Abstract
Mutations in the neuronal protein α-synuclein cause familial Parkinson disease. Phosphorylation of α-synuclein at serine 129 is prominent in Parkinson disease and influences α-synuclein neurotoxicity. Here we report that α-synuclein is also phosphorylated at tyrosine 125 in transgenic Drosophila expressing wild-type human α-synuclein and that this tyrosine phosphorylation protects from α-synuclein neurotoxicity in a Drosophila model of Parkinson disease. Western blot analysis of fly brain homogenates showed that levels of soluble oligomeric species of α-synuclein were increased by phosphorylation at serine 129 and decreased by tyrosine 125 phosphorylation. Tyrosine 125 phosphorylation diminished during the normal aging process in both humans and flies. Notably, cortical tissue from patients with the Parkinson disease–related synucleinopathy dementia with Lewy bodies showed less phosphorylation at tyrosine 125. Our findings suggest that α-synuclein neurotoxicity in Parkinson disease and related synucleinopathies may result from an imbalance between the detrimental, oligomer-promoting effect of serine 129 phosphorylation and a neuroprotective action of tyrosine 125 phosphorylation that inhibits toxic oligomer formation.
Introduction
α-Synuclein has been strongly implicated in the pathogenesis of Parkinson disease both genetically and pathologically. A missense mutation in α-synuclein, A53T, was the first defined genetic lesion in familial Parkinson disease (1). Two additional point mutations linked to autosomal dominant early-onset Parkinson disease have subsequently been described, A30P (2) and more recently E46K (3). Duplications (4) and triplications (5) of the α-synuclein gene locus have also been identified in familial forms of Parkinson disease. The observation that increased gene dosage of normal-sequence α-synuclein can cause Parkinson disease has given strong support to the hypothesis that increased levels of α-synuclein may predispose to disease in the more common forms of the disorder that do not have an obvious familial component. Mechanisms that might control such increases in α-synuclein levels are not currently clear but may include transcription factor dysregulation (6) and inability of normal degradatory pathways to function adequately (7, 8).
A central role for α-synuclein in the pathogenesis of Parkinson disease is supported pathologically by the presence of α-synuclein in Lewy bodies. Initial reports of a mutation in the gene encoding α-synuclein in familial Parkinson disease were followed quickly by identification of α-synuclein protein as a major component of Lewy bodies (9). These filamentous protein aggregates represent the pathological hallmark of Parkinson disease, including not only the rare familial forms of the disorder caused by α-synuclein mutations, but the much more common later-onset variant, which in most cases does not involve a clear family history of the disease. In addition, Lewy bodies are also seen in a group of related disorders with pathological and some clinical similarities. These disorders are commonly termed synucleinopathies and may share important pathways of pathogenesis with Parkinson disease. Dementia with Lewy bodies is one of the more common synucleinopathies and has a number of clinical and pathological similarities to Parkinson disease but is characterized by more extensive pathological involvement of cortical areas with concomitant mental impairment (10).
Given the genetic and neuropathological importance of α-synuclein in Parkinson disease and related synucleinopathies, significant effort has been expended in investigating mechanisms that may control aggregation and neurotoxicity of the protein. Several posttranslational modifications of α-synuclein have been described in Lewy bodies, including phosphorylation at Ser129 (11), nitration at tyrosine residues (12), and C-terminal truncation (13). We have previously identified Ser129 phosphorylation as a key event in α-synuclein neurotoxicity (14) using a Drosophila model of α-synuclein neurotoxicity (15). In our system, Ser129 phosphorylation conferred toxicity to α-synuclein without a substantial increase in the number of fibrillar deposits, suggesting that nonfibrillar species of α-synuclein may be neurotoxic.
Studies in cell culture systems have shown that in addition to Ser129, 3 adjacent tyrosine residues (Tyr125, Tyr133, and Tyr136) are phosphorylated (16, 17). Phosphorylation of tyrosine residues in the carboxyterminal segment of α-synuclein suppresses eosin-induced oligomerization (18). Here we report that Tyr125 phosphorylation is present in both human and Drosophila brain. Further, experimental manipulation of tyrosine phosphorylation supports a role in protecting from α-synuclein neurotoxicity.
Results
α-Synuclein is phosphorylated at Tyr125.
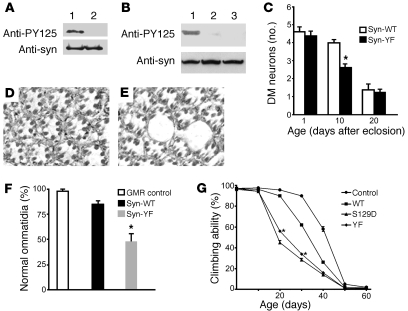
We used an antibody that specifically recognizes α-synuclein phosphorylated at Tyr125 (PY125 antibody) to demonstrate tyrosine phosphorylation in transgenic Drosophila expressing wild-type human α-synuclein (α-synWT; Figure 1A). We next created transgenic Drosophila lines carrying mutant α-synuclein complementary DNA constructs in which the COOH-terminal codons for Tyr125 alone (α-synY125F) or all 3 tyrosine residues (Y125, Y133, and Y136) had been replaced by codon for phenylalanine to prevent phosphorylation (α-synYF) (18). Specificity of the antibody was supported by lack of immunoreactivity following substituting Tyr125 to phenylalanine (Figure 1A). In addition, pretreatment of the homogenate from the brains of flies expressing wild-type α-synuclein with phosphatase to remove phosphate groups also abolished immunoreactivity (Figure 1B). These findings taken together strongly support the specificity of the PY125 antibody in these studies.
Figure 1. Blocking tyrosine phosphorylation increases α-synuclein toxicity.
