Abstract
Learning impairments and the instability of memory are defining characteristics of cognitive aging. However, it is unclear if deficits in the expression of new memories reflect an accelerated decay of the target memory or a consequence of inefficient learning. Here, aged mice (19–21-mo old) exhibited acquisition deficits (relative to 3–5-mo old mice) on three learning tasks, although these deficits were overcome with additional training. When tested after a 30-d retention interval, the performance of aged animals was impaired if initial learning had been incomplete. However, if trained to equivalent levels of competence, aged animals exhibited no retention deficits relative to their young counterparts. These results suggest that age-related “memory” impairments can be overcome through a more effective learning regimen.
Aging is associated with broad deficits in the acquisition of new knowledge (Matzel et al. 2008; see, for review, Gallagher and Rapp 1997; Rosenzweig and Barnes 2003), as well as impairments in the retrieval of both old and newly acquired information. While it is clear that old memories (i.e., ones obtained prior to age-related cognitive declines) do in fact become less stable with age (Gallagher 1997), it is less clear whether newly attained memories are inherently less stable in aged animals, or whether age-related memory deficits reflect a secondary consequence of inefficient learning.
The majority of published data regarding cognitive aging describes impairments of animals' learning abilities (Gage and Dunnett 1984; Markowska et al. 1994; Meliska et al. 1997; Nalbantoglu et al. 1997; Vogel et al. 2002; Matzel et al. 2008), although a smaller percentage of these studies also report animals' performances after long retention intervals. Of those studies that report retention deficits, in most of those studies the initial learning upon which the long-term memory was based was impaired relative to young animals (e.g., Barnes and McNaughton 1985; Kinney et al. 2001a,b; Gould and Feiro 2005). Interestingly, in those few studies in which initial learning was equated across young and old animals, including studies of spatial water maze performance and appetitive instrumental responding, no retention deficits were observed, even after retention intervals as long as 21 d (Soffie and Lejeune 1991; Martinez-Serrano et al. 1996; Port et al. 1996).
Although suggestive, the above experiments were not systematically designed to assess the stability of memory in aged animals as a function of the level of initial learning. In the present study, young (3–5 mo) and old (19–21 mo) male Balb/C mice were trained in three learning tasks (a spatial water maze, an egocentric Lashley III maze, and a three-choice odor discrimination). When trained to pre-asymptotic levels, aged animals exhibited both learning and retention deficits (assessed 30 d after initial training). However, when aged animals were trained to levels of competence comparable to their young counterparts, both young and old animals exhibited statistically indistinguishable levels of retention.
Sixty Balb/C mice arrived in our laboratory at 2.5 mo (n = 30) or 18.5 mo (n = 30) of age. Each age category was divided into two groups of 15, one of which would receive subasymptotic training on each of the three learning tasks, and one of which would receive extended training on those tasks. Two aged mice became ill during the course of testing and were excluded from all analyses. Young mice ranged from 19.8 to 29.1 g, and aged mice from 26.2 to 37.3 g. Maintenance, food deprivation, and training conditions were as previously described (Matzel et al. 2006, 2003). Behavioral testing of young and aged mice was concluded at ∼5 and 21 mo of age, respectively.
All animals were tested in three independent learning tasks. Briefly, the spatial water maze encourages mice to integrate spatial information to efficiently escape from a pool of water. In odor discrimination, animals must use a target odor (from a group of three odors) to guide their search for food. In the Lashley III maze, animals learn an egocentric sequence of turns to obtain a food reinforcer. Training on each task required 2–10 d (depending on the task and the level of training), and animals received four days of rest between tasks. A retention test was administered 30 d after the last training trial of each task.
