Abstract
The hippocampus has been proposed to support a cognitive map, a mental representation of the spatial layout of an environment as well as the nonspatial items encountered in that environment. In the present study, we recorded simultaneously from 43 to 61 hippocampal pyramidal cells as rats performed an object recognition memory task in which novel and repeated objects were encountered in different locations on a circular track. Multivariate analyses of the neural data indicated that information about object identity was represented secondarily to the primary information dimension of object location. In addition, the neural data related to performance on the recognition memory task. The results suggested that objects were represented as points of interest on the hippocampal cognitive map and that this map was useful in remembering encounters with particular objects in specific locations.
The hippocampus plays an important role in spatial memory for both humans and rodents (O'Keefe 1999; Burgess et al. 2002). Findings from many studies in rodents indicate that the hippocampus supports memory for locations referenced to external landmarks, a capacity that O'Keefe and Nadel (1978) described over 30 yr ago as a “cognitive map” (using a term they borrowed from Tolman 1948). In the time since that pioneering thesis, it has become clear that the rodent hippocampus is also important for nonspatial memory (Eichenbaum et al. 1999). Damage to the rat hippocampus (defined here as CA fields, dentate gyrus, and subiculum) leads to impairments on nonspatial tasks, including object recognition memory (Clark et al. 2000; Fortin et al. 2004), transitive odor associations (Bunsey and Eichenbaum 1996), memory for temporal order (Fortin et al. 2002; Kesner et al. 2002), and social transmission of food preference (Alvarez et al. 2001; Clark et al. 2002).
The circuitry by which information arrives at and exits from the hippocampus is consistent with the idea that the hippocampus is important for both spatial and nonspatial memory. In both rats and macaques, detailed anatomical studies have indicated that spatial information arrives at the hippocampus via the postrhinal cortex (parahippocampal cortex in primates) and the medial entorhinal cortex, whereas nonspatial information takes a path largely through the perirhinal cortex and lateral entorhinal cortex (Witter and Amaral 1991; Suzuki and Amaral 1994; Witter et al. 2000). Thus, the hippocampus is ideally situated to combine spatial and nonspatial information in the service of remembering item–location associations (Manns and Eichenbaum 2006).
Single-unit recording studies in the rat hippocampus have largely focused on the spatial correlates of hippocampal pyramidal neuron firing rates. Fewer studies have investigated nonspatial correlates of hippocampal activity during memory tasks for nonspatial items. However, in one such study, Wood et al. (1999) found that some individual hippocampal pyramidal neurons responded to particular odors and that others responded to particular odors in specific locations during an odor recognition memory task. Thus, activity of individual cells appeared to contain information about nonspatial items as well as spatial locations.
An important question is how the activity of individual hippocampal neurons combine to represent item–location associations as a neural ensemble. In particular, how is an encounter with an object in a particular location represented in the pattern of spiking among many hippocampal pyramidal neurons? How might this representation relate to memory for the object or for the location? In the present study, we recorded simultaneously from 43 to 61 hippocampal pyramidal cells as rats performed an object recognition memory task in which novel and repeated objects were encountered in different locations on a circular track. Multivariate analyses of the neural data indicated that information about object identity was represented secondarily to the primary information dimension of object location. In addition, the analyses indicated that the neural data related to performance on the recognition memory task. The results suggest that objects were represented as points of interest on the hippocampal cognitive map and that this map was useful in remembering encounters with particular objects in specific locations.
Results
Exploration behavior
The task involved rats encountering objects at various locations as they completed laps on a circular track for small pieces of chocolate (for a full description of the task, see Materials and Methods). Figure 1 shows a schematic of the testing procedure. The basic design for each block of laps was that, after every three laps, new objects were added to the track in previously unoccupied locations, and existing objects (actually, copies of the objects) were moved to new locations. Novel objects were used for each block of trials, and rats encountered up to 40 objects over 10 blocks of laps on each testing day (rat 4 completed only nine blocks).
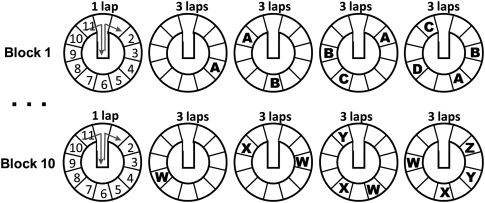
Figure 1.
Schematic of the recognition memory task procedure. Ten blocks of trials were completed in each recording session. On each block of trials, four objects (indicated by letters) were encountered in various locations as rats completed clockwise laps on a circular track. Each block started with an empty lap, and the numbers on the drawing of the empty lap indicate the o'clock positions in which objects were encountered.
Previous studies using a similar task in rats (Save et al. 1992; Clark et al. 2000; Mumby et al. 2002), monkeys (Zola et al. 2000; Bachevalier and Nemanic 2008), and humans (Pascalis et al. 2004; Pihlajamäki et al. 2004) have found that incidental memory for objects and their locations depends on the same hippocampal memory system that supports other types of recognition memory tasks. In addition, performance in humans on a similar task correlated with overt recognition memory judgments but not perceptual priming (Manns et al. 2000). Thus, performance on the present task likely relied on a hippocampus-dependent type of recognition memory.
The overall question was whether the rats' behavior (and the pattern of neuronal activity in the hippocampus) would reflect memory for encounters with the objects. Based on numerous previous studies using a similar task (e.g., Ennaceur and Delacour 1988; Mumby et al. 2002), we expected that memory for repeated objects would be reflected in shorter durations of exploration for the repeated objects compared with the novel objects. In addition, we expected durations of exploration for objects repeated in a new location to be longer than for objects repeated in the same location.
