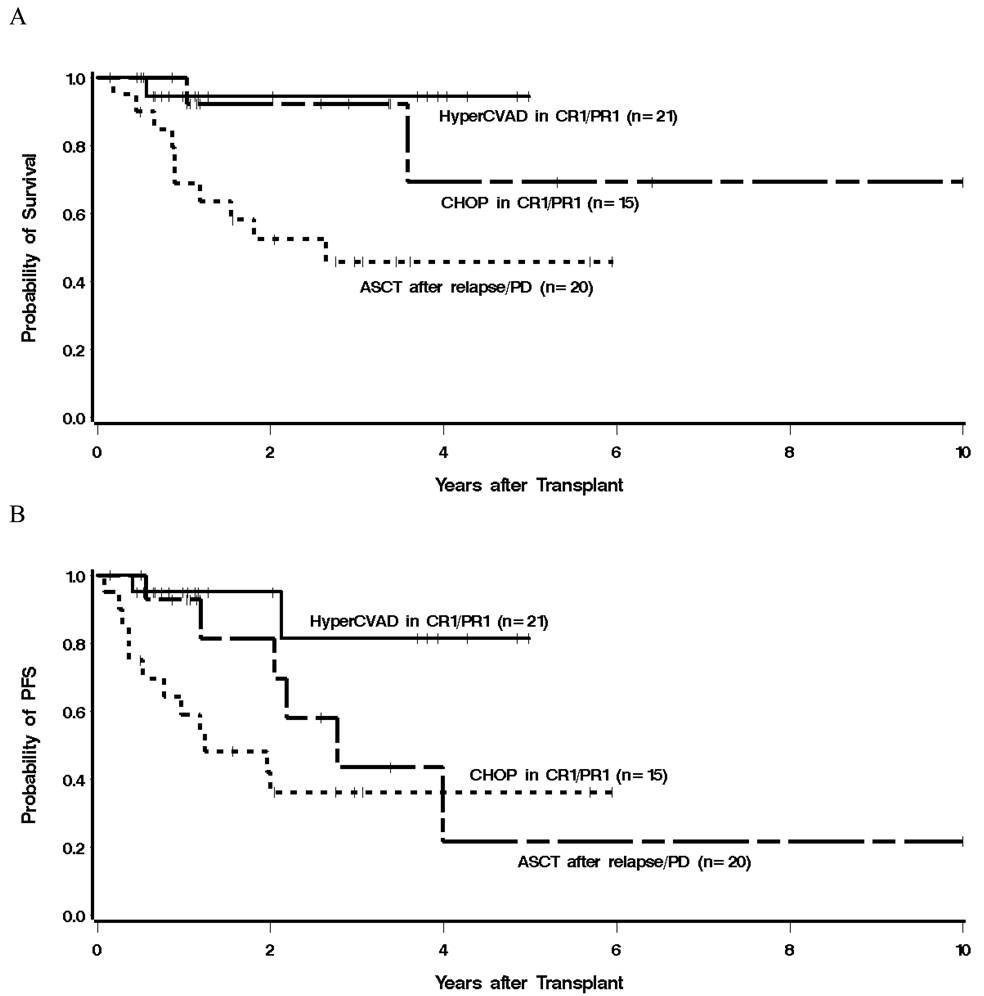
Given the low number of deaths among patients transplanted in CR1/PR1 (2 in the CHOP group and 1 in the HyperCVAD group), a statistical comparison of mortality between these 2 induction regimens is not meaningful. Among patients treated with HyperCVAD (±R) followed by ASCT in first remission, the median OS and PFS have not been reached. Among patients treated with CHOP (±R) followed by ASCT in CR1/PR1, the median OS was not yet reached, and the median PFS was 33.2 months. The estimated 3-year PFS was 81% for patients in the HyperCVAD group and 44% for patients in the CHOP group (Figure 3). The hazard of failure for PFS among CHOP (±R) patients transplanted in CR1/PR1 was 3.67 (95% CI 0.77–18.24) times that of patients in the HyperCVAD group, but this difference did not reach statistical significance (P = .11).