(A) The PY125 antibody specifically recognizes phosphorylated Tyr125. Phenylalanine substitution at Tyr125 eliminates immunoreactivity. Lane 1, α-synWT; lane 2, α-synY125F. The same membrane was stripped and reprobed with a phosphorylation-independent α-synuclein antibody (anti-syn). (B) The α-synYF mutant effectively eliminates anti-PY125 immunoreactivity. Compare lane 1 (α-synWT) with lane 3 (α-synY125F). Lane 2 (α-synWT) is the same as lane 1 except for pretreatment with phosphatase. Flies were 20 days old. (C) Quantitative analysis of TH-immunoreactive dorsomedial dopamine neurons over time in transgenic flies. Values represent mean ± SEM. Accelerated loss of TH-immunoreactive neurons is observed in 10-day-old α-synYF transgenic flies (*P < 0.05, multivariant ANOVA with supplementary Newman-Keuls test). The driver in A–C was elav-GAL4. (D and E) Blocking tyrosine phosphorylation enhances retinal degeneration. Tangential section through the retina of an aged adult fly expressing α-synWT shows mild architectural distortion (D), whereas expression of α-synYF produced a greater loss of retinal integrity with large vacuoles (E). Original magnification in D and E, ×400. (F) Quantitative comparison demonstrating significant reduction in the percentage of normal photoreceptor clusters in α-synYF transgenic flies (*P < 0.01, multivariant ANOVA with supplementary Newman-Keuls test). Flies were 30 days old. The driver in D–F was GMR-GAL4. (G) Accelerated loss of climbing ability in transgenic flies expressing α-synS129D and α-synYF throughout the nervous system (elav-GAL4 driver). Asterisks indicate climbing scores that are significantly different from the control and wild-type α-synuclein transgenic scores (*P < 0.01, multivariant ANOVA with supplementary Newman-Keuls test). Control genotype: elav-GAL4/+. Error bars in F and G represent SEM.
Tyrosine phosphorylation protects from α-synuclein neurotoxicity.
To determine the role of tyrosine phosphorylation in α-synuclein neurotoxicity and aggregation, we used the binary GAL4/UAS system to direct tissue-specific transgene expression (19). Lines expressing equivalent levels of α-synWT and α-synYF were used in subsequent studies (Figure 1B and Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI39088DS1). First, the elav-GAL4 driver was used to target expression in a pan-neural pattern. Brains from transgenic flies 1, 5, 10, 15, 20, and 30 days after eclosion were then immunostained with an antibody against tyrosine hydroxylase (TH), which specifically identifies dopaminergic neurons. We focused our analysis on the dorsomedial cluster of dopaminergic neurons, because these neurons are readily identifiable and are preferentially sensitive to α-synuclein toxicity (15, 20, 21). In 1-day-old and 5-day-old transgenic flies expressing α-synYF, the number of dopaminergic neurons in the dorsomedial clusters was not significantly different from the number present in young flies expressing α-synWT or in nontransgenic controls (Figure 1C). Thus, expression of tyrosine phosphorylation–incompetent α-synuclein does not disrupt development of dopaminergic neurons. However, onset of neurodegeneration was significantly accelerated. On average, 2 TH-immunoreactive neurons were observed in 10-day-old flies expressing α-synYF, whereas dorsomedial dopaminergic neurons were relatively well preserved in wild-type α-synuclein transgenic lines at the same time point (Figure 1C). Similar to flies expressing α-synWT, those expressing α-synYF exhibited a marked loss of TH-immunoreactive neurons by 20 days of age (Figure 1C). No further loss was noted at 30 days (data not shown).
With the pan-neural elav-GAL4 driver we have not previously seen significant toxicity of α-synuclein in brain cells other than dopaminergic neurons (15), even when we have analyzed a mutant form of α-synuclein with increased neurotoxicity or genetic modifiers that enhance dopaminergic cell death (14, 22). To determine whether the enhanced toxicity of α-synYF retained specificity for dopaminergic neurons, we prepared serial sections through the whole brain and stained the sections with hematoxylin to identify neuronal and glial cell bodies in the cortex and eosin to highlight neuropil structures. These studies revealed normal overall development of the brain, with appropriate size and configuration of major brain structures. In addition, there was no clear loss of nuclei in the cortex, sign of DNA fragmentation, or loss of neuropil integrity (data not shown). Thus, enhanced α-synuclein toxicity to dopaminergic neurons was not accompanied by widespread, general neurodegeneration in the brain.
Expression of α-synWT targeted to the retina using a glass gene promoter construct (GMR-GAL4 driver) caused mild adult-onset retinal degeneration manifested by disruption of the normal arrangement of ommatidia (Figure 1D) (15). As would be predicted based on the increased toxicity seen to dopamine neurons, expression of α-synYF produced a more prominent retinal degeneration, with vacuole formation and marked loss of tissue integrity (Figure 1E). To evaluate retinal degeneration on a quantitative basis, we determined the percentage of normal ommatidia, which was significantly decreased in flies expressing α-synYF, compared with α-synWT transgenics or control flies not expressing α-synuclein (Figure 1F).
As an additional means to assess the toxicity of α-synYF, we evaluated the locomotor performance of flies expressing the mutant variant. Transgenic flies that express either the wild-type or familial Parkinson disease–linked mutant form of α-synuclein in their central nervous systems, via the pan-neural elav-GAL driver (15), or specific dopaminergic drivers (23, 24), show an age-dependent reduction in climbing ability when compared with controls. We found that flies expressing α-synYF showed a greater reduction in climbing ability compared with flies that expressed wild-type α-synuclein (Figure 1G), demonstrating the ability of α-synYF to accelerate functional decline as well as neuropathology.
