Abstract
Ethanol induces neuronal cell injury and death by dysregulating several signaling events that are controlled, in part, by activation of MAPK/ERK1/2 and/or inactivation of its corresponding phosphatase, PP1. Recently, we have purified a novel protein of 38 kDa in size, p38SJ, from a callus culture of Hypericum perforatum, which belongs to an emerging DINGG family of proteins with phosphate binding activity. Here, we show that treatment of neuronal cells with p38SJ protects cells against injury induced by exposure to ethanol. Furthermore, pre-treatment of neuronal cells with p38SJ diminishes the level of the pro-apoptotic protein Bax and some events associated with apoptosis such as caspase 3 cleavage. In addition, by inducing stress, alcohol can elevate production of reactive oxygen species (ROS) that leads to a decrease in the activity of superoxide dismutase (SOD). Our results showed that p38SJ restores the activity of SOD in the ethanol treated neuronal cells. These observations provide a novel biological tool for developing new approaches for preventing neuronal cell death induced by ethanol and possibly treament of neurological disorders associated with alcohol abuse.
Keywords: neural apoptosis, alcohol, St. John’s Wort
INTRODUCTION
Alcohol abuse is associated with multiple neurological and behavioral deficits including neuropathy and encephalopathy, cerebellar degeneration, cognitive changes, and impairment in judgment and memory (for review see Alderazi and Brett 2007; Brun and Andersson 2001; Brust 2008; Johnson et al., 1986). Ethanol damages the nervous system and causes neuronal cell injury by affecting several signal transduction pathways, including MAPK (Sanna et al, 2002, Logrip 2008). Activation of MAPK by ethanol is receptor-dependent. Among the receptors affected by ethanol are GABA receptors (Lee et al, 2007a, Ueno 2001). It has been shown that the cellular effects of ethanol that occur via modulating the PKA and CREB transcription pathways are activated through GABA receptors (Criswell and Breese, 2005); increased expression of PKA and CREB was observed following ethanol treatment (Pandey et al., 2001).
Earlier studies have demonstrated that ethanol can cause neuronal cell death through oxidative stress (Antonio et al., 2008; Haorah et al., 2008a; Heaton et al., 2002, 2003; Lee et al., 2007; Ramachandran et al., 2003; Watts et al., 2005). Recent studies have also indicated increased levels of ROS in the CNS of alcoholics, probably due to the metabolism of ethanol (Haorah et al., 2008a). Alcohol-induced oxidative stress in brain has been studied extensively as a novel pathway of neurodegeneration associated with alcohol abuse (Haorah et al., 2008a and b). Many well-developed studies point at the importance of activation of kinases and inhibition of phosphatases in cell injury caused by ethanol-induced oxidative stress in the brain (Haorah et al., 2005, 2007, 2008b; Lohmann 2004). The potential mechanisms leading to induction of oxidative stress and alcohol-induced ROS production, and thus to neuronal injury are not fully understood.
Recently we identified a novel 38 kDa protein, p38SJ, from an in vitro cultivated callus culture of Hypericum perforatum and cloned its partial cDNA, p27SJ. p27SJ belongs to the DINGG family of proteins, as it contains a conserved sequence DINGG at the N-terminus (Darbinian et al., 2008; Perera et al., 2008). p27SJ exhibits the capacity to modulate expression of viral and cellular genes including HIV-1, MCP-1 (Darbinian-Sarkissian et al., 2006; Mukerjee et al., 2008),
In humans, a peptide containing DINGG was first identified in synovial fluid and was found to be part of a larger protein known as p205 synovial T-cell stimulating protein (Blass et al, 1999; Hain et al, 1996). Subsequent studies led to the identification of another member of the human DINGG family with growth-promoting effects in normal and tumor cells (Adams et al, 2002; Belenky et al, 2003; Morales et al, 2006). In addition to human tissue, DINGG proteins have been isolated from various fungi, animal and plant tissues, and exhibit close homology with Pseudomonas proteins (for review see Ahn et al, 2007; Berna et al, 2002, 2008; Chen et al, 2007; Lewis and Crowther, 2005; Moniot et al, 2007; Pantazaki et al, 2007; Riah et al, 2000; Scott and Wu, 2005).