Figure 3.
Kaplan-Meier estimates of overall survival (A) and progression-free survival (B) from the time of ASCT, with respect to primary comparison group. The estimated 3 year OS and PFS for patients treated with HyperCVAD (±R) followed by ASCT in CR1/PR1 were 94% and 81%, respectively, compared with 92% and 44%, in patients treated with a CHOP (±R) regimen followed by ASCT in CR1/PR1. Patients who received a different induction regimen or underwent ASCT with refractory disease or after relapse had the lowest OS and PFS at 46% and 36%.
One patient in the relapsed/refractory group developed a melanoma 5 years after ASCT. Excluding non-melanoma skin malignancies, no other secondary malignancies were reported among the study patients after ASCT.
Prognostic Factors Other than Induction Regimen and Remission Status at Transplant
A number of factors other than induction regimen and remission status at transplant were examined for their association with overall mortality and failure for PFS (Table III). Because of the limited number of events (13 deaths, 20 deaths or relapses), the number of factors that could be included in any regression model is limited. In univariate models for overall mortality, elevated LDH was associated with an increased risk of death (44%, compared to 0%, P = .0002). Patients with an IPI of 0–1 were less likely to die compared to patients with higher IPI scores (9% of patients with IPI of 0–1, compared to 33% with higher IPI, HR 0.23 [0.05–1.03], P = .05), and patients whose induction regimen contained rituximab were also less likely to die compared to those whose regimen did not contain rituximab (15% compared to 47%, HR 0.33 [0.11–1.00], P = .05). Similarly, patients who received rituximab after transplantation had a reduced hazard ratio for mortality (0.13 [0.02–1.03], P = .05). No other factors were statistically significantly associated with mortality. After adjusting for IPI score (0–1 vs. 2–5), the hazard of mortality among patients transplanted with relapsed/refractory disease remained statistically significantly higher than among patients transplanted in first remission, although the magnitude of the difference was reduced (HR 5.12, P = .02). A similar finding held after adjusting for use of rituximab in the induction regimen (HR 5.13, P = .02). Adjusting for conditioning regimen (modeled as TBI-containing vs. I-131-containing vs. high-dose chemotherapy only) likewise did not reduce the association of ASCT in first remission with improved survival (HR 13.37, P = .005), and adjusting for the use of rituximab following ASCT led to the same qualitative conclusion with respect to ASCT in first remission (HR 4.62, P = .03).
Table III.
Univariate regression analysis results for overall mortality
| Factor | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age* | Older, more failure | --- | .65 |
| Age < 60 | 1 | --- | .87 |
| Age ≥ 60 | 1.11 | 0.34–3.64 | .87 |
| Stage III† | 1 | --- | --- |
| Stage IV | 1.42 | 0.18–10.94 | .74 |
| PS 0–1 | 1 | --- | --- |
| PS 2–3 | 1.99 | 0.26–15.45 | .51 |
| Normal LDH‡ | 1 | --- | --- |
| High LDH | 1 | .0005 | |
| ENS 0–1 | 1 | --- | --- |
| ENS 2–3 | 2.02 | 0.60–6.78 | .26 |
| No B Symptoms | 1 | --- | --- |
| B Symptoms | 1.96 | 0.66–5.85 | .23 |
| IPI 0–1 | 1 | --- | --- |
| IPI 2–3 | 4.83 | 1.07–21.85 | .04 |
| IPI 4–5 | 0 | --- | 23 |
| Female | 1 | --- | --- |
| Male | 1.50 | 0.33–6.77 | .60 |
| No Splenomegaly | 1 | --- | --- |
| Splenomegaly | 3.06 | 0.84–11.14 | .09 |
| No BM Involvement | 1 | --- | --- |
| BM Involvement | 0.92 | 0.20–4.19 | .92 |
| No GI Involvement | 1 | --- | --- |
| GI Involvement | 1.24 | 0.34–4.53 | .75 |
| Hb < 12 | 1 | --- | --- |
| Hb ≥ 12 | 0.49 | 0.15–1.60 | .23 |