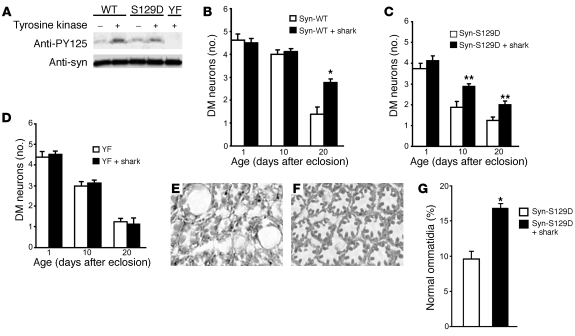
α-Synuclein can be phosphorylated at C-terminal tyrosines by a number of protein tyrosine kinases, including Syk (18) and Src family members (16). To explore the role of kinases in controlling α-synuclein toxicity in vivo and to confirm that the enhanced toxicity of α-synYF was mediated through reduced tyrosine phosphorylation, we used the GAL4/UAS system to overexpress shark, a Drosophila homolog of Syk (25). Overexpression of shark increased phosphorylation at Tyr125 on α-synWT (Figure 2A). In addition, overexpression of shark was also able to elevate Tyr125 phosphorylation of a mutant version of α-synuclein that mimics phosphorylation at Ser129 (α-synS129D). Levels of total α-synuclein were unchanged by the expression of shark (Figure 2A). Coexpression of shark significantly rescued the neurotoxicity of both α-synWT and α-synS129D (Figure 2, B and C). Thus, increasing the expression of Src tyrosine kinase increased phosphorylation of α-synuclein in vivo and ameliorated its selective neurotoxicity.
Figure 2. Overexpression of Drosophila tyrosine kinase shark increases α-synuclein phosphorylation and ameliorates its neurotoxicity.
(A) Increased tyrosine phosphorylation in flies coexpressing shark and α-synuclein in the brain compared with flies expressing α-synuclein alone. Flies were 1 day old. (B–D) Quantitative analysis of TH-immunoreactive neurons in flies coexpressing shark and α-synuclein and flies expressing α-synuclein alone. Values represent mean ± SEM. Asterisks indicate that the difference in TH-immunoreactive dopaminergic neuronal number between the 2 genotypes at the time points examined is statistically significant (*P < 0.01, **P < 0.05, multivariant ANOVA with supplementary Newman-Keuls test). The driver in A–D was elav-GAL4. (E–G) Augmented tyrosine phosphorylation suppresses retinal toxicity. Retinal degeneration in flies expressing α-synS129D (E) is largely prevented by coexpressing shark (F). Original magnification in E and F, ×400. (G) Quantitative comparison demonstrating significant rescue of the percentage of normal photoreceptor clusters in α-synS129D transgenic flies expressing shark (*P < 0.01, Student’s t test). Error bars represent SEM. The driver in E–G was GMR-GAL4. Flies were 30 days old.
In contrast, no significant rescue was observed upon coexpression of shark and α-synYF (Figure 2D), indicating that the rescue was indeed mediated via tyrosine residues and supporting the specificity of shark action. Similarly, targeting shark expression to the eye decreased the retinal toxicity of α-synuclein. Retinal cell loss was increased in flies expressing α-synS129D compared with α-synWT (Figure 2E). Retinal degeneration was attenuated by coexpression of shark, as demonstrated by representative histological sections (Figure 2F) and quantitative analysis (Figure 2G).
Opposing effects on soluble oligomer formation.
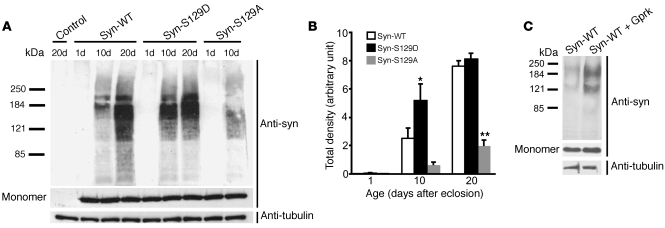
We have recently identified soluble oligomeric species of α-synuclein in transgenic Drosophila (22). Formation of these oligomer species requires the central NAC aggregation domain of α-synuclein (26). To determine the role that α-synuclein phosphorylation plays in controlling oligomer formation in vivo, we first characterized the levels of oligomeric species in flies expressing α-synS129D and the Ser129 phosphorylation–incompetent mutant α-synS129A, which is not toxic in our system (14). Western blot analysis of high-speed supernatants of fly brain homogenates did not identify oligomeric forms in 20-day-old nontransgenic controls, or in 1-day-old α-synuclein transgenic flies. In contrast, oligomeric species appeared in 10-day-old α-synuclein transgenic flies and accumulated with age. Levels of soluble oligomers correlated well with α-synuclein neurotoxicity. Soluble oligomers accumulated rapidly in flies expressing α-synS129D and were significantly less abundant in flies expressing α-synS129A (Figure 3, A and B). Preventing Ser129 phosphorylation with the α-synS129A mutant did not completely block oligomer formation, supporting the ability of α-synS129A to have toxicity under other circumstances (see Discussion). Coexpression of wild-type α-synuclein and the serine kinase Gprk2 produced an enhancement of α-synuclein oligomerization similar to that seen with expression of α-synS129D (Figure 3C), consistent with a role for endogenous phosphorylation at Ser129 in controlling oligomerization.
Figure 3. α-Synuclein toxicity correlates with accumulation of soluble high-molecular-weight oligomers.
(A) Representative immunoblot of soluble fractions from brain homogenates run on a 4%–12% gradient gel shows increased oligomer formation in flies expressing α-synS129D and decreased oligomer formation in flies expressing α-synS129A. Aliquots of the same protein preparations were loaded on a separate 10%–20% gel to visualize monomeric α-synuclein and tubulin. Control genotype: elav-GAL4. (B) Quantitative analysis of oligomer formation using densitometry. Values represent mean ± SEM of 3 independent experiments. Single asterisk indicates the significantly increased accumulation of high-molecular-weight species in flies expressing α-synS129D (*P < 0.01, multivariant ANOVA with supplementary Newman-Keuls test) at 10 days. Double asterisks indicate the significantly decreased accumulation in α-synS129A flies at 20 days (**P < 0.01, multivariant ANOVA with supplementary Newman-Keuls test). (C) Coexpression of the serine kinase Gprk2 with α-synWT increases high-molecular-weight α-synuclein species. Flies were 10 days old.
