Abstract
Much about the etiology, pathophysiology, natural course and optimal treatment of cystic disease of the biliary tree remains under debate. Gastroenterologists, surgeons and radiologists alike still strive to optimize their roles in the management of choledochal cysts. To that end, much has been written about this disease entity, and the purpose of this 3-part review is to organize the available literature and present the various theories currently argued by the experts. In part 1, we discuss the background of the disease, describing the etiology, classification, pathogenesis and malignant potential of choledochal cysts.
Abstract
Dans une large mesure, l’étiologie, la pathophysiologie, le cours naturel et le traitement optimal de la maladie kystique de l’arbre biliaire continuent de faire l’objet de débats. Les gastroentérologues, les chirurgiens et les radiologues cherchent toujours à optimiser leur rôle respectif dans la prise en charge des kystes du cholédoque. C’est pourquoi les chercheurs ont beaucoup écrit à propos de cette entité morbide, et le présent examen en 3 parties a pour objet d’organiser les études publiées et de présenter les diverses théories que font actuellement valoir les experts. Dans la première partie, nous discutons du contexte de cette maladie, en décrivant l’étiologie, la classification, la pathogenèse et le potentiel cancéreux des kystes du cholédoque.
Although cystic disease of the biliary tree has been described since 1723, much about its etiology, pathophysiology, natural course and optimal treatment remains under debate.1 Almost 300 years later, gastroenterologists, surgeons and radiologists alike still strive to optimize their roles in the management of choledochal cysts. To that end, much has been written about this disease entity in multiple attempts to unravel the enigma.
Epidemiology
Choledochal cysts (CCs) are rare medical conditions with an incidence in the western population of 1 in 100 000–150 000 live births, although the incidence has been reported to be as high as 1 in 13 500 births in the United States and 1 in 15 000 births in Australia.2,3 The rate is remarkably higher in Asian populations with a reported incidence of 1 in 1000, and about two-thirds of cases occur in Japan.4 The reason for this Asian preponderance is still unclear. There is also an unexplained female:male preponderance, commonly reported as 4:1 or 3:1.1–4 Distribution of the different types of CCs are as follows: 50%–80% are type I, 2% type II, 1.4%–4.5% type III, 15%–35% type IV and 20% type V.
Classification
Alonso-Lej and colleagues5 proposed the first classification system for CCs in 1959, describing 3 types of bile duct dilation, which has gained wide acceptance. Todani and colleagues6 expanded this system in 1977 to include the occurrence of intrahepatic and multiple cysts, and this modified classification is now most commonly used by clinicians. Type-I cysts have subsequently been subclassified into 3 types. Type IA shows marked cystic dilation of the entire extrahepatic biliary tree, with sparing of the intrahepatic ducts. The cystic duct and the gallbladder arise from the dilated common bile duct (CBD). Type IB is defined by focal, segmental dilation of the extrahepatic bile duct. Although by definition the cyst can arise from anywhere within the extrahepatic biliary tree, it is most commonly distal, with the cystic duct branching off a normal CBD. The biliary tree proximal to the gallbladder is usually normal. Type-IC cysts are smooth fusiform dilations of the entire extrahepatic bile duct, usually extending from the pancreaticobiliary junction to the intrahepatic biliary tree.7
Type-II cysts are discrete diverticuli of the extrahepatic duct with a narrow stalk connection to the CBD. Type-III cysts are also called choledochocele owing to their similarity in morphology, and postulated etiology, to ureteroceles. They consist of dilation of the distal CBD that is confined to the wall of the duodenum, and often bulge into the duodenal lumen. Although the outer lining of the cyst is always lined by duodenal mucosa, the inner lining can either be duodenal or biliary epithelium.8 Sarris and colleagues9 have further subdivided choledochoceles into 5 types based on the cysts’ relations to the ampulla of Vater and the pancreatic duct. Although this system identifies the different configurations in which choledochoceles occur, the presentation and management of all subtypes are identical. Thus further characterizing type-III cysts into their subclassifications has not gained popularity among clinicians.
Type-IV cysts are multiple in nature and are further subdivided based on intrahepatic duct development. Type-IVA cysts are multiple intrahepatic and extrahepatic dilations. The intrahepatic duct dilation can be cystic, fusiform or irregular.7 Todani and colleagues10 have recommended further description of type-IVA cysts as cystic–cystic, cystic–fusiform or fusiform–fusiform to better delineate the nature of their intrahepatic and extrahepatic morphologies. Type-IVB cysts refer to multiple dilations of the extrahepatic biliary tree only, described radiographically as either a “string of beads” or “bunch of grapes” appearance.7
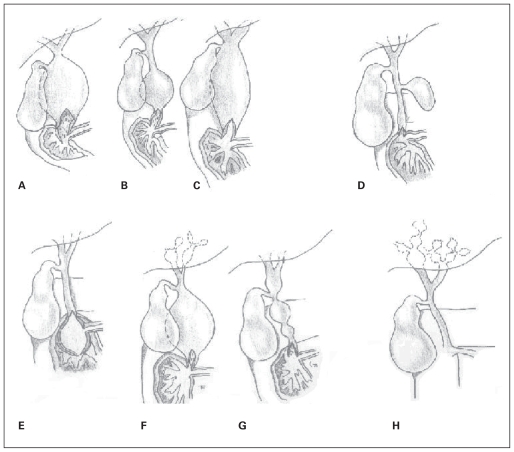
Type-V CCs refer to Caroli disease, also known as communicating cavernous ectasia, which is multiple saccular or cystic dilations of the intrahepatic bile ducts. Simple Caroli disease is isolated biliary dilation, whereas Caroli syndrome is cystic disease associated with congenital hepatic fibrosis.11 Some authors have described Caroli disease with associated extrahepatic CC, but the distinction between this and type-IVA cysts is unclear. Levy and colleagues7 state that saccular dilation of the intrahepatic bile ducts and diffuse fusiform extrahepatic bile duct dilation less than 3 cm marks Caroli disease as separate from type-IVA cysts.12 Figure 1 shows the different types of CCs.