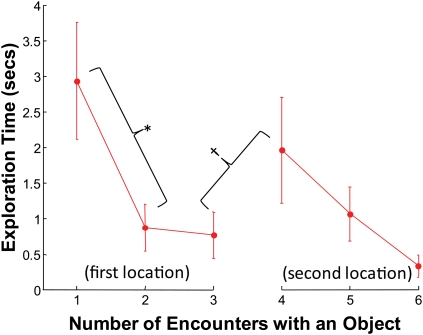
Rats' pattern of object exploration indicated memory for both object identity and object location. Figure 2 shows the average object exploration time as a function of the number of times an object was encountered. The data are plotted for the objects that were encountered in at least two locations (i.e., the fourth object from each block is not included). Only the first six encounters are shown because some objects were encountered only six times. For the first three times a rat encountered an object, the object remained in the same place. A repeated-measures ANOVA for the first three encounters revealed a statistically significant linear contrast (F(1,3) = 13.88, P < 0.05), indicating that exploration decreased with repetition. We more specifically defined a “repetition effect” as the difference in exploration between the first and second encounters. This measure also indicated that rats explored repeated objects for a shorter duration than novel objects, indicating a memory for the repeated objects (paired-sample t-test: t(3) = 2.75, P < 0.05). For this and all subsequent paired-sample t-tests, one-tailed P-values are used because we had clear a priori predictions (Ennaceur and Delacour 1988; Mumby et al. 2002) and because a two-tailed P-value might be underpowered for n = 4. In between the third and fourth encounter, objects were moved to a new location on the track. We defined a “relocation effect” as the difference in exploration time between the third and fourth encounters. This measure revealed that rats reinitiated exploration of repeated objects moved to a new location (paired-sample t-test: t(3) = 2.48, P < 0.05). This effect suggested that the rats had memory for not only the object itself but also the object's previous location.
Figure 2.
Performance on the recognition memory task. Duration of exploration is plotted for the first six encounters with each object. For the first three encounters, the object remained in the same location. (*) Indicates a statistically significant reduction in exploration between the first and second encounter with an object (see Results), presumably reflecting memory for the repeated object. Between the third and fourth encounter, the object was moved to a new location. (†) Indicates a statistically significant increase in exploration between the third and fourth encounter (see Results), presumably reflecting memory for the object and its previous location. Error bars, SEM across the four rats.
Objects were removed and copies were repositioned on the track after every three laps, and thus the delay between laps was somewhat longer in these instances compared with instances in which objects were not moved. As a result, the time elapsed since a rat last saw a particular object was somewhat longer for laps after objects had been moved compared with laps in which the objects had not been moved (mean interstimulus interval ± SEM = 73.6 ± 8.6 sec vs. 22.5 + 5.3 sec, respectively; t(3) = 11.76, P < 0.01). We therefore asked whether there was a relationship between the interstimulus interval and duration of exploration that might account for some of the rats' tendency to inspect moved objects longer than unmoved objects. For each rat, we calculated the correlation between interstimulus interval and exploration duration for all encounters with objects after the objects had been moved. However, none of the correlations reached statistical significance, and there was no overall trend across the four rats (r = 0.24, −0.17, −0.28, and 0.21 for rats 1, 2, 3, and 4, respectively; all Ps > 0.05). Thus, the somewhat longer interstimulus interval did not seem to account for the rats' tendency to explore moved objects longer than objects that were not moved.
Single-unit analyses
A total of 205 hippocampal pyramidal neurons were recorded (149 CA1 neurons and 56 CA3 neurons). The numbers of neurons recorded in the four test sessions were 61 (43 CA1, 18 CA3), 43 (18 CA1, 25 CA3), 44 (44 CA1, 0 CA3), and 57 (44 CA1, 13 CA3) for rats 1, 2, 3, and 4, respectively.
Although the main goal of the study was to ask how object–location associations were represented by multiple neurons, we first asked whether information about both object location and object identity would be detectable in the activity of individual hippocampal pyramidal cells. Firing rates were calculated for each 0.5-sec object encounter event (see Materials and Methods). For each cell, the firing rates were then separately entered into two ANOVAs. The first ANOVA was a one-way ANOVA with location as the factor (there were 10 possible locations). With an α level of 0.01, the effect of location reached statistical significance for 123 of 205 pyramidal cells (60.0%; 96 of 149 CA1 cells and 27 of 56 CA3 cells). Based on an α level of 0.01, approximately two of the 205 ANOVA tests (1%) would, in principle, be expected to yield a statistically significant result due to chance alone. However, due to the fact that the data were skewed (many encounters yielded a firing rate of zero for many of the neurons), we sought to verify that the ANOVA tests did not overestimate the incidence of statistically significant location information. We randomly reshuffled the location assignments for each trial in such a way that all three encounters with an object within a trial were randomly assigned the same location. We reshuffled the data 100 times and recalculated the number of neurons that showed statistically significant location information for each shuffle. Across the 100 shuffles, the average number of statistically significant neurons was 2.7 (1.3%), and thus the ANOVA tests did not appear to overestimate the incidence of statistically significant location information in the original data. We also verified the results from the ANOVA tests by conducting parallel nonparametric Kruskal-Wallis tests on object location for each neuron, which also resulted in 123 of 205 neurons (96 of 149 in CA1and 27 of 56 in CA3) reaching statistical significance with an α level of 0.01. Thus, the single-unit analyses indicated that over half of the individual pyramidal cells showed significant information about object location.
