Abstract
Mutations in the enzyme superoxide dismutase 1 (SOD1) have been linked to the neurodegenerative disease amyotrophic lateral sclerosis (ALS). In this issue of the JCI, Zhong et al. report that the endogenous anticoagulant activated protein C (APC) is able to cross the blood–spinal cord barrier in mice and signal to both neuronal and non-neuronal cells (see the related article beginning on page 3437). This signaling resulted in the suppression of mutant SOD1 synthesis and retarded disease progression in a murine model of ALS. Here we discuss the potential importance of these data and possible relevance to human neurodegenerative diseases.
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease that strikes in midlife, causing progressive weakness, disability, and death. Unfortunately, the cause of ALS is unknown, and the treatment is largely palliative. Research into the pathogenesis of and potential treatments for ALS focuses heavily on experimental models that use human genetic mutations in transgenic animals or on cellular models that express mutant proteins known to impart a high risk of developing ALS in people who carry these mutations. Mutant superoxide dismutase 1 (SOD1) is the most common protein known to cause ALS in humans; however, the mechanisms underlying mutant SOD1–related ALS are unknown. People with mutant SOD1–related ALS represent only about 20% of inherited (i.e., familial) ALS cases and about 2% of all patients with ALS (1). Nevertheless, the animal models of mutant SOD1–related ALS develop a neurological disorder that mimics the human disease, and investigations using these models have taught us a lot about motor neuron biology as well as the potential interactions between motor neurons and their environment that support or destabilize their normal function and survival.
Recent publications by Garbuzova-Davis et al. (2) and Zhong et al. (3) reported evidence of abnormalities in blood-brain barrier (BBB) function in mutant SOD1 animal models of ALS. These investigators hypothesized that compromise of the BBB allows exposure of motor neurons to potentially neurotoxic proteins from the blood, such as hemoglobin (Hb), that could either initiate or accelerate the process of motor neuron degeneration. In the current issue of the JCI, Zhong and colleagues (4) have followed up on the findings of these previous studies by carefully investigating BBB leakage in a mouse model of ALS. The authors introduced a therapeutic intervention, the anticoagulant protease activated protein C (APC), which reduced the vascular leak of Hb-derived products into the spinal cords of transgenic mice expressing ALS-linked mutant SOD1 (SOD1G93A) and had the added effect of downregulating production of the mutant SOD1 protein in both neuronal and non-neuronal cells (Figure 1), resulting in slower disease progression and extension of survival time in this animal model.
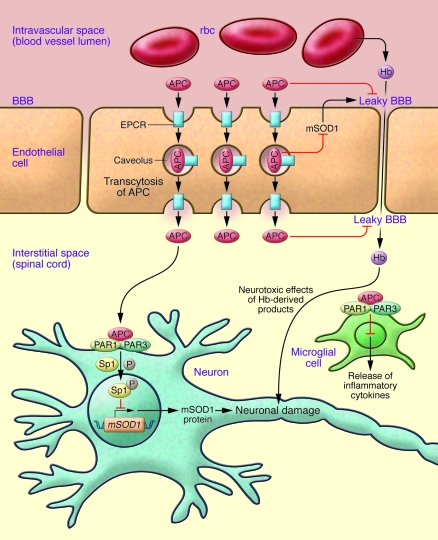
Figure 1. Potential mechanism for the protection of neural tissue in ALS.
APC binds to the EPCR located in caveolae. Caveolae translocate the endothelium and allow deposition of APC across the BBB, consistent with the results reported in this issue by Zhong et al. (4). Once across the BBB, APC can dissociate from EPCR and signal neuronal cells through activation of PAR1 in a process dependent on PAR3 (4), and this in turn increases the phosphorylation of the nuclear transcription factor Sp1, thereby downregulating the production of mutant SOD1 (mSOD1), a mediator of neuronal damage in this animal model of familial ALS. As described by Zhong et al. (4), APC can also provide neuroprotective effects by reducing leakage through the BBB, inhibiting release of inflammatory cytokines from microglia as well as lessening the oxidative damage to neurons by Hb products entering the extravascular space through the endothelium.