Fig. 1.
Choledochal cyst classification. (A) Type-IA cystic dilation of the extrahepatic duct. (B) Type-IB focal segmental dilation of the extrahepatic duct. (C) Type-IC fusiform dilation of the entire extrahepatic bile duct. (D) Type-II simple diverticula of the common bile duct. (E) Type-III cyst/choledochocele distal intramural dilation of the common bile duct within the duodenal wall. (F) Type-IVA combined intrahepatic and extrahepatic duct dilation. (G) Type-IVB multiple extrahepatic bile duct dilations. (H) Type-V/Caroli disease multiple intrahepatic bile duct dilation.
Lilly and colleagues13 described an entity that they called “form fruste” CCs. Patients with these cysts present with typical symptoms of abdominal pain and obstructive jaundice, without bile duct dilation, but exhibiting an abnormal pancreaticobiliary duct junction. These patients have the same symptoms, histological evidence of inflammation and malignancy potential as those with CCs, and so some authors believe they should be included within the spectrum of disease.14–16
Kaneyama and colleagues17 described 4 patients, an incidence of 1.1% in that series, with a combination of type-I and type-II cysts. Intraoperatively, all 4 patients were morphologically identical, with a fusiform type-IC cyst with a type-II diverticulum arising from the middle portion of the cyst and the cystic duct draining into the right side of the diverticulum. The authors suggested that this may be a new clinical subtype. Four cases have also been reported of diverticular cysts of the cystic duct, which the authors suggested might be another subtype.18 The question arises, however, whether this is just a variant type-II cyst.
Visser and colleagues19 recently challenged the traditional classification system, stating that it grouped together separate disease entities, marked by differing etiologies, natural courses, surgical options and complication profiles. They also contended that type-I and type-IVA cysts are simply variations of the same disease, as in their experience all type-I cysts had some element of intrahepatic dilatation, and the degree of intrahepatic dilation defining one type versus the other was arbitrary. They advocated using descriptive nomenclature instead of the traditional alphanumeric classification, and this has been supported by subsequent authors.20
Pathogenesis
The etiology of CCs is still unclear, although many theories have been put forth. Babbitt’s21 theory of cysts caused by an abnormal pancreaticobiliary duct junction (APBDJ) such that the pancreatic duct and the common bile duct meet outside the ampulla of Vater, thus forming a long common channel, has gained much popularity. This theory postulates that the long common channel allows mixing of the pancreatic and biliary juices, which then activates pancreatic enzymes. These active enzymes cause inflammation and deterioration of the biliary duct wall, leading to dilation.21 Furthermore, greater pressures in the pancreatic duct can further dilate weak-walled cysts.22 Many studies have measured the amylase level in CC bile, which is always higher in patients than in controls.23,24 Furthermore, higher levels of amylase were significantly associated with younger age of symptom onset and higher grade of dysplasia. This lends credence to the theory that pancreaticobiliary reflux not only exists in these patients, but also leads to inflammation and dysplasia.24–28 The authors also postulated that high levels of reflux (and thus amylase) results in earlier symptoms, whereas low levels result in chronic, insidious disease that presents with complications later in life. Although amylase may be a marker for pancreatic reflux, it is more likely that the other enzymes actually cause epithelial breakdown. Therefore further studies have been conducted to assess trypsinogen and phospholipase A2 levels in CC bile, which were also found to be elevated.24,29–35 Interestingly, 61% of the trypsinogen in the bile duct and 65% of the trypsinogen in the gallbladder was activated to trypsin, which can only be accomplished by the presence of enterokinase.24 Although normal epithelium does not produce enterokinase outside of the duodenum, it is secreted by dysplastic biliary epithelium, including the epithelium of patients with APBDJ.29,31 Therefore it is theorized that enterokinase from diseased biliary epithelium activates trypsinogen to trypsin, which in addition to its digestive and irritating effects activates phospholipase A2. Activated phospholipase A2 hydrolyzes epithelial lecithin to lysolecithin, resulting in further inflammation and bile wall breakdown.34,35 Also supporting this theory are animal studies in which both ligation of the common bile duct and surgical creation of APBDJ lead to cystic dilation of the biliary tree in canine and murine models.36 Administration of secretin, which increases pancreatic secretion, has been shown to dilate the CBD and gallbladder in patients with CC, whereas controls showed duodenal filling only. This demonstrates pancreaticobiliary reflux in these patients.37,38 As described previously, the existence of form fruste CC supports the belief that APBDJ is related to the pathogenesis, symptoms and complications of overt CC.8
Skeptics of this theory call it into question because only 50%–80% of CCs are associated with APBDJ, and immature neonatal acini do not make sufficient pancreatic enzymes to explain antenatally diagnosed CC.39,40 Counterarguments by supporters of Babbitt’s theory state that long common channels are arbitrarily defined in terms of length, with wide variation in measured length based on imaging modality and angles.41 In fact, different authors have defined a long common channel as anywhere from 10 to 45 mm. Therefore, APBDJ and a common channel may in fact exist in a much larger proportion of patients with CC, but may be underestimated owing to unrealistic long common channel definitions or inadequate imaging methods. Okada and colleagues35 recommend defining a long common channel as any pancreaticobiliary junction that lies outside of the duodenal wall and thus could result in pancreobiliary reflux and mixing.