Here we demonstrate that the treatment of neuronal cells with p38SJ protects them from ethanol-induced apoptosis.
MATERIALS AND METHODS
Cell culture
Rat cortical neurons were propagated following enzymatic and mechanical treatment of Sprague Dawley rat embryonic tissue at day 17 (E17) using TrypleExpress enzyme (Invitrogen, Carlsbad, CA) at 37 °C for 10 min, followed by three washes with Hibernate E medium. After mechanical treatment of tissue with a fire-polished glass Pasteur pipette, single cell suspension was diluted with culturing medium and cells were plated on poly-D-lysine-coated 60 mm dishes at a density of 2.5 × 106/plate and cultured in 3 ml Neurobasal medium containing B27 supplement, 0.25 mM Glutamax, and 0.25 mM L-glutamine (allfrom Invitrogen). Cells were maintained at 37 °C in a humidified incubator containing 7% CO2.
Microscopy
Phase contrast images of neuronal cells were visualized with an inverted Olympus fluorescence microscope using IPLAB software. Contrast and brightness were adjusted equally for all images using Adobe Photoshop version 5.5.
Plant extract preparation
One hundred milligrams of dried H. perforatum were dissolved in 1 ml of lysis buffer containing 30 mM Tris (pH 7.4), 167 mM NaCl, 0.1% Nonidet P-40 and protease inhibitors cocktail (Sigma, St. Louis, MO USA). Cell debris was removed by centrifugation at 14,000 rpm for 5 min at 4 °C. Total soluble proteins from the callus were centrifuged at 10,000 rpm for 5 min and the supernatant was recovered and fractionated through 3, 30 and 50 kDa MilliPore Microcon filters (Millipore, Billerica, MA USA), to separate the 38 kDa protein from the low molecular weight proteins and other plant organic components. The purity of the 38 kDa protein was determined by SDS-PAGE.
Preparation of protein extracts and immunoblot analysis
For preparation of whole cell protein extracts, following treatment with ethanol and/or p38SJ, cells were washed with cold phosphate-buffered saline (PBS) and solubilized in lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 0.1% Nonidet P-40, and 1% protease inhibitors cocktail (Sigma, St. Louis, MO USA). Cell debris was removed by centrifugation at 10,000 rpm for 5 min at 4 °C. Fifty micrograms of protein were resolved in Laemmli sample buffer and fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For Western blot analysis, protein samples were resolved by SDS-PAGE and after transfer to membrane, reacted with specific antibodies and the proteins visualized with the enhanced chemiluminescence detection system ECL+ according to the manufacturer’s instructions (GE Healthcare, Piscataway NJ), and exposed to X-ray film.
Caspase-GLO 3/7 assay
Apoptosis was determined by analysis of activation of caspase-3 using the substrate DEVD-aminoluciferin from Caspase-Glo™ 3/7 assay kit (Promega, Madison, WI, USA), according to the manufacturer’s instruction. Luminescence was recorded on a Turner Designs Luminometer TD-20/20 Data were analyzed using Excel software.
Methylthiazoletetrazolium (MTT) assay
For the methylthiazoletetrazolium (MTT) assay, we used a cell proliferation kit (MTT) according to the manufacturer’s protocol (Roche, Indianapolis, IN USA). Cells were plated onto 96-well plates in triplicate in two sets at a density of 15,000 cells/well and pre-incubated with p38SJ (for 2 hours) then co-incubated with ethanol. After 24 hours, 10 μl MTT (5 mg/ml) were added to the wells (final concentration, 0.5 mg/ml) for 4 h, and the reaction was stopped by the addition of 100 μl of solubilization solution. Viable cells with active mitochondria cleave the tetrazolium ring into a visible dark blue formazan reaction product, which was quantified by spectrophotometry in a microplate reader at 570 nm with a reference wavelength of 650 nm. The relative cell viability (percent) was determined as the ratio of average absorbance for treated cells to that for mock, untreated cells.