The second ANOVA was a one-way ANOVA with object identity as the factor. It was not feasible to conduct an ANOVA with all 40 objects (or 36 in the case of rat 4) because some objects were encountered only three times. Thus, we restricted the analysis to the first object encountered on each block (e.g., the “A” object for the first block), each of which was encountered 12 times. This approach also had the effect of making the analysis of object identity and object location more similar: Each of 10 locations was sampled 30 times and each of 10 objects was sampled 12 times. With an α level of 0.01, the effect of object identity reached statistical significance for 34 of 205 pyramidal cells (16.6%; 29 of 149 in CA1 and five of 56 in CA3). Over 100 random shuffles of object identity, an average of 0.6 cells (0.3%) reached statistical significance, indicating that the ANOVA did not overestimate the incidence of statistically significant object identity information. Parallel Kruskal-Wallis tests yielded statistical significance for 27 of 205 neurons (13.2%; 22 of 149 in CA1 and five of 56 in CA3), a similar number to that obtained from the ANOVA tests. Thus, although the activity of individual pyramidal cells appeared to contain much less information about object identity compared with object location, the amount of information about object identity appeared to be greater than one would expect by chance. A two-way ANOVA (object identity by object location) was not conducted because each object appeared in at most four locations, and thus there was insufficient data to conduct this type of analysis using univariate statistics.
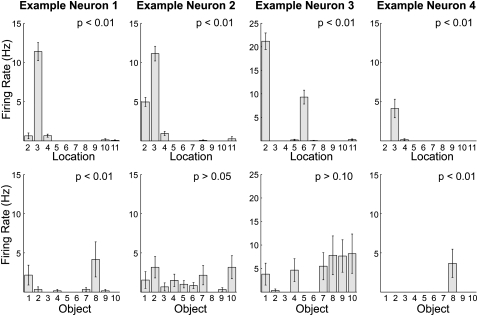
Figure 3 shows four example neurons firing rates during object encounter events as a function of object identity and as a function of object location, parallel to the two ANOVAs reported previously (as in the prior analyses, the location plots include all objects whereas the object identity plots include only the first object, e.g., “A,” from each block). Reflecting the prominence of location coding in the single-unit analyses, all four example neurons show a significant effect of object location. Reflecting the lesser prominence of object identity coding, only two of the four neurons show a significant effect of object identity.
Figure 3.
Firing rates from four example neurons as a function of object location (top row) or object identity (bottom row). The four example neurons are taken from rats 1, 2, 3, and 4, respectively. Example neurons 1 and 3 are CA1 pyramidal neurons, and neurons 2 and 4 are CA3 pyramidal neurons. Separate one-way ANOVAs were conducted on the data to test for main effects of object location and for object identity. All four neurons showed a significant effect of location (Ps < 0.01), whereas only two showed a significant effect of object identity (Ps < 0.01), reflecting the lesser prominence of object coding observed across all cells (see text for details).
Multiple-unit analyses
We next asked whether detectable information about object location and object identity would be apparent when considering the pattern of activity across all pyramidal neurons recorded during the test session. We expected to observe reliable information about object location based on the results with the single-unit analyses, but we were also interested in whether the neural representation of object identity would be more prominent when inspected by multiple-unit analyses. We were also interested in whether an interaction would exist between object location and object identity, a question that was not feasible for single-unit analyses. Similar to the single-unit analyses, we observed no significant differences between neurons recorded in CA1 versus CA3, and the following multiple-unit analyses combine both types of cells.
Our aim was to determine whether the pattern of activity for one object encounter was similar to another encounter in the same location, irrespective of object identity. We also examined whether the pattern of activity for one object encounter would be similar to another encounter with the same object, irrespective of location. Finally, we asked whether the similarity would be greatest for another encounter with the same object in the same location. For each 0.5-sec object encounter event, a multivariate data point was obtained by first calculating the firing rate for each pyramidal neuron in that session. The data point was then represented as a point in multidimensional space such that each axis represented the firing rate of one neuron. Thus, for the four sessions, the number of dimensions ranged from 43 to 61 (i.e., the total number of pyramidal neurons for that session). We then asked whether the next closest data point for each object encounter was from an encounter with the same object or from an encounter in the same location (or both). Distance was measured as simple Euclidean distance. Proximity of multivariate data points was taken as a measurement of similarity between the pattern of firing rates for object encounters and, to the extent that patterns were reliable across repetitions of object location or object identity, was taken as an indicator of the level of information about objects and their locations. The approach is formally a k-nearest neighbor classification approach (where k = 1), which has been used frequently in situations in which the underlying distribution is not known (Cover and Hart 1967).
This nearest neighbor approach identified the correct (i.e., the same) location for 72.7%, on average, of all object encounters (77.2%, 61.3%, 74.4%, and 77.8% for rats 1, 2, 3, and 4, respectively). The likelihood of this result being obtained due to chance was determined for each session by randomly reshuffling location assignment across all points 1000 times and repeating the nearest neighbor analysis on each reshuffle. A percentage as high as the actual data was never observed in the reshuffled data, indicating that the P-value for each session was less than 0.001. In addition, for the randomly reshuffled data, on average 9.7% of all object encounters were nearest to another encounter from the same location, a chance level that reflects the 10 possible locations. As a group, the nearest neighbor object location classification for all four rats significantly differed from this chance level (t(3) = 16.30, P < 0.001). Thus, information about object location was prominent in the multiple-unit pattern of firing rate activity.