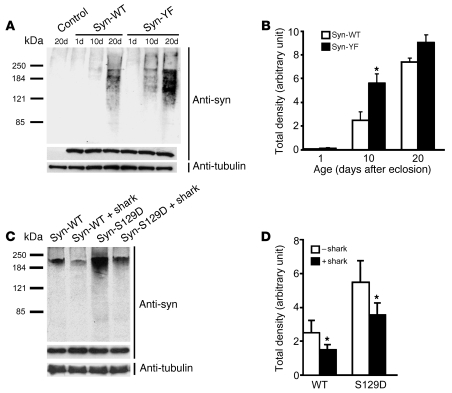
To determine whether Tyr125 phosphorylation also altered α-synuclein oligomerization, we compared transgenic lines expressing α-synWT and α-synYF. Preventing Tyr125 phosphorylation with the α-synYF mutation promoted earlier accumulation of oligomeric species compared with flies expressing the wild-type protein (Figure 4, A and B). Conversely, increasing Tyr125 phosphorylation by expression of shark kinase decreased soluble oligomer formation of both α-synWT and α-synS129D (Figure 4, C and D). Thus, the formation of soluble oligomers was regulated in an antagonistic fashion by phosphorylation of Ser129 and tyrosines in the carboxyterminal domain of α-synuclein. Consistent with a role for phosphorylation of Ser129 in promoting oligomer formation and phosphorylation of Tyr125 in preventing oligomerization, oligomers were preferentially phosphorylated at Ser129 compared with Tyr125 (Supplemental Figure 2).
Figure 4. Tyr125 phosphorylation status alters α-synuclein oligomerization.
(A) Increased high-molecular-weight α-synuclein species are evident in flies expressing α-synYF. A lighter exposure than in Figure 3A is shown to demonstrate the increase in oligomeric species in α-synYF. Control genotype: elav-GAL4. (B) Quantitative analysis of oligomer formation. Values represent mean ± SEM of 3 independent experiments. Asterisk indicates significant difference compared with α-synWT (*P < 0.01, multivariant ANOVA with supplementary Newman-Keuls test). (C) Coexpression of the tyrosine kinase shark decreases high-molecular-weight α-synuclein species formation of both α-synWT and α-synS129D. (D) Quantitative analysis of shark kinase inhibition of oligomer formation. Values represent mean ± SEM of 3 independent experiments. Asterisks indicate significant decrease in flies coexpressing shark and α-synuclein (*P < 0.05, Student’s t test). Flies were 10 days old.
To further investigate the role of Tyr125 phosphorylation in modulating the solubility of α-synuclein, we monitored the formation of large insoluble protein aggregates, or inclusion bodies, by immunostaining fly brains with antibodies directed against α-synuclein (15, 22). We saw no clear alteration in the number of large inclusions in α-synYF transgenic flies. Nor was there any detectable increase in proteinase K resistance, a typical feature of fibrillar protein aggregates (27) (data not shown).
To determine whether sufficient tyrosine phosphorylation of α-synuclein is present in our system to support a plausible role for tyrosine phosphorylation in disease pathogenesis, we evaluated the amount of α-synuclein that was phosphorylated at Tyr125 in vivo. We performed 2-dimensional gel electrophoresis followed by Western blotting using fresh fly brain homogenates prepared in the presence of phosphatase inhibitors. We found that approximately 30% of total α-synuclein was phosphorylated at Tyr125 (Supplemental Figure 3), supporting the plausibility of a role for Tyr125 phosphorylation in controlling α-synuclein aggregation and neurotoxicity in vivo. A previous study of human postmortem tissue failed to identify tyrosine-phosphorylated α-synuclein (28). We therefore evaluated the sensitivity of Tyr125 phosphorylation in our system by incubating postmortem tissue in the absence of phosphatase inhibitors for various periods of time. We observed that the reactivity of α-synuclein to anti-PY125 decreased with increasing intervals of incubation, consistent with postmortem dephosphorylation at Tyr125 (Supplemental Figure 4).
Possible mechanisms of neuroprotection.
Although we demonstrate that tyrosine phosphorylation decreases oligomer formation, the way in which phosphorylation controls aggregation is not clear. One possibility is that phosphorylation of α-synuclein at Tyr125 might protect from neurotoxicity and oligomer formation by decreasing phosphorylation at Ser129. We thus compared the degree of Ser129 phosphorylation in flies with varying levels of Tyr125 phosphorylation. We detected no clear differences in the levels of phospho-Ser129 among flies expressing α-synWT, α-synYF, and α-synWT coexpressed with shark kinase to increase Tyr125 phosphorylation (Supplemental Figure 5, A and C). We also detected no influence of Ser129 phosphorylation on phosphorylation of Tyr125. Similar levels of phospho-Tyr125 were present in flies expressing α-synWT, α-synS129A, and α-synS129D (Supplemental Figure 5, B and D). The ability of the PY125 antibody to recognize α-synS129A and α-synS129D to the same degree as α-synWT further supports the specificity of the antibody.
Alternatively, phosphorylation of α-synuclein at Tyr125 might influence proteolytic processing of α-synuclein. However, we found no clear alteration in levels of C-terminally truncated α-synuclein in flies expressing α-synYF or flies coexpressing shark kinase along with α-synWT to increase phosphorylation of Tyr125 (Supplemental Figure 6). To further explore the role of phosphorylation in controlling C-terminal proteolysis of α-synuclein, we examined the levels of truncated protein in flies expressing α-synS129A or α-synS129D, as well as flies coexpressing α-synWT and the kinase Gprk2 to increase phosphorylation at Ser129. In these experiments, we found no evidence that modification of Ser129 altered levels of C-terminally truncated α-synuclein (Supplemental Figure 6).