There is a theory that CCs are instead purely congenital in nature.42,43 This theory states that embryologic overproliferation of epithelial cells results in dilation during the cannulation period of development. Davenport and Basu44 noted that all neonatal CCs they reviewed were cystic in nature, and pathologically had fewer neurons and ganglions. Their theory was that round cysts are congenital in nature, with distal obstruction due to aganglionosis and proximal dilation (similar to Hirschprung disease). In this case, chronic inflammation and symptoms occur owing to biliary stasis within the dilation rather than pancreatic reflux. They believe that fusiform dilations are acquired lesions due to APBDJ.44 Ohkawa and colleagues45 discovered that elastin fibres in the biliary tree do not develop until 1 year of age. They assert that increased neonatal tendency for round dilation is due to APBDJ and increased pressure within the bile duct, which yields round dilation before 1 year of age with the absence of elastin and fusiform dilation after the age of 1 year.44–46 Contradicting this is Xeijong’s observation that neonatal CCs are round, whereas cysts associated with biliary atresia are fusiform, suggesting that round lesions are congenital and fusiform dilations are due to distal obstruction and thus acquired.10 Other authors speculate that all adult cysts are acquired due to distal obstruction, with longer, narrower stenosis leading to round lesions and shorter wider stenosis leading to fusiform lesions.10,44 The distal obstruction may be due to sphincter of Oddi dysfunction or scarring and stone formation from an APBDJ.47,48 The same theorists contend that type-IVA cysts result from combined distal as well as hilar and intrahepatic stenosis.10
Choledochal cysts are associated with many different developmental anomalies, which have given to rise some additional etiological theories. Such associations include colonic atresia, duodenal atresia, imperforate anus, pancreatic arteriovenous malformation, multiseptate gallbladder, OMENS plus syndrome, ventricular septal defect, aortic hypoplasia, pancreatic divisum, pancreatic aplasia, focal nodular hyperplasia, congenital absence of the portal vein, heterotopic pancreatic tissue and familial adenomatous polyposis.47–63 Embryologically, the pancreas forms when the ventral and dorsal pancreatic buds rotate, fuse and form connections with the biliary tree. Abnormal rotation and fusion may result in APBDJ and CC, pancreatic divisum and pancreatic aplasia.40,50,51,56,59,64 Although the relation with enteric atresia is not clear, hypotheses include common developmental malformations or embryological cyst compression of either the gastrointestinal tract itself or its blood supply.52,53,58,60 Familial adenomatous polyposis is associated with mutations in the adenomatosis polyposis coli tumour suppression gene, which leads to interference with normal biliary cell–cell adherence, and therefore may lead to cystic dilation.49 Reasons for the other associations remain unclear.
The above theories may explain the formation of type-I and type-IV cysts, but some authors contend that the etiologies of the other types are quite distinct. As described previously, type-II cysts are true diverticuli of the common bile duct, with histological evidence of little inflammation and carcinogenic potential. There also have been reports of “diverticular” cysts with no apparent communication with the biliary tree.65 Therefore the question arises as to whether this is truly a cystic dilation caused by the above mechanisms or if it simply reflects a biliary duplication cyst. The etiology of choledochoceles is also not clear. Wheeler66 suggested that obstruction of the ampulla of Vater may result in localized dilation of the distal intramural bile duct. Others believe that increased pressure owing to sphincter of Oddi dysfunction leads to such dilation. As mentioned previously, the inner lining of a choledochocele can be biliary or duodenal epithelium, leading some authors to believe that these reflect either duodenal or biliary duplication cysts.67,68
Type-V CCs, or Caroli disease, is a disease entity quite separate from other CCs, with very different theories of etiology. Embryology of the intrahepatic biliary tree is as follows: a single layer of cells called a ductal plate forms around the portal branches, which then duplicates to form a double layer. Remodelling and selective resorption of the ductal plate commences in the 12th week and progresses to form the large bile ducts at the hilum to the small ductules in the periphery. Arrest of this remodelling results in Caroli disease. When such duct plate malformation occurs at the level of the large ducts, Caroli disease results. Malformation that continues to later stages of development such that the peripheral ductules are affected results in Caroli syndrome, with intrahepatic cysts reflecting large duct arrest and congenital hepatic fibrosis reflecting ductule arrest.7,69 Caroli disease is associated with biliary atresia, which is also thought to be due to duct plate malformation. Caroli disease also is associated with both autosomal recessive and, less commonly, autosomal dominant polycystic kidney disease.70–72 It is postulated that the genetic mutations responsible for the renal malformations also result in hepatic duct plate malformation.71–73