The nearest neighbor approach identified another encounter with the same object for 15.3%, on average, of all object encounters (15.5%, 13.2%, 16.7%, and 15.6% for rats 1, 2, 3, and 4, respectively). The same reshuffling procedure as described for object location analyses indicated that the object identity P-value for each session was less than 0.001 (average chance level was 2.8% for the reshuffled data). As a group, the nearest neighbor object identity classification significantly differed from the chance level (t(3) = 16.93, P < 0.001).
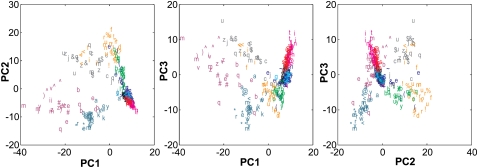
Since it was not possible to plot the multivariate data points in the total number of dimensions, we used principal components analysis (PCA) as a dimension reduction technique to enable plotting. Figure 4 shows for rat 1 a three-panel plot in which each panel shows a combination of the first three principal components. PCA was used only for plotting purposes, and all analyses were based on measurements of Euclidean distance in the original multidimensional space in which axes represented firing rates of individual neurons. Each point in the plot is labeled according to both object location and object identity.
Figure 4.
Multivariate representation of hippocampal activity for each object encounter for one example session (rat 1; see Supplemental Fig. 1 for graphs for the other three rats). The three panels show the three possible parings of the first three principal components of the pattern of activity across 61 simultaneously recorded hippocampal pyramidal neurons. The data point for each encounter is depicted by both a letter (or symbol), which represents the identity of the specific object, and a color, which represents the position in which it was encountered on the circular track. Data points tended to cluster first by object location and then by object identity (see Results). It should be noted that principal components were used only for plotting purposes and that all analyses were performed on the raw data.
The appearance of the PCA plot confirmed the results of the nearest neighbor analyses regarding object location. Data points for object encounters in the same location showed a strong tendency to cluster together. Thus, location appeared to be primary in determining the ensemble pattern of firing rates (the pattern was the same for the other three rats) (see Supplemental Fig. 1). Less clear was whether data points tended also to cluster according to object identity. The previous nearest neighbor analyses suggested that object identity significantly influenced firing patterns. However, the prominence of object location information raises a concern with this previous analysis. In particular, objects were encountered in the same location more than once, and thus the proximity of data points for the same object might have been due to location coding rather than identity coding.
The appearance of the PCA plots suggested to us additional nearest neighbor analyses related to object identity, analyses that would not be confounded by object location. In particular, we hypothesized that object identity might be represented as secondary clusters of data points within primary clusters of object location. Accordingly, we asked if there would still be an above-chance tendency for the pattern of firing rates to be similar for encounters with the same object if the analysis was restricted to objects encountered in the same location. For all four sessions, the average number of object encounters for which the nearest neighbor was another encounter with the same object in the same location was 19.9% (22.4%, 16.8%, 21.1%, and 19.3%, for rats 1, 2, 3, and 4, respectively). The likelihood of this result occurring due to chance was obtained by randomly reshuffling object identity assignment within each location 1000 times. Using this approach, the P-value was observed to be less than 0.001 for each of the four sessions (average chance level, 7.7%). As a group, this nearest neighbor object identity classification for all four rats significantly differed from the chance level (t(3) = 10.06, P < 0.01). Thus, there was statistically significant information about object identity that appeared to be represented secondary to the primary dimension of object location.
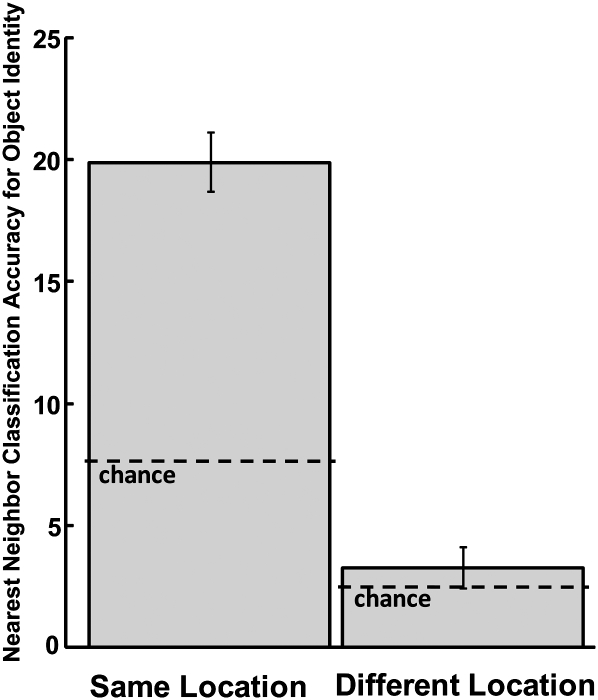
Furthermore, we reasoned that if object identity was completely secondary to object location, data points for encounters with the same object in different locations should have been no closer together than one would expect by chance. Across the four sessions, the average percentage of nearest neighbors that represented the same object encountered in different locations was only 3.3%. The likelihood of this result occurring due to chance was obtained by randomly reshuffling the object identity assignments within each location 1000 times and finding the nearest neighbor for each point for each reshuffle. Using this approach, the average chance level was 2.5%, less than 1% different than the actual value. Despite these small values, the P-value for the individual sessions was observed to be less than 0.01 for three of the four rats. However, a comparison of the actual values for the four to chance indicated that as a group they did not significantly differ from chance (2.3%, 1.4%, 4.5%, and 4.9% vs. 2.5%; t(3) = 0.19, P > 0.1). Figure 5 shows a bar graph of these results along with the above results regarding the percentage of nearest neighbor data points for the same objects in the same location. The graph is meant to emphasize that the pattern of hippocampal spiking activity distinguished between different objects encountered in the same location but that there appeared to be little to no significant object identity coding independent of object location coding.