Tyr125 phosphorylation diminishes during human aging.
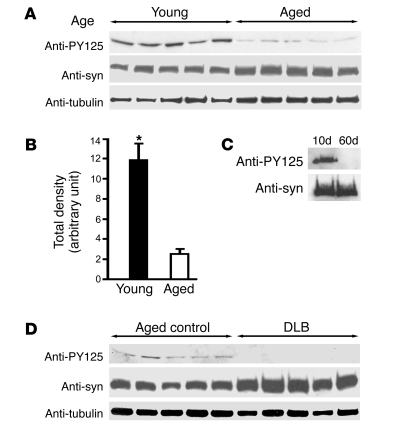
To explore the relevance of Tyr125 phosphorylation of α-synuclein in humans, we examined the levels of phosphorylation in frontal cortical homogenates from young (18–28 years) and aged (69–86 years) normal adult controls. We found a striking reduction in Tyr125 phosphorylation with aging (Figure 5, A and B). Remarkably, we saw a similar aging-related decline in α-synuclein phosphorylation in transgenic flies (Figure 5C). The age-related reduction in tyrosine phosphorylation of α-synuclein appears to be specific, because overall tyrosine phosphorylation was not significantly decreased in aged Drosophila (data not shown). Increasing age is an important risk factor for developing symptomatic α-synuclein neurotoxicity in both humans and flies (15, 29).
Figure 5. α-Synuclein is phosphorylated at Tyr125, and phosphorylation is reduced in aging human and Drosophila brain.
(A) Age-related tyrosine phosphorylation levels in humans. Shown are immunoblots from 5 young (≤28 years old) and 5 aged (≥69 years old) frontal cortical samples as detected by the antibody specific for α-synuclein phosphorylated at Tyr125. (B) The densitometry intensities of anti-PY125–positive bands pooled from both young and aged groups. Values represent mean ± SEM of 3 independent experiments (*P ≤ 0.01, Student’s t test). (C) The PY125 blot demonstrates age-related decline in tyrosine-phosphorylated wild-type α-synuclein in Drosophila brain extracts. The driver was elav-GAL4. Days after eclosion are indicated. (D) Tyr125 phosphorylation is further reduced in dementia with Lewy body (DLB) patients. Shown are immunoblots of frontal cortical samples from 5 DLB cases (≥65 years old) and 5 aged controls (≥69 years old).
We next compared the levels of Tyr125 phosphorylation in brain homogenates from normal aged controls and patients with dementia with Lewy bodies, a synucleinopathy with prominent cortical Lewy bodies (30). We did not detect phosphorylation at Tyr125 in frontal cortical homogenates from 5 patients (65–82 years) (Figure 5D), in contrast to clear immunoreactivity in aged controls (69–86 years). Thus, loss of protective tyrosine phosphorylation may predispose to clinically relevant α-synuclein neurotoxicity in human disease.
Discussion
Here we document opposing effects on neurotoxicity by different C-terminal phosphorylation events on the α-synuclein molecule. We have previously demonstrated that phosphorylation of Ser129 increases neurotoxicity in our transgenic Drosophila model (14). We now find the opposite effect of tyrosine phosphorylation: C-terminal tyrosine phosphorylation reduces toxicity of α-synuclein toward dopaminergic and retinal neurons (Figure 1). We further suggest that tyrosine phosphorylation can act downstream of Ser129 phosphorylation because overexpression of shark kinase rescued the neurotoxicity of a pseudophosphorylated version of α-synuclein (α-synS129D).
To determine the mechanism by which tyrosine phosphorylation protects from α-synuclein neurotoxicity, we examined a number of properties of α-synuclein that have been linked to neurotoxicity. Phosphorylation of C-terminal tyrosines might plausibly alter other C-terminal posttranslational modifications of α-synuclein. However, in our model, we found no evidence for changes in Ser129 phosphorylation mediated by tyrosine phosphorylation (Supplemental Figure 5, A and C). Nor did serine phosphorylation influence tyrosine phosphorylation (Supplemental Figure 5, B and D). C-terminal truncation of α-synuclein occurs in humans and in mice and flies expressing human α-synuclein (13). C-terminal truncation is associated with enhanced neurotoxicity in mice and Drosophila (31). We found no evidence that tyrosine phosphorylation alters the amount of C-terminally truncated α-synuclein (Supplemental Figure 5). Similarly, phosphorylation at Ser129 did not influence C-terminal truncation.
Aggregation has been strongly implicated in the pathogenesis of Parkinson disease, first by the association of Lewy bodies and Lewy neurites with the disorder (32) and later by many studies exploring biophysical and cell biological determinants of α-synuclein solubility (33). We did not find changes in the numbers or distribution of large α-synuclein–containing aggregates when we manipulated tyrosine phosphorylation. However, we did observe significant alterations in smaller aggregated species (Figure 4), or oligomers. We further found that phosphorylation of Ser129 also influenced oligomerization of α-synuclein in vivo, with a correlation between the degree of neurotoxicity and propensity to form oligomers (Figure 3). Of note, our prior studies on phosphorylation of Ser129 dissociated the formation of large inclusions with neurotoxicity (14), although we do find that aggregation is required for toxicity in our system (22). Our overall findings thus support the hypothesis that the antagonistic actions of neurotoxic Ser129 phosphorylation and neuroprotective Tyr125 phosphorylation are mediated through opposite effects on the formation of small toxic aggregates.
However, the precise identity of the toxic species of α-synuclein remains controversial. In human tissue, the number of large aggregates generally correlates with neuronal dysfunction (34). On the other hand, electron microscopic studies have documented well-preserved organelles in inclusion-bearing nigral neurons, suggesting that neurons with inclusions are healthy (35). Experimental studies in a variety of cell culture and animal models have produced conflicting results regarding the nature of the toxic species of α-synuclein. Indeed, it is possible that a variety of aggregated species have neurotoxicity (36, 37).