Superoxide Dismutase (SOD) activity assay
To measure SOD activity in primary neurons, following treatment with p38SJ for two hours and ethanol, cytosolic fraction was prepared and incubated with Xantine Oxidase Solution for 1 hour at 37 °C. Absorbance was read at 490 nm to generate superoxide anions. The activity of SOD is determined as the inhibition of chromagen reduction. In the presence of SOD, superoxide anion concentration is reduced, yielding less colorimetric signal. SOD activity was shown in %.
Antibodies
Antibody specific for phospho-p44/42 mitogen-activated protein kinase (MAPK/Erk1/2), anti-p44/42 MAPK, rabbit polyclonal, and anti-GRB2 rabbit polyclonal antibodies were purchased from Cell Signaling Technologies (Danvers, MA). Anti-caspase-3 rabbit polyclonal and anti-Bax antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
RESULTS AND DISCUSSION
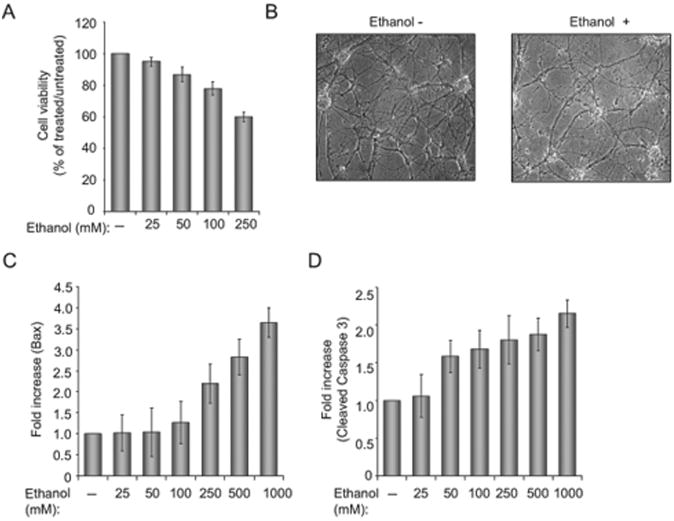
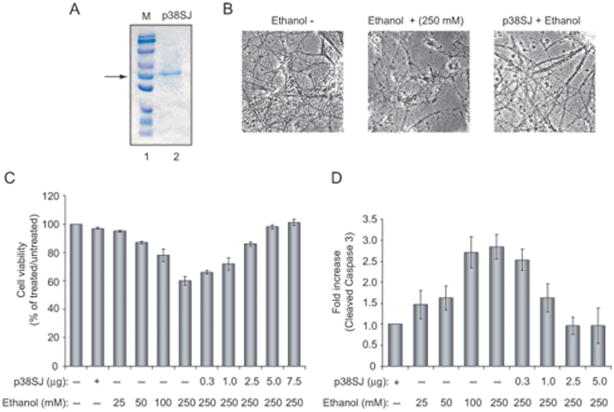
To examine the effect of ethanol on neuronal cells, we prepared primary cultures of rat neurons, and examined cell viability in response to ethanol treatment. As shown in Figure 1A, increasing the concentration of ethanol results in decreased viability of neuronal cells. At 250 mM ethanol treatment, approximately 40% of the cells were dead and the remaining cells showed reduced levels of arborization and processes (Fig. 1B). Examination of pro-apoptotic proteins revealed the induction of BAX and increased levels of caspase 3. Figure 1 illustrates quantitative analysis of Bax (Panel C) and cleaved caspase 3 (Panel D) as determined by immunoblot assay. To evaluate the ability of p38SJ to protect neuronal cells from apoptosis upon ethanol treatment, cells were incubated with highly purified p38SJ (Fig. 2A) for 24 hours prior to analysis of cell viability and cleavage of caspases. As shown in Fig. 2, treatment of cells with p38SJ drastically improved the amount of arborization of the ethanol treated cells (Panel B) and cell viability (Panel C), and decreased the level of cleavage of caspase 3 (Panel D), indicating that p38SJ has neuroprotective activity in response to ethanol treatment.
Figure 1. Effect of ethanol on neuronal cell morphology and apoptotic pathways.