Carcinogenesis
It is well accepted that a CC is a premalignant state, with cancer not only occurring more often in these patients but also 10–15 years earlier.74 The overall risk of cancer has been reported to be 10%–15%, and increases with age.35,75 The risk rises from 2.3% in patients aged 20–30 years to 75% in patients aged 70–80 years, and histopathology shows increasing dysplasia with increasing age.76,77 Distribution of the types of cancer found in patients with CC are as follows: adenocarcinoma 73%–84%, anaplastic carcinoma 10%, undifferentiated cancer 5%–7%, squamous cell carcinoma 5% and other carcinoma 1.5%.78–80 The site of cancer is the extrahepatic bile duct in 50%–62% of patients, gallbladder in 38%–46%, intrahepatic bile ducts in 2.5%, and the liver and pancreas in 0.7% each.77 A review by Todani and colleagues79 found that 68% of cancers were associated with type-I, 5% type-II, 1.6% type-III, 21% type-IV and 6% type-V CCs. Abnormal pancreaticobiliary duct junction has a 16%–55% risk of malignancy with or without bile duct dilation, and cancer has been reported in 12%–39% of form fruste patients.77,81,82 Cancer usually occurs within the cyst in CC and in the gallbladder in form fruste CC, leading some authors to postulate that malignancy occurs at the site of bile stasis, irritation and inflammation (within the dilated cyst in CC and within the gallbladder when no cyst exists).77,82–84 Caroli disease is associated with a cancer risk of 7%–15%.64,69,85 The incidence of malignancy with choledochocele is usually reported as 2.5%, but 1 study reported a 27% incidence.86 Although not typically associated with APBDJ, some authors claim that the choledochocele itself may be a site of pancreatic and biliary juice mixing, as the pancreatic duct and the CBD may both open into the cyst, thus creating the same inflammatory and precancerous milieu as with an APBDJ.86,87 Cancer occurs as a result of chronic inflammation, cell regeneration and DNA breaks, leading to dysplasia. The inflammation can be from either recurrent cholangitis or pancreaticobiliary reflux.75,76,88–90 Chronic inflammation also destroys the protective mucin-producing epithelial cells.75 Furthermore, chronic postobstructive infection by gram-negative bacteria such as Escherichia coli metabolizes bile acids into carcinogens.75 K-ras mutations and overexpression of p53, which have been demonstrated in many other malignancies, are also present in malignant, precancerous dysplastic and chronically inflamed bile ducts in CC and APBDJ. This suggests that pancreatic reflux causes K-ras mutation, cellular atypia, p53 overexpression and carcinogenesis.25,91 Although most CC-associated malignancy presents with abdominal pain, weight loss and obstructive jaundice, many can be asymptomatic, and therefore vigilant surveillance is necessary.74,75,84,90
Conclusion
As described, CCs are part of a complicated disease entity about which many debates still ensue. Although the modified Alonso-Lej classification system is widely used by clinicians now, its validity is questionable. Apart from existing in the same anatomic area, the different subtypes of CCs have quite varied characteristics. As described in this article, and in the upcoming parts 2 and 3 of this review, the subtypes have different etiologies, carcinogenecity, ideal imaging modalities and optimal treatment strategies. Therefore clustering all of them within the same disease modality, based solely on anatomy, seems simplistic. Furthermore, the alphanumeric naming is esoteric, and universal comprehension of the pathology involved will be facilitated by descriptive nomenclature instead. The evidence supporting APBDJ as the common etiology for CCs is impressive, ranging from pathological to biochemical to animal models. The distinction should be made between the term “long common channel” and APBDJ, as pancreaticobiliary fluid mixing and enzyme activation seems to be the factor leading to cystic dilatation, and this may occur in the absence of a common channel that exceeds an arbitrarily defined “normal” common channel length. The patients with CC who fail to demonstrate a long common channel may be such patients who have a normal common channel length yet also have APBDJ and premature pancreaticobiliary mixing. In subsequent articles, we will examine the diagnosis and treatment of biliary cystic disease.
Footnotes
Competing interests: None declared.
Contributors: All authors contributed to study design and writing the article and approved its publication. Dr. Singham acquired and analyzed the data. Drs. Yoshida and Scudamore reviewed the article.