Tyrosine phosphorylation of α-synuclein has been documented in several tissue culture systems (16–18, 38). In the studies of Mirzaei et al. (38), tyrosine phosphorylation occurs in response to rotenone treatment, suggesting a response to oxidative stress conditions, which have been strongly implicated in Parkinson disease (39). However, a mass spectrometric study using postmortem tissue from patients failed to identify tyrosine phosphorylation of α-synuclein (28). Since the antibody we use is specific for α-synuclein phosphorylated at Tyr125 (Figure 1) and we document substantial decay of Tyr125 phosphorylation with increasing postmortem period (Supplemental Figure 4), postmortem loss of phosphorylation at Tyr125 may have limited prior attempts to visualize detect phosphorylation in human tissue. A detailed understanding of the role of tyrosine phosphorylation of α-synuclein may require the development of additional immunological reagents because we have not been successful in using the current PY125 antibody to localize tyrosine-phosphorylated α-synuclein in tissue sections.
Our findings are consistent with a number of studies that have associated phosphorylation of Ser129 with increased toxicity of α-synuclein in mouse models and tissue culture systems (40–43). However, two reports from studies in rat models using retrovirus-mediated expression of α-synWT, α-synS129A, and α-synS129D in substantia nigra neurons yielded conflicting results (44, 45). The α-synS129A variant showed toxicity in both studies, while results for α-synS129D were variable. Effects of the mutants on aggregation were not consistent, with both increases and decreases in the numbers of large aggregates suggested for both variants. A more recent report failed to demonstrate differences in neurotoxicity or aggregation when comparing α-synWT, α-synS129A, and α-synS129D (46). The reason for these discrepancies is not clear. One possibility is that dose-dependent interactions between rat α-synuclein and virally expressed mutant human α-synuclein alter the aggregation properties of the protein, as has been demonstrated in vitro for mixtures of mouse and human α-synuclein (47). Since we and others have implicated oligomeric species in α-synuclein neurotoxicity, it would also be of interest to analyze oligomer levels in the rat system.
The report of Paleologou et al. suggesting that the α-synS129A and α-synS129D variants do not accurately recapitulate all aspects of the in vitro biochemical behavior of authentically nonphosphorylated and phosphorylated α-synuclein raises the possibility that the phosphorylation mutants fail to replicate aspects of α-synuclein biology in vivo (48). To address this important issue, we have not only relied on mutant forms of α-synuclein, but have also altered kinase levels and documented changes in phosphorylation levels of α-synuclein (ref. 14; Figures 2–4; Supplemental Figures 5 and 6). In our studies, altering α-synuclein phosphorylation using genetic methods produces effects consistent with those seen with expression of mutant forms of α-synuclein.
Advancing age is the most important risk factor for developing neurodegenerative disorders, including Parkinson disease and other α-synucleinopathies (29). Similarly, dopaminergic neurodegeneration and inclusion formation are seen with increasing age in our α-synuclein transgenic Drosophila model (15). We show here that protective tyrosine phosphorylation of α-synuclein progressively decreases with aging in both flies and humans. These findings suggest that aging-related changes in kinase and phosphatase regulatory networks controlling α-synuclein tyrosine phosphorylation are conserved between vertebrates and invertebrates. In addition to the specific implications for Parkinson disease and related disorders, our results raise the more general possibility that aging-related alterations in posttranslational modifications influencing protein aggregation may be important in the development of neurodegenerative disorders.
Although the normal role of α-synuclein is not yet clearly established, a number of studies have suggested a function in controlling synaptic neurotransmission (49–51), possibly by regulating SNARE complex integrity (52). If tyrosine phosphorylation is important for the normal synaptic function of α-synuclein, loss of tyrosine-phosphorylated α-synuclein might be relevant to aging-related alterations in processes such as synaptic plasticity and vesicular transport that could impact cognitive function in the elderly (53). Regardless of the impact on the normal function of α-synuclein, our data suggest that decreased neuroprotective tyrosine phosphorylation during aging may contribute to the increased incidence of Parkinson disease and related synucleinopathies that occurs in older individuals. These findings emphasize the importance of investigating the kinases responsible for phosphorylating α-synuclein in vivo, as they hold promises for novel therapeutic targets in preventing and treating α-synucleinopathies, including Parkinson disease.
Methods
Transgenic Drosophila.
Fruit flies were grown on standard cornmeal medium at 25°C. The coding sequences for α-synuclein carboxyterminal tyrosine residues (TAT or TAC) were mutated to TTT or TTC for phenylalanine (18), and mutant cDNAs were cloned into the GAL4-responsive pUAST expression vector. Transgenic strains were created by embryo injection. At least 4 independent transgenic lines were derived for each construct. The UAS-shark line was a gift from E.R. Stanley (Albert Einstein College of Medicine, New York, New York, USA; ref. 54).
Quantitative real-time PCR.
RNA was isolated from fly heads using TRIzol solution (Invitrogen) as indicated by the manufacturer, except an additional centrifugation was performed at 11,000 g for 10 minutes to remove cuticles prior to the addition of chloroform. RNA quality was evaluated on 1% agarose gel. One microgram of total RNA was used in the reverse-transcription reaction (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems). Real-time PCR amplifications were performed with an Applied Biosystems StepOne system. Primers were designed using Primer Express 3.0 and were used at a final concentration of 150 nM. PCR reactions were set up in a 16-μl reaction volume using SYBR Green PCR Master Mix, and RpL32 ribosomal protein L32 transcript was used as the endogenous control.
Histological and immunocytochemical analysis.
