Abstract
Ethanol-induced inhibition of myocyte BK current causes cerebrovascular constriction, yet the molecular targets mediating ethanol action remain unknown. Using BK channel-forming (cbv1) subunits from cerebral artery myocytes, we demonstrate that ethanol potentiates and inhibits current at Ca2+i lower and higher than ~15 μM, respectively. By increasing cbv1’s apparent Ca2+i-sensitivity, accessory BK β1 subunits shift the activation-to-inhibition crossover of ethanol action to <3 μM Ca2+i, with consequent inhibition of current under conditions found during myocyte contraction. Knocking-down KCNMB1 suppresses ethanol-reduction of arterial myocyte BK current and vessel diameter. Therefore, BK β1 is the molecular effector of alcohol-induced BK current inhibition and cerebrovascular constriction.
Keywords: channel auxiliary subunits, KCNMB1, maxiK channel, cerebral artery, alcohol, vasoconstriction
Ethanol at concentrations obtained in circulation during heavy episodic alcohol intake, such as in binge drinking, constricts cerebral arteries in many species, including humans, resulting in reduced brain blood flow [1-4]. Using a rodent model, we demonstrated that alcohol-induced cerebrovascular constriction is linked to ethanol-induced reduction of Spontaneous Transient Outward Currents (STOCs) in the cerebral artery myocyte [2]. In these cells, STOCs result from activation of large conductance, voltage- and Ca2+-gated potassium (BK) channels [5-6]. Notably, acute exposure to clinically-relevant ethanol concentrations (50 mM) may depress BK channel activity [2]. However, the molecular targets and mechanisms determining alcohol inhibition of arterial smooth muscle BK current and, eventually, reduced arterial diameter, remain unknown.
BK channels result from the association of four identical subunits encoded by the Slo1 (KCNMA1) gene [7]. In most tissues, however, slo1 subunits interact with a variety of membrane-bound proteins, including small, accessory β(1-4) subunits (encoded by four genes, KCNMB1-4) that modify BK current phenotype [8-9]. Remarkably, KCNMB1-4 are differentially expressed across tissues, β1 being very abundant in vascular smooth muscle yet scarce in other tissues [9]. Taking advantage of our cloning of a BK channel-forming slo1 isoform that is particularly abundant in rat cerebrovascular myocytes (“cbv1”, AY330293 [10]) and its associated β1 subunit (FJ154955), complemented with the KCNMB1 K/O mouse model, we set to address the specific role of BK channel subunits in alcohol actions on smooth muscle BK channel function and its impact on the diameter of resistance-size, small cerebral arteries that control brain blood flow.
Materials and Methods
Cbv1 and β1 cloning
Rat cerebral artery myocyte isolation and total RNA purification were performed as described [10]. The cloning and functional characterization of cbv1 is provided elsewhere [10-11]. BK β1 was cloned by PCR using the primers: forward, GCC ATG GGG AAG AAG CTG GTG ATG; reverse, CTA CTT CTG AGC TGC CAA GAC AGA GAG. After initialization at 95°C for 2 min, 30 cycles (94°C for 30 sec, 58°C for 30 sec, 72°C for 1 min) were completed, followed by final elongation at 72°C for 5 min. Amplified PCR products were purified with ultrafree-DA (Millipore) and ligated into PCR-TOPO (Invitrogen). After sequencing 16 clones, the most prevalent BK β1 isoform from arterial myocytes was ligated into pOX (Aguan Wei; Washington University). Correct insertion and ligation were verified by sequencing at UTennessee HSC Molecular Rsch. Ctr. pOX-β1 cDNA was linearized with NotI and transcribed in vitro using T3 (mMessage-mMachine; Ambion).
cRNA injection into Xenopus oocytes
Oocytes were removed from Xenopus laevis (NASCO) and prepared as described elsewhere [12]. cRNA was dissolved in diethyl polycarbonate-treated water at 5 (cbv1) and 15 (β1) ng/μl; 1 μl aliquots were stored at −70°C. Cbv1 (2.5 ng/μl) and β1 (7.5 ng/μl) cRNAs were coinjected, giving molar ratios ≥6:1 (β:α). Cytosolic cRNA injection (23 nl/oocyte) was conducted using a modified micropipette (Drummond). The interval between injection and patch-clamp recordings was 48-72 h.