References
- 1.Vater A. Dissertation in auguralis medica poes diss qua Scirrhis viscerum dissert. c. s. ezlerus . Vol. 70. Edinburgh: University Library; 1723. p. 19. [Google Scholar]
- 2.Howard ER. Choledochal cysts. In: Howard ER, editor. Surgery of liver disease in children. Oxford: Butterworth-Heinemann; 1991. pp. 78–90. [Google Scholar]
- 3.Gigot J, Nagorney D, Farnell M, et al. Bile duct cysts: a changing spectrum of disease. J Hepatobiliary Pancreat Surg. 1996;3:405–11. [Google Scholar]
- 4.O’Neill JA., Jr Choledochal cyst. Curr Probl Surg. 1992;29:361–410. doi: 10.1016/0011-3840(92)90025-x. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Lej F, Rever WB, Pessango DJ. Congenital choledochal cyst, with a report of 2, and analysis of 94 cases. Int Abstr Surg. 1959;108:1–30. [PubMed] [Google Scholar]
- 6.Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263–9. doi: 10.1016/0002-9610(77)90359-2. [DOI] [PubMed] [Google Scholar]
- 7.Levy AD, Rohrman CA. Biliary cystic disease. Curr Probl Diagn Radiol. 2003;32:233–63. doi: 10.1016/j.cpradiol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Yamaoka K, Tazawa J, Koizumi K, et al. Choledochocele with obstructive jaundice: a case report and a review of the Japanese literature. J Gastroenterol. 1994;29:661–4. doi: 10.1007/BF02365453. [DOI] [PubMed] [Google Scholar]
- 9.Sarris GE, Tsang D. Choledochocele: case report, literature review and a proposed classification. Surgery. 1989;105:408–14. [PubMed] [Google Scholar]
- 10.Todani T, Watanabe Y, Toki A, et al. Classification of congenital biliary cystic disease: special reference to type Ic and IVA cysts with primary ductal stricture. J Hepatobiliary Pancreat. 2003;10:340–4. doi: 10.1007/s00534-002-0733-7. [DOI] [PubMed] [Google Scholar]
- 11.Keane F, Hadzic N, Wilkinson ML, et al. Neonatal presentation of Caroli’s disease. Arch Dis Child Fetal Neonatal Ed. 1996;77:F145–6. doi: 10.1136/fn.77.2.f145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy AD, Rohrman CA, Murukata LA, et al. Caroli’s disease: radiologic spectrum with pathologic correlation. AJR Am J Roentgenol. 2002;179:1053–7. doi: 10.2214/ajr.179.4.1791053. [DOI] [PubMed] [Google Scholar]
- 13.Lilly JR, Stellin GP, Karrer FM. Forme fruste choledochal cyst. J Pediatr Surg. 1985;20:449–51. doi: 10.1016/s0022-3468(85)80239-6. [DOI] [PubMed] [Google Scholar]
- 14.Sarin YK, Sengar M, Puri AS. Forme fruste choledochal cyst. Indian Pediatr. 2005;42:1153–5. [PubMed] [Google Scholar]
- 15.Shimotakahara A, Yamataka A, Kobayashi H, et al. Forme fruste choledochal cyst. Long-term follow-up with special reference to surgical technique. J Pediatr Surg. 2003;38:1833–6. doi: 10.1016/j.jpedsurg.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Thomas S, Sen S, Zachariah N, et al. Choledochal cyst sans cyst — experience with six “form fruste” cases. Pediatr Surg Int. 2002;18:247–51. doi: 10.1007/s003830100675. [DOI] [PubMed] [Google Scholar]
- 17.Kaneyama K, Yamataka A, Kobayashi H, et al. Mixed type I and II choledochal cyst: A new clinical subtype. Pediatr Surg Int. 2005;21:911–3. doi: 10.1007/s00383-005-1510-x. [DOI] [PubMed] [Google Scholar]
- 18.Loke TKL, Lam SH, Chan CS. Choledochal cyst. An unusual type of cystic dilatation of the cystic duct. AJR Am J Roentgenol. 1999;173:619–20. doi: 10.2214/ajr.173.3.10470889. [DOI] [PubMed] [Google Scholar]
- 19.Visser BC, Suh I, Wy LW, et al. Congenital choledochal cysts in adults. Arch Surg. 2004;139:855–62. doi: 10.1001/archsurg.139.8.855. [DOI] [PubMed] [Google Scholar]
- 20.Wiseman K, Buczkowski AK, Chung SW, et al. Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg. 2005;189:527–31. doi: 10.1016/j.amjsurg.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Babbitt DP. Congenital choledochal cyst: new etiological concept based on anomalous relationships of the common bile duct and pancreatic bulb. Ann Radiol (Paris) 1969;12:231–40. [PubMed] [Google Scholar]
- 22.Han SJ, Hwang EH, Chung KS, et al. Acquired choledochal cyst from anomalous pancreatobiliary duct union. J Pediatr Surg. 1997;32:1735–8. doi: 10.1016/s0022-3468(97)90519-4. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama M, Haradome H, Takahara T, et al. Biliopancreatic reflux via anomalouspancreaticobiliary junction. Surgery. 2004;135:457–9. doi: 10.1016/s0039-6060(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 24.Todani T, Narusue M, Watanabe Y, et al. Management of congenital choledochal cyst with intrahepatic involvement. Ann Surg. 1978;187:272–80. doi: 10.1097/00000658-197803000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong IH, Jung YS, Kim H, et al. Amylase level in extrahepatic bile duct in adult patients withcholedochal cyst plus anomalous pancreaticobiliary ductal union. World J Gastroenterol. 2005;11:1965–70. doi: 10.3748/wjg.v11.i13.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhan JH, Hu XL, Dai GJ, et al. Expressin of p53 and inducible nitric oxide synthase in congenital choledochal cysts. Hepatobiliary Pancreat Dis Int. 2004;3:120–3. [PubMed] [Google Scholar]