Adult flies were fixed in formalin and embedded in paraffin. To assess brain morphology, we stained serial 4-μm sections with hematoxylin and eosin. Immunostaining on paraffin sections was performed using an avidin-biotin-peroxidase complex method as described previously (14). For evaluation of dopaminergic neurons, the number of TH-immunoreactive cells (anti-TH, 1:500; ImmunoStar) at the level of the giant interneuron commissure was determined in well-oriented frontal sections. At least 8 hemibrains were examined per genotype and time point.
For histological examination of retinas, heads were fixed in glutaraldehyde and embedded in paraffin. Tangential retinal sections were prepared at a thickness of 2 μm and stained with toluidine blue. For quantitative analysis, areas including 20 ommatidia (photoreceptor clusters) were selected, and the number of normal ommatidia was determined. Ommatidia were considered abnormal if the number of rhabdomeres was fewer than the normal 7 or if vacuoles disrupted the normal structure significantly enough to efface at least 50% of the ommatidia. Retinas from at least 6 animals were examined per genotype and time point.
Western blot analysis and oligomer detection.
Adult fly heads were analyzed by standard SDS-PAGE and immunoblotted as described previously (14). Human postmortem brain tissues from age- and postmortem interval–matched patients (Massachusetts Alzheimer’s Disease Research Center Brain Bank; experiments performed according to protocols approved by the Institutional Review Board of Partners HealthCare, Boston, Massachusetts, USA) were homogenized in lysis buffer (50 mM Tris-HCl at pH 7.4, 170 mM NaCl, 5 mM EGTA) containing protease inhibitors (Complete Mini; Roche) and phosphatase inhibitor (PhosSTOP tablet; Roche). The lysate was centrifuged at 15,000 g at 4°C for 15 minutes, and the resulting supernatant was collected and analyzed. After exposure, band intensities were quantified using NIH ImageJ (http://rsbweb.nih.gov/ij/). Primary antibodies used were anti–α-synuclein (clone 42, 1:20,000; BD Biosciences), anti–phospho-Ser129 (1:5,000; Wako Chemicals), anti–phospho-Tyr125 (1:5,000; Abcam ab10789). For phosphatase treatment, frozen heads were first homogenized in homogenization buffer (10 mM Tris, pH 7.4, 800 mM NaCl, 1 mM EGTA, and 10% sucrose) and then incubated with lambda protein phosphatase (New England Biolabs) at 37°C for 2 hours before mixing with the SDS-PAGE loading buffer.
To compare oligomer levels, 200 heads for each genotype and time point were homogenized at 4°C in TNE buffer (10 mM Tris-HCl at pH 7.5, 150 mM NaCl, 5 mM EGTA) containing protease inhibitors (Complete Mini; Roche), phosphatase inhibitors (PhosSTOP tablet; Roche), and detergent (0.5% Nonidet P-40) (55). The homogenate was centrifuged at 100,000 g at 4°C for 1 hour. The resulting supernatant was loaded without heating onto a 4%–12% Tris-glycine gradient gel. The primary antibody, clone 42, was used at a concentration of 1:5,000 to identify oligomers. Corresponding monomer blots to assess total α-synuclein levels were performed with clone 42 at a concentration of 1:20,000. Adjustment of the primary antibody concentration was made to ensure adequate detection of the lower-abundance oligomeric species. Controls were run for both oligomer and monomer blots to ensure that detection conditions were within the linear range of detection for quantitative analysis.
Insoluble α-synuclein (Supplemental Figure 5) was prepared by the method of Lee et al. (55). Briefly, 25 heads for each genotype at 20 days of age were homogenized at 4°C in fractionation buffer containing protease inhibitors, phosphatase inhibitors, and Nonidet P-40. The homogenate was centrifuged at 100,000 g for 1 hour at 4°C. The resulting pellet was resuspended in the same TNE/NP-40 buffer followed by centrifugation at 100,000 g for 1 hour. The pellet was taken as the insoluble fraction.
Two-dimensional gel electrophoresis.
The first dimension by isoelectric focusing was performed using the Bio-Rad PROTEAN IEF cell (Bio-Rad). Adult fly heads were homogenized in rehydration buffer (8 M urea, 2% CHAPS, 50 mM dithiothreitol, 0.2% ampholytes) containing protease and phosphatase inhibitors; 0.25 mg total protein was applied to an 11-cm IPG strip (pH 4–7); and electrophoresis was performed for 5 hours at 8,000 V. The separated proteins were then resolved in the second dimension by standard PAGE on a 12% gel, which was subsequently subjected to immunoblot analysis.
Climbing assay.
The climbing assay was performed as described previously (15). The experiment was carried out under red light (Kodak safelight filter 1A). Twenty flies were placed in a plastic vial and gently tapped to the bottom of the vial. The number of flies at the top the vial was determined after 18 seconds of climbing. Five trials were performed for each time point, and the experiment was repeated 3 times.
Statistics.
Statistical analyses were performed using multivariant ANOVA with supplementary Newman-Keuls test or 2-tailed Student’s t test. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank D. Rennie (Cutaneous Biology Research Center, Massachusetts General Hospital) for fly embryo injection. We also acknowledge the Massachusetts General Hospital ADRC Brain Bank. We appreciate the assistance of Katherine Sharpe and Paola Merlo with real-time PCR measurements. This work was supported by NIH grants R01-NS41536 (to M.B. Feany) and P50-NS38372 (to B.T Hyman) and by the Parkinson’s Disease Foundation (to L. Chen).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 119:3257–3265 (2009). doi:10.1172/JCI39088.