A Lashley III maze was constructed from black Plexiglas. A 3-cm-diameter white disk was located in the center of the goal box, and a 45 mg Bio-Serv food pellet (dustless rodent grain) was placed at the center of the disk and served as the reinforcer. Food-deprived animals received a day of acclimation to the maze, followed by either one or two days of training (four trials/day). On the day prior to the acclimation, animals received three Bio-Serv pellets in their home cage (thus mitigating any neophobia to the food on subsequent exposures). On the acclimation day, each mouse was confined in each of the first three alleys of the maze for 4 min, and in the final alley (containing the goal box) for 6 min. On this acclimation day, three Bio-Serv pellets were placed in the goal box. On the subsequent training day(s), each animal was placed in the start box and allowed to freely navigate the maze, during which time the number of errors to reach the goal box were recorded. (An error was constituted by a turn in the wrong direction or a retracing of a previously completed path.) Upon consuming the food pellet, the animal was returned to its home cage for a 25-min intertrial interval (ITI). All animals completed four trials during the first training day. Half of those animals then received an additional four training trials on the following day. Twenty-nine days after the last training trial, all animals received three Bio-Serv pellets in their home cages, and on the subsequent day were again tested in the maze.
For the water maze, a round pool (140 cm diameter, 56 cm deep) was filled to within 20 cm of the top with water that was clouded with a water soluble black paint. A hidden 14-cm-diameter black platform was located in a fixed position 1 cm below the surface of the water. The pool was enclosed by a ceiling high black curtain on which five different light patterns (which served as spatial cues) were fixed at various positions. These light cues provided the only illumination of the maze, which was 60 Lux at the water's surface.
On the day prior to training, each animal was confined for 360 sec to the platform by a clear Plexiglas cylinder that fits around the platform. For either one or two training days (six trials Day 1, five trials Day 2), the animals were started from one of three positions, such that no consecutive trials started from the same position. After locating the platform or swimming for 90 sec, the animals were left or placed on the platform for 10 sec, after which they were placed in a holding box (for 12 min) before the start of the next trial. After the sixth or 11th training trial, animals were returned to their home cages for 3 h, and were then administered a 30-sec “probe” test in which the escape platform was removed from the maze and the time spent searching in the target quadrant was recorded. One hour later each animal received an additional training trial (intended to re-establish the search strategy employed by the animal prior to the probe test). Animals were then returned to their home cages, where a 30-d retention interval began.
In odor discrimination, mice navigate through a field using unique odors to guide them. The animals learn to choose the food cup that contains the target smell when given three choices. The food cup locations are rearranged on each trial, but the accessible food is always marked by the same target odor (in this case mint). The chamber consisted of a black Plexiglas 60-cm-square field with 30-cm-high walls, which was located in a dimly lit room with high ventilation. A food cup was located in three corners. The target cup had accessible food (30 mg of chocolate puffed rice), while the remaining cups contained food that could not be accessed. A cotton tipped swab (2-cm long) was loaded with 25 μL of lemon-, mint- (the target odor), or almond-flavored extract and extended vertically from the back corner of each cup.
Each animal had one day of acclimation and one day of training (consisting of four training trials). (In this task, both young and old animals reached asymptotic levels of performance [near errorless] within four training trials.) On Day 1 (adaptation), each mouse was placed in the box for 20 min with no food cups present. On the subsequent training day, a food cup was placed in three corners of the field, but only the cup associated with the mint odor contained accessible food. Each animal received four trials in which they were placed in the corner of the training chamber that did not contain a food cup. A trial continued until the animal obtained the food from the target location, at which time the animal was returned to its home cage to begin a 20 min ITI. At the end of each trial the food cups were rearranged, but mint always remained as the target odor. For each trial, the number of errors (contact with or sniffing within 2 cm of an incorrect food cup) was recorded. After the fourth training trial, the animal was returned to its home cage for a 30-d retention interval. On the 29th retention day, all animals received three pieces of chocolate flavored rice in their home cages, and on the subsequent day were again tested as in original training.