Mouse cerebrovascular myocyte isolation
Mice were decapitated using sharp scissors following a procedure approved by the Institutional Animal Care and Use Committee from The University of Tennessee Health Science Center, an AAALAC-accredited institution. Basilar and middle cerebral arteries were dissected out from each brain under a stereozoom microscope (Nikon) and placed in ice-cold dissociation medium (DM) (mM): 0.16 CaCl2, 0.49 EDTA, 10 HEPES, 5 KCl, 0.5 KH2PO4, 2 MgCl2, 110 NaCl, 0.5 NaH2PO4, 10 NaHCO3, 0.02 phenol red, 10 taurine, 10 glucose. Each artery was cut into 1-2 mm long rings (~30 rings/preparation). Rings were put in 3 ml DM containing 0.00075% papain, 0.05% bovine serum albumin (BSA) and 0.004% dithiothreitol (DTT) for 12 min at 37 °C in a shaking water bath at 60 oscillations/min. Then the incubation solution was replaced with the 3 ml DM that contained 0.06% soybean trypsin inhibitor (STI), 0.05% BSA and 2% collagenase (26.6 units/ml). Incubation was performed for 10 min at the same conditions as before. Finally, the tissue was transferred into 3 ml DM with 0.075% STI and pipetted using a series of borosilicate Pasteur pipettes having fire-polished, diminishing internal diameter tips. BSA was added to the preparation, reaching a final concentration of 0.0625%. The procedure rendered a cell suspension containing relaxed, individual myocytes (≈2 myocytes/field using a 40X objective) that could be easily identified under microscope (Olympus IX-70) due to the cell characteristic, elongated shape (50-100 μm long). Myocyte viability was verified immediately before electrophysiological recordings by evaluating their reversible contractile responses to the application of noradrenaline from a pressure ejection pipette ([noradrenaline] in the pipette = 100 μM) [13]. The cell suspension was stored on ice, and the cells were used for patch-clamping up to 4 h after being isolated.
Electrophysiology
Oocytes were prepared for patch-clamp recordings as described [12]. Single-channel and macroscopic currents were recorded from inside-out (I/O) patches. For experiments with oocytes, both bath and electrode solutions contained (mM): 130 Kgluconate, 5 EGTA, 2.28 MgCl2, 15 HEPES, pH 7.35. For experiments with myocytes, KCl substituted for Kgluconate. In all experiments, free Ca2+ in solution was adjusted to the desired value by adding CaCl2. In the experiments where the free Ca2+i was set to ≥1 μM, 1.6 mM HEDTA was added. In all experiments, varying amounts of CaCl2 were used to set the free Ca2+ at the desired level, keeping free Mg2+ constant at 1 mM [14]. Free Ca2+ and Mg2+ were calculated using Max Chelator (C. Patton, Stanford University, CA) and experimentally validated using Ca2+-sensitive/reference electrodes (Corning) as described elsewhere [14].
Patch electrodes were pulled from glass capillaries (Drummond) as previously described [12]. The procedure gave tip resistances of 2-5 MΩ (for macropatch recordings) or 5-10 MΩ (for conventional I/O single channel recordings) when filled with electrode solution. An Ag/AgCl electrode was used as ground electrode. After excision from the oocyte, the inner side of the membrane patch was exposed to a stream of bath solution containing each agent at final concentration. Solutions were applied onto the patches using a DAD12 pressurized system (ALA Scientific) via a micropipette tip with an internal diameter of 100 μm. Experiments were carried out at room temperature (21°C).
Both macroscopic and unitary currents were acquired using an EPC8 (HEKA Electronics) amplifier, and digitized using a 1320 interface and pCLAMP8 or pCLAMP9 software (Molecular Devices). Macroscopic currents were evoked from I/O macropatches held at −80 mV by 200 ms-long, 10 mV depolarizing steps from −100 to +160 mV. Currents were low-pass filtered at 1 kHz with an 8-pole Bessel filter 902LPF (Frequency Devices) and sampled at 5 kHz. Average current amplitude was determined 175-200 ms after the start of the depolarizing step. Unitary currents were low-pass filtered at 7-10 kHz with an 8-pole Bessel filter and sampled at 35-50 kHz.