(A) Cell viability assay in rat primary neurons, incubated with increasing concentrations of ethanol (from 25 to 250 mM). Equal numbers of cells were plated in duplicate, and then incubated with ethanol. Cell viability was evaluated by Trypan blue exclusion assay. Bar 1 represents untreated cells set as 100%. (B)Phase images (magnification 200×) of neuronal cells incubated in the absenceand presence of 250 mM ethanol. (C)Quantification of the differences in Bax levels as determined by densitometric analysis of the band corresponding to Bax (as determined by Western blot assay) that was normalized to the level of Grb2. (D) Quantification of the differences in cleaved caspase 3 after being normalized to the level of Grb2.
Figure 2. Effect of p38SJ on neuronal cell injury by ethanol.
(A)SDS-PAGE illustrating highly purified p38SJ that was obtained upon size fractionation of crude protein extracts from the callus culture of St. John’s Wort (Darbinian-Sarkissian et al., 2006). The position of the p38SJ is shown by an arrow.(B) Phase images of neuronal cells incubated with ethanol (250 mM) and/or p38SJ (300 ng/ml). Reduction in number of neurons and neuronal processes caused by ethanol was reversed in the presence of p38SJ. (C) Cell viability assay in rat primary neurons pre-incubated with the increasing concentrations of p38SJ, as indicated in Figure 2B, for 2 hours and then treated with ethanol. Equal numbers of cells were plated in duplicate, and cell viability was evaluated by Trypan blue exclusion assay. Lane 1 contains untreated cells set as 100%. (D) p38SJ prevents caspase-3 cleavage in ethanol-treated neuronal cells. Cell lysates prepared from untreated, pre-treated with p38SJ for 2 hours prior to ethanol treatment, and ethanol-treated cells at 24 hours of incubation were analyzed by immunoblot analysis for cleaved caspase-3. Equal loading was verified by using anti-tubulin antibody. Bar graphs demonstrate the quantified density of bands, presented as a histogram for cleaved caspase-3, normalized to tubulin.
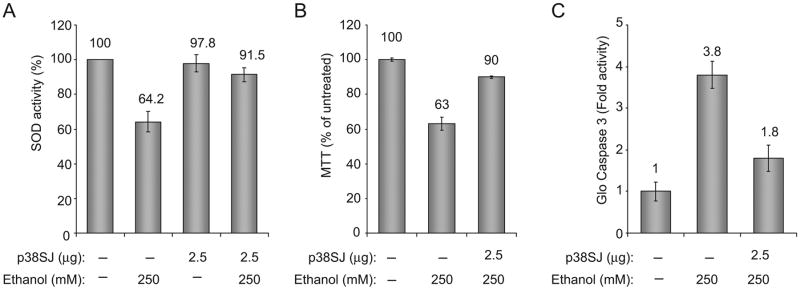
Next, we investigated the effect of p38SJ on oxidative stress in neuronal cells. To this end, we determined the activity of superoxide dismutase (SOD) in cells incubated with p38SJ prior to ethanol treatment. To measure SOD activity in primary neurons, we utilized the OxiSelect SOD Activity Assay system (Cell Biolabs, San Diego, CA). Data from SOD assay indicate that ethanol induces oxidative stress in neurons by inhibiting SOD activity (Fig. 3A). p38SJ was able to prevent ethanol-induced oxidative stress, and restore SOD activity in ethanol treated cells. Under identical experimental conditions, results from MTT cell metabolism/activity assay showed restoration of cells by p38SJ in ethanol treated cells (Fig. 3B) and a decrease in the caspase 3 activity as evaluated by Glo assay (Fig. 3C).
Figure 3. Oxidative stress, cell viability, and caspase-3 assays.
(A) Neuronal cells treated with ethanol for 24 h or pretreated with p38SJ for 2 h prior to exposure to ethanol. Cells were analyzed by SOD assay. SOD activity is shown as % of untreated control (bar 1).(B)Cell viability determined by MTT assay.(C)Caspase-3 activity determined by Caspase-3/7 assay.