- 27.Iwai N, Yanagihara J, Tokiwa K, et al. Congenital choledochal dilatation with emphasis on pathophysiology of the biliary tract. Ann Surg. 1992;215:27–30. doi: 10.1097/00000658-199201000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao X, Li H, Wang O, et al. Abnormal pancreatic isoamylases in the serum of children with choledochal cysts. Eur J Pediatr Surg. 2003;13:26–30. doi: 10.1055/s-2003-38291. [DOI] [PubMed] [Google Scholar]
- 29.Ochiai K, Kaneko K, Kitagawa M, et al. Activated pancreatic enzyme and pancreatic stone protein (PSP/reg) in bile of patients with pancreaticobiliary maljunction/choledochal cysts. Dig Dis Sci. 2004;49:1953–6. doi: 10.1007/s10620-004-9599-7. [DOI] [PubMed] [Google Scholar]
- 30.Narita H, Hashimoto T, Suzuki T, et al. Clinical and experimental studies on the activation mechanism of pancreatic enzymes refluxing into the biliary tract with an anomalous pancreaticobiliary ductal junction. Nippon Shonika Gakkai Zasshi. 1990;26:609–15. [Google Scholar]
- 31.Shimada K, Yanagisawa J, Nakayama F. Increased lysophosphatidylcholine and pancreatic enzyme content in bile of patients with anomalous pancreaticobiliary ductal junction. Hepatology. 1991;13:438–44. [PubMed] [Google Scholar]
- 32.Yagi T, Miyoshi Y, Komi N. An activation mechanism of pancreatic proenzymes refluxing into the biliary tract in APBD patients. Shikoku Igakkai Zasshi. 1993;49:106–13. [Google Scholar]
- 33.Yamashiro Y, Sato M, Shimizu T, et al. How great is the incidence of truly congenital common bile duct dilatation. J Pediatr Surg. 1993;28:622–5. doi: 10.1016/0022-3468(93)90673-9. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno M, Kato T, Koyama K. An analysis of mutagens in the contents of the biliary tract in pancreaticobiliary maljunction. Surg Today. 1996;26:597–602. doi: 10.1007/BF00311663. [DOI] [PubMed] [Google Scholar]
- 35.Okada A, Hasegawa T, Oguchi Y, et al. Recent advances in pathophysiology and surgical treatment of congenital dilatation of the bile duct. J Hepatobiliary Pancreat Surg. 2002;9:342–51. doi: 10.1007/s005340200038. [DOI] [PubMed] [Google Scholar]
- 36.Tajiri T, Tate G, Inagaki T, et al. Mucinous cystadenoma of the pancreas 17 years after excision of gallbladder because of a choledochal cyst. J Gastroenterol. 2004;39:181–7. doi: 10.1007/s00535-003-1271-z. [DOI] [PubMed] [Google Scholar]
- 37.Oguchi Y, Okada A, Nakamura T, et al. Histopathologic studies of congenital dilatation of the bile duct as related to an anomalous junction of the pancreaticobiliary ductal system: clinical and experimental studies. Surgery. 1988;103:168–73. [PubMed] [Google Scholar]
- 38.Matos C, Nicaise N, Deviere J, et al. Choledochal cysts: comparison of findings at MR cholangiopancreatography and endoscopic retrograde cholangiopancreatography in eight patients. Radiology. 1998;209:443–8. doi: 10.1148/radiology.209.2.9807571. [DOI] [PubMed] [Google Scholar]
- 39.Imazu M, Iwai N, Tokiwa K, et al. Factors of biliary carcinogenesis in choledochal cysts. Eur J Pediatr Surg. 2001;11:24–7. doi: 10.1055/s-2001-12190. [DOI] [PubMed] [Google Scholar]
- 40.Hosoki T, Hasuike Y, Michita T, et al. Visualization of pancreaticobiliary reflux in anomalous pancreaticobiliary junction by secretin stimulated dynamic magnetic resonance cholangiopancreatography. Acta Radiol. 2004;45:375–82. doi: 10.1080/02841850410005462. [DOI] [PubMed] [Google Scholar]
- 41.Davenport M, Stringer MD, Howard ER. Biliary amylase and congenital choledochal dilatation. J Pediatr Surg. 1995;130:474–7. doi: 10.1016/0022-3468(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 42.Cheng SP, Yang TL, Jeng KS, et al. Choledochal cyst in adults: aetiological considerations to intrahepatic involvement. ANZ J Surg. 2004;74:964–7. doi: 10.1111/j.1445-1433.2004.03221.x. [DOI] [PubMed] [Google Scholar]
- 43.Yotsuyanagi S. Contribution to aetiology and pathology of idiopathic cystic dilatation of the common bile duct with report of three cases. Gann. 1936;30:601–752. [Google Scholar]
- 44.Davenport M, Basu R. Under pressure: choledochal malformation manometry. J Pediatr Surg. 2005;40:331–5. doi: 10.1016/j.jpedsurg.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Ohkawa H, Sawaguchi S, Yamazaki Y, et al. Experimental analysis of the ill effect of anomalous pancreatobiliary ductal union. J Pediatr Surg. 1982;17:7–13. doi: 10.1016/s0022-3468(82)80316-3. [DOI] [PubMed] [Google Scholar]
- 46.Tao KS, Lu YG, Wang T, et al. Procedure for congenital choledochal cysts and curative effect analysis in adults. Hepatobil Pancr Dis Int. 2002;1:442–5. [PubMed] [Google Scholar]