APC biology
APC is a serine protease generated from the inactive plasma zymogen, protein C, via proteolysis mediated by the complex of thrombin, thrombomodulin, and endothelial protein C receptor (EPCR) primarily on the surface of the endothelium. Originally identified primarily as an anticoagulant, both because of its potent in vitro anticoagulant activity and because of the dramatic thrombotic complications that arise in infants deficient in protein C (5), APC has recently drawn attention for its ability to protectively modulate a variety of disease processes, including human sepsis (6) and rodent models of Crohn disease (7), diabetic nephropathy (8), stroke (9), tumor metastasis (10), multiple sclerosis (11), and reperfusion injury (12). Most of these protective effects are primarily or potentially independent of the anticoagulant activity of APC. The common feature of most of these protective effects is dependency on the EPCR, found primarily on endothelial cells (13), and on protease-activated receptor 1 (PAR1), a 7-transmembrane G protein–coupled receptor expressed by most cell types (14). Thrombin is the prototypical activator of PAR1, and when it proteolytically activates PAR1, it elicits a variety of proinflammatory responses leading to expression of adhesion molecules on the cell surface and loss of endothelial cell barrier function. APC can also activate PAR1 (15), but APC-mediated activation gives rise to a different response, which protects against loss of barrier function and decreases proinflammatory responses through downregulation of NF-κB. The mechanisms underlying the pro- versus antiinflammatory effects of PAR1 activation remain the subject of investigation. One model from the literature (16) suggests that the location of APC bound to EPCR within caveolae (small invaginations of the plasma membrane) of luminal endothelial cells may help to dictate which G protein is linked to PAR1 and hence the nature of the downstream signaling. The ability of APC to elicit cellular responses that are cytoprotective and antiinflammatory through PAR1 activation may explain why APC is effective in preventing disease progression in many of the disease models mentioned above (6–12). Indeed, variants of APC with low anticoagulant activity have been generated that retain cytoprotective activity and remain protective in many animal models of disease, including sepsis (17). One of these variants was used in the present study by Zhong et al. (4). In most of these disease models, the target cells are located within the intravascular space, but several of the diseases cited above, including ALS, involve extravascular cells. Key to extravascular signaling is the fact that APC needs to reach the extravascular tissue from the intravascular space. Because EPCR binds APC reversibly and can be observed in caveolae (16), and because these organelles are known for their ability to transcytose the cell (18), it is likely that APC bound to EPCR located on the luminal surface of the endothelium is carried across the endothelium during transcytosis of the caveolae, resulting in the delivery of proteins either present in the caveolae or bound to other proteins present therein, resulting in the delivery of proteins that potentially activate PAR1 to the interstitial space (i.e., spinal cord; Figure 1), as reported by Zhong et al. in their current study (4). Then, signaling through PAR1— with the participation of PAR3 — would elicit cellular responses (4), including downregulation of mutant SOD1 through decreases in the levels of the nuclear transcription factor Sp1, manifested at least in part through increased phosphorylation of Sp1.
Novel features of the proposed APC signaling mechanism in ALS
The features of the signaling mechanism reported by Zhong et al. (4) differ from those seen previously because the target neuronal cells appear to lack EPCR, raising the issue of how this signaling occurs mechanistically. Several proteases can activate PAR1, but the presence of EPCR is usually required for the protective signaling observed through PAR1 activation. The previously described function of PAR3 is to bind thrombin (but not signal directly) and facilitate PAR4 activation, leading to mouse platelet activation (14). Therefore, to our knowledge, the PAR1-PAR3 interaction observed by Zhong et al. (4) is new and of unknown mechanistic significance. Perhaps activation of the PAR1-PAR3 complex on neuronal cells leads to the altered signaling specificity normally observed with APC activation of PAR1 on EPCR-expressing cells. A second issue is that the binding of APC to EPCR will concentrate APC near the cell surface, thus facilitating activation of PAR1; thus, an unresolved question arises as to why APC is an efficient activator of neuronal cells lacking EPCR.
Limitations of the mouse model of ALS
The concept of BBB dysfunction in ALS is not new, and was suggested in a few human studies of spinal fluid characteristics that demonstrated an elevated CSF albumin/serum albumin ratio in a large percentage of ALS patients (19, 20). However, enthusiasm for treating ALS patients with APC might be dampened by several factors, including the disappointing experience of trying to translate therapeutic successes in mutant SOD1 animals to ALS patients (21). Clearly, mice that express multiple copies of the mutant human SOD1 gene are not a faithful recapitulation of the genotype found in people carrying SOD1 mutations who express the mutant and wild-type SOD1 proteins at similar levels. Moreover, there is little evidence supporting the hypothesis that common mechanisms are involved in mutant SOD1–related ALS and sporadic ALS. Furthermore, pathological evidence of leakage of toxic substances through the BBB, as was demonstrated in mutant SOD1–ALS mice (2, 3), has not been reported in humans. Regardless, current treatments for ALS are woefully inadequate, and any therapeutic intervention that is well supported by a clear hypothesis and experimental evidence should be seriously considered for a clinical trial. APC, with its ability to downregulate the production of mutant SOD1, should perhaps be tried first in ALS patients with known SOD1 mutations. However, clinical trials in this very rare population are hindered by the limited numbers of potential participants. The bonus of APC may be its ability to reduce BBB leakage in ALS patients, and a trial of APC therapy for ALS would not only address its possible therapeutic effect, but perhaps provide data on the role of BBB leakage in ALS progression.