Figure 5.
Nearest neighbor classification accuracy for object identity. Accuracy is shown as the percentage of object encounters for which the multivariate data point was closest to the data point of another encounter with the same object. The left bar shows the accuracy when considering only objects in the same location. The right bar shows the accuracy when considering only objects encountered in a different location. The accuracy for objects in the same location was well above chance (indicated by a dashed line) but was not significantly above chance for objects in different locations (see Results). Error bars, SEM for the four rats.
To further investigate the interaction between object and location coding, we calculated an average similarity measurement for each object encounter event related to each of four categories: (1) all other events involving the same location and the same object, (2) all other events involving the same location and different objects, (3) all other events involving a different location and the same object, and (4) all other events involving a different location and a different object. For each object encounter event, the similarity measurement was obtained by averaging all Euclidean distances in multidimensional firing rate space between the event and all other events in each of the four categories. The reasoning is that shorter average distances would reflect greater similarity (greater clustering) within each category and thus more prominent information coding. This approach differs from the nearest neighbor approach in that it considers all data points with a category rather than just the closest data point.
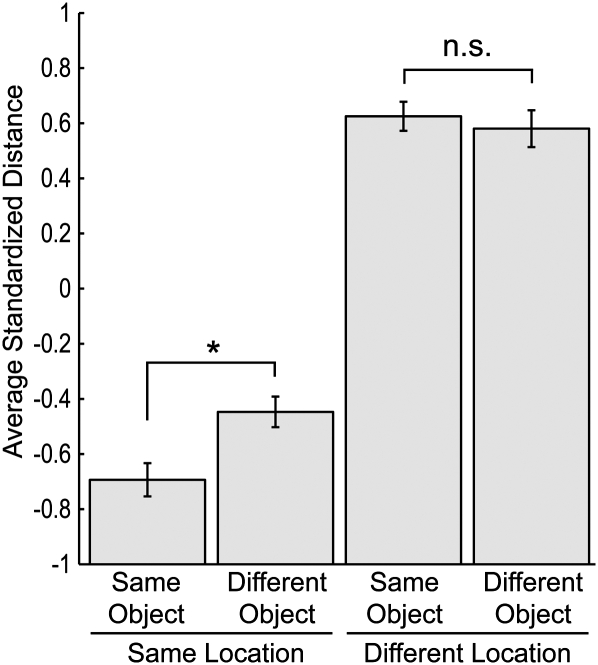
Figure 6 shows the average similarity (average multivariate distances) for object encounter events as a function of whether the events involved the same location and/or same object. The distances for each session were transformed to Z-scores before averaging across sessions because distance measurements would have differed across sessions according to the total number of neurons recorded and because distances could have been influenced by baseline firing rate differences. Thus, negative scores represented the shortest distances (i.e., the greatest similarity). A two-factor repeated-measures ANOVA (same object/different object by same location/different location) revealed a significant interaction between object identity and object location (F(1,3) = 75.96, P < 0.01) as well as partial effects of object identity (F(1,3) = 42.19, P < 0.01) and object location (F(1,3) = 102.45, P < 0.01). Follow-up paired-samples t-tests revealed a significant difference between similarity scores (average standardized distances) for encounters with the same object in the same location and encounters with different objects in the same location (t(3) = 8.81, P < 0.01) but not between similarity scores for encounters with the same object in different locations and encounters with different objects in the different locations (t(3) = 2.80, P = 0.07; although this latter result approached statistical significance, it was in the opposite direction of the previous result: Encounters with different objects in different locations were numerically more similar to one another than encounters with the same object in different locations). Thus, the results indicate that hippocampal representations of encounters with the same object were more similar than encounters with different objects but only when the encounters were in the same location, similar to the findings with the nearest neighbor classification accuracy depicted in Figure 5.
Figure 6.
Average similarity of hippocampal representations of object encounter events. The similarity of hippocampal representations was represented as the average standardized distances between multivariate data points for each object encounter event and all other events in one of four categories: encounters with the same object in the same location, encounters with a different object in the same location, encounters with the same object in a different location, and encounters with a different object in a different location. Shorter distances indicate greater similarity of neural patterns. Encounters with the same object were more similar than encounters with different objects (* indicates a P-value less than 0.01 from a paired samples t-test) but only when the encounters were in the same location (n.s. indicates not statistically significant; P > 0.05; for full statistical analyses, see text). Error bars, SEM across the four rats.
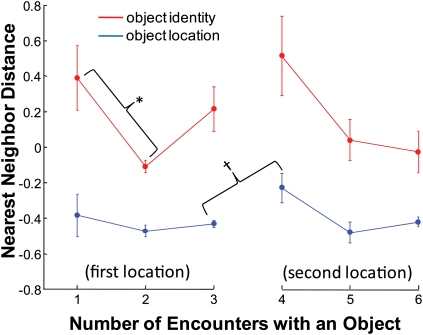
We next sought to relate patterns of firing rate activity to object exploration. For these analyses, we again used the nearest neighbor approach. Figure 7 shows the average multivariate distance between nearest neighbors for both object location (i.e., distance between an encounter's data point and the closest data point representing the same location) and object identity (i.e., distance between an encounter's data point and the closest data point representing the same object). Based on the previous results, we defined information about object identity here as the nearest neighbor distance between two encounters with the same object in the same location. The data are plotted as a function of the number of times an object was encountered, similar to the way that the behavioral data were plotted in Figure 2. The distances for each session were transformed to Z-scores before averaging across sessions because distance measurements would have differed across sessions according to the total number of neurons recorded and because distances could have been influenced by baseline firing rate differences. A shorter distance indicated greater similarity in the pattern of firing rates, and due to the Z-transformation, the shortest distances (the greatest similarity) were represented by negative numbers. Overall, nearest neighbor distances between multivariate data points representing encounters in the same location were much shorter than distances between points representing encounters with the same object, a trend that is consistent with the idea that location was the primary dimension of information coding (repeated-measures ANOVA; main effect of location vs. identity: F(1,3) = 66.77, P < 0.01).
