Abstract
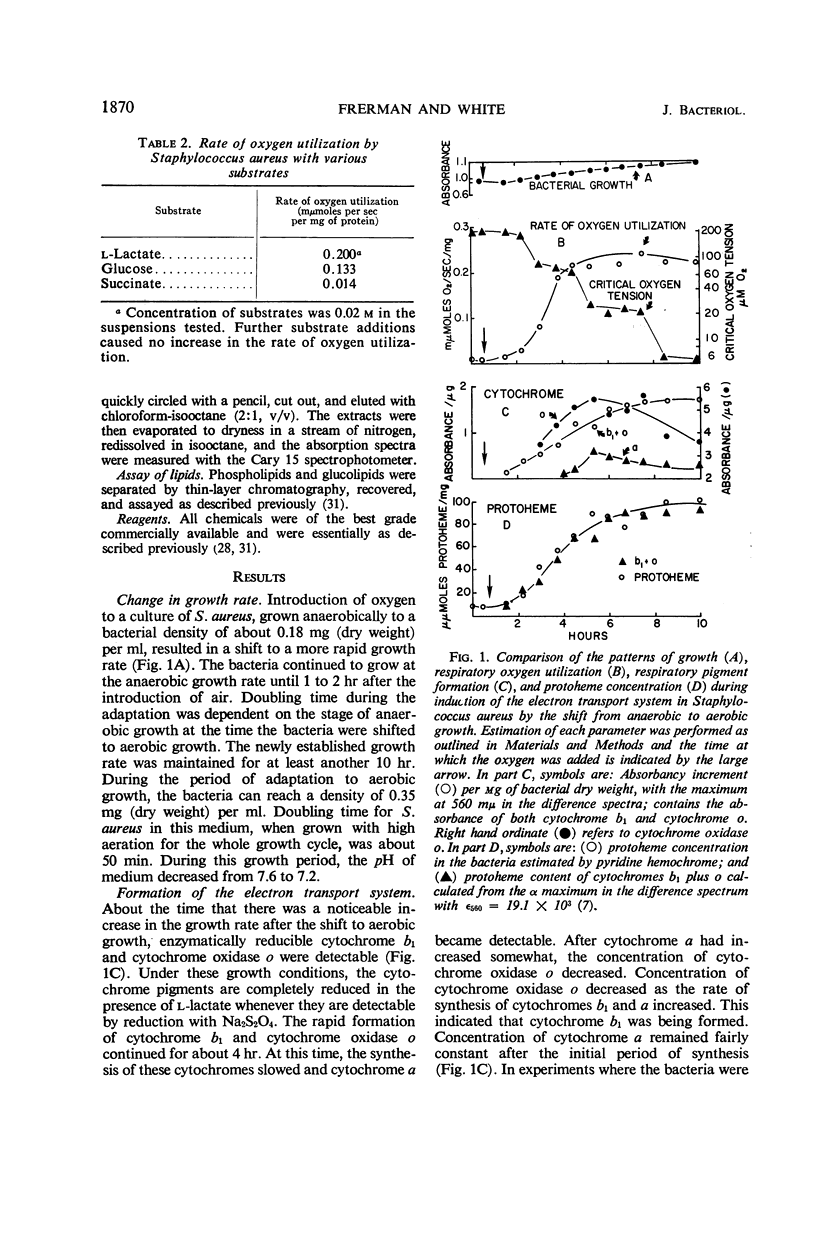
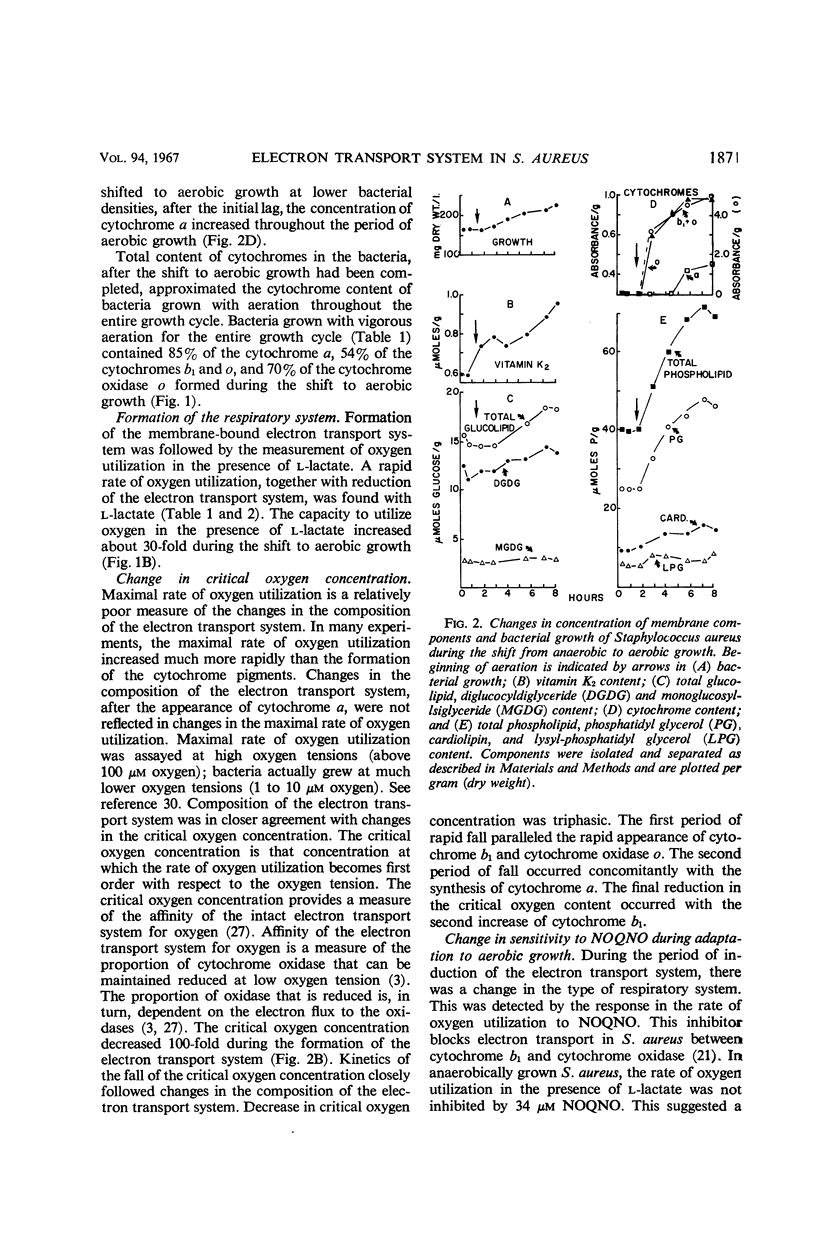
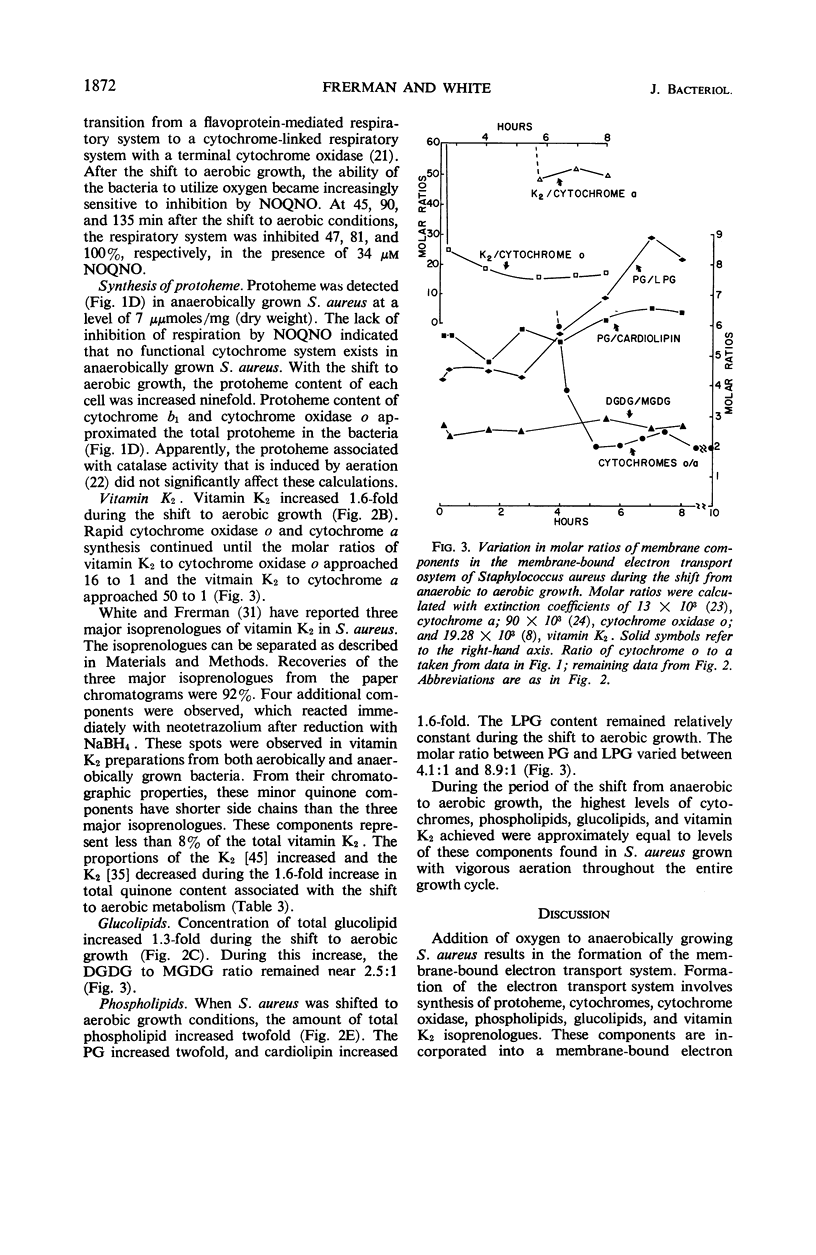
Addition of oxygen to a culture of anaerobically growing Staphylococcus aureus results in the formation of a membrane-bound, functional electron transport system. With the shift to aerobic growth, there is at least a 15-fold increase in cytochrome a and at least a 55-fold increase in cytochrome oxidase o. At the completion of the shift to aerobic growth, the cytochrome levels equal those found in bacteria grown with aeration throughout the entire growth cycle. Cytochromes b1 and o are formed first. Their synthesis slows when cytochrome a becomes detectable. Concentrations of cytochromes b1 and sometimes cytochrome a increase late in the adaptive period. Concomitant with this is a decrease in the oxygen tension at which the rate of oxygen utilization becomes dependent on the oxygen concentration. During the shift to aerobic growth, the protoheme content increases ninefold, and all the protoheme can be accounted for in enzymatically reducible cytochrome b1 and cytochrome oxidase o. Protoheme, but not a functional cytochrome system, is synthesized by anaerobically growing S. aureus. Heme a appears only after a period of aerobic growth. During the shift to aerobic growth, there is a 1.6-fold increase in the vitamin K2 content, with an alteration in the ratios of the 35 and 45 carbon side chain isoprenologues. A twofold increase in phosphatidyl glycerol and a 1.6-fold increase in cardiolipin occur with the shift to aerobic growth. Lysyl-phosphtidyl glycerol remains essentially constant in this period. Concentrations of mono- and diglucosyl diglycerides increase coordinately 1.3-fold during the shift to aerobic growth at a 2.5 to 1 m ratio.