Macroscopic conductance (G)-V plots were fitted to a Boltzmann function of the type G(V) = Gmax/1+exp[(−V+V1/2)/k]. Boltzmann fitting routines were run using the Levenberg-Marquardt algorithm to perform nonlinear least squares fits. Single-channel analysis was initially performed using pCLAMP9 (Molecular Devices). The product of the number of channels in the patch (N) and the probability that a channel is open (Po) was used as an index of the channel steady-state activity. NPo was calculated from the area under the curve of the Gaussian fit of all-points amplitude histograms [15-16]. NPo values were obtained from gap-free recording of single channel activity for 1 to 3 min under each condition.
Artery diameter determination
Middle cerebral arteries were isolated from adult male 8-12 week-old KCNMB1 knockout and C57BL/6 (control) mice. Pressurization of arteries was performed as described [2]. Endothelium was removed by passing an air bubble into the vessel lumen for 90 sec. Diameter changes were monitored through an inverted microscope (Nikon Corp.), recorded on camera (Sanyo Electric Corp.), and stored on computer. Diameter data were acquired and analyzed using IonWizard 4.4 (IonOptics Corp.).
Pressurized arteries were extraluminally perfused with physiological saline solution (PSS) (composition in [2]) at a constant rate of 3.75 ml/min using a Dynamax RP-1 peristaltic pump (Rainin). Drugs were dissolved to make stock solutions (see Chemicals) and diluted in PSS to final concentration.
Chemicals
Except ethanol (American Bioanalytical) and iberiotoxin (Alomone), all chemicals were purchased from Sigma. Ethanol (100% pure) was freshly added to the bath solution immediately before experiments. Bath solution with urea iso-osmotically substituting for ethanol was used as control perfusion, with the solution osmolarity ranging 301-322 mM/kg (Wescor Vapro). Iberiotoxin was dissolved in distilled water to 20 μM and further diluted in PSS to final concentration.
Statistics
Electrophysiological and arterial diameter data were analyzed with pCLAMP 8.0 (Molecular Devices) and IonWizard 4.4 (IonOptics), respectively. Further analysis, plotting and fitting were conducted using Origin 7.0 (Originlab) and InStat 3.0 (GraphPad). Statistical analysis was conducted using one-way ANOVA and Bonferroni’s multiple comparison test; significance was set at P<0.05.
Results
Beta1 subunits determine ethanol final action on recombinant cerebrovascular BK channels
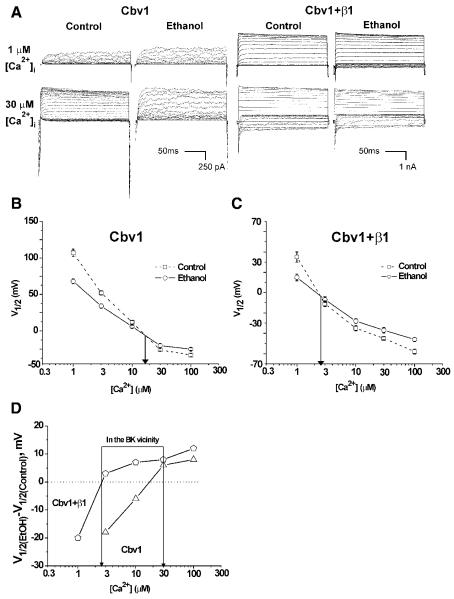
Voltage-clamp electrophysiology on cell-free membrane patches after expression of recombinant BK channel subunits in Xenopus oocytes is a widely used approach to study ethanol actions on the BK channel complex [17]. Macroscopic BK currents mediated by cbv1±β1 subunits were evoked by depolarizing voltage steps (Materials and Methods) in I/O macropatches exposed to 0.3, 1, 3, 10, 30, and 100 μM Ca2+i, in absence and presence of 50 mM ethanol. This ethanol concentration corresponds to 3% (v/v) (or 30‰), which is found in circulation after heavy binge drinking [18] and reported to inhibit rat cerebrovascular myocyte BK currents [2]. Then, we obtained G/Gmax-V plots [15], from which we derived the voltage needed to achieve half-maximal conductance (V1/2). At Ca2+i below 20 μM, ethanol caused BK current potentiation (Fig. 1A, top left two panels), which is evident by a reduction in V1/2 (Fig. 1B). Our data also show that as Ca2+i increases, ethanol potentiation diminishes, turning to inhibition of current at Ca2+i≥30 μM (Fig. 1A, bottom left two panels). Thus, ethanol action on cerebrovascular myocyte homomeric cbv1-mediated current is a function of the channel-activating ligand (Ca2+i), resulting in alcohol final opposite effects: potentiation of current at resting, submicromolar Ca2+i vs. inhibition of current at high (>10 μM) Ca2+i.