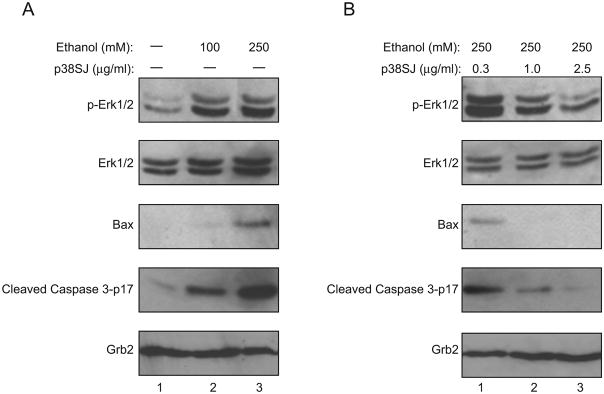
In light of earlier studies pointing to the capacity of ethanol to dysregulate signaling events involving phosphorylation of ERK1/2 (Glotin et al., 2006; Ku et al., 2007; Lee et al., 2006; Luo et al., 2006; Zhuang et al., 2007), we examined the levels of phosphorylated and total ERK1/2 in neuronal cells upon treatment with ethanol. As seen in Fig. 4A, ethanol treatment induces phosphorylation of ERK1/2 (pERK1/2) with no major impact on the total level of ERK1/2. As before, the level of BAX and cleaved caspase were increased upon ethanol treatment. Treatment of these cells with p38SJ reduced the level of the phosphorylated form of pERK1/2, had no impact on the total level of ERK1/2, and significantly decreased levels of BAX and cleaved caspase 3. These observations indicate that p38SJ has the ability to inhibit ethanol-induced phosphorylation of ERK1/2 and activation of apoptotic pathways. The decrease in phosphorylation of ERK1/2 may reduce the level of the pro-apoptotic BAX and caspase 3.
Figure 4. Disruption of ERK 1/2 and its phosphorylated form by p38SJ.
(A) Expression of ERK1/2 and proteins involved in apoptosis in ethanol treated neuronal cells. Western blot analysis for ERK1/2 and phospho-Erk 1/2 in rat primary neuronal cells after treatment with 100 and 250 mM ethanol for 24 h. The level of total ERK1/2 and Grb2 expression serve as a control for protein loading. (B). Effect of p38SJ on ERK1/2 phosphorylation in cells incubated with ethanol. Western blot analysis for ERK1/2 and phospho-ERK1/2 in the neuronal cells upon exposure to ethanol for 24 h after pre-treatment of cells with p38SJ for 2 h.
Hypericum perforatum, also known as St John’s Wort, has received special attention due to its pharmacological properties (Diwu 1995; Roth 2004; Wagner 1994,). Extracts from this plant contain active secondary metabolites including hypericin, a photosensitive red-colored naphthodianthron which is a bioactive compound that can act as a kinase inhibitor. Hypericum perforatum extracts contain other flavonoids such as rutin, with a free radical scavenging activity, and a potential antioxidant activity (Saija et al., 1995). While these secondary metabolites have been intensively investigated, the spectrum of proteins found in Hypericum perforatum still remains very poorly understood. Recently we identified a DINGG family protein named p38SJ and have cloned its truncated protein, p27SJ (Darbinian et al., 2008; Darbinian-Sarkissian et al., 2006; Perera et al., 2008). p38SJ has a phosphate binding domain similar to bacterial DINGG protein, yet the importance of this binding in the biological activity of p38SJ remains to be established. Our results presented here show that p38SJ inhibits the phosphorylated form of ERK1/2, an event that is seen upon the treatment of neurons with alcohol. Earlier studies have demonstrated that ethanol and reactive oxygen species (ROS) produced by ethanol modulate intracellular signaling pathways including mitogen-activated protein kinase (MAPK) cascades. Evidently, ethanol causes activation of all three MAPKs, including ERK, p38MAPK, and JNK; and ROS accumulation (Ku et al., 2007). ERK1/2 signalinghas differential effects on cell physiology such that the persistent activation of ERK1/2 promotes cell death mediated by oxidative stress, while inhibition of ERK1/2 leads to cell survival (Glotin 2006; Lee 2006; Luo 2006; Zhuang 2007). Our results show that inhibition of ERK1/2 phosphorylation by p38SJ promotes cell survival in ethanol treated cells as evidenced by the reduced levels of pro-apoptotic proteins. Our recent studies demonstrate that p27SJ, a variant of p38SJ with a C-terminal deletion, has a phosphatase activity, and its presence can dysregulate signaling events that control cell proliferation (Darbinian et al., 2009). Thus, one can envision a model in which p38SJ, by enforcing its phosphatase activity most likely on ERK1/2 and others, interferes with signaling pathways that are involved in ethanol-mediated neuronal cell death.