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP D. H., PANDYA K. P., KING H. K. Ubiquinone and vitamin K in bacteria. Biochem J. 1962 Jun;83:606–614. doi: 10.1042/bj0830606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Shaw N., Baddiley J. Bacterial glycolipids. Glycosyl diglycerides in gram-positive bacteria. Biochem J. 1966 Jun;99(3):546–549. doi: 10.1042/bj0990546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Cellular oxygen requirements. Fed Proc. 1957 Sep;16(3):671–680. [PubMed] [Google Scholar]
- COLLINS F. M., LASCELLES J. The effect of growth conditions on oxidative and dehydrogenase activity in Staphylococcus aureus. J Gen Microbiol. 1962 Nov;29:531–535. doi: 10.1099/00221287-29-3-531. [DOI] [PubMed] [Google Scholar]
- ENGLESBERG E., GIBOR A., LEVY J. B. Adaptive control of terminal respiration in Pasteurella pestis. J Bacteriol. 1954 Aug;68(2):146–151. doi: 10.1128/jb.68.2.146-151.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Perdue J. F. Membranes as expressions of repeating units. Proc Natl Acad Sci U S A. 1966 May;55(5):1295–1302. doi: 10.1073/pnas.55.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS N. J., CONTI S. F. EFFECT OF HEMIN ON THE FORMATION OF THE CYTOCHROME SYSTEM OF ANAEROBICALLY GROWN STAPHYLOCOCCUS EPIDERMIDIS. J Bacteriol. 1965 Mar;89:675–679. doi: 10.1128/jb.89.3.675-679.