Fig. 1. β1 subunits determine that ethanol inhibits recombinant BK current at physiological Ca2+ and voltage.
A) BK currents from I/O macropatches recorded at 1 and 30 μM Ca2+i, in absence and presence of 50 mM ethanol, following cbv1±β1 expression in Xenopus oocytes; B) β1 shifts the activation-to-inhibition crossover of ethanol responses to <3 μM Ca2+i. Thus, at physiological Ca2+i found in the cerebral artery myocyte, ethanol causes inhibition of heteromeric cbv1+β1 current (D) (in D, points above and below the dotted line indicate inhibition and potentiation of current, respectively).
The ethanol “opposing” effects on macroscopic current (Fig. 1A) were matched by the results describing drug behavior at single-channel resolution, with the alcohol increasing BK channel steady-state activity (NPo) at 0.3 μM Ca2+i while inhibiting activity at 30 μM Ca2+i (Suppl. Fig.1). These changes occurred without alteration of channel unitary current amplitude, indicating that ethanol action on cbv1 channels is limited to that of a gating modifier, with the alcohol final effect on current being determined by the Ca2+ level that is effectively sensed by the channel-forming subunit itself.
On the other hand, heteromeric cbv1+β1–mediated currents were characterized by amplitudes significantly higher than those of homomeric cbv1, a reflection of the increase in the channel apparent Ca2+ sensitivity introduced by β1 when co-expressed with slo1 [8,19-21]. Remarkably, β1 subunits significantly shifted the “crossover” from ethanol-evoked activation to inhibition towards lower Ca2+i levels, namely, ≤3 μM Ca2+ (Fig. 1C). Therefore, at Ca2+ levels found in the vicinity of the BK channel during cerebral artery myocyte contraction (i.e., 4-30 μM) [22], the presence of β1 subunits determines that ethanol inhibits BK current (Fig. 1D).
KCNMB1 ablation blunts ethanol-induced inhibition of native cerebrovascular BK channels and endothelium-independent vasoconstriction
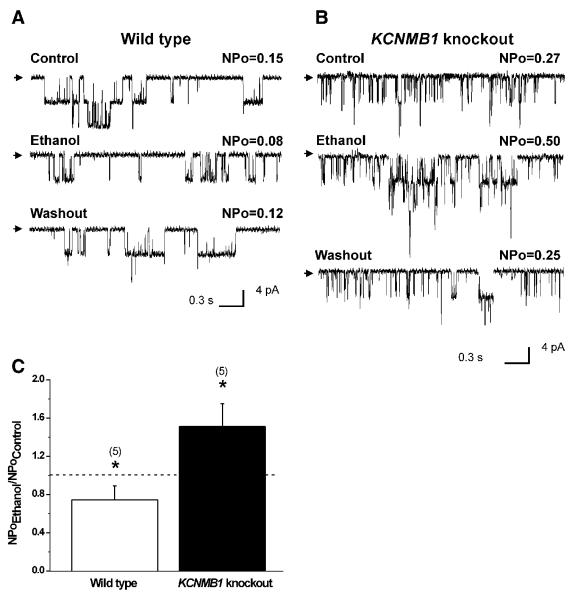
To determine whether β1 subunits determine ethanol final action on BK channels not only when the drug is probed on heterologously expressed recombinant channel subunits but also when the alcohol is evaluated on BK channels in their native environment, we next applied ethanol to native channels in myocytes freshly isolated from wt vs. KCNMB1 knockout mice cerebral arteries. Recordings were obtained in I/O patches to match the configuration used with recombinants, with the membrane potential (−40 to −20mV) and Ca2+i (10 μM) set to levels reached near the native channel during myocyte contraction [22-23]. In myocytes from wt mouse, where native BK are heteromeric α+β1 complexes [9], 50 mM ethanol consistently caused a modest yet significant decrease in NPo (~ −26+/−9%; P<0.05), which was fully