Lashley III maze
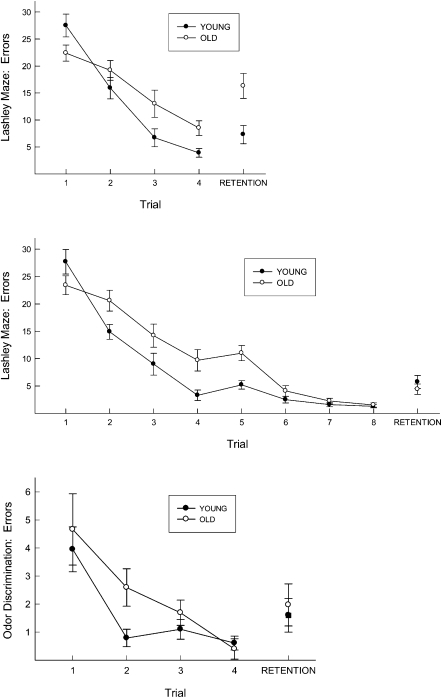
Errors to reach the goal box are depicted in Figure 1. The top panel of Figure 1 illustrates the acquisition of animals trained with four trials, F(3,78) = 64, P < 0.0001. Aged animals were impaired relative to their young counterparts, as indicated by a trial × group interaction, F(3,78) = 4.77, P < 0.01, and were still significantly impaired on the fourth training trial, F(1,26) = 5.95, P < 0.05. When performance was assessed 30 d after four training trials, aged animals exhibited a significant retention deficit, F(1,26) = 17.37, P < 0.001. The middle panel of Figure 1 illustrates acquisition during eight training trials, F(7,196) = 88, P < 0.0001. However, by the eighth trial, the performance of young and old animals did not differ, F(1,28) = 0.01, ns. When retention was assessed 30 d later, no difference between young and old animals was detected, F(1,28) = 1.6, ns. These results indicate that the memory deficits exhibited by aged animals in the Lashley III maze can be mitigated if young and old animals are initially trained to equivalent levels of competence.
Figure 1.
(Top) Errors to locate food in the Lashley III maze across four training trials and after 30 d of retention in both young and old mice. A significant deficit in 30-d retention was observed in old, relative to young, mice after four training trials. Brackets indicate standard errors. (Middle) Errors to locate food in the Lashley III maze across eight training trials and after 30 d of retention in both young and old mice. Both young and old animals exhibited comparable retention. Brackets indicate standard errors. (Bottom) Errors to locate food in the odor discrimination task. Both young and old animals exhibited comparable performance after four training trials, and no differences in retention between young and old animals were observed after 30 d. Brackets indicate standard errors.
Odor discrimination
The bottom panel of Figure 1 illustrates the mean errors to locate the target food cup for young and old animals. All animals exhibited rapid acquisition across four trials, although aged animals reached asymptotic levels more slowly, as indicated by a trial × group interaction, F(3,168) = 8.99, P < 0.001. Nevertheless, both young and aged animals reached an equivalent level of performance by the fourth training trial F(1,28) = 0.55, ns. Given the comparable level of performance by the end of the first training session, no additional training was administered, and animals were again tested after a 30-d retention interval (bottom panel of Fig. 1), where young and old animals exhibited comparable levels of performance, F(1,56) = 1.54, ns. These results indicate that no retention deficits in odor discrimination arise if young and old animals are initially trained to equivalent levels of competence.
Spatial water maze
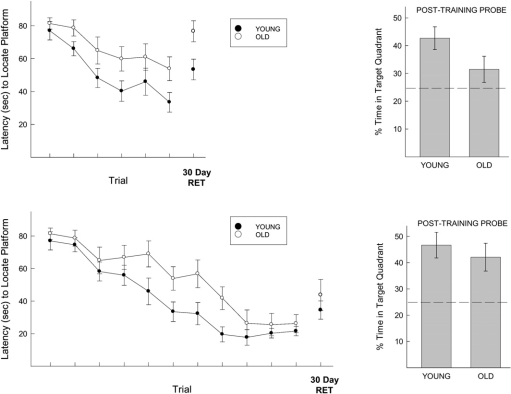
Figure 2 (top left panel) illustrates the latency to locate the hidden platform recorded across six training trials, F(5,130) = 32, P < 0.0001. Inspection of the acquisition curves indicates that aged animals were impaired relative to their young counterparts, as indicated by a planned comparison of the groups' performances on the sixth trial, F(1,26) = 11.95, P < 0.01. This deficit in latencies was confirmed by the groups' performances on a probe test (of search patterns) administered 3 h after the last trial (Fig. 2, top right panel), F(1,26) = 6.32, P < 0.05. When tested 30 d later, aged animals exhibited a significant retention deficit, F(1,26) = 11.82, P < 0.01. When trained with 11 trials, aged animals ultimately reached a level of performance comparable to their young counterparts, as latencies to locate the platform did not differ on Trial 11, F(1,28) = 1.51, ns. This was confirmed by the lack of a difference in probe trial performance 3 h after the completion of the 11th trial, F(1,28) = 1.65, ns. When retention was assessed 30 d later, no differences were observed between the young and old animals, F(1,28) = 2.51, ns. These results indicate that the memory deficits exhibited by aged animals in the water maze can be mitigated if young and old animals are initially trained to equivalent levels of competence.