Figure 7.
Similarity of hippocampal representations of object identity and object location for the first six encounters with an object. The similarity of hippocampal representations was represented as nearest neighbor distances between multivariate data points representing the pattern of neural activity across all simultaneously recorded cells (see Materials and Methods). Information about object location for each object encounter was calculated as the distance between the data point representing that encounter and the nearest data point representing an encounter in the same location (shown in blue). Information about object identity for each object encounter was calculated as the distance between the data point representing that encounter and the nearest data point representing an encounter with the same object in the same location (shown in red). (*) Indicates that nearest neighbor distances for object identity were significantly shorter for the first repetition of an object in the same location, reflecting a greater amount of object information for the second time it was encountered compared with the first time it was encountered. (†) Indicates that nearest neighbor distances for object location were significantly longer for the first encounter for an object repeated in a new location, reflecting a decreased amount of location information for the object's new location compared with the previous location. The overall shorter distances for object location compared with object identity reflects the primacy of location coding (see Results). Distances were converted to Z-scores for each session. Error bars, SEM across the four rats.
The neural data in Figure 7 also showed evidence of the same memory effects that were observed in the behavioral data in Figure 2. First, a “repetition effect” was observed for the neural representation of object identity. The nearest neighbor distances between data points of the same object were significantly shorter for the second encounter with an object compared with the first (t(3) = 3.19, P < 0.05). This finding suggests repetition of an object led to greater information about the object's identity in the pattern of hippocampal activity. The repetition effect did not reach statistical significance for object location (t(3) = 0.98, P > 0.1), perhaps because the rat had already encountered each location on the track many times during training. Second, a “relocation effect” was observed for the neural representation of object location. The nearest neighbor distances between data points of the same location were significantly longer for the first encounter with a repeated object in a new location compared with the last encounter in the previous location (t(3) = 2.37, P < 0.05). This finding suggests that moving an object led to less or disrupted information about the object's location in the pattern of hippocampal activity. The relocation effect did not reach statistical significance for object identity (t(3) = 1.20, P > 0.1), perhaps due to the fact that the object itself stayed the same, even though it was moved to a new location.
Discussion
In the present object recognition memory task, we found that the pattern of activity among many simultaneously recorded hippocampal pyramidal neurons reflected information about both object location and object identity. Moreover, we found that the neural information structure of object encounters represented object–location associations by coding object location as the primary dimension of information and by coding object identity only secondarily. Specifically, hippocampal representations of encounters with the same object in the same location tended to be similar to each other (more so than different objects in the same location), whereas representations of the same object in different locations were no more similar than one would expect by chance. Indeed, because information about location was so prominent in hippocampal activity, representations of different objects encountered in the same location showed much greater similarity than representations of the same object encountered in different locations. We also found that object identity and object location information in the pattern of hippocampal activity corresponded to the recognition memory task. Repetition of an object in the same location corresponded to increased information about object identity, and moving an object to a new location corresponded to decreased information about object location.
The results of the present study are consistent with O'Keefe and Nadel's (1978) proposal that the hippocampus supports a cognitive map of the external world. Their idea was that the hippocampus was part of a “memory system, which contains information about places in the organism's environment, their spatial relations, and the existence of specific objects in specific places” (O'Keefe and Nadel 1978, p. 2). The prominence of spatial coding in the rat hippocampus was a key observation in the formation of their idea, and many studies since then have revealed much about how the hippocampus and adjacent structures represent this spatial information and relate it to the rat's movements (McNaughton et al. 2006; Moser et al. 2008). The results of the present study now reveal how the memory for “specific objects in specific locations” is represented within this cognitive map. In particular, the results suggest that objects were represented as points of interest on the hippocampal cognitive map and that this map was useful in remembering encounters with particular objects in specific locations.
The results of the present study are consistent with the anatomical and electrophysiological data indicating that partially distinct spatial and nonspatial information pathways converge on the mammalian hippocampus (Witter et al. 2000; Knierim et al. 2006; Manns and Eichenbaum, 2006). Cortical areas important for spatial information connect with the hippocampus largely via the postrhinal cortex (parahippocampal cortex in primates) and medial entorhinal cortex, whereas cortical areas important for nonspatial information connect with the hippocampus largely via the perirhinal cortex and lateral entorhinal cortex (Witter and Amaral 1991; Suzuki and Amaral 1994; Witter et al. 2000). In addition, recording studies in the medial entorhinal cortex have identified prominent spatial correlates (Fyhn et al. 2004; Hafting et al. 2005), whereas recording studies in the lateral entorhinal cortex or perirhinal cortex have failed to find significant spatial information (Burwell et al. 1998; Hargreaves et al. 2005). Instead, these types of studies have shown that perirhinal and lateral entorhinal neurons encode specific nonspatial items (Miller et al. 1993; Suzuki et al. 1997; Young et al. 1997).