Acknowledgments
The authors wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for sharing of reagents and ideas, and their continued support. We thank Dr. Yuri Popov from Yerevan State University in Yerevan, Armenia for his collaboration and providing the initial callus culture of H. perforatum. We also thank C. Schriver for editorial assistance. This work was made possible by grants awarded by NIH to SA.
Contract grant sponsor: NIH; Contract grant number: R01MH074392
References
- Adams L, Davey S, Scott K. The DING protein: an autocrine growth-stimulatory protein related to the human synovial stimulatory protein. Biochim Biophys Acta. 2002;1586:254–264. doi: 10.1016/s0925-4439(01)00104-1. [DOI] [PubMed] [Google Scholar]
- Ahn S, Moniot S, Elias M, Chabriere E, Kim D, Scott K. Structure-function relationships in a bacterial DING protein. FEBS Lett. 2007;581:3455–3460. doi: 10.1016/j.febslet.2007.06.050. [DOI] [PubMed] [Google Scholar]
- Alderazi Y, Brett F. Alcohol and the nervous system. Curr Diag Pathol. 2007;13:203–209. [Google Scholar]
- Antonio AM, Druse MJ. Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res. 2008;1204:16–23. doi: 10.1016/j.brainres.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky M, Prasain J, Kim H, Barnes S. DING, a genistein target in human breast cancer: a protein without a gene. J Nutr. 2003;133(7 Suppl):2497S–2501S. doi: 10.1093/jn/133.7.2497S. [DOI] [PubMed] [Google Scholar]
- Berna A, Bernier F, Scott K, Stuhlmuller B. Ring up the curtain on DING proteins. FEBS Lett. 2002;524:6–10. doi: 10.1016/s0014-5793(02)03053-3. [DOI] [PubMed] [Google Scholar]
- Berna A, Bernier F, Chabriere E, Perera T, Scott K. DING proteins; novel members of a prokaryotic phosphate-binding protein superfamily which extends into the eukaryotic kingdom. Int J Biochem Cell Biol. 2008;40:170–175. doi: 10.1016/j.biocel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Blass S, Schumann F, Hain NA, Engel JM, Stuhlmuller B, Burmester GR. p205 is a major target of autoreactive T cells in rheumatoid arthritis. Arthritis Rheum. 1999;42:971–980. doi: 10.1002/1529-0131(199905)42:5<971::AID-ANR16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Brun A, Andersson J. Frontal dysfunction and frontal cortical synapse loss in alcoholism-the main cuase of alcohol dementia? Dement Geriatr Cogn Disord. 2001;12:289–294. doi: 10.1159/000051271. [DOI] [PubMed] [Google Scholar]
- Brust JCM. A 74 year old man with memory loss and neuropathy who enjoys alcoholic beverages. JAMA. 2008;299:1046–1054. doi: 10.1001/jama.299.5.jrr80000. [DOI] [PubMed] [Google Scholar]
- Chen Z, Franco CF, Baptista RP, Cabral JM, Coelho AV, Rodrigues CJ, Jr, Melo EP. Purification and identification of cutinases from Colletotrichum kahawae and Colletotrichum gloeosporioides. Appl Microbiol Biotechnol. 2007;73:1306–1313. doi: 10.1007/s00253-006-0605-1. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Darbinian N, Popov Y, Khalili K, Amini S. Creation of a bi-directional protein transduction system for suppression of HIV-1 expression by p27SJ. Antiviral Res. 2008;79:136–141. doi: 10.1016/j.antiviral.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbinian N, Czernik M, Darbinyan A, Elias M, Chabriere E, Bonasu S, Khalili K, Amini S. Evidence for phosphatase activity of p27SJ and its impact on the cell cycle. J Cell Biochem. 2009 doi: 10.1002/jcb.22135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbinian-Sarkissian N, Darbinyan A, Otte J, Radhakrishnan S, Sawaya BE, Arzumanyan A, Chipitsyna G, Popov Y, Rappaport J, Amini S, Khalili K. p27(SJ), a novel protein in St John’s Wort, that suppresses expression of HIV-1 genome. Gene Ther. 2006;13:288–295. doi: 10.1038/sj.gt.3302649. [DOI] [PubMed] [Google Scholar]