References
- 1.Polymeropoulos M.H., et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 2.Kruger R., et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 3.Zarranz J.J., et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 4.Chartier-Harlin M.C., et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 5.Singleton A.B., et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 6.Scherzer C.R., et al. GATA transcription factors directly regulate the Parkinson’s disease-linked gene alpha-synuclein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olanow C.W. The pathogenesis of cell death in Parkinson’s disease — 2007. Mov. Disord. 2007;22(Suppl. 17):S335–S342. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Vicente M., et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spillantini M.G., et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 10.McKeith I.G., et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara H., et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 12.Giasson B.I., et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 13.Li W., et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L., Feany M.B. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 15.Feany M.B., Bender W.W. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 16.Ellis C.E., Schwartzberg P.L., Grider T.L., Fink D.W., Nussbaum R.L. alpha-synuclein is phosphorylated by members of the Src family of protein-tyrosine kinases. J. Biol. Chem. 2001;276:3879–3884. doi: 10.1074/jbc.M010316200. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T., Yamashita H., Takahashi T., Nakamura S. Activated Fyn phosphorylates alpha-synuclein at tyrosine residue 125. Biochem. Biophys. Res. Commun. 2001;280:1085–1092. doi: 10.1006/bbrc.2000.4253. [DOI] [PubMed] [Google Scholar]
- 18.Negro A., Brunati A.M., Donella-Deana A., Massimino M.L., Pinna L.A. Multiple phosphorylation of alpha-synuclein by protein tyrosine kinase Syk prevents eosin-induced aggregation. FASEB J. 2002;16:210–212. doi: 10.1096/fj.01-0517fje. [DOI] [PubMed] [Google Scholar]
- 19.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 20.Auluck P.K., Chan H.Y., Trojanowski J.Q., Lee V.M., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y., Nishimura I., Imai Y., Takahashi R., Lu B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron. 2003;37:911–924. doi: 10.1016/S0896-6273(03)00143-0. [DOI] [PubMed] [Google Scholar]
- 22.Periquet M., Fulga T., Myllykangas L., Schlossmacher M.G., Feany M.B. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J. Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haywood A.F., Staveley B.E. Parkin counteracts symptoms in a Drosophila model of Parkinson’s disease. BMC Neurosci. 2004;5:14. doi: 10.1186/1471-2202-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botella J.A., Bayersdorfer F., Schneuwly S. Superoxide dismutase overexpression protects dopaminergic neurons in a Drosophila model of Parkinson’s disease. Neurobiol. Dis. 2008;30:65–73. doi: 10.1016/j.nbd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Ferrante A.W., Jr., Reinke R., Stanley E.R. Shark, a Src homology 2, ankyrin repeat, tyrosine kinase, is expressed on the apical surfaces of ectodermal epithelia. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1911–1915. doi: 10.1073/pnas.92.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giasson B.I., Murray I.V., Trojanowski J.Q., Lee V.M. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J. Biol. Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 27.Neumann M., et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J. Clin. Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson J.P., et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 29.Moghal S., Rajput A.H., D’Arcy C., Rajput R. Prevalence of movement disorders in elderly community residents. Neuroepidemiology. 1994;13:175–178. doi: 10.1159/000110376. [DOI] [PubMed] [Google Scholar]
- 30.Lippa C.F., et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 31.Tofaris G.K., et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J. Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holdorff B. Fritz Heinrich Lewy (1885-1950). J. Neurol. 2006;253:677–678. doi: 10.1007/s00415-006-0130-2. [DOI] [PubMed] [Google Scholar]
- 33.Waxman E.A., Giasson B.I. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim. Biophys. Acta. 2009;1792:616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurtig H.I., et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 35.Forno L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Lansbury P.T., Lashuel H.A. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 37.Lee V.M., Trojanowski J.Q. Mechanisms of Parkinson’s disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Mirzaei H., Schieler J.L., Rochet J.C., Regnier F. Identification of rotenone-induced modifications in alpha-synuclein using affinity pull-down and tandem mass spectrometry. Anal. Chem. 2006;78:2422–2431. doi: 10.1021/ac051978n. [DOI] [PubMed] [Google Scholar]
- 39.Savitt J.M., Dawson V.L., Dawson T.M. Diagnosis and treatment of Parkinson disease: molecules to medicine. J. Clin. Invest. 2006;116:1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwatsubo T. Pathological biochemistry of alpha-synucleinopathy. Neuropathology. 2007;27:474–478. doi: 10.1111/j.1440-1789.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 41.Ihara M., et al. Sept4, a component of presynaptic scaffold and Lewy bodies, is required for the suppression of alpha-synuclein neurotoxicity. Neuron. 2007;53:519–533. doi: 10.1016/j.neuron.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Sugeno N., et al. Serine 129 Phosphorylation of {alpha}-synuclein induces unfolded protein response-mediated cell death. J. Biol. Chem. 2008;283:23179–23188. doi: 10.1074/jbc.M802223200. [DOI] [PubMed] [Google Scholar]
- 43.Zabrocki P., et al. Phosphorylation, lipid raft interaction and traffic of alpha-synuclein in a yeast model for Parkinson. Biochim. Biophys. Acta. 2008;1783:1767–1780. doi: 10.1016/j.bbamcr.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Azeredo da Silveira S., et al. Phosphorylation Does Not Prompt, Nor Prevent, the Formation of {alpha}-synuclein Toxic Species in a Rat Model of Parkinson’s Disease. Hum. Mol. Genet. 2008;18:872–887. doi: 10.1093/hmg/ddn417. [DOI] [PubMed] [Google Scholar]
- 45.Gorbatyuk O.S., et al. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFarland N.R., et al. alpha-Synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2009;68:515–524. doi: 10.1097/NEN.0b013e3181a24b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rochet J.C., Conway K.A., Lansbury P.T., Jr. Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39:10619–10626. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- 48.Paleologou K.E., et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J. Biol. Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abeliovich A., et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 50.Cabin D.E., et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy D.D., Rueter S.M., Trojanowski J.Q., Lee V.M. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O.M., Sudhof T.C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Yankner B.A. A century of cognitive decline. Nature. 2000;404:125. doi: 10.1038/35004673. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez R., et al. The Drosophila shark tyrosine kinase is required for embryonic dorsal closure. Genes Dev. 2000;14:604–614. [PMC free article] [PubMed] [Google Scholar]
- 55.Lee M.K., et al. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.