However, these anatomical and physiological data do not provide an answer for why nonspatial object information is represented secondarily to location information. The arrival of prominent inputs of both types of information could have been used to predict several other types of coding, including hypothetical frameworks in which object location and object identity were on equal footing or in which object location was represented as a secondary attribute of object identity. One possibility for the primacy of location coding in the rat hippocampus could be due to the possibility that rats attend to spatial features more so than nonspatial features. If so, perhaps the hierarchy of information coding might be different in the hippocampus of other species that attend less to spatial information.
Another possibility for the relegation of object identity information as secondary to object location information is that encounters with the objects were incidental to the rats' task of completing laps on the circular track. Perhaps coding of object identity would have been more prominent if the rats had been rewarded in some way for inspecting and remembering the objects. Nevertheless, what is clear from the behavioral data in the present study is that the rats did voluntarily inspect the objects without overt rewards and that they did remember those encounters.
An additional question regards the temporal dimension of the data. Objects were encountered not only in different points in space but also in different points in time. Thus, it is possible that the neural classification of object identity benefitted from the fact that objects were encountered across blocks of laps spread over an hour-long testing session. Indeed, a recent study found that the pattern of activity across simultaneously recorded hippocampal neurons gradually changed across the course of a testing session, and that the rats used this temporal information to guide memory judgments about the order in which odors had been encountered (Manns et al. 2007). In the present study, objects were presented in only one block of laps, and memory for temporal order was not assessed. Thus, the question would be difficult to answer with the present design of the task. However, what can be answered is the question of whether a simple shift in overall firing rate or a change in running speed across blocks of trials (which could impact firing rates) could account for the present results. First, only 12 of the 205 pyramidal neurons reported in the present study showed a significant (P < 0.01) effect of block when their firing rates from all object encounter events were entered into a one-way ANOVA with block number as the factor, in a manner similar to the analyses of object location or object identity (11 of 205 showed a significant effect of block when entered into a parallel Kruskal-Wallis test). This number (5.8%) is close to the number (1%) one would expect by chance. Thus, it does not seem that there was a tendency for firing rates to differ as a function of block that could explain the tendency for firing rates to differ as a function of object identity. Second, rats' running speeds during object encounter events remained relatively constant across blocks of laps (repeated-measures ANOVA: F(8,3) = 0.54, P > 0.1) (for a plot of the average running speed across blocks of laps, see Supplemental Fig. 2). Thus, there was no change in running speed across blocks of laps that could account for the differences in hippocampal activity between object encounter events.
A relevant consideration for the results of the present study is the fact that all recordings were taken from the dorsal hippocampus. A recent study of hippocampal pyramidal cells found that the real-world scale of spatial receptive fields (“place fields”) systematically differed as a function of where along the dorsal-ventral axis the neuron was located in the hippocampus (Kjelstrup et al. 2008; see also Jung et al. 1994). Pyramidal cells in the dorsal hippocampus tended to have small spatial receptive fields, cells in the middle hippocampus tended to have medium spatial receptive fields, and cells in the ventral hippocampus tended to have large spatial receptive fields. Based on the findings from this study, one would predict that if cells were recorded from the ventral hippocampus in the present task, the spatial receptive fields would have encompassed a large portion (if not all) of the circular track. Thus, information in the pattern of neural activity about the specific location of the objects on the circular track might be much less prominent in this case. If so, these large-scale spatial representations in the ventral hippocampus might be viewed as useful in representing a general spatial context rather than a specific location.
A final question is why damage to the hippocampus has resulted in impairments on nonspatial tasks in previous studies (Bunsey and Eichenbaum 1996; Clark et al. 2000, 2002; Alvarez et al. 2001; Fortin et al. 2002, 2004; Kesner et al. 2002). In the present study, no information about object identity was observed independent of object location, and thus one might be led to conclude that the hippocampus is unimportant for nonspatial memory. However, this finding does not mean that there was no information about object identity at all. In fact, the pattern of hippocampal activity distinguished between different objects encountered in the same location at levels well above chance. Indeed, the modulation of location-based spiking activity by objects or events occurring in these locations might represent an effective neural strategy for remembering episodic memories, a possibility previously considered by others (Wood et al. 2000; Leutgeb et al. 2005). These findings are also consistent with the view that even simple recognition of objects is in part supported by recollection of items in the context in which they were experienced (Zola et al. 2000; Fortin et al. 2004).
Materials and Methods
Four male Long–Evans rats weighing between 375 and 425 g were trained to complete clockwise laps on a circular track by rewarding them with a small piece of chocolate for each lap. Data were taken from one test session for each rat. The outside diameter of the circular track was 91.4 cm. The track width was 7.6 cm. On testing days, objects were placed on the track, and rats were allowed to explore objects voluntarily. Objects were attached to the outside edge of the track such that the objects were immediately adjacent to the track but did not encroach on the track (i.e., rats' paths did not need to deviate to inspect objects). The track was divided into 12 equal sections, and objects were placed in 10 of the 12 possible locations. The section at the end of the central runway (the 12 o'clock position) and the section immediately clockwise to it (the 1 o'clock position) never contained objects. The objects were a collection of plastic, wood, metal, or ceramic junk objects or toys that were typically larger than 7 cm × 7 cm × 7 cm but smaller than 12 cm × 12 cm × 12 cm. Rats were not exposed to the test objects prior to the testing session.