Figure 2.
(Top left) Latency to locate hidden platform in the spatial water maze across six training trials and 30 d of retention. A significant impairment in acquisition was observed among aged animals (an effect also seen in the search strategy exhibited during a short-term probe test, top right). A significant deficit in 30-d retention was observed in old relative to young mice after six training trials. Brackets indicate standard errors. (Bottom left) Latency to locate hidden platform in the spatial water maze across 11 training trials and 30 d of retention. Both young and old animals exhibited comparable performance after 11 trials (a pattern also seen in the search strategy exhibited during a short-term probe test, bottom right). After 11 training trials, no differences in retention between young and old animals were observed after 30 d. Brackets indicate standard errors.
Analysis of retention deficits
In the two cases above where different levels of training were administered (the Lashley III maze and water maze), aged animals exhibited “retention” deficits relative to young animals if initial training was subasymptotic. That is, performance after 30 d of retention was deficient in old, relative to young, animals. It is reasonable to ask whether this deficit reflected differential decay of the memory per se, or simply reflected differences of a magnitude similar to that which existed at the end of training. To address this question, performance on the last day of subasymptotic training was compared to that observed at the time of the 30-d retention test. An interaction of performance at these two time points with the age of the subjects would reflect differential decay of the memory in aged animals. In the Lashley III maze, performance of young and old animals differed at the time of retention testing relative to the performance during the fourth (subasymptotic) training trial, F(1,26) = 85.3, P < 0.0001, indicative of a decay in performance across the 30-d retention interval. Furthermore, age interacted with performance across the last trial of training and retention testing, F(1,26) = 13.9, P < 0.01, suggesting that in this task, the memory of aged animals decayed at an accelerated rate. The same was not true of performance in the water maze. In this task, performance of young and old animals again differed at the time of retention testing relative to the performance during the sixth (subasymptotic) training trial, F(1,27) = 172.9, P < 0.0001, indicative of a decay in performance across the 30-d retention interval. However, age did not interact with performance across the last trial of training and retention testing, F(1,27) = 0.4, ns, suggesting that memory in young and old animals decayed at a similar rate. Based on these combined results, one could conclude that “retention” deficits in aged animals can reflect impaired learning and/or differential decay of a weak (subasymptotic) memory. It is notable that regardless of the source of the retention deficit, these deficits can be overcome with sufficient initial training.
The present results again indicate that aged mice exhibit both learning and retention deficits relative to their young counterparts. However, we find that memory deficits in aged animals were absent if the animals were initially trained to a level of competence comparable to young animals. These results suggest that much of the deficit in retention of new information that accompanies aging reflects deficient learning, not a failure of the memory per se.
The experiments described here were intended to address the nature of memory failures that accompany cognitive aging by equating the efficacy of learning in young and old animals. A similar approach has previously been taken to assess the basis for memory failure at very young ages (i.e., “infantile amnesia”). It is commonly observed that memories acquired during early postnatal stages of development are rapidly “lost.” However, early work in this field failed to recognize the degree to which the learning upon which memories were based differed in young and mature animals (Campbell 1967). Using procedures that equated initial learning, it has since been determined that even under conditions in which immature animals exhibit better learning than their adult counterparts, immature animals continue to express a relatively rapid decline of long-term retention. Unlike the nominal results presented here, the results of memory tests of infant animals suggest that their memory failures may reflect real deficits that are independent of variations in learning (although it is noted that the memory “failure” exhibited by young animals may severely underestimate their actual capacity for memory; e.g., Campbell and Jaynes [1966]; Galluccio and Rovee-Collier [2005]; Hsu and Rovee-Collier [2006]). It has thus been argued that infantile amnesia reflects (at least in part) the consequences of the immaturity of the nervous system at the time of learning (Campbell and Spear 1972). Similar classes of influences are sure to impact memory storage in aged subjects, and it is certainly the case that not all memory deficits in aged animals simply reflect an impairment of initial learning. For instance, Barnes and McNaughton (1985) trained young and old animals to similar levels of performance in a spatial maze task (although it is noted that on select measures of performance, levels of acquisition differed across the ages). Despite similar levels of acquisition, Barnes and McNaughton (1985) observed more rapid and extensive retention deficits in their aged sample. Thus, while it is reasonable to assume that real deficits in the storage of new memories can accompany cognitive aging, the present results indicate that memory failures in aged animals are not a necessary consequence of aging and can, under certain circumstances, be overcome with more effective training. Although often ignored, this issue represents a recurring problem in studies of both human and animal memory and memory failure (see, for critical discussion, Underwood 1964).