reversible (Fig. 2A,C). In contrast, ethanol caused a fully reversible increase in NPo when probed onto KCNMB1 K/O myocytes (≈150% of control) (Figs. 2B,C). Ethanol responses from wt and KCNMB1 K/O mouse match those obtained with recombinant cbv1+β1 and cbv1, respectively (Suppl. Fig. 1), making it unlikely that putative factors compensatory to KCNMB1 deletion could play a significant role in ethanol action on native BK current. More generally, the correspondence between oocyte and myocyte results seems to indicate that possible differences in proteolipid composition or membrane organization between frog oocyte and mouse myocyte membranes do not play a significant role in ethanol action on cerebral artery BK current. Collectively, data from recombinant and native BK channels indicate that BK accessory β1 subunits are responsible for ethanol-induced current inhibition at physiological conditions of membrane voltage and Ca2+ found in the vicinity of the channel during cell contraction.
Fig. 2.
Single-channel recordings demonstrate that 50 mM ethanol inhibits cerebral artery myocyte BK channels in wt (A) while potentiating activity in KCNMB1 knockout mice (B). Channel openings are downward deflections; arrows indicate the baseline. V=−30 mV; Ca2+i=10 μM; C) Averaged ethanol responses. **P<0.05, ethanol vs. control.
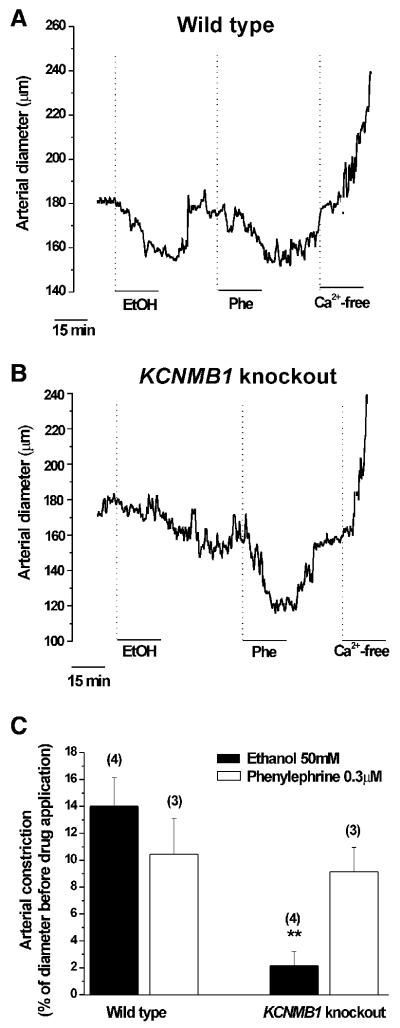
To determine the impact of β1-mediated, ethanol inhibition of BK channels on organ function, we evaluated ethanol action on pressurized, de-endothelized, resistance-size cerebral arteries from KCNMB1 knockout vs. wt mice. The presence/absence of a functional endothelium was determined from the vessel responses to endothelium-dependent and independent vasodilators, as described [2, 24]. As control, we probed the vessels with 3 μM phenylephrine [25], which causes vasoconstriction without involving myocyte BK channels. Phenylephrine caused a similar reversible reduction in arterial diameter (~−10%) in wt and KCNMB1 knockout animals (Fig. 3), indicating that KCNMB1 ablation does not alter fundamental contractile and relaxation processes in the myocyte. In wt animals, 50 mM ethanol caused a robust decrease in diameter (average: −14±2%; Figs. 3A,C). Moreover, iberiotoxin (Ibtx) at concentrations that selectively block BK channels (100 nM) significantly prevented ethanol from causing endothelium-independent constriction (Suppl. Fig. 2). These data and those shown in Fig. 2 indicate that in the mouse species ethanol-induced cerebrovascular constriction is primarily determined by the alcohol inhibition of myocyte BK channels. This ethanol action is similar to that underlying rat cerebral artery constriction [2], buttressing the idea that the primary mechanisms mediating ethanol action on brain arteries are conserved among species.