Rats completed up to 10 blocks of 13 laps (130 laps total) within a testing session. Figure 1 shows a schematic of the testing procedure. Each block of laps began with a lap around an empty track. The rat then completed three laps in which one object (“A,” in the first example block depicted in Fig. 1) occupied one of the 10 possible locations. On the next three laps, a copy of the original object (“A”) was placed in a new location, and a new object (“B”) was placed in a third location. On the next three laps, copies of the first two objects were placed in new positions on the track along with a new object (“C”), which was placed in a previously unoccupied location. On the final three laps, copies of the first three objects were placed on the track in new positions along with the final object (“D”), which was placed in a previously unoccupied location. Thus, on each block, rats encountered four objects, three of which were encountered in at least two different locations. Novel objects were used for each block of trials. Thus, rats encountered up to 40 objects over 10 blocks of laps on each testing day (rat 4 completed only nine blocks). The locations of the objects were randomized for each block, with the stipulation that a particular location was occupied only once per block. Objects were chosen to appear together on a block in a way that roughly equated size. The order in which objects were presented within a block (e.g., which was assigned to be “A,” “B,” “C,” or “D”) was randomly determined.
Performance was recorded and scored using digital video. The time was recorded for each instance in which a rat's nose first came within 0.5 cm of an object. For analysis of neural data, the time window for an object encounter event was defined as 0.25 sec before this time to 0.25 sec after this time (0.5 sec in duration). We refer to these instances as object encounter events or more simply as encounters. For analysis of behavioral data, the duration of exploration for each object was calculated as the length between the point at which the rat's nose first came within 0.5 cm of an object and the point at which the rat broke off active exploration. Instances in which a rat encountered an object but did not stop to actively explore the object were assigned an exploration duration of zero. The fixed 0.5-sec window for neural data analysis was chosen because it provided a consistent sampling window across events and because we reasoned that, if rats used memory information to guide their decision about whether or not to explore an object, that information must have been available at the outset of exploration. For behavioral analysis, we used the entire bout of exploration as an indication of the rat's memory for the object. Based on considerable prior data (e.g., Ennaceur and Delacour 1988; Mumby et al. 2002), we expected that a rat would explore remembered objects for a shorter duration on average compared with new or forgotten objects, but when objects were subsequently relocated to new places, the rat would re-explore an object for a longer duration.
Rats were implanted with a chronic recording headstage above the left dorsal hippocampus (centered at 3.8 mm posterior and 2.9 mm lateral to bregma). Eight to 9 tetrodes were used to record data from CA1 and CA3 in the dorsal hippocampus (for one rat, data were recorded from only CA1). Each tetrode was composed of 4 12.5-μm nichrome wires whose tips were plated with gold to bring the impedance to 200 kΩ at 1 kHz. Animals were allowed to recover for 5–7 d, and the tetrodes were then moved down slowly, over the course of 2–3 wk, until the tips reached the pyramidal cell layer of CA1 or CA3. Tetrodes were never turned on the day of the recording. The placement of the tetrode tips was verified by several electrophysiological hallmarks (CA1: prominent theta oscillations in local field potential; CA3: prominent gamma oscillations in local field potential; CA1 and CA3: complex spikes of pyramidal cells, theta-modulated spiking, multi-unit bursts accompanied by 200 Hz “ripples” in the field potential) and by histology. A 40-μA current was passed through each recording tetrode for 20 sec immediately prior to euthanizing the rat, and the resulting brain lesions served as confirmation of tetrode position. Pyramidal neurons were distinguished from interneurons by spike waveform and by baseline firing rate. During testing, spike activity was filtered (600–6000 Hz), and above-threshold spikes were saved for offline analysis. Activity of individual neurons was obtained by using software (Offline Sorter, Plexon Inc.) to define clusters of spikes determined by visually inspecting several waveform characteristics across the four wires (e.g., spike amplitude or waveform shape). All analyses were conducted with MATLAB (MathWorks).
Acknowledgments
We thank Kimberly Ong, Lisa Pytka, Carolyn Pearson, and Hannah Dalke for their assistance. This research was supported by grants NIH MH079564 (J.R.M), NIH MH51570 (H.E.), and NSF SBE0354378 (H.E.).
Footnotes
[Supplemental material is available online at http://www.learnmem.org.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1484509.
References
- Alvarez P, Lipton PA, Melrose R, Eichenbaum H. Differential effects of damage within the hippocampal region on memory for a natural, nonspatial odor-odor association. Learn Mem. 2001;8:79–86. doi: 10.1101/lm.38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Shapiro ML, O'Malley MT, Eichenbaum H. Positional firing properties of perirhinal cortex neurons. Neuroreport. 1998;9:3013–3018. doi: 10.1097/00001756-199809140-00017. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. J Neurosci. 2002;22:4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TM, Hart PE. Nearest neighbor pattern classification. IEEE Trans Inf Theory. 1967;13:21–27. [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: Parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Stark CE, Squire LR. The visual paired-comparison task as a measure of declarative memory. Proc Natl Acad Sci. 2000;97:12375–12379. doi: 10.1073/pnas.220398097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the “cognitive map.”. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in the anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J. Do hippocampal pyramidal cells signal non-spatial as well as spatial information? Hippocampus. 1999;9:352–364. doi: 10.1002/(SICI)1098-1063(1999)9:4<352::AID-HIPO3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford University Press; Oxford, UK: 1978. [Google Scholar]
- Pascalis O, Hunkin NM, Holdstock JS, Isaac CL, Mayes AR. Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and relatively intact item recognition. Neuropsychologia. 2004;42:1293–1300. doi: 10.1016/j.neuropsychologia.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Muhot M. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey. V: Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]