| Number of Cycles of Induction Chemo* | Higher, less failure | --- | .15 |
| No Rituximab in Induction | 1 | --- | --- |
| Rituximab in Induction | 0.33 | 0.11–1.00 | .05 |
| 0–1 pre-ASCT Chemo | 1 | --- | --- |
| ≥2 Chemo | 8.38 | 1.85–37.96 | .006 |
| CR to Induction | 1 | --- | --- |
| No CR to Induction | 5.65 | 1.25–25.64 | .02 |
| CR1 at ASCT | 1 | --- | --- |
| PR1 at ASCT | 2.93 | 0.27–32.41 | .38 |
| Neither CR1 nor PR1 at ASCT | 10.85 | 1.38–85.11 | .02 |
| No Post-ASCT | 1 | --- | --- |
| Rituximab Post-ASCT Rituximab | 0.13 | 0.02–1.03 | .05 |
| Group 1 (HyperCVAD ) | 1 | --- | --- |
| Group 2 (CHOP) | 2.28 | 0.21–25.26 | .50 |
| Group 3 (relapsed/refractory) | 9.76 | 1.24–76.98 | .03 |
Age, number of cycles, and number of pre-ASCT chemo regimens modeled as a continuous linear variable
No patients with Stage II disease, only 1 patient with Stage I disease
Fourteen patients missing data, 6 (43%) have died
The same factors listed above associated with mortality were associated with PFS (Table IV). Additionally, a better response to induction was associated with improved PFS; patients achieving a PR as opposed to a CR had a hazard ratio (HR) of 5.88 for relapse or mortality (1.67–20.67, P = .006), and patients that did not achieve either a CR or PR had a HR of 16.43 (3.51–76.84, P = .0004). Adjusting for IPI or use of rituximab during induction did not change the conclusion with respect to the association seen between remission status at ASCT and PFS, although the magnitude of the association was reduced (HR 2.73, P = .03 after adjusting for IPI; HR 2.62, P = .04 after adjusting for rituximab). The association fell below the level of statistical significance after adjusting for conditioning regimen (HR 2.46, P = .08), as it did after separately adjusting for rituximab use following ASCT (HR 2.30, P = .10). No other factors examined were statistically significantly associated with the risk of relapse/mortality.
Table IV.
Univariate regression analysis results for failure for PFS
| Factor | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age* | Older, more failure | --- | .25 |
| Age < 60 | 1 | 0.77–4.70 | --- |
| Age ≥ 60 | 1.91 | 0.77–4.70 | .16 |
| Stage III† | 1 | 0.13–1.63 | --- |
| Stage IV | 0.47 | 0.13–1.63 | .23 |
| PS 0–1 | 1 | --- | --- |
| PS 2–3 | 4.52 | 0.99–20.66 | .05 |
| Normal LDH‡ | 1 | --- | --- |
| High LDH | 7.41 | 1.97–27.80 | .003 |
| ENS 0–1 | 1 | --- | --- |
| ENS 2–3 | 1.96 | 0.66–5.77 | .22 |
| No B Symptoms | 1 | --- | --- |
| B Symptoms | 2.21 | 0.90–5.40 | .08 |
| IPI 0–1 | 1 | --- | --- |
| IPI 2–3 | 5.74 | 1.65–19.91 | .006 |
| IPI 4–5 | 5.20 | 0.51–52.97 | .16 |
| Female | 1 | --- | --- |
| Male | 1.21 | 0.27–5.18 | .81 |
| No Splenomegaly | 1 | --- | --- |
| Splenomegaly | 2.46 | 0.94–6.44 | .07 |
| No BM Involvement | 1 | --- | --- |
| BM Involvement | 0.80 | 0.23–2.78 | .72 |
| No GI Involvement | 1 | --- | --- |
| GI Involvement | 1.23 | 0.40–3.77 | .72 |
| Hb < 12 | 1 | --- | --- |
| Hb ≥ 12 | 0.47 | 0.18–1.23 | .12 |
| Number of Cycles of Induction Chemo* | Higher, less failure | --- | .64 |
| No Rituximab in Induction | 1 | --- | --- |
| Rituximab in Induction | 0.28 | 0.12–0.69 | .005 |
| 0–1 pre-ASCT Chemo | 1 | --- | --- |
| ≥2 Chemo | 4.04 | 1.55–10.53 | .004 |
| CR to Induction | 1 | --- | --- |
| PR to Induction | 5.88 | 1.67–20.67 | .006 |
| Neither CR nor PR | 16.43 | 3.51–76.84 | .0004 |
| CR1 at ASCT | 1 | --- | --- |
| PR1 at ASCT | 4.69 | 0.94–23.32 | .06 |
| Neither CR1 nor PR1 at ASCT | 7.66 | 1.71–34.29 | .008 |
| No Post-ASCT Rituximab | 1 | --- | --- |
| Post-ASCT Rituximab | 0.21 | 0.05–0.90 | .04 |
| Group 1 (HyperCVAD ) | 1 | --- | --- |
| Group 2 (CHOP) | 3.67 | 0.74–18.20 | .11 |