Fig. 3.
Diameter trace showing that ethanol constricts endothelium-denuded cerebral arteries in wt (A) but not in KCNMB1 knockout mice (B). Phenylephrine (0.3 μM), a BK channel-independent vasoconstrictor, constricts cerebral arteries similarly in wt and KCNMB1 knockout mice; C) Averaged vasoconstrictive responses. **P<0.01, KCNMB1 knockout vs. wt mice.
In contrast to the wt mouse data, ethanol consistently (n=4) failed to constrict cerebral arteries from KCNMB1 knockout mouse (Fig. 3B,C), occasionally evoking up to ~10% increase in diameter, which could be blocked by 100 nM Ibtx (not shown). These results indicate that smooth muscle BK β1 subunits are the primary actuators of alcohol-induced, endothelium-independent cerebrovascular constriction, and buttress the idea that ethanol modulation of myocyte BK current determines ethanol final effect on cerebral artery diameter.
Discussion
Our study demonstrates that knocking out KCNMB1 totally abolishes alcohol-induced constriction of pressurized, resistance-size cerebral arteries, these arteries remaining responsive to vasoconstrictors that do not act via BK inhibition. Consistent with the key role of BK channels in controlling cerebrovascular tone [5] and buttressing their role as relevant targets of ethanol-induced cerebral artery constriction [2], KCNMB1 deletion also suppresses acute ethanol inhibition of native BK channels in arterial myocytes exposed to levels of voltage and Ca2+ reached in the cerebral artery myocyte during contraction. Remarkably, in absence of KCNMB1, myocyte channels were not inhibited but activated by acute ethanol, a response that is characteristic of native BK channels in several neuronal preparations, whether the neuronally-abundant accessory β4 subunit is present or not [26], vascular endothelium and other tissues [17, 27-28] where KCNMB1 expression is negligible [9].
Ethanol-induced cerebrovascular constriction via myocyte BK channels has been previously conceptualized into direct and indirect mechanisms. The former corresponds to a decrease in BK Po [2] resulting from alcohol actions on Ca2+-driven gating of channel-forming slo1 [16]. The latter corresponds to ethanol inhibition of ryanodine receptor-mediated sparks [2], a vasodilatory local Ca2+ signal that activates BK channels in vascular smooth muscle [5]. In vascular myocytes, KCNMB1 ablation has been reported to alter the coupling between Ca2+-sparks and BK currents [29]. Thus, whether ethanol acts via direct or indirect mechanisms to cause cerebrovascular constriction, the central role of the BK β1 subunit as a transducer that couples increases in Ca2+i to BK activation explains the efficacy of KCNMB1 deletion in totally blunting ethanol-induced inhibition of myocyte BK channels and the resulting cerebrovascular constriction. The molecular underpinnings of BK β1 subunit-induced control of ethanol effect on BK channel gating remain to be addressed fully. However, data from recombinant cerebrovascular BK channels (Fig. 1) did demonstrate that the β1-driven switch in the cbv1 channel’s ethanol response from activation to inhibition corresponds to the β1 subunit-induced leftward shift in the Ca2+ sensitivity of the channel complex. Using mouse brain recombinant slo1 (mslo; mbr5) channels, we recently studied the Ca2+-dependence of ethanol effect on homomeric BK channels, and found that such dependence of alcohol action involves both the “Ca2+-bowl” and the high-affinity Regulatory of Conductance for Potassium I (RCKIH) domains for Ca2+i recognition in mslo [16]. The similar Ca2+-dependence of ethanol action on cbv1 (current study) and mslo currents [16] is consistent with the involvement of these slo1 Ca2+-recognition sites in alcohol action, as the primary sequences of these two high affinity, Ca2+i-sensing sites show 100% identity between cbv1 (AAP82453) and mslo (mbr5; AAA39746).