- 47.Elton E, Hanson BL, Biber BP, et al. Dilated common channel syndrome: endoscopic diagnosis, treatment, and relationship to choledochocele formation. Gastroinest Endosc. 1998;47:471–8. doi: 10.1016/s0016-5107(98)70247-0. [DOI] [PubMed] [Google Scholar]
- 48.Craig AG, Chen LD, Saccone GT, et al. Sphincter of Oddi dysfunction associated with choledochal cyst. J Gastroenterol Hepatol. 2001;16:230–4. doi: 10.1046/j.1440-1746.2001.02365.x. [DOI] [PubMed] [Google Scholar]
- 49.Behrns KE, Shaheen NJ, Grimm IS. Type I choledochal cyst in association with familial adenomatous polyposis. Am J Gastroenterol. 1998;93:1337–8. doi: 10.1111/j.1572-0241.1998.425_f.x. [DOI] [PubMed] [Google Scholar]
- 50.Kinjo T, Aoki H, Sunagawa H, et al. Congenital absence of the portal vein associated with focal nodular hyperplasia of the liver and congenital choledochal cyst: a case report. J Pediatr Surg. 2001;36:622–5. doi: 10.1053/jpsu.2001.22303. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki K, Uchida T, Nakayama H. Heterotopic pancreatic tissue associated with intra- and extrahepatic choledochal cysts. Pathol Int. 1999;49:759–62. doi: 10.1046/j.1440-1827.1999.00930.x. [DOI] [PubMed] [Google Scholar]
- 52.Shih HS, Ko SF, Chaung JH. Is there an association between duodenal atresia and choledochal cyst. J Pediatr Gastroenterol Nutr. 2005;40:378–81. doi: 10.1097/01.mpg.0000148773.80213.03. [DOI] [PubMed] [Google Scholar]
- 53.Arbell D, Orkin B, Naveh Y, et al. Duodenojejunal atresia with absent dorsal mesentery, choledochal cyst, and malrotation in a premature newborn — a case report. J Pediatr Surg. 2006;41:E11–3. doi: 10.1016/j.jpedsurg.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 54.Kapoor S, Mukherjee SB, Paul R, et al. OMENS-plus syndrome. Indian J Pediatr. 2005;72:707–8. doi: 10.1007/BF02724084. [DOI] [PubMed] [Google Scholar]
- 55.Rayamajhi A, Singh R, Prasad R, et al. An unusual case of Type IV A choledochal cyst with subaortic ventricular septal defect. Pediatr Int. 2006;48:187–9. doi: 10.1111/j.1442-200X.2006.02187.x. [DOI] [PubMed] [Google Scholar]
- 56.Li L, Yamataka A, Segawa O, et al. Coexistence of pancreas divisum and septate common channel in a child with choledochal cyst. J Pediatr Gastroenterol Nutr. 2001;32:602–4. doi: 10.1097/00005176-200105000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Dalvi AN, Pramesh CS, Prasanna GS, et al. Incomplete pancreatic divisum with anomalous choledochopancreatic duct junction with choledochal cyst. Arch Surg. 1999;134:1150–2. doi: 10.1001/archsurg.134.10.1150. [DOI] [PubMed] [Google Scholar]
- 58.Komuro H, Takahashi MI, Matoba K, et al. Rare association of severe hypoplasia of the abdominal aorta with imperforate anus, colonic atresia, and choledochal cyst. Pediatr Surg Int. 2006;22:289–92. doi: 10.1007/s00383-005-1604-5. [DOI] [PubMed] [Google Scholar]
- 59.Oyachi N, Ohhama N, Take H, et al. Aplasia of the dorsal pancreas and choledochal cyst. Pediatr Surg Int. 2006;22:557–9. doi: 10.1007/s00383-006-1652-5. [DOI] [PubMed] [Google Scholar]
- 60.Al-Wafi A, Morris-Stiff G, Lari A. Colonic atresia secondary to a choledochal cyst. Pediatr Surg Int. 1998;13:422–3. doi: 10.1007/s003830050355. [DOI] [PubMed] [Google Scholar]
- 61.Mizuno K, Itoh K, Monoe T, et al. Pancreaticobiliary arteriovenous malformation with common bile duct dilation in a patient with hemobilia. J Clin Gastroenterol. 2001;33:61–3. doi: 10.1097/00004836-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Turkvatan A, Erden A, Celik M, et al. Ectopic hypoplastic and multiseptate gallbladder with coexisting choledochal cyst: evaluation with sonography and magnetic resonance cholangiopancreaticography. J Clin Ultrasound. 2006;34:88–91. doi: 10.1002/jcu.20207. [DOI] [PubMed] [Google Scholar]
- 63.Ramnarine IR, Mulpur AK, McMahon MJ, et al. Pleurobiliary fistula from a ruptured choledochal cyst. Eur J Cardiothorac Surg. 2001;19:216–8. doi: 10.1016/s1010-7940(00)00632-1. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka T. Pathogenesis of choledochal cyst. Am J Gastroenterol. 1995;90:685. [PubMed] [Google Scholar]
- 65.Jindal RM, Harris N, McDaniel HM, et al. Presentation of choledochal cysts without intrabiliary communication on endoscopic retrograde cholangiopancreatography. Liver Transpl Surg. 1996;2:468–71. doi: 10.1002/lt.500020610. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler W. An unusual case of obstruction of the common bile duct (choledochocele?) Br J Surg. 1940;27:446–8. [Google Scholar]
- 67.Kagiyama S, Okazaki K, Yamamoto Y. Anatomic variants of choledochocele and manometric measurements of pressure in the cele and the orifice zone. Am J Gastroenterol. 1987;82:641–9. [PubMed] [Google Scholar]