| Group 3 (relapsed/refractory) | 6.93 | 1.55–31.00 | .01 |
Age, number of cycles, and number of pre-ASCT chemo regimens modeled as a continuous linear variable
No patients with Stage II disease, only one patient with Stage I disease
Fourteen patients missing data, 8 (57%) have died and/or relapsed
DISCUSSION
Historically, the use of ASCT in patients with MCL began as a salvage therapy for relapsed or refractory disease, although it later became clear that survival results were poor with this approach [4,5,26]. Several studies subsequently emerged suggesting improved clinical outcomes for patients transplanted in first remission compared with historical controls or patients receiving delayed transplants [8,27–29]. This evidence was supported by a prospective trial showing improved PFS for patients randomized to ASCT rather than interferon maintenance following induction chemotherapy [15]. Based on these data, and encouraged by technical advances in the management of complications related to stem cell transplantation, ASCT as consolidation therapy in first remission has become the preferred treatment for MCL for many oncologists and hematologists, although this practice remains controversial.
In this study we evaluated outcomes of patients with MCL after ASCT with respect to their remission status at the time of transplant and the induction regimen they received. We found that patients who underwent ASCT in first remission had significantly better OS and PFS after ASCT compared to patients who had relapsed/refractory disease at ASCT. Although more patients with relapsed/refractory disease had an IPI score greater than 2 compared with patients in CR1/PR1 at ASCT, this did not appear to account for the observed difference between the groups, as a multivariable analysis adjusting for IPI continued to show an OS and PFS benefit for the patients transplanted in first remission. The OS benefit also remained significant after adjusting for conditioning regimen, although the PFS benefit was reduced. We also found that patients achieving less than a CR with induction therapy had a significantly higher risk of mortality compared with patients who achieved a CR.
These results, along with studies demonstrating better outcomes among patients transplanted in CR1 as opposed to PR1 [23,27], highlight the importance of optimizing the initial response to chemotherapy and remission status at ASCT. It is important to emphasize that the current data do not, and cannot, address the question of whether ASCT is preferred over not being transplanted, as only patients who proceeded to ASCT are currently considered.
Recent years have seen a shift in the most commonly employed induction regimens for previously untreated MCL as data on newer regimens have emerged. More intensive regimens such as a HyperCVAD/MTX-Ara-C regimen, in combination with rituximab, are increasingly being used in favor of older regimens such as R-CHOP and R-CVP, although the optimal induction regimen has not yet been firmly established. One series by Conde et al. compared post-ASCT outcomes of patients from an international database with respect to induction regimen, and found a 4-year disease-free survival of 68% for patients treated with HyperCVAD compared with 33% for patients treated with a CHOP-like regimen [22]. In a more recent abstract by Vose et al. of 80 patients who underwent ASCT in CR1/PR1, patients who were treated with a HyperCVAD (±R) induction regimen had a significantly better 3-year OS (97% vs. 68%) than patients who were treated with a CHOP-like (±R) induction regimen [23]. Additionally, a subset analysis of a study by Ganti et al. suggested that a HyperCVAD (±R) induction regimen was associated with fewer relapses [30].