- Diwu Z. Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Invited review Photochem Photobiol. 1995;61:529–539. doi: 10.1111/j.1751-1097.1995.tb09903.x. [DOI] [PubMed] [Google Scholar]
- Glotin AL, Calipel A, Brossas JY, Faussat AM, Tréton J, Mascarelli F. Sustained versus transient ERK1/2 signaling underlies the anti- and proapoptotic effects of oxidative stress in human RPE cells. Invest Ophthalmol Vis Sci. 2006;47:4614–4623. doi: 10.1167/iovs.06-0297. [DOI] [PubMed] [Google Scholar]
- Hain NA, Stuhlmuller B, Hahn GR, Kalden JR, Deutzmann R, Burmester GR. Biochemical characterization and microsequencing of a 205-kDa synovial protein stimulatory for T cells and reactive with rheumatoid factor containing sera. J Immunol. 1996;157:1773–1780. [PubMed] [Google Scholar]
- Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol. 2005;78:1223–1232. doi: 10.1189/jlb.0605340. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med. 2008a;45:1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haorah J, Schall K, Ramirez SH, Persidsky Y. Activation of protein tyrosine kinases and matrix metalloproteinases causes blood-brain barrier injury: Novel mechanism for neurodegeneration associated with alcohol abuse. Glia. 2008b;56:78–88. doi: 10.1002/glia.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Mayer J, Miller R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci Lett. 2002;334:83–86. doi: 10.1016/s0304-3940(02)01123-0. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Mayer J, Moore DB. Effects of ethanol on neurotrophic factors, apoptosis-related proteins, endogenous antioxidants, and reactive oxygen species in neonatal striatum: relationship to periods of vulnerability. Dev Brain Res. 2003;140:237–252. doi: 10.1016/s0165-3806(02)00610-7. [DOI] [PubMed] [Google Scholar]
- Johnson RH, Eisenhofer G, Lambie DG. The effects of acute and chronic ingestion of ethanol on the autonomic nervous system. Drug Alcohol Depend. 1986;18:319–328. doi: 10.1016/0376-8716(86)90094-3. [DOI] [PubMed] [Google Scholar]
- Ku BM, Lee YK, Jeong JY, Mun J, Han JY, Roh GS, Kim HJ, Cho GJ, Choi WS, Yi GS, Kang SS. Ethanol-induced oxidative stress is mediated by p38 MAPK pathway in mouse hippocampal cells. Neurosci Lett. 2007;419:64–67. doi: 10.1016/j.neulet.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH, Kim BW, Choi HY, Jeong MY, Cho SG. Interplay between PI3K/Akt and MAPK signaling pathways in DNA-damaging drug-induced apoptosis. Biochim Biophys Acta. 2006;1763:958–968. doi: 10.1016/j.bbamcr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Lee HY, Li SP, Park MS, Bahk YH, Chung BC, Kim MO. Ethanol’s effect on intracellular signal pathways in prenatal rat cortical neurons is GABAB1 dependent. Synapse. 2007a;61:622–628. doi: 10.1002/syn.20416. [DOI] [PubMed] [Google Scholar]
- Lee JH, Tajuddin NF, Le PT, Druse MJ. A serotonin-1A agonist provides protection against the ethanol-induced decrease of endogenous antioxidant enzymes in fetal rhombencephalic neurons. Alcohol Clin Exp Res. 2007b;31:37A. [Google Scholar]
- Lewis AP, Crowther D. DING proteins are from Pseudomonas. FEMS Microbiol Lett. 2005;252:215–222. doi: 10.1016/j.femsle.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Lohman C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995:184–186. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J Biol Chem. 2006;281:16436–16442. doi: 10.1074/jbc.M512430200. [DOI] [PubMed] [Google Scholar]