Acknowledgments
This work was supported by grants from the National Institute of Aging (PHS AG022698 and AG029289) and the Busch Foundation to L.D.M.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1503209.
References
- Barnes CA, McNaughton CL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Campbell BA. In: Early behavior: Comparative and developmental approaches. Stevenson HW, et al., editors. Wiley; New York: 1967. [Google Scholar]
- Campbell BA, Jaynes J. Reinstatement. Psychol Rev. 1966;73:478–480. doi: 10.1037/h0023679. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Spear NE. Ontogeny of memory. Psychol Rev. 1972;79:215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SSBA. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M. Animal models of memory impairment. Philos Trans R Soc Lond B Biol Sci. 1997;352:1711–1717. doi: 10.1098/rstb.1997.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Galluccio L, Rovee-Collier C. Updating reactivated memories in infancy: II. Time passage and repetition effects. Dev Psychobiol. 2005;47:18–30. doi: 10.1002/dev.20072. [DOI] [PubMed] [Google Scholar]
- Gould TH, Feiro O. Age-related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behav Brain Res. 2005;165:160–171. doi: 10.1016/j.bbr.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Hsu VC, Rovee-Collier C. Memory reactivation in the second year of life. Infant Behav Dev. 2006;29:91–107. doi: 10.1016/j.infbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001a;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001b;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci. 1994;14:4815–4824. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Serrano A, Fischer W, Soderstrom S, Ebendal T, Bjorklund A. Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc Natl Acad Sci. 1996;93:6355–6360. doi: 10.1073/pnas.93.13.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a “general” learning ability in mice. J Neurosci. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobiol Learn Mem. 2006;86:228–240. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Grossman H, Light K, Townsend DA, Kolata S. Variations in age-related declines in general cognitive abilities of Balb/C mice are associated with disparities in working memory span/capacity and body weight. Learn Mem. 2008;15:733–746. doi: 10.1101/lm.954808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliska CJ, Burke PA, Bartke A, Jensen RA. Inhibitory avoidance and appetitive learning in aged normal mice: Comparison with transgenic mice having elevated plasma growth hormone levels. Neurobiol Learn Mem. 1997;68:1–12. doi: 10.1006/nlme.1997.3772. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- Port RL, Murphy HA, Magee RA. Age-related impairment in instrumental conditioning is restricted to initial acquisition. Exp Aging Res. 1996;22:73–81. doi: 10.1080/03610739608253998. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Soffie M, Lejeune H. Acquisition and long-term retention of a two-lever DRL schedule: Comparison between mature and aged rats. Neurobiol Aging. 1991;12:25–30. doi: 10.1016/0197-4580(91)90035-i. [DOI] [PubMed] [Google Scholar]
- Underwood BJ. Degree of learning and the measurement of forgetting. J Verbal Learn Verbal Behav. 1964;3:112–129. [Google Scholar]
- Vogel RW, Ewers M, Ross C, Gould TJ, Woodruf-Pak DS. Age-related impairment in the 250-millisecond delay eyeblink classical conditioning procedure in C57BL/6 mice. Learn Mem. 2002;9:321–336. doi: 10.1101/lm.50902. [DOI] [PMC free article] [PubMed] [Google Scholar]