In this study we compared the outcomes of patients treated with ASCT in CR1/PR1 after receiving either a HyperCVAD/MTX-Ara-C (±R) or CHOP (±R) induction regimen. A statistical comparison of these 2 groups with respect to OS is difficult, since there were only 3 deaths among patients transplanted in first remission (2 in the CHOP group and 1 in the HyperCVAD group). With regard to PFS, however, there was a higher risk of failure among patients in the CHOP (±R) group, primarily through a higher risk of relapse, compared to those treated with HyperCVAD (±R). The difference did not reach statistical significance, but the power to detect such a difference was limited by the small number of events. That the estimated 3-year OS and PFS of patients in this group were very similar to those reported by Vose et al., however, increases one’s confidence that this PFS trend reflects a true superiority of HyperCVAD (±R) in MCL. Furthermore, there was a significantly better CR/CRu rate among patients in the HyperCVAD group compared with those in the CHOP group (81% vs. 27%). As with any retrospective study, however, there were limitations. For example, more patients in the CHOP group had high-risk disease based on IPI score, and fewer patients received rituximab as part of induction compared with patients in the HyperCVAD group, which may have contributed to their lower CR rate with induction and PFS. We must also acknowledge that the median follow-up of patients in the CHOP group is longer than patients in the HyperCVAD group, and that it is possible that this difference might have influenced our findings. The above differences mainly reflect changes in treatment preference over time; in the earlier years of the study, more patients received a CHOP (±R) induction regimen, with fewer patients receiving rituximab, whereas in more recent years, patients were more frequently treated with a HyperCVAD induction regimen that included rituximab.
Although HyperCVAD appears to be associated with higher toxicity than CHOP [16,20,21] and requires hospitalization for infusion, it was not associated with a higher rate of mortality during subsequent transplantation. Only one patient died without a preceding relapse (1.8% non-relapse mortality). It should be noted, however, that patients who died during induction or were otherwise not able to tolerate ASCT were not included in this study. The incidence of secondary malignancies was 1 of 56 (1.8%), though a longer follow-up time will be needed to address this concern adequately.
An unresolved question concerning MCL is whether ASCT is curative. Previous series present conflicting results, with some studies showing apparent plateaus on PFS curves, whereas others do not [6–11,13–15,28,31–33]. Nevertheless, it is clear that many patients achieve long-term disease-free intervals following ASCT in first remission. One patient on this study remains in a molecular CR more than 10 years after ASCT, and is likely cured. Further studies are needed to identify pre-treatment characteristics predictive of long-term survival with induction plus ASCT or intensive induction alone.
In conclusion, the results of this study suggest that treating MCL patients with ASCT in first remission affords a survival benefit when compared to ASCT after relapse or progression. Our data also show that among patients who are transplanted in CR1/PR1, those who receive HyperCVAD (±R) followed by ASCT may have improved PFS compared to those treated with CHOP (±R) followed by ASCT. In view of the higher toxicity and need for hospitalization associated with the HyperCVAD regimen, randomized trials to confirm these results are warranted.
ACKNOWLEDGEMENTS
This research was supported by NIH Grant P01 CA44991 (O. Press), Lymphoma Research Foundation Mantle Cell Lymphoma Research Initiative, K23CA85479 (A. Gopal), and gifts from the Hext Family Foundation, the Edson Foundation, The Jose Carreras Foundation Against Leukemia, David and Patricia Giuliani, Mary and Geary Britton-Simmonds, James and Sherry Raisbeck, and Frank and Betty Vandermeer.