Inhibition of mslo current is caused by ethanol favoring channel dwelling into a Ca2+-driven, low-activity gating mode [16]. Notably, β1 subunit’s most notorious effect on BK current phenotype is a robust increase in the channel apparent Ca2+ sensitivity [19-20]. Conceivably, β1 presence would favor ethanol’s facilitation of channel dwelling into the low activity mode, which would be evident by a shift in the crossover from ethanol-induced activation to inhibition toward lower Ca2+ levels, as shown in Fig. 1. Consistent with this interpretation, 1) the neuronally-abundant β4 subunit, which in contrast to β1 does not modify the channel apparent Ca2+-sensitivity at relevant levels of Ca2+ (~10 μM) [8], does not modify the crossover of ethanol actions on mslo(mbr5) channels [16]; and 2) β1 subunits fail to modulate bovine aortic smooth muscle slo1 (bslo) NPo responses to ethanol when evaluated at 0.3 μM Ca2+ [14], a Ca2+ level at which β1 modification of channel gating does not translate effectively in observable (N)Po modification [21].
Significance and health-relatedness
Present (Fig. 3) and previous data [2] show that ethanol-targeting of myocyte BK currents causes a 10-15% reduction in the diameter of resistance-size, cerebral arteries. This action would lead to ~30% reduction in cerebral blood flow, as diameter and blood flow are related by a third power relationship [30]. It is noteworthy that ethanol has been probed in middle cerebral arteries, which are particularly affected by stroke (www.strokecenter.org), using concentrations obtained in circulation following heavy binge drinking. Binge drinking, the prevalent form of alcohol intake in developed countries, is an independent risk for stroke morbimortality [31]. Our current identification of the molecular target of ethanol-inhibition of myocyte BK current with resulting cerebrovascular constriction brings new insights into ethanol effect on BK channel, and opens a door for novel therapeutic approaches to treat cerebrovascular disease associated with alcohol intake.
Supplementary Material
Acknowledgements
Authors thank Maria Asuncion-Chin for technical assistance. Supported by NIH grants HL77424 and AA11560 (A.M.D.).
Abbreviations
- BK
large conductance, calcium- and voltage-gated potassium
- N
number of channels present in the membrane patch
- Po
channel open probability
- STOC
Spontaneous Transient Outward Current
- PSS
physiological saline solution
- EtOH
ethanol
References
- 1.Altura BM, Altura BT. Alcohol, the cerebral circulation and strokes. Alcohol. 1984;1(4):325–31. doi: 10.1016/0741-8329(84)90056-9. [DOI] [PubMed] [Google Scholar]
- 2.Liu P, Ahmed A, Jaggar J, Dopico A. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci U S A. 2004;101:18217–22. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson P, Cremona A, Paton A, Turner C, Wallace P. The risk of alcohol. Addiction. 1993;88(11):1493–508. doi: 10.1111/j.1360-0443.1993.tb03135.x. [DOI] [PubMed] [Google Scholar]
- 4.Christie IC, Price J, Edwards L, Muldoon M, Meltzer CC, Jennings JR. Alcohol consumption and cerebral blood flow among older adults. Alcohol. 2008;42(4):269–75. doi: 10.1016/j.alcohol.2008.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278(2):C235–56. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 6.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34(3):211–29. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 7.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7(12):921–31. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 8.Brenner R, Jegla T, Wickenden A, Liu Y, Aldrich R. Cloning and characterization of novel large conductance Ca2+-activated potassium channel β subunits hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–61. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 9.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–61. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 10.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97(8):805–12. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Liu P, Crowley J, Asuncion-Chin M, Dopico A. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. Cloning and functional characterization of BKCa channel-forming (rslo cbv) subunits from rat cerebral artery smooth muscle cells. Program No. 960.13. [Google Scholar]