Potential promise and complications of APC therapy for ALS
A trial of APC therapy in people with ALS would not be without risk. A potential complication of treating nonthrombotic diseases with wild-type APC is the increased risk of bleeding due to APC’s anticoagulant functions, especially if the treatment requires prolonged infusion. Extensive structural and structure-function studies of APC led to the identification of APC variants (17) that retained signaling capacity but lost most of their anticoagulant function, thus reducing the risk of hemorrhage. These APC variants (17), used in the present study (4), provide a good example of the power of this approach. A concern with the use of mutant proteins in treating diseases, especially in the form of prolonged therapy, is the potential to elicit epitope spreading (22), in which antibodies to epitopes on the mutant proteins ultimately spread to react with the distinct epitopes on the native molecule, which in this case could lead to massive thrombotic complications.
The present study may have importance beyond its potential relevance to ALS. Zhong and colleagues illustrate that EPCR can be used to transport APC across the BBB (4). If their results from these mouse studies are reproducible in humans, either EPCR or derivatized APC could be used to transport therapeutic agents into the brain. Given that APC is a serine protease, it is relatively simple to design targeting agents to fill the active site reversibly and thus provide a delivery vehicle.
Acknowledgments
C.T. Esmon is an investigator of the Howard Hughes Medical Institute and is a recipient of a Transatlantic Network for Excellence in Cardiovascular Research grant from the Leducq Foundation. J.D. Glass is supported by grants from the NIH and from the Packard Center for ALS Research.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 119:3205–3208 (2009). doi:10.1172/JCI40682
See the related article beginning on page 3437.
References
- 1. Mitsumoto, H., Chad, D.A., and Pioro, E.P. 1998.Amyotrophic lateral sclerosis. F.A. Davis. Philadelphia, Pennsylvania, USA. 480 pp. [Google Scholar]
- 2.Garbuzova-Davis S., et al. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;1157:126–137. doi: 10.1016/j.brainres.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Zhong Z., et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong Z., et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esmon C.T., Schwarz H.P. An update on clinical and basic aspects of the protein C anticoagulant pathway. Trends Cardiovasc. Med. 1995;5:141–148. doi: 10.1016/1050-1738(95)00054-D. [DOI] [PubMed] [Google Scholar]
- 6.Bernard G.R., et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 7.Scaldaferri F.F., et al. Crucial role of the protein C pathway in governing microvascular inflammation in inflammatory bowel disease. J. Clin. Invest. 2007;117:1951–1960. doi: 10.1172/JCI31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isermann B., et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T., et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 10.Van Sluis G.L., et al. Endogenous activated protein C limits cancer cell extravasation through sphingosine-1-phosphate receptor 1 mediated vascular endothelial barrier enhancement. Blood. 2009;114:1968–1973. doi: 10.1182/blood-2009-04-217679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han M.H., et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1083. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani A., Okajima K., Uchiba M., Noguchi T. Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood. 2000;95:3781–3787. [PubMed] [Google Scholar]
- 13.Laszik Z., Mitro A., Taylor F.B., Jr., Ferrell G., Esmon C.T. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation. 1997;96:3633–3640. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi-Matsui M., et al. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 15.Riewald M., Petrovan R.J., Donner A., Ruf W. Activated protein C signals through the thrombin receptor PAR1 in endothelial cells. . J. Endotoxin Res. 2003;9:317–321. doi: 10.1179/096805103225002584. [DOI] [PubMed] [Google Scholar]
- 16.Bae J.S., Yang L., Manithody C., Rezaie A.R. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosnier L.O., Zlokovic B.V., Griffin J.H. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh D.P., Tan X.Y., Oh P., Schnitzer J.E. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1996–2001. doi: 10.1073/pnas.251662398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apostolski S., et al. Serum and CSF immunological findings in ALS. Acta Neurol. Scand. 1991;83:96–98. doi: 10.1111/j.1600-0404.1991.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi A., Abbruzzese G., Arata L., Cocito L., Vische M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J. Neurol. 1984;231:75–78. doi: 10.1007/BF00313720. [DOI] [PubMed] [Google Scholar]
- 21.Glass J.D., Benatar M., Polak M. Selecting promising ALS therapies in clinical trials. . Neurology. 2007;68:1545–1546. doi: 10.1212/01.wnl.0000265319.46474.f6. [DOI] [PubMed] [Google Scholar]
- 22.James J.A., Gross T., Scofield R.H., Harley J.B. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B’-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J. Exp. Med. 1995;181:453–461. doi: 10.1084/jem.181.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]