- Moniot S, Elias M, Kim D, Scott K, Chabriere E. Crystallization, diffraction data collection and preliminary crystallographic analysis of DING protein from Pseudomonas fluorescens. Acta Crystallograph Sect F Struct Biol Cryst Commun. 2007;63(Pt 7):590–592. doi: 10.1107/S1744309107028102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R, Berna A, Carpentier P, Contreras-Martel C, Renault F, Nicodeme M, Chesne-Seck ML, Bernier F, Dupuy J, Schaeffer C, Diemer H, Van-Dorsselaer A, Fontecilla-Camps JC, Masson P, Rochu D, Chabriere E. Serendipitous discovery and X-ray structure of a human phosphate binding apolipoprotein. Structure. 2006;14:601–609. doi: 10.1016/j.str.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Mukerjee R, Deshmane SL, Darbinian N, Czernik M, Khalili K, Amini S, Sawaya BE. St. John’s Wort protein, p27(SJ), regulates the MCP-1 promoter. Mol Immunol. 2008;45:4028–4035. doi: 10.1016/j.molimm.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Mittal N. Effects of chronic ethanol intake and its withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat cortex. J Pharmacol Exp Ther. 2001;296:857–868. [PubMed] [Google Scholar]
- Pantazaki AA, Tsolkas GP, Kyriakidis DA. A DING phosphatase in Thermus thermophilus. Amino Acids. 2007;34:437–448. doi: 10.1007/s00726-007-0549-5. [DOI] [PubMed] [Google Scholar]
- Perera T, Berna A, Scott K, Lemaitre-Guillier C, Bernier F. Proteins related to St. John’s Wort p27SJ, a suppressor of HIV-1 expression, are ubiquitous in plants. Phytochemistry. 2008;69:865–872. doi: 10.1016/j.phytochem.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G. Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res. 2003;74:577–588. doi: 10.1002/jnr.10767. [DOI] [PubMed] [Google Scholar]
- Riah O, Dousset JC, Bofill-Cardona E, Courriére P. Isolation and microsequencing of a novel cotinine receptor. Cell Mol Neurobiol. 2000;20:653–664. doi: 10.1023/a:1007094623775. [DOI] [PubMed] [Google Scholar]
- Roth BL, Lopez E, Beischel S, Westkaemper RB, Evans JM. Screening the receptorome to discover the molecular targets for plant-derived psychoactive compounds: a novel approach for CNS drug discovery. Pharmacol Ther. 2004;102:99–110. doi: 10.1016/j.pharmthera.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radical Biology & Medicine. 1995;19:481–486. doi: 10.1016/0891-5849(94)00240-k. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Scott K, Wu L. Functional properties of a recombinant bacterial DING protein: comparison with a homologous human protein. Biochim Biophys Acta. 2005;1744:234–244. doi: 10.1016/j.bbamcr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ueno S, Harris RA, Messing RO, Sanchez-Perez AM, Hodge CW, McMahon T, Wang D, Mehmert KK, Kelley SP, Haywood A, Olive MF, Buck KJ, Hood HM, Blednov Y, Findlay G, Mascia MP. Alcohol actions on GABA(A) receptors: from protein structure to mouse behavior. Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):76S–81S. doi: 10.1097/00000374-200105051-00014. [DOI] [PubMed] [Google Scholar]
- Wagner H, Bladt S. Pharmaceutical quality of hypericum extracts. J Geriatr Psych Neurol. 1994;7:65–68. doi: 10.1177/089198879400700118. [DOI] [PubMed] [Google Scholar]
- Watts LT, Rathinam ML, Schenker S, Henderson GI. Astrocytes protect neurons from ethanol-induced oxidative stress and apoptotic death. J Neurosci Res. 2005;80:655–666. doi: 10.1002/jnr.20502. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292:F440–447. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]