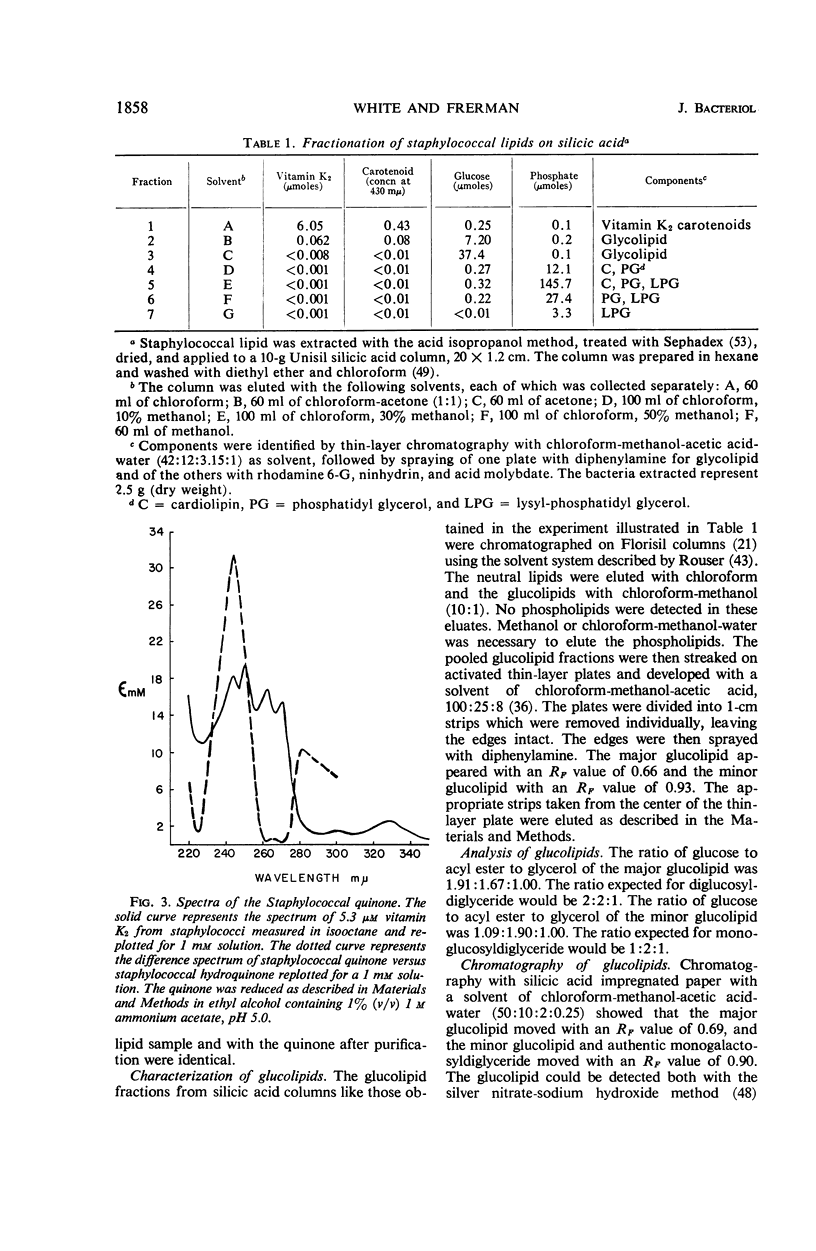
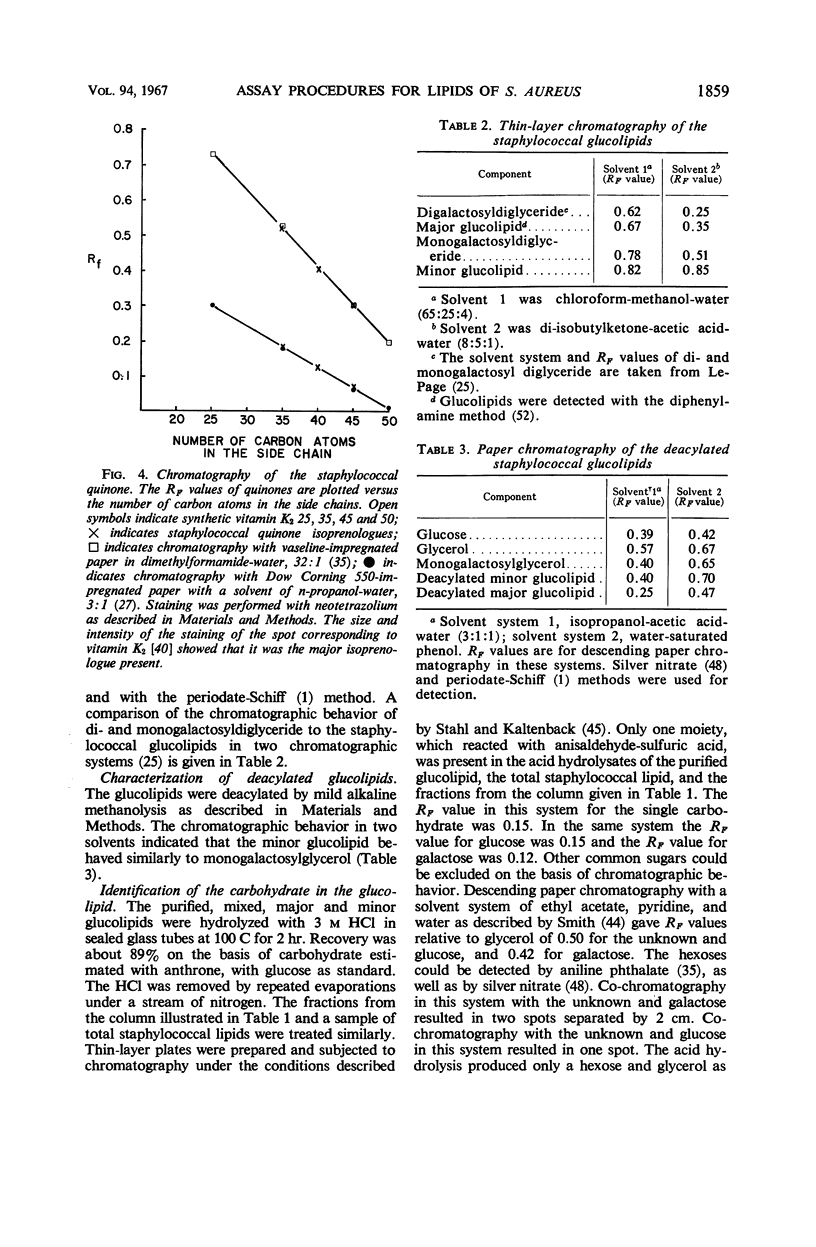
Abstract
Satisfactory extraction and assay procedures have been developed for the lipids of Staphylococcus aureus. The following lipids have been characterized in detail: the vitamin K2, which is shown to exist as isoprenologues with side chains of 35, 40, and 45 carbon atoms; monoglucosyldiglyceride and diglucosyldiglyceride, which account for all the carbohydrate in the lipid extracts; the lysyl ester of phosphatidyl glycerol, phosphatidyl glycerol, and cardiolipin, which account for 98% of the phosphate in the lipid extract. The extraction procedure removes 98% of the total bacterial fatty acids. Acidification of the medium before harvest and refluxing in isopropanol are critical in the extraction procedure for the maximal recovery of lysyl-phosphatidyl glycerol and the glucolipids. The lipids have been shown to be a part of the same membrane as the respiratory pigments.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bonsen P. P., de Haas G. H., van Deenen L. L. Synthetic and structural investigations on 3-phosphatidyl-1'-(3'-O-L-lysyl)glycerol. Biochemistry. 1967 Apr;6(4):1114–1120. doi: 10.1021/bi00856a021. [DOI] [PubMed] [Google Scholar]
- COLLINS F. M., LASCELLES J. The effect of growth conditions on oxidative and dehydrogenase activity in Staphylococcus aureus. J Gen Microbiol. 1962 Nov;29:531–535. doi: 10.1099/00221287-29-3-531. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. The measurement of 32P labelling of individual kephalins and lecithin in a small sample of tissue. Biochim Biophys Acta. 1954 Jul;14(3):374–379. doi: 10.1016/0006-3002(54)90195-x. [DOI] [PubMed] [Google Scholar]
- FERRARI R. A., BENSON A. A. The path of carbon in photosynthesis of the lipids. Arch Biochem Biophys. 1961 May;93:185–192. doi: 10.1016/0003-9861(61)90248-x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Frerman F. E., White D. C. Membrane lipid changes during formation of a functional electron transport system in Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1868–1874. doi: 10.1128/jb.94.6.1868-1874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. THE INCORPORATION OF GLYCEROL AND LYSINE INTO THE LIPID FRACTION OF STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Feb;94:390–400. doi: 10.1042/bj0940390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER J. F., LASCELLES J. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J Gen Microbiol. 1962 Sep;29:157–164. doi: 10.1099/00221287-29-1-157. [DOI] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- HOUTSMULLER U. M., VAN DEENENL ON THE ACCUMULATION OF AMINO ACID DERIVATIVES OF PHOSPHATIDYLGLYCEROL IN BACTERIA. Biochim Biophys Acta. 1964 Feb 24;84:96–98. doi: 10.1016/0926-6542(64)90106-4. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- LASCELLES J., WOODS D. D. The synthesis of folic acid by Bacterium coll and Staphylococcus aureus and its inhibition by sulphonamides. Br J Exp Pathol. 1952 Jun;33(3):288–303. [PMC free article] [PubMed] [Google Scholar]
- LEA C. H., RHODES D. N. The ninhydrin reaction of unhydrolysed phospholipids. Biochim Biophys Acta. 1955 Jul;17(3):416–423. doi: 10.1016/0006-3002(55)90391-7. [DOI] [PubMed] [Google Scholar]
- LEPAGE M. THE SEPARATION AND IDENTIFICATION OF PLANT PHOSPHOLIPIDS AND GLYCOLIPIDS BY TWO-DIMENSIONAL THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Jan;13:99–103. doi: 10.1016/s0021-9673(01)95078-2. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., RAMASARMA T. Chromatography of the coenzyme Q family of compounds on silicone-impregnated paper. J Biol Chem. 1959 Mar;234(3):672–676. [PubMed] [Google Scholar]
- LESTER R. L., WHITE D. C., SMITH S. L. THE 2-DESMETHYL VITAMIN K2'S. A NEW GROUP OF NAPHTHOQUINONES ISOLATED FROM HEMOPHILUS PARAINFLUENZAE. Biochemistry. 1964 Jul;3:949–954. doi: 10.1021/bi00895a018. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lennarz W. J., Nesbitt J. A., 3rd, Reiss J. The participation of sRNA in the enzymatic synthesis of O-L-lysyl phosphatidylgylcerol in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):934–941. doi: 10.1073/pnas.55.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVAK M. COLORIMETRIC ULTRAMICRO METHOD FOR THE DETERMINATION OF FREE FATTY ACIDS. J Lipid Res. 1965 Jul;6:431–433. [PubMed] [Google Scholar]