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS N. J., JOHANTGES J., DEIBEL R. H. EFFECT OF ANAEROBIC GROWTH ON NITRATE REDUCTION BY STAPHYLOCOCCUS EPIDERMIDIS. J Bacteriol. 1963 Apr;85:782–787. doi: 10.1128/jb.85.4.782-787.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N. J., Maclosky E. R., Conti S. F. Effects of oxygen and heme on the development of a microbial respiratory system. J Bacteriol. 1967 Jan;93(1):278–285. doi: 10.1128/jb.93.1.278-285.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASCELLES J., SZILAGYI J. F. PHOSPHOLIPID SYNTHESIS BY RHODOPSEUDOMONAS SPHEROIDES IN RELATION TO THE FORMATION OF PHOTOSYNTHETIC PIGMENTS. J Gen Microbiol. 1965 Jan;38:55–64. doi: 10.1099/00221287-38-1-55. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., WHITE D. C., SMITH S. L. THE 2-DESMETHYL VITAMIN K2'S. A NEW GROUP OF NAPHTHOQUINONES ISOLATED FROM HEMOPHILUS PARAINFLUENZAE. Biochemistry. 1964 Jul;3:949–954. doi: 10.1021/bi00895a018. [DOI] [PubMed] [Google Scholar]
- MOSS F. Adaptation of the cytochromes of Aerobacter aerogenes in response to environmental oxygen tension. Aust J Exp Biol Med Sci. 1956 Oct;34(5):395–405. doi: 10.1038/icb.1956.48. [DOI] [PubMed] [Google Scholar]
- POLONOVSKI J., WALD R., PAYSANT-DIAMENT M. [The lipids of Staphylococcus aureus]. Ann Inst Pasteur (Paris) 1962 Jul;103:32–42. [PubMed] [Google Scholar]
- POLONOVSKI J., WALD R., PETEK F. ISOLEMENT DES GLUCOLIPIDES DE STAPHYLOCOCCUS AUREUS ET IDENTIFICATION DU DIGLUCOSYLDIGLYC'ERIDE. Bull Soc Chim Biol (Paris) 1965;47:409–416. [PubMed] [Google Scholar]