- 12.Dopico A, Anantharam V, Treistman S. Ethanol increases the activity of Ca2+-dependent K+ (mslo) channels: Functional interaction with cytosolic Ca2+ J Pharmacol Exp Ther. 1998;284:258–68. [PubMed] [Google Scholar]
- 13.Dopico AM, Kirber MT, Singer JJ, Walsh JV. Membrane stretch directly activates large conductance Ca2+-activated K+ channels in mesenteric artery smooth muscle cells. Am J Hypetens. 1994;7(1):82–9. doi: 10.1093/ajh/7.1.82. [DOI] [PubMed] [Google Scholar]
- 14.Dopico AM. Ethanol sensitivity of BK(Ca) channels from arterial smooth muscle does not require the presence of the beta 1-subunit. Am J Physiol Cell Physiol. 2003;284(6):C1468–80. doi: 10.1152/ajpcell.00421.2002. [DOI] [PubMed] [Google Scholar]
- 15.Dopico AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca(2+)-activated K+ channels in isolated neurohypophysial terminals. Mol Pharmacol. 1996;49(1):40–8. [PubMed] [Google Scholar]
- 16.Liu J, Vaithianathan T, Manivannan K, Parrill A, Dopico AM. Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol Pharmacol. 2008;74(3):628–40. doi: 10.1124/mol.108.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodie MS, Scholz A, Weiger TM, Dopico AM. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res. 2007;31(10):1625–32. doi: 10.1111/j.1530-0277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 18.Diamond I. In: Cecil Textbook of Medicine. Wyngaarden JB, Smith LH Jr., Plum F, editors. Saunders, PA: 1992. pp. 44–7. [Google Scholar]
- 19.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance Ca2+-activated potassium channels. Neuron. 1995;14(3):645–50. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 20.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energeticsMechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol. 2000;116(3):411–32. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimigean CM, Magleby KL. Functional coupling of the beta(1) subunit to the large conductance Ca(2+)-activated K(+) channel in the absence of Ca(2+)Increased Ca(2+) sensitivity from a Ca(2+)-independent mechanism. J Gen Physiol. 2000;115(6):719–36. doi: 10.1085/jgp.115.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–75. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 23.Knot HJ, Nelson M. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1995;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol. Pharmacol. 2007;72(2):359–69. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- 25.de Chantemèle E.J. Belin, Retailleau K, Pinaud F, Vessières E, Bocquet A, Guihot AL, Lemaire B, Domenga V, Baufreton C, Loufrani L, Joutel A, Henrion D. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler. Thromb. Vasc. Biol. 2008;28(12):2216–24. doi: 10.1161/ATVBAHA.108.171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin GE, Hendrickson LM, Penta KL, Friesen RM, Pietrzykowski AZ, Tapper AR, Treistman SN. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proc. Natl. Acad. Sci. U S A. 2008;105(45):17543–8. doi: 10.1073/pnas.0801068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papassotiriou J, Köhler R, Prenen J, Krause H, Akbar M, Eggermont J, Paul M, Distler A, Nilius B, Hoyer J. Endothelial K(+) channel lacks the Ca(2+) sensitivity-regulating beta subunit. FASEB J. 2000;14(7):885–94. [PubMed] [Google Scholar]
- 28.Kuhlmann CR, Li F, Lüdders DW, Schaefer CA, Most AK, Backenköhler U, Neumann T, Tillmanns H, Waldecker B, Erdogan A, Wiecha J. Dose-dependent activation of Ca2+-activated K+ channels by ethanol contributes to improved endothelial cell functions. Alcohol Clin Exp Res. 2004;28(7):1005–11. doi: 10.1097/01.alc.0000130811.92457.0d. [DOI] [PubMed] [Google Scholar]
- 29.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87(11):E53–60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 30.Gourley J, Heistad D. Characteristics of reactive hyperemia in the cerebral circulation. Am J Physiol. 1984;246:52–8. doi: 10.1152/ajpheart.1984.246.1.H52. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289(5):579–88. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.