- PAGE A. C., Jr, GALE P., WALLICK H., WALTON R. B., McDANIEL L. E., WOODRUFF H. B., FOLKERS K. Coenzyme Q. 17. Isolation of coenzyme Q10 from bacterial fermentation. Arch Biochem Biophys. 1960 Aug;89:318–321. doi: 10.1016/0003-9861(60)90062-x. [DOI] [PubMed] [Google Scholar]
- PELICK N., WILSON T. L., MILLER M. E., ANGELONI F. M., STEIM J. M. SOME PRACTICAL ASPECTS OF THIN-LAYER CHROMATOGRAPHY OF LIPIDS. J Am Oil Chem Soc. 1965 May;42:393–399. doi: 10.1007/BF02635574. [DOI] [PubMed] [Google Scholar]
- POLONOVSKI J., WALD R., PAYSANT-DIAMENT M. [The lipids of Staphylococcus aureus]. Ann Inst Pasteur (Paris) 1962 Jul;103:32–42. [PubMed] [Google Scholar]
- RADIN N. S., BROWN J. R., LAVIN F. B. The preparative isolation of cerebrosides. J Biol Chem. 1956 Apr;219(2):977–983. [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- RENKONEN O. Determination of glycerol in phosphatides. Biochim Biophys Acta. 1962 Jan 29;56:367–369. doi: 10.1016/0006-3002(62)90580-2. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. The rate of formation of hyaluronidase, coagulase and total extracellular protein by strains of Staphylococcus aureus. J Gen Microbiol. 1954 Apr;10(2):209–220. doi: 10.1099/00221287-10-2-209. [DOI] [PubMed] [Google Scholar]
- STRASTERS K. C., WINKLER K. C. CARBOHYDRATE METABOLISM OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Nov;33:213–229. doi: 10.1099/00221287-33-2-213. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. Detection of phosphate esters on paper chromatograms. Nature. 1953 Mar 21;171(4351):529–530. doi: 10.1038/171529a0. [DOI] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- WHITE D. C. Respiratory systems in the hemin-requiring Haemophilus species. J Bacteriol. 1963 Jan;85:84–96. doi: 10.1128/jb.85.1.84-96.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. THE FUNCTION OF 2-DEMETHYL VITAMIN K2 IN THE ELECTRON TRANSPORT SYSTEM OF HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1965 Mar;240:1387–1394. [PubMed] [Google Scholar]
- White D. C. The obligatory involvement of the electron transport system in the catabolic metabolism of Haemophilus parainfluenzae. Antonie Van Leeuwenhoek. 1966;32(2):139–158. doi: 10.1007/BF02097454. [DOI] [PubMed] [Google Scholar]