REFERENCES
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Bosch F, Lopez-Guillermo A, Campo E, Ribera JM, Conde E, Piris MA, et al. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998;82:567–575. doi: 10.1002/(sici)1097-0142(19980201)82:3<567::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Oinonen R, Franssila K, Teerenhovi L, Lappalainen K, Elonen E. Mantle cell lymphoma: clinical features, treatment and prognosis of 94 patients. Eur J Cancer. 1998;34:329–336. doi: 10.1016/s0959-8049(97)10056-9. [DOI] [PubMed] [Google Scholar]
- 4.Freedman AS, Neuberg D, Gribben JG, Mauch P, Soiffer RJ, Fisher DC, et al. High-dose chemoradiotherapy and anti-B-cell monoclonal antibody-purged autologous bone marrow transplantation in mantle-cell lymphoma: no evidence for long-term remission. J Clin Oncol. 1998;16:13–18. doi: 10.1200/JCO.1998.16.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Vose JM, Bierman PJ, Bierman PJ, Weisenburger DD, Lynch JC, Bociek Y, Chan WC, et al. Autologous hematopoietic stem cell transplantation for mantle cell lymphoma. Biol Blood Marrow Transplant. 2000;6:640–645. doi: 10.1016/s1083-8791(00)70030-9. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF, Saliba RM, Okoroji GJ, Acholonu SA, Champlin RE. Long-term follow-up of autologous stem cell transplantation in patients with diffuse mantle cell lymphoma in first disease remission: the prognostic value of beta2-microglobulin and the tumor score. Cancer. 2003;98:2630–2635. doi: 10.1002/cncr.11838. [DOI] [PubMed] [Google Scholar]
- 7.Lefrere F, Delmer A, Levy V, Delarue R, Varet B, Hermine O. Sequential chemotherapy regimens followed by high-dose therapy with stem cell transplantation in mantle cell lymphoma: an update of a prospective study. Haematologica. 2004;89:1275–1276. [PubMed] [Google Scholar]
- 8.Mangel J, Leitch HA, Connors JM, Buckstein R, Imrie K, Spaner D, et al. Intensive chemotherapy and autologous stem-cell transplantation plus rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Ann Oncol. 2004;15:283–290. doi: 10.1093/annonc/mdh069. [DOI] [PubMed] [Google Scholar]
- 9.Oinonen R, Jantunen E, Itala M, Lehtinen T, Kuittinen O, Franssila K, et al. Autologous stem cell transplantation in patients with mantle cell lymphoma. Leuk Lymphoma. 2002;43:1229–1237. doi: 10.1080/10428190290026286. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie DS, Seymour JF, Grigg AP, Roberts AW, Hoyt R, Thompson S, et al. The hyper-CVAD-rituximab chemotherapy programme followed by high-dose busulfan, melphalan and autologous stem cell transplantation produces excellent event-free survival in patients with previously untreated mantle cell lymphoma. Ann Hematol. 2007;86:101–105. doi: 10.1007/s00277-006-0193-2. [DOI] [PubMed] [Google Scholar]
- 11.Vigouroux S, Gaillard F, Moreau P, Harousseau JL, Milpied N. High-dose therapy with autologous stem cell transplantation in first response in mantle cell lymphoma. Haematologica. 2005;90:1580–1582. [PubMed] [Google Scholar]
- 12.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, Conde E, Lopez-Guillermo A, Gisselbrecht C, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 13.Haas R, Brittinger G, Meusers P, Murea S, Goldschmidt H, Wannenmacher M, Hunstein W. Myeloablative therapy with blood stem cell transplantation is effective in mantle cell lymphoma. Leukemia. 1996;10:1975–1979. [PubMed] [Google Scholar]
- 14.de Guibert S, Jaccard A, Bernard M, Turlure P, Bordessoule D, Lamy T. Rituximab and DHAP followed by intensive therapy with autologous stem-cell transplantation as first-line therapy for mantle cell lymphoma. Haematologica. 2006;91:425–426. [PubMed] [Google Scholar]