- 68.Venu RP, Geenen JE, Hogan WJ, et al. Role of endoscopic retrograde cholangiopancreatography in the diagnosis and treatment of choledochocele. Gastroenterology. 1984;87:1144–9. [PubMed] [Google Scholar]
- 69.Fulcher AS, Turner MA, Sanyal AJ. Case 38: Caroli disease and renal tubular ectasia. Radiology. 2001;220:720–2. doi: 10.1148/radiol.2203000825. [DOI] [PubMed] [Google Scholar]
- 70.Mousson C, Rabec M, Cercueil JP, et al. Caroli’s disease and autosomal dominant polycystic kidney disease: a rare association. Nephrol Dial Transplant. 1997;12:1481–3. doi: 10.1093/ndt/12.7.1481. [DOI] [PubMed] [Google Scholar]
- 71.Ninan VT, Nampoory MRN, Johny KV, et al. Caroli’s disease of the liver in a renal transplant patient. Nephrol Dial Transplant. 2002;17:1113–5. doi: 10.1093/ndt/17.6.1113. [DOI] [PubMed] [Google Scholar]
- 72.Sgro M, Rosetti S, Barozzino T, et al. Caroli’s disease: prenatal diagnosis, postnatal outcome and genetic analysis. Ultrasound Obstet Gynecol. 2004;23:73–6. doi: 10.1002/uog.943. [DOI] [PubMed] [Google Scholar]
- 73.Aguilera V, Rayón M, Pérez-Aguilar F, et al. Caroli’s syndrome and imaging: report of a case. Rev Esp Enferm Dig. 2004;96:74–6. doi: 10.4321/s1130-01082004000100009. [DOI] [PubMed] [Google Scholar]
- 74.Tsuchiya R, Harada N, Ito T, et al. Malignant tumors in choledochal cysts. Ann Surg. 1977;186:22–8. doi: 10.1097/00000658-197707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bismuth H, Krissat J. Choledochal cystic malignancies. Ann Oncol. 1999;10(Suppl 4):94–8. [PubMed] [Google Scholar]
- 76.Benjamin IS. Biliary cystic disease: the risk of cancer. J Hepatobiliary Pancreat Surg. 2003;10:335–9. doi: 10.1007/s00534-002-0696-8. [DOI] [PubMed] [Google Scholar]
- 77.Todani T, Watanabe Y, Fujii M, et al. Carcinoma arising from the bile duct in choledochal cyst and anomalous arrangement of the pancreatobiliary ductal union. Biliary Tract Pancreas. 1985;6:525–35. [Google Scholar]
- 78.Todani T, Watanabe Y, Tokai A, et al. Carcinoma related to choledochal cysts with internal drainage operations. Surg Gynecol Obstet. 1987;164:61–4. [PubMed] [Google Scholar]
- 79.Todani T, Tabuchi K, Watanabe Y, et al. Carcinoma arising in the wall of congenital bile duct cysts. Cancer. 1979;44:1134–41. doi: 10.1002/1097-0142(197909)44:3<1134::aid-cncr2820440350>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 80.Fieber SS, Nance FC. Choledochal cyst and neoplasm: a comprehensive review of 106 cases and presentation of two original cases. Am Surg. 1997;63:982–7. [PubMed] [Google Scholar]
- 81.Okamura K, Hayakawa H, Kuze M. Triple carcinomas of the biliary tract associated with congenital choledochal dilatation and pancreaticobiliary maljunction. J Gastroenterol. 2000;35:465–71. doi: 10.1007/s005350070093. [DOI] [PubMed] [Google Scholar]
- 82.Miyano G, Yamataka A, Shimotakahara A. Cholecystectomy alone is inadequate for treatment of form fruste choledochal cyst: evidence from a rare but important case. Pediatr Surg Int. 2005;21:61–3. doi: 10.1007/s00383-004-1266-8. [DOI] [PubMed] [Google Scholar]
- 83.Sugiyama M, Haradome H, Takahara T, et al. Anomalous pancreaticobiliary junction shown on multidetector CT. AJR Am J Roentgenol. 2003;180:173–5. doi: 10.2214/ajr.180.1.1800173. [DOI] [PubMed] [Google Scholar]
- 84.Franko J, Nussbaum ML, Morris JB. Choledochal cyst cholangiocarcinoma arising from adenoma: case report and a review of the literature. Curr Surg. 2006;63:281–4. doi: 10.1016/j.cursur.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Totkas S, Hohenberger P. Cholangiocellular carcinoma associated with segmental Caroli’s disease. Eur J Surg Oncol. 2000;26:520–1. doi: 10.1053/ejso.1999.0936. [DOI] [PubMed] [Google Scholar]
- 86.Ohtsuka T, Inoue K, Ohuchida J. Carcinoma arising in choledochocele. Endoscopy. 2001;33:614–9. doi: 10.1055/s-2001-15324. [DOI] [PubMed] [Google Scholar]
- 87.Horaguchi J, Fujita N, Kobayashi G. Clinical study of choledochocele: Is it a risk factor for biliary malignancies. J Gastroenterol. 2005;40:396–401. doi: 10.1007/s00535-005-1554-7. [DOI] [PubMed] [Google Scholar]
- 88.Lipsett PA, Pitt HA. Surgical treatment of choledochal cysts. J Hepatobiliary Pancreat Surg. 2003;10:352–9. doi: 10.1007/s00534-002-0797-4. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe Y, Toki A, Todani T. Bile duct cancer developed after cyst excision for choledochal cyst. J Hepatobiliary Pancreat Surg. 1999;6:207–12. doi: 10.1007/s005340050108. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida H, Itai Y, Minami M. Biliary malignancies occurring in choledochal cysts. Radiology. 1989;173:389–92. doi: 10.1148/radiology.173.2.2678253. [DOI] [PubMed] [Google Scholar]
- 91.Iwasaki Y, Shimoda M, Furihata T, et al. Biliary papillomatosis arising in a congenital choledochal cyst: report of a case. Surg Today. 2002;32:1019–102. doi: 10.1007/s005950200206. [DOI] [PubMed] [Google Scholar]