- SCHAEFFER P. Recherches sur le métabolisme bactérien des cytochromes et des porphyrines. I. Disparition partielle des cytochromes par culture anaérobie chez certaines bactéries aérobies facultatives. Biochim Biophys Acta. 1952 Sep;9(3):261–270. doi: 10.1016/0006-3002(52)90160-1. [DOI] [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H. Molecular proportion of the fixed cytochrome components of the respiratory chain of Keilin-Hartree particles and beef heart mitochondria. Biochim Biophys Acta. 1966 Jan 11;113(1):175–178. doi: 10.1016/s0926-6593(66)80132-7. [DOI] [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. FACTORS AFFECTING THE AFFINITY FOR OXYGEN OF CYTOCHROME OXIDASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1963 Nov;238:3757–3761. [PubMed] [Google Scholar]
- WHITE D. C. Respiratory systems in the hemin-requiring Haemophilus species. J Bacteriol. 1963 Jan;85:84–96. doi: 10.1128/jb.85.1.84-96.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. SYNTHESIS OF 2-DEMETHYL VITAMIN K2 AND THE CYTOCHROME SYSTEM IN HAEMOPHILUS. J Bacteriol. 1965 Feb;89:299–305. doi: 10.1128/jb.89.2.299-305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. The purification and properties of cytochrome o from Vitreoscilla. J Biol Chem. 1966 Jul 25;241(14):3308–3315. [PubMed] [Google Scholar]
- White D. C. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. J Bacteriol. 1967 Feb;93(2):567–573. doi: 10.1128/jb.93.2.567-573.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. The obligatory involvement of the electron transport system in the catabolic metabolism of Haemophilus parainfluenzae. Antonie Van Leeuwenhoek. 1966;32(2):139–158. doi: 10.1007/BF02097454. [DOI] [PubMed] [Google Scholar]