- 15.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 16.Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, Metzner B, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 17.Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Trumper L, Reiser M, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer. 2006;107:1014–1022. doi: 10.1002/cncr.22093. [DOI] [PubMed] [Google Scholar]
- 18.Zucca E, Roggero E, Pinotti G, Pedrinis E, Cappella C, Venco A, Cavalli F. Patterns of survival in mantle cell lymphoma. Ann Oncol. 1995;6:257–262. doi: 10.1093/oxfordjournals.annonc.a059155. [DOI] [PubMed] [Google Scholar]
- 19.Zinzani PL, Magagnoli M, Moretti L, De Renzo A, Battista R, Zaccaria A, et al. Randomized trial of fludarabine versus fludarabine and idarubicin as frontline treatment in patients with indolent or mantle-cell lymphoma. J Clin Oncol. 2000;18:773–779. doi: 10.1200/JCO.2000.18.4.773. [DOI] [PubMed] [Google Scholar]
- 20.Howard OM, Gribben JG, Neuberg DS, Grossbard M, Poor C, Janicek MJ, Shipp MA. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–1294. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 21.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- 22.Conde E, Marco F, Caballero D, et al. Autologous stem cell transplantation (ASCT) for mantle cell lymphoma (MCL) Blood. 2002;100:2529a. [abstract] [Google Scholar]
- 23.Vose J, Loberiza FR, Bierman PJ, Bociek G, Armitage JO. Mantle cell lymphoma (MCL): Induction therapy with HyperCVAD/High dose methotrexate and cytarabine (M-C) (+/− rituximab) improves results of autologous stem cell transplant in first remission. J Clin Oncol. 2006;24:7511a. [Google Scholar]
- 24.Jaffe ES, Harris NL, Stein H, Vardiman J, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 25.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:2454–2460. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed]
- 26.Ketterer N, Salles G, Espinouse D, Dumontet C, Neidhardt-Berard EM, Moullet I, et al. Intensive therapy with peripheral stem cell transplantation in 16 patients with mantle cell lymphoma. Ann Oncol. 1997;8:701–704. doi: 10.1023/a:1008278605751. [DOI] [PubMed] [Google Scholar]
- 27.Dreger P, Martin S, Kuse R, Sonnen R, Glass B, Kroger N, et al. The impact of autologous stem cell transplantation on the prognosis of mantle cell lymphoma: a joint analysis of two prospective studies with 46 patients. Hematol J. 2000;1:87–94. doi: 10.1038/sj.thj.6200007. [DOI] [PubMed] [Google Scholar]
- 28.Gianni AM, Magni M, Martelli M, Di Nicola M, Carlo-Stella C, Pilotti S, et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen) Blood. 2003;102:749–755. doi: 10.1182/blood-2002-08-2476. [DOI] [PubMed] [Google Scholar]
- 29.Kasamon YL, Jones RJ, Diehl LF, Nayer H, Borowitz MJ, Garrett-Mayer E, et al. Outcomes of autologous and allogeneic blood or marrow transplantation for mantle cell lymphoma. Biol Blood Marrow Transplant. 2005;11:39–46. doi: 10.1016/j.bbmt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Ganti AK, Bierman PJ, Lynch JC, Bociek RG, Vose JM, Armitage JO. Hematopoietic stem cell transplantation in mantle cell lymphoma. Ann Oncol. 2005;16:618–624. doi: 10.1093/annonc/mdi107. [DOI] [PubMed] [Google Scholar]
- 31.Andersen NS, Pedersen L, Elonen E, Johnson A, Kolstad A, Franssila K, et al. Primary treatment with autologous stem cell transplantation in mantle cell lymphoma: outcome related to remission pretransplant. Eur J Haematol. 2003;71:73–80. doi: 10.1034/j.1600-0609.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 32.Thieblemont C, Antal D, Lacotte-Thierry L, Delwail V, Espinouse D, Michallet AS, et al. Chemotherapy with rituximab followed by high-dose therapy and autologous stem cell transplantation in patients with mantle cell lymphoma. Cancer. 2005;104:1434–1441. doi: 10.1002/cncr.21313. [DOI] [PubMed] [Google Scholar]
- 33.Gopal AK, Rajendran JG, Petersdorf SH, Maloney DG, Eary JF, Wood BL, et al. High-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002;99